Abstract
Objective: Adipose tissue is a robust source of adipose-derived stem cells (ADSCs) that may be able to provide secreted factors that promote the ability of wounded tissue to heal. However, adipocytes also have the potential to dedifferentiate in culture to cells with stem cell-like properties that may improve their behavior and functionality for certain applications.
Approach: ADSCs are adult mesenchymal stem cells that are cultured from the stromal vascular fraction of adipose tissue. However, adipocytes are capable of dedifferentiating into cells with stem cell properties. In this case study, we compare ADSC and dedifferentiated fat (DFAT) cells from the same patient and fat depot for mesenchymal cell markers, embryonic stem cell markers, ability to differentiate to adipocytes and osteoblasts, senescence and telomerase levels, and ability of conditioned media (CM) to stimulate migration of human dermal fibroblasts (HDFs).
Innovation and Conclusions: ADSCs and DFAT cells displayed identical levels of CD90, CD44, CD105, and were CD34- and CD45-negative. They also expressed similar levels of Oct4, BMI1, KLF4, and SALL4. DFAT cells, however, showed higher efficiency in adipogenic and osteogenic capacity. Telomerase levels of DFAT cells were double those of ADSCs, and senescence declined in DFAT cells. CM from both cell types altered the migration of fibroblasts. Despite reports of ADSCs from a number of human depots, there have been no comparisons of the ability of dedifferentiated DFAT cells from the same donor and depot to differentiate or modulate migration of HDFs. Since ADSCs were from an obese diabetic donor, reprogramming of DFAT cells may help improve a patient's cells for regenerative medicine applications.

Denise R. Cooper, PhD
Introduction
Potential applications of adipose-derived stem cells (ADSCs) in regenerative medicine have been demonstrated conceptually by numerous investigations. No marker specifically identifies an ADSC, especially those that have been passaged in culture. Despite this, ADSC still posses the potential to differentiate into multiple cell types depending upon culture media additions.1 Mature adipocytes from the same patient sample can also be a source of stem-like cells that are derived by dedifferentiation using the ceiling culture method.1 These cells, called dedifferentiated fat (DFAT) cells, are a candidate source of stem cells like ADSCs since they can differentiate into multiple cell types. Early studies suggest that DFAT cells have a partial stem cell signature less robust than ADSCs.2 However, these studies reported on DFAT cells and ADSCs from early passages3,4 and did not compare DFAT cells from the same patient and fat depot.
Stem cell markers and function in vitro have not been demonstrated for ADSCs and DFAT cells isolated from the same patient's lipid depot. Presently, we derived both cells from subcutaneous fat from an obese diabetic patient. A subset of the embryonic stem cell (ESC) and lineage markers were characterized following multiple passages in culture. The ability of cells to differentiate to adipocytes and to osteoblasts, and the capacity of conditioned media (CM) from cells to alter migration of human dermal fibroblasts (HDFs), reflecting the potential for wound healing, was compared. We hypothesized that the DFAT cells would be comparable in adipogenic and osteogenic potential in culture as the ADSC due to the reprogramming.
Clinical Problem Addressed
The potential for CM from either ADSCs or DFAT cells to impact cell migration in cells undergoing wound healing was compared in ADSCs and DFAT cells from the same fat depot of an obese diabetic patient to determine whether functional changes occurred during the reprogramming of adipose cells.
Materials and Methods
Adipose samples
Subcutaneous adipose tissue, collected as a by-product at the site of the incision, was harvested during Roux-en-y bypass surgery for weight loss from a human adult female patient at Tampa General Hospital, Tampa, Florida. The de-identified sample was obtained under an Institutional Review Board–approved exemption (# 108360, University of South Florida), and was transported to the laboratory and processed within 24 h of receipt.
Preparation of adipose stromal vascular fraction
The tissue was washed with modified phosphate-buffered saline (PBS) containing 5% penicillin/streptomycin/amphotericin B (P/S/A). Next, it was placed in sterile tissue culture plates with 0.075% collagenase Type 1 (Worthington) in modified PBS. Single-cell suspensions were prepared by mincing the tissue into small pieces using scalpels and pipetting several times to further disrupt it. Pieces were incubated for 2 h, 37°C, with shaking to facilitate digestion. The collagenase activity was stopped by adding 5 mL of alpha–minimum essential medium (α-MEM) complete media with 20% heat inactivated fetal bovine serum (FBS; Atlanta Biological). Disaggregated tissue was pipetted up and down to promote a cell suspension, and then filtered through a 100-μm cell strainer (BD Falcon) with several rinses. Mature adipocytes were collected by centrifugation in a 50-mL conical tube (400 g, ambient temperature, 5 min) and removed as the supernatant. The cell pellet, containing stromal vascular cells, was vigorously shaken to mix stromal cells. This completes the separation of the stromal vascular fraction (SVF) from primary adipocytes. The centrifugation step is repeated and the collagenase solution above the pellet is aspirated off without disturbing the SVF. The pellet was resuspended in 1 mL of the erythrocyte lysis buffer (Easy Sep™ 10× RBC Lysis Buffer #20120, Stem Cell Technologies) for 10 min at ambient temperature, and washed in 20 mL of PBS with 2% P/S/A before centrifugation, 300–500 g, 5 min. The supernatant was aspirated and the cell pellet resuspended in a 3 mL stromal medium (α-MEM; Mediatech) with 20% FBS, 1% l-glutamine (Mediatech), 1% P/S/A, and the cell suspension filtered through a 70-μm cell strainer (BD Falcon) with several rinses in the stromal medium. SVF cells were plated for initial cell culture at 37°C in an atmosphere of 5% CO2 in humid air in the stromal medium with 100 mg/dL glucose. The medium was replaced every 2 days. Subconfluent cells were passaged by trypsinization. For experiments, we used 5–8 passage ADSCs from a female donor (age 45.6 years, BMI 48 kg/m2) with diabetes mellitus.
