Abstract
Canine adenovirus type 2 vectors (CAV-2) are promising tools to treat global central nervous system (CNS) disorders because of their preferential transduction of neurons and efficient retrograde axonal transport. Here we tested the potential of a helper-dependent CAV-2 vector expressing β-glucuronidase (HD-RIGIE) in a mouse model of mucopolysaccharidosis type VII (MPS VII), a lysosomal storage disease caused by deficiency in β-glucuronidase activity. MPS VII leads to glycosaminoglycan accumulation into enlarged vesicles in peripheral tissues and the CNS, resulting in peripheral and neuronal dysfunction. After intracranial administration of HD-RIGIE, we show long-term expression of β-glucuronidase that led to correction of neuropathology around the injection site and in distal areas. This phenotypic correction correlated with a decrease in secondary-elevated lysosomal enzyme activity and glycosaminoglycan levels, consistent with global biochemical correction. Moreover, HD-RIGIE-treated mice show significant cognitive improvement. Thus, injections of HD-CAV-2 vectors in the brain allow a global and sustained expression and may have implications for brain therapy in patients with lysosomal storage disease.
Introduction
Mucopolysaccharidosis type VII (MPS VII or Sly Syndrome) is an autosomal recessive disease that belongs to a group of lysosomal storage disorders (LSD), referred to collectively as mucopolysaccharidoses (MPS), caused by the loss of function of one of several lysosomal enzymes. MPS VII is caused by a deficiency in β-glucuronidase (βgluc) activity (EC 3.2.1.31), a lysosomal hydrolase involved in the stepwise degradation of glucuronic acid-containing glycosaminoglycans (GAGs) dermatan sulfate, heparan sulfate, and chondroitin sulfate (Vogler et al., 1994). Lysosomal enzymes are essentially ubiquitously expressed; thus, multiple organs are impaired because of cells accumulating undegraded substrates. MPS VII patients display a range of clinical variability, from the most severe with hydrops fetalis to an attenuated phenotype with late onset and almost normal intelligence (Muenzer, 2011). The features of MPS VII include coarse facies, hydrocephaly, and multiple skeletal abnormalities. Affected individuals also frequently develop hepatosplenomegaly, heart valve abnormalities, developmental delay, and progressive intellectual disability (Shipley et al., 1993). The MPS VII mouse has been extensively used as a model of the human LSD as it shares clinical, biochemical, and pathological symptoms, including growth retardation (Birkenmeier et al., 1989; Vogler et al., 1998). Thus, MPS VII mouse is a useful tool for the evaluation of the effectiveness of experimental therapies for MPS VII disorders.
Among the treatments tested for MPS VII, bone marrow transplants, particularly in neonatal mice, can correct widespread lysosomal storage of MPS VII mice in bone, bone marrow, visceral organs, and brain; increase the lifespan to approach that found in normal mice; and correct cardiac abnormalities (Soper et al., 2001; Schuldt et al., 2004). Another therapeutic approach for peripheral LSD symptoms is enzyme replacement therapy (ERT). ERT has improved pathologies in patients with Gaucher disease (Grabowski et al., 1998), Fabry disease (Eng et al., 2001; Wilcox et al., 2004), Pompe disease (Thurberg et al., 2006), MPS I (Kakkis et al., 2001), MPS II (Muenzer, 2011), and MPS VI (Harmatz et al., 2006). For MPS VII, data from animal models (O'Connor et al., 1998; LeBowitz et al., 2004) have supported the approval of a phase 1/2 clinical trial (NCT01856218). However, this approach is limited by the permeability of the blood–brain barrier (BBB). As many LSD, including MPS VII, affect the central nervous system (CNS), a strategy that can cross the BBB is necessary.
One approach to address long-term CNS therapy is gene transfer via viral vectors that confer stable and long-term correction. This could provide sustained therapy if a sufficient level of enzyme was secreted in the brain. We and others have demonstrated the potential of different vectors in correcting neuronal pathologies in MPS II (Cardone et al., 2006), MPS IIIA and B (Cressant et al., 2004; Langford-Smith et al., 2012), and MPS VII mice (Bosch et al., 2000a,b; Liu et al., 2007) as well as in larger animal models for the disease (Ciron et al., 2006; Ellinwood et al., 2011). However, clinically relevant gene therapy using common human pathogens as vectors may be complicated by the high incidence of preexisting humoral and cellular immunity (Chirmule et al., 1999; Perreau et al., 2007a).
Human adenoviral vectors induce both innate and adaptive immune responses that trigger the elimination of transgene expression in a relatively short term. Helper-dependent (HD) adenovirus can circumvent the immune response once reaching the nucleus, although they could have been previously neutralized by antiadenovirus antibodies (reviewed by Lowenstein et al., 2007). Canine adenovirus type 2 (CAdV2, or commonly referred to as CAV-2) vectors preferentially transduce neurons, and retrograde axonal transport is efficient, leading to expression of the transgene in many areas of the brain after a single injection (Soudais et al., 2001; Salinas et al., 2009). Compared with human adenovirus serotype 5 vectors, CAV-2 vectors induce a low level of innate response and do not activate the human complement pathways (Keriel et al., 2006; Perreau et al., 2007b). In addition, limited presence and titers of neutralizing antibodies against CAV-2 are found in the human population (Kremer et al., 2000; Perreau and Kremer, 2005). In addition, HD-CAV-2 vectors lead to long-term transgene expression in rodents (Soudais et al., 2004), and have a cloning capacity of ∼30 kb. This is an advantage compared with adeno-associated viral (AAV) vectors, as it allows the possibility of modulating therapeutic genes with large, endogenous, or inducible promoters and/or regulatory sequences.
The aim of this study was to test the therapeutic efficacy of intrastriatal injection of an HD-CAV-2 vector expressing βgluc (HD-RIGIE) in MPS VII mice. We achieved global, long-term correction in MPS VII mouse brains with bilateral striatal injections of HD-RIGIE. We show recovery of biochemical and neuropathological abnormalities throughout the forebrain and midbrain, which led to significant cognitive improvement.
Materials and Methods
Animals
We used a tolerant mouse model for MPS VII (Sly et al., 2001) developed from the original βgluc-deficient mouse (Levy et al., 1996). Heterozygous (Gusmps/+) mice, kindly provided by Dr. William S. Sly (St. Louis University School of Medicine, St. Louis, MO), were bred and mutants were identified at 1 month of age by the absence of βgluc activity from tail clip homogenates. Animal care and experimental procedures were performed in accordance with 86/609/EEC regarding the care and use of animals for experimental procedures and were approved by the Biosafety and the Ethics Committees of the Universitat Autònoma de Barcelona.
First-generation CAV-2 vectors
E1-deleted CAVGFP has been previously described (Kremer et al., 2000). Vector particles were produced in canine E1 trans-complementing cells (DKZeo), originally derived from the canine kidney cell line DK (ATCC CRL6247) (Kremer et al., 2000). Virus from the supernatant were concentrated by precipitation with ammonium sulfate (Schagen et al., 2000) and pooled with the cellular fraction to maximize recovery. This pool was purified using two CsCl density ultracentrifugation steps and CsCl was removed by size exclusion chromatography using PD-10 columns (GE Healthcare), and the virus was stored in 10% glycerol phosphate-buffered saline. Titers were 1.44×1012 physical particles (pp)/ml with a pp to infectious particle (ip) ratio of 4:1.
Production of HD-RIGIE and HD-GFP vectors
HD-RIGIE expressed the human GUSB cDNA and GFP under the control of a Rous sarcoma virus promoter. The RIGIE cassette (RSV-IVS-GUSB-