Establishment of dedifferentiated adipocyte cells
Adipocytes, obtained by the top layer of cells following centrifugation of the digested fat tissue, were placed in 75-mm culture flasks filled with the α-MEM with 10% FBS and a modified ceiling culture method1 was used to isolate adhered adipocytes at the end of 2 weeks followed by another 2–3 weeks in bottom culture (or longer) in hypoxia to improve the dedifferentiation process.
Conditioned media
The medium for cell migration assays was collected after culture of cells for 48 h in a serum-free defined medium, the mesenchymal stem cell basal medium (MSC-BM-CD™ from Lonza #00190620). After collection, CM were frozen at −80°C in 1.5-mL aliquots.
Stem cell markers using RT-qPCR
Total RNA was extracted from DFAT cells and ADSCs using RNABee reagent according to the manufacturer's instructions. RNA was primed with oligo(dT) and reverse transcribed using Superscript II (Life Technologies) according to the manufacturer's directions. Quantitative (q)PCR was carried out using 50 ng cDNA, Maxima SYBER Mix (Fermentas) primer mix, and RNAse-free water. Quantification of the PCR fragment was performed by the 7900 Real Time PCR System (Applied Biosystems). Relative quantification was by ΔΔCT with β-actin for normalization. The calibrator was the ADSC sample in all cases. The level of the gene expression was calculated using 2−ΔΔCT. Product specificity was verified by dissociation curve analysis. Primers have been described previously.5
Multilineage differentiation
Multipotency of DFAT cells and ADSCs was assayed in vitro. Differentiation to osteoblasts was performed by culturing cells in the osteoblast differentiation medium (DM) for 4 weeks with changes every 5 days (ZenBio™). The medium contained Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 (1:1, v/v), FBS, β-glycerophosphate, ascorbate-2-phosphate, dexamethasone, 1,25 (OH)2 Vitamin D3, penicillin, and streptomycin. Differentiation to adipocytes was performed by culturing cells in adipocyte DM for 12 days (with changes in the medium every 5 days) (ZenBio). Adipocye DM-2 contained DMEM/Ham's F-12 (1:1, v/v), HEPES pH7.4, FBS, biotin, pantothenate, human insulin, dexamethasone, isbutylmethylxanthine, PPARg agonist, penicillin, streptomycin, and amphotericin B. Positive staining of Alizarin Red (2% Alizarin Red Solution [CM-0058]; Lifeline Cell Technology) for osteogenesis. The cells were washed once with PBS and fixed with phosphate-buffered formalin for 20 min. Fixed cells were washed once with distilled water and subsequently stained with 1% Alizarin Red S dissolved in distilled water for 5 min. The remaining dye was washed out with distilled water, and the cells were washed once more. Images of the stained cells were captured using a light microscope and its image analyzing software. Afterward, for quantification of staining, 800 μL 10% (v/v) acetic acid was added to each well, and the plate was incubated at room temperature for 30 min with shaking. The monolayer, now loosely attached to the plate, was then scraped from the plate with a cell scraper (Fisher Life Sciences) and transferred with 10% (v/v) acetic acid to a 1.5-mL microcentrifuge tube with a wide-mouth pipette. After vortexing for 30 s, the slurry was overlaid with 500 μL mineral oil, heated to 85°C for 10 min, and transferred to ice for 5 min. Care was taken at this point to avoid opening of the tubes until fully cooled. The slurry was then centrifuged at 20,000 g for 15 min and 500 μL of the supernatant was removed to a new 1.5-mL microcentrifuge tube. Then, 200 μL of 10% (v/v) ammonium hydroxide was added to neutralize the acid. In some cases, the pH was measured at this point to ensure that it was between 4.1 and 4.5. Aliquots (150 μL) of the supernatant were read in triplicate at 450 nm.6 Oil Red O for adipogenesis was used according to the manufacturer's instructions. The medium was removed from the culture dish and 10% formalin was incubated for 5 min at RT. The same volume of fresh formalin was replaced and cells were fixed for at least 1 h. After removing formalin, wells were washed with 60% isopropanol and dried completely. The Oil Red O working solution was added for 10 min. The solution was removed and monolayers were washed 4×. Images were acquired. Oil Red O was eluted by adding 100% isopropanol and incubating 10 min. The soluble Oil Red O was transferred to 1.5-mL tubes and OD at 490 nm was read using isopropanol for the blank. Images of differentiated cells were captured brightfield and phase contrast on a Nikon Eclipse fluorescent microscope using 10× or 20× objective with a 0.38 μM/pixel.
Senescence
ADSCs and DFAT cells were grown in 35-mm plates. Media were removed and plates rinsed with 1× PBS. Cells were fixed for 15 min at room temperature with a 1× fixative solution (20% formaldehyde, 2% glutaraldehyde in 10× PBS). After rinsing, 1 mL of the β-galactosidase staining solution (400 mM citric acid/sodium phosphate, 1.5 M NaCl, 20 mM MgCl2, 500 mM potassium ferrocyanide, and 20 mg/mL X-gal in dimethyl formamide) was added to the cells and incubated overnight in a dry incubator.7 Images of color on cells were captured as described above.
Telomerase levels
Whole cell lysates were collected and Western blot analysis was performed as described.8 The membrane was immunoblotted using an antitelomerase antibody (Cell Signaling). After incubation with anti-rabbit IgG-HRP, enhanced chemiluminescence (Pierce) was used for detection.
Flow cytometry
Immunophenotypical analysis of cultured cells was performed using the FITC-, PE-, or APC-conjugated monoclonal antibodies against CD31, CD34, CD44, CD45, CD73, CD90, CD105, CD106, and CD117. Cells were detached using the EDTA buffer, washed, and resuspended at a concentration of 106 cells/mL. Cells were incubated at 4°C for 10 min in PBS with 10% FBS. Cells were centrifuged for 5 min at 200 g. The cell pellet was resuspended in the binding buffer (PBS/2% FBS/0.01% sodium azide) followed by incubation with the specific mAbs at 4°C for 30 min, then washed with the binding buffer, and resuspended in 0.5 mL of the same buffer and analyzed by flow cytometry (BD Accuri C6).
Cell migration
Primary HDFs were isolated from skin obtained from consenting young adults (ages 20–39 years) undergoing elective surgery under an IRB approved protocol. Discarded skin was placed in a decontaminating solution consisting of DMEM GlutaMax with 4.5 g/L d-glucose, 25 mM HEPES (Gibco®; Life Technologies), 20 μg/mL gentamicin reagent, and 1% Pen/Strep and incubated at 4°C for at least 1 h or up to 24 h. Skin was then rinsed 3× in 1× Dulbecco's PBS (DPBS), trimmed of fat and then cut into 3–5-mm-wide strips, which were incubated in 1.83 U/mg dispase (used 0.010 g/mL in sterile DPBS) at 4°C overnight on a rotator. The epithelium was removed from the dermis and the dermis was minced with sterile scissors and further digested in the AccuMax solution (Sigma) for 1–2 h at 37°C. The growth medium (DMEM with 10% FBS and 1% Pen/Strep) was added to the digested dermis and the solution was put through a 70-μm cell strainer, collected, centrifuged, and the cell pellet resuspended in a fresh growth medium. The cell (HDF) suspension was plated and HDF colonies appeared over the next 10 days in culture at 37°C, 95% air, and 5% CO2. Subcultured HDFs were used for experiments between passage 2 and 7.
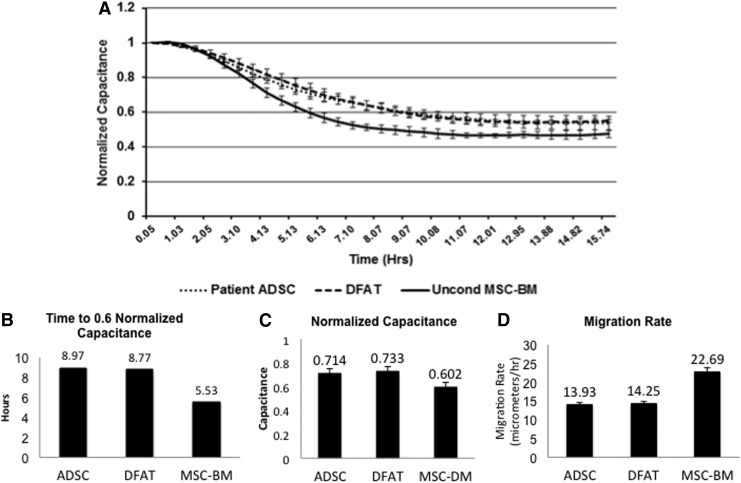
Electric Cell-substrate Impedance Sensing- (ECIS™ model Zθ [Applied Biophysics]) was used to assay the migration of HDFs exposed to 48 h CM obtained from ADSCs, DFAT cells, or unconditioned MSC-BM (Lonza). HDFs were subcultured onto 8W10E ECIS arrays (Applied Biophysics) that were previously incubated with 10 mM cysteine in ddH2O and stabilized with growth media (DMEM, 10% FBS, and 1% Pen/Strep). At 95% HDF confluence, the medium was replaced with a low serum medium (2% FBS) for 18 h. HDFs were then incubated in 10 μg/mL Mitomycin C (Sigma) in low serum media for 2 h to prevent HDFs from proliferation. HDFs were then incubated in CM (n=4 wells per CM type) or unconditioned MSC-BM and the arrays were placed on the ECIS platform and electrodes were checked for initial cell resistance, impedance, and capacitance readings before applying an alternating current (AC) of 1 μA at multiple frequencies (62.5 to 64,000 Hz) to the 250-μm electrodes for 90 min. To uniformly wound the monolayers on the 250-μm electrodes, a current of 6,000 μA at 40,000 Hz was applied for 20 s causing the cells to die and lift from the surface of the electrode. AC (1 μA, multiple frequencies) was continuously applied to the electrodes to measure capacitance (nF), representing cell coverage/confluence and follow wound closure for 20 h at 37°C, 95% air and 5% CO2. Data are presented as normalized capacitance with mean±SD (Fig. 5). At zero time, a capacitance of 1 signifies 0 coverage/confluence. Data were collected using ECIS Software version 1.2.104.0 PC (7 Aug 2012).
Figure 5.
Measurement of wound closure by electric cell-substrate impedance sensing (ECIS™). (A) Normalized capacitance over a time course for HDFs exposed to 48 h of conditioned media (CM) from ADSCs and DFAT cells, or unconditioned mesenchymal stem cell basal medium (MSC-BM). A capacitance of 1 indicates 0 coverage/confluence at the time of wounding (0). Data are represented as mean±SD (n=4 wells of HDFs per CM group). (B) The time taken for HDFs to reach a normalized capacitance of 0.6. (C) Normalized capacitance values of the HDFs at 5.5 h after wounding. (D) The HDF migration rate as calculated by v=r/t, where r=radius of the electrodes (125 μm) and t=time (hours to reach a normalized capacitance of 0.6). DFAT cells were passage 5 and ADSC were passage 7.
Statistics
Data are mean±SD. Statistical comparisons between groups were performed using the Student's t-test. A value of p<0.05 was considered statistically significant unless noted otherwise.
Results
Isolation and appearance of ADSCs and DFAT cells after 5–7 passages
To isolate ADSCs, adipose tissue was processed as described and the fat cells from the same biopsy were subjected to ceiling culture for 6 weeks to DFAT cells. We selected passages (P)5–7 of cells7 as this represented a homogeneous culture of both cell types. DFAT cells required more time for reprogramming, so they are a lower passage than ADSCs. In Fig. 1, light microscopy of plastic adherent subcutaneous ADSCs and DFAT cells demonstrated undifferentiated fibroblast-like morphology growing in directional patterns.
Figure 1.
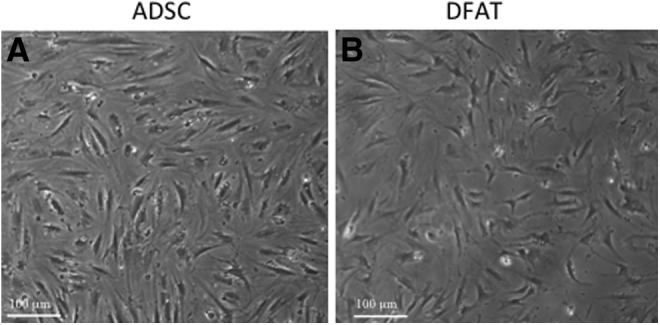
Morphology of adipose-derived stem cells (ADSCs) and dedifferentiated fat (DFAT) cells from subcutaneous fat. (A) Morphology of ADSCs in culture (P7) at 4× under light microscopy with phase contrast. (B) Morphology of DFAT cells (P5) at 4× under light microscopy with phase contrast. DFAT cells were passage 5 and ADSCs were passage 7.
Analysis of expression of pluripotency-associated gene cells by RT-qPCR
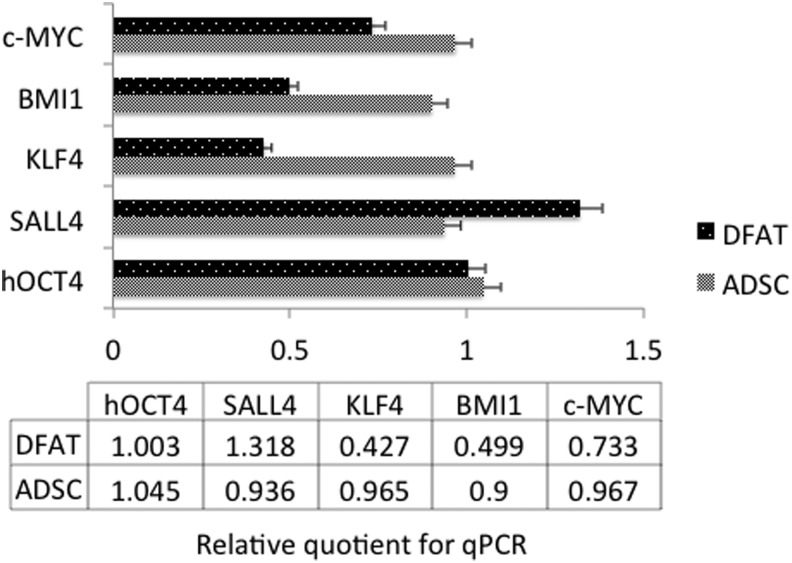
Pluripotency-associated genes are important for the maintenance of stem cells. To compare their pluripotent states, we performed RT-qPCR for four ESC markers.1 DFAT cells and ADSCs expressed similar levels of Oct4 and SALL4, however, ADSCs expressed slightly higher levels of BMI1 and KLF4 (Fig. 2).
Figure 2.
Comparison of embryonic stem cell markers between ADSCs and DFAT cells from subcutaneous fat. The relative amounts were detected by quantitative PCR as described in Materials and Methods. DFAT cells were passage 5–6 and ADSCs were passage 7–8. **p<0.05 vs. ADSC group.
Flow cytometry
DFAT cells and ADSCs from the same patient and fat depot, subcutaneous, demonstrated similar surface markers in culture (Table 1). Both cell types were negative for CD34, a MSC-associated marker, but were positive for CD44, CD73, CD90, and CD105, also MSC-associated markers. DFAT cells were negative for CD106, while ADSCs expressed extremely low levels. The only marker showing any difference between the two was CD31 with ADSCs expressing a higher percentage than DFAT cells. This indicates that DFAT cells re-established multipotency during the dedifferentiation process, and that they resembled MSCs.
Table 1.
Comparison of the surface antigens of dedifferentiated fat cells and adipose-derived stem cells from subcutaneous depot of female diabetes mellitus patient
| Marker | Other Names | sc-DFAT | sc-ADSC |
|---|---|---|---|
| CD31 | PECAM-1 | 1.1% | 8.3% |
| CD34 | L-selectin ligand | Negative | Negative |
| CD44 | Pgp-1 | 89.1% | 87.9% |
| CD45 | LCA | 0.1% | 1.8% |
| CD73 | eNT | 88.1 | 89.1 |
| CD90 | Thy-1 | 99.4% | 98.5% |
| CD105 | Endoglin | 17.9% | 19% |
| CD106 | VCAM-1 | Negative | 1.1% |
| CD117 | c-Kit | 0.3% | 0.3% |
Flow cytometry analysis of P5-7 cells as indicated were stained with CD31, CD34, CD44, CD45, CD73, CD90, CD105, CD106, and CD117 cell surface markers. Results are percentage of positive cells and are expressed as mean for three separate experiments. DFAT cells were passages 5–6 and ADSCs were passages 7–8. Adipose-derived stem cells from an obese patient (BMI: 47.66 kg/m2) were compared from two different fat depots (subcutaneous and omental) with regard to expression of embryonic stem cell factors and surface markers. Additionally, the dedifferentiated adipocytes from both depots were analyzed. Although sc-ADSCs and sc-DFAT cells were similar in their expression of surface markers and stem cell markers, the om-ADSCs and om-DFAT cells showed more variability with regard to both types of markers. Sc-ADSCs were not as effective in regard to adipocyte differentiation or osteoblast transdifferentiation as the sc-DFAT cells. Similar trends are being seen for om-ADSCs and om-DFAT cells. The reversal of adipogenesis capacity with dedifferentiation suggests that this model may be good for studying the effects of weight loss on the multipotency of adipocyte stem cells.
Multilineage differentiation ability
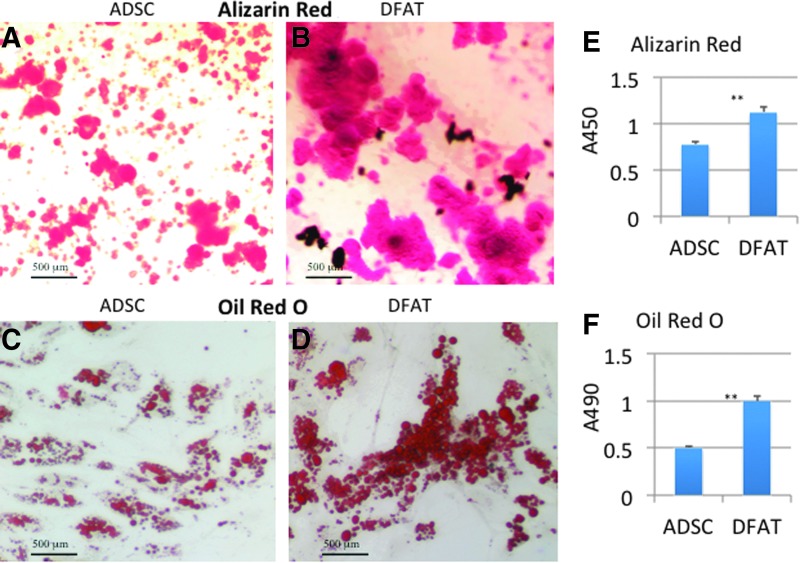
There are reports of DFAT cells differentiating into multiple mesenchymal lineages, including osteogenic, adipogenic, chondrogenic, and myogenic lines.1,2 Osteogenic differentiations of DFAT cells can be induced by all-trans retinoic acid, an analog of retinol that interacts with bone morphogenetic proteins to inhibit adipogenesis and enhance osteogenesis.9 Here we used DM from ZenBio (OB-1). Cells were placed in the medium for 4 weeks before staining with Alizarin Red S for calcified matrix deposition. Both ADSCs and DFAT cells accumulated the dye (Fig. 3A, B). DFAT cells accumulated larger deposits of Alizarin Red than ADSCs by >50% as shown in Fig. 3E.
Figure 3.
Alizarin Red and Oil Red O staining of ADSCs and DFAT cells from subcutaneous fat. (A) ADSCs were cultured under osteogenic conditions for 4 weeks; Alizarin Red staining for matrix mineralization and quantification was performed. (B) DFAT cells were cultured under osteogenic conditions for 4 weeks; Alizarin Red staining for matrix mineralization and quantification was performed as described. (C) ADSCs were stained with Oil Red O to detect intracellular lipid accumulation. The lipid retained dye was eluted in isopropanol and quantified by spectrophotometric analysis. The dye is visualized at 10× under light microscopy with brightfield. (D) DFAT cells were stained as above and the lipid retained dye quantified as detailed. The differentiation was performed on four different dishes of cells with similar results. DFAT cells were passage 5 and ADSCs were passage 7. **p<0.05 vs. ADSC group. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
DFAT cells proliferated for numerous passages without detectable establishment of lipid droplets as reported by others.2 However, redifferentiation of DFAT cells toward adipocytes occurred after 5–7 days of DM with significant lipid accumulation observed after 2 weeks. ADSCs were slower to differentiate to adipocytes. Figure 3C and D shows the appearance of the droplets in both cell types. Analysis of the Oil Red O indicated that DFAT cells accumulated 50% more dye than the ADSCs (Fig. 3F).
Senescence and telomerase levels
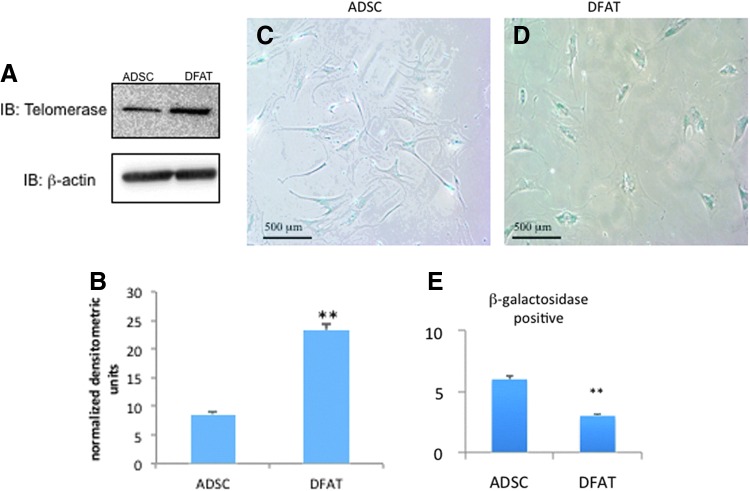
During DNA synthesis and repeated cell division, telomeres shorten as a result of the incomplete replication of linear chromosomes. Telomeres, a complex of guanine-rich repeat sequences with associated proteins, protect the eukaryotic chromosome ends against chromosomal fusion, recombination, and terminal DNA degradation. In ESCs, telomerase maintains the telomere length and cellular immortality. In cultured ADSCs, telomerase is reported to be both present and absent.10,11 DFAT cells have a higher telomerase activity than ADSCs.1 Our data corroborate that DFAT cells expressed 2.5-fold more telomerase than ADSCs (Fig. 4A, B).
Figure 4.
Telomerase levels and senescence in ADSCs and DFAT cells. (A) Protein from 35-mm culture dishes was isolated and subjected to Western blot analysis. The analysis was repeated three times with similar results. (B) DFAT cells expressed 2.5-fold more telomerase. (C, D) Cells were stained for β-galactosidase as described in methods to detect senescence as labeled. Cells were then visualized under light microscopy at 10×. (E) The proportion of cells staining positive was calculated by counting five areas of the same size from each field. The graph is an average of β-gal-positive cells for each field. DFAT cells were passage 5 and ADSCs were passage 7. **p<0.05 vs. ADSC group. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Cellular senescence occurs when normal diploid cells lose the ability to divide in vitro. The β-galactosidase activity is correlated with senescence. Detection of senescence-associated (SA) β-gal activity is visualized as a blue stain in cells.7 Here a larger proportion of cells positive for SA β-gal were in ADSCs than DFAT cells (Fig. 4C, D). When cells were scored for blue staining relative to the cell number, there were 50% more positive ADSCs than DFAT cells (Fig. 4E).
DFAT cell and ADSC CM altered migration of HDFs after wounding at similar rates
To further characterize function, we utilized a uniform wound-healing assay measured by ECIS HDFs, exposed to CM from ADSCs and DFAT cells, was wounded by uniform electroporation, and coverage/confluence was measured as normalized capacitance (Fig. 5A). At wounding (0 time), all groups have a normalized capacitance of 1, which indicates 0 coverage/confluence. An AC of 1 μA at multiple frequencies was then applied and capacitance was monitored for 20 h. HDFs in the unconditioned medium (MSC-BM) migrated faster over time as indicated by a decreased time to reach a normalized capacitance of 0.6 (5.5 vs. 8.97 and 8.77 h for ADSC and DFAT cell CM, respectively) (Fig. 5B), and by an increased calculated migration rate of 22.59 versus 13.93 and 14.25 μm/h (Fig. 5D). A capacitance value of 0.6 was chosen as the ADSC and DFAT cell CM groups both stabilized near this value. Capacitance values at 5.5 h were significantly higher for ADSC (0.714, p=0.001) and DFAT cell (0.733, p=0.029) CM groups indicating a lower HDF coverage of the electrodes compared to HDFs (0.602) in the unconditioned MSC-BM (Fig. 5C).
Discussion
In the past decade, the use of ADSCs as a source for preclinical application has increased exponentially. Two fractions have been evaluated extensively. First, SVF cells containing a variety of cell types, including B and T lymphocytes, endothelial cells, fibroblasts, macrophages, pericytes, preadipocytes, and related populations have been described.12 Second, the culture of SVF cells on plastic surfaces yields an adherent subpopulation of ADSCs. These cells are more homogeneous based on surface markers and are similar to bone marrow mesenchymal stem cells.13 Mature adipocytes are considered a terminally differentiated lineage without the ability to proliferate. Ceiling culturing using the buoyancy of adipocytes gives rise to the plasticity of adipocytes to dedifferentiate. These reprogrammed fibroblast-like cells resume proliferation and have multipotent abilities.
Here we compared ESC markers in later passages of ADSCs and DFAT cells from the same patient's subcutaneous fat. OCT4 and SALL4 levels were similar, whereas ADSCs expressed higher levels of BMI1 and KLF4 that DFAT cells. These markers are commonly expressed in human stem cells capable of self-renewal. BMI1 is related to senescence regulation and self-renewal. Overexpression of BMI1 promoted a proliferative advantage and extended murine embryonic fibroblast (MEF) lifespan, and immortalized MEFs.14 BMI1 was lower in DFAT cells and indicates that other factors must be related to the increased telomerase levels we noted. SALL4 is required for stabilization of ESCs.15 DFAT cells had more SALL4 mRNA, which may reflect increased stability. In ESCs, Klf4 was a good indicator of the stem-like capacity. This transcription factor interacts with the CREB-binding protein.16 Klf4 is present at the promoter of an enzymatic subunit of telomerase, where it forms a complex with β-catenin where it is required for accumulation of β-catenin.17 Whether Klf4 mRNA is related to the telomerase activity here is not known.
DFAT cells re-establish MSC markers during the dedifferentiation process as reported.2 The percentages of cells staining positive for seven MSC markers were the same for DFAT cells and ADSCs. Coexpression of CD73, CD90, CD40, and CD44 was high and CD31, CD34, CD45, and CD106 was low. The finding that both cells expressed identical levels of markers after roughly the same time in culture indicates the cells share common phenotypes that are stable. However, DFAT cells are known to remain stable for well over 30 passages.2 The absence of CD31, CD34, and CD106 was anticipated as these markers are low in cells that have been passaged several times. CD73 and CD44 are markers related to MSCs and are highly expressed as are CD90 and CD105 in subcutaneous ADSCs.18 CD117 should be low as we noted. Our finding that positive markers are maintained at high levels during passage and with dedifferentiation shows an enrichment of stem cell populations with culture.
DFAT cells were more osteogenic than ADSCs. DFAT cells were also more adipogenic, with significant lipid accumulation observed after 14 days. ADSCs accumulated lipids at a slower rate, demonstrating only 50% of the Oil Red O that DFAT cells accumulated. Hence, the differentiation potential of DFAT cells was greater than the same patient's ADSCs. This may reflect the fact that the patient was obese and diabetic, and ADSCs from obese patients are less likely to differentiate.19–22 However, increasing stem cell potential for adipocyte and osteoblast formation by dedifferentiating mature adipocytes to DFAT cells is a novel finding. It is mimetic of the effect of weight loss in postgastric bypass subjects.21
Senescence was also greater in ADSCs than in DFAT cells, indicating that the DFAT cells have a greater self-renewal capacity as well as improved stem cell qualities as assessed by telomerase levels. Obese adipose tissue and preadipocytes show an increased SA β-gal activity.23 This suggests that a senescent-like stage occurs in differentiating adipocytes from obese donors. Our data suggest that DFAT cells overcome this and show less senescence. This may imply that reprogramming cells through dedifferentiation confers protection against cellular senescence, which could protect them from a proinflammatory phenotype. Hence, DFAT cells behave more like lean preadipocytes than ADSCs from the obese patient.
ADSCs secrete paracrine mediators that influence cell behavior, with the potential to promote wound healing.24,25 Specifically, CM from cultured hADSCs have been shown to increase collagen synthesis, enhance fibroblast migration, reduce the wound area, and to have potent antioxidant activity that may protect the surrounding cells from oxidative stress.24,26,27 However, the source of ADSCs may contribute to their functionality.12 Most studies use ADSCs derived from young healthy animals or from young healthy female humans, applied to tissue culture models or normal mice. El-ftesi et al. demonstrated that ADSCs from aged and diabetic mice have a decreased ability to stimulate neovascularization and that this capacity is further blunted when the ADSCs are cultured in hypoxia.28 Cianfarani et al., report that diabetes altered and diminished the function of ADSCs in wound assays.29 Our study is unique in that we isolated the ADSCs and DFAT cells from an obese, diabetic donor and after characterization for stem cell markers, applied CM to primary human fibroblasts. Using the highly sensitive ECIS system to measure migration, we showed that this donor source is indistinguishable with regard to whether the CM was from DFAT cells or ADSCs. Comparison to ADSC and DFAT cell CM from normal weight and nondiabetic donors is currently under investigation, but beyond the intent of this study.
Innovation
The primary innovation of this study was the comparison of cells from the same donor. Despite studies of ADSC from other sources, there are no comparisons of the ability of dedifferentiated adipocytes from the same donor to alter migration of HDFs. CM from ADSCs or DFAT cells of the same donor stimulated cell migration from a young donor at the same rate in an assay that measures the ability of secreted factors to maintain the cell proliferation.10 Other studies tested different media for ADSC expansion, and then compared proliferation and morphology.30 This study used a fully defined medium with two cell preparations in a defined migration assay. Both cell types are likely to secrete tumor necrosis factor-alpha, which stimulates cell migration through mechanisms involving IL-6 and IL-8. The study is innovative in that it suggests that dedifferentiated adipocytes are an alternative source of ADSCs for cell therapy and many of the DFAT cell properties are improved with the reprogramming.
Key Findings.
Our case study details differences and similarities between subcutaneous ADSCs and DFAT cells from an obese diabetic donor:
• Dedifferentiated fat cells (DFAT) demonstrated homogeneity with similar stem cell and surface markers to ADSCs after multiple passages.
• The most notable differences were in increased telomerase levels and an increased ability to redifferentiate and transdifferentiate to adipocytes and osteoblasts by DFAT cells versus ADSCs.
• There was similarity in the ability of CM from both cell types to alter cell migration of HDFs, a parameter important for wound healing.
• Since ADSCs were from an obese diabetic donor, improvements of dedifferentiated adipose cells noted with the reprogramming process may provide a means of improving a patient's cells for regenerative medicine applications.
Abbreviations and Acronyms
- AC
alternating current
- ADSC
adipose-derived stem cell
- CM
conditioned media
- DFAT
dedifferentiated fat cells
- DM
differentiation medium
- ECIS™
Electric Cell-substrate Impedance Sensing
- ESC
embryonic stem cell
- FBS
fetal bovine serum
- HDF
human dermal fibroblast
- MEF
mouse embryonic fibroblast
- MEM
minimum essential medium
- PBS
phosphate-buffered saline
- SVF
stromal vascular fraction
Acknowledgments and Funding Sources
This work was supported by the Medical Research Service of the Department of Veterans Affairs Merit Review (D.R.C., N.A.P., L.J.G., P.B.), and NIH grant RO1 NIDDK054393 (D.R.C.). The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
James E. Watson provided technical assistance. His ability to isolate, differentiate, and dedifferentiate fat cells, and collect media was essential for this study. Niketa A. Patel's laboratory provided assays of senescence, telomerase, ESC markers, and microscopy in addition to help in writing. Gay Carter performed flow cytometry and RT-qPCR for ESC markers. Andrea Moor, basic scientist, performed the cell migration assays and assisted in writing. Rekha Patel quantified cell senescence and performed microscopy, Western blots, and quantifying differentiation. Tomar Ghansah, basic scientist, developed this method for isolating stromal vascular cells from human fat and initial basic flow cytometry antibody panel for immunophenotyping of ADSCs. Abhishek Mathur, surgery fellow, obtained tissue and assisted in deriving stromal vascular cells. Michel M. Murr, surgeon, performed the bypass surgery and provided guidance for the study. Paula Bickford, basic scientist, supported flow cytometry and provided suggestions for the study. Lisa J. Gould, plastic surgeon, suggested the use of CM for wound healing and use of adipocyte-derived stem cells from older, obese patients as a source. She assisted in writing. Denise R. Cooper, basic scientist, conceptualized the study of using cells from the same fat depot of a donor to dedifferentiate and compare characteristics and function. She planned the experimental design and wrote the manuscript.
References
- 1.Gao Q, Zhao L, Song Z, and Yang G: Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol Biol Rep 2012; 39:5791. [DOI] [PubMed] [Google Scholar]
- 2.Shen JF, Sugawara A, Yamashita J, Ogura H, and Sato S: Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues. Int J Oral Sci 2011; 3:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono H, Oki Y, Bono H, and Kano K: Gene expression profiling in multipotent DFAT cells derived from mature adipocytes. Biochem Biophys Res Commun 2011; 407:562. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, and Mugishima H: Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 2008; 215:210. [DOI] [PubMed] [Google Scholar]
- 5.Forte A, Schettino MT, Finicelli M, Cipollaro M, Colacurci N, Cobellis L, and Galderisi U: Expression pattern of stemness-related genes in human endometrial and endometriotic tissues. Mol Med 2009; 15:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregory CA, Gunn WG, Peister A, and Prockop DJ: An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 2004; 329:77. [DOI] [PubMed] [Google Scholar]
- 7.Mimura T. and Joyce NC: Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci 2006; 47:1387. [DOI] [PubMed] [Google Scholar]
- 8.Patel NA, Apostolatos HS, Mebert K, Chalfant CE, Watson JE, Pillay TS, Sparks J, and Cooper DR: Insulin regulates protein kinase CβII alternative splicing in multiple target tissues: development of a hormonally responsive heterologous minigene. Mol Endocrinol 2004; 18:899. [DOI] [PubMed] [Google Scholar]
- 9.Oki Y, Watanabe S, Endo T, and Kano K: Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct 2008; 33:211. [DOI] [PubMed] [Google Scholar]
- 10.Madonna R, Willerson JT, and Geng YJ: Myocardin a enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells 2008; 26:202. [DOI] [PubMed] [Google Scholar]
- 11.Katz AJ, Tholpady A, Tholpady SS, Shang H, and Ogle RC: Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 2005; 23:412. [DOI] [PubMed] [Google Scholar]
- 12.Gimble JM, Bunnell BA, and Guilak F: Human adipose-derived cells: an update on the transition to clinical translation. Regen Med 2012; 7:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, and Hedrick MH: Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13:4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park IK, Morrison SJ, and Clarke MF: Bmi1, stem cells, and senescence regulation. J Clin Invest 2004; 113:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuri S, Fujimura S, Nimura K, Takeda N, Toyooka Y, Fujimura Y, Aburatani H, Ura K, Koseki H, Niwa H, and Nishinakamura R: Sall4 is essential for stabilization, but not for pluripotency, of embryonic stem cells by repressing aberrant trophectoderm gene expression. Stem Cells 2009; 27:796. [DOI] [PubMed] [Google Scholar]
- 16.Geiman DE, Ton-That H, Johnson JM, and Yang VW: Transactivation and growth suppression by the gut-enriched Kruppel-like factor (Kruppel-like factor 4) are dependent on acidic amino acid residues and protein-protein interaction. Nucleic Acids Res 2000; 28:1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, Del Valle I, Hein K, Vogt R, and Kemler R: Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012; 336:1549. [DOI] [PubMed] [Google Scholar]
- 18.Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Gelmini S, Guasti D, Benvenuti S, Annunziato F, Bani D, Liotta F, Francini F, Perigli G, Serio M, and Luconi M: Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J 2009; 23:3494. [DOI] [PubMed] [Google Scholar]
- 19.Tchkonia T, Giorgadze N, Pirtskhalava T, Thomou T, DePonte M, Koo A, Forse RA, Chinnappan D, Martin-Ruiz C, von Zglinicki T, and Kirkland JL: Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes 2006; 55:2571. [DOI] [PubMed] [Google Scholar]
- 20.Cignarelli A, Perrini S, Ficarella R, Peschechera A, Nigro P, Giorgino F: Human adipose tissue stem cells: relevance in the pathophysiology of obesity and metabolic diseases and therapeutic applications. Expert Rev Mol Med 2012; 14:e19. [DOI] [PubMed] [Google Scholar]
- 21.Chen JG, Spagnoli A, and Torquati A: Adipogenic differentiation of adipose tissue-derived human mesenchymal stem cells: effect of gastric bypass surgery. Surg Endosc 2012; 26:3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, and Kirkland JL: Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol 2002; 282:R1286. [DOI] [PubMed] [Google Scholar]
- 23.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, and Komuro I: A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 2009; 15:1082. [DOI] [PubMed] [Google Scholar]
- 24.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS: Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007; 48:15. [DOI] [PubMed] [Google Scholar]
- 25.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, and March KL: Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004; 109:1292. [DOI] [PubMed] [Google Scholar]
- 26.Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, and Sung JH: Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci 2008; 49:133. [DOI] [PubMed] [Google Scholar]
- 27.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, and Sung JH: Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 2009; 17:540. [DOI] [PubMed] [Google Scholar]
- 28.El-Ftesi S, Chang EI, Longaker MT, and Gurtner GC: Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg 2009; 123:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, and Odorisio T: Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen 2013; 21:545. [DOI] [PubMed] [Google Scholar]
- 30.Lindroos B, Boucher S, Chase L, Kuokkanen H, Huhtala H, Haataja R, Vemuri M, Suuronen R, and Miettinen S: Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy 2009; 11:958. [DOI] [PubMed] [Google Scholar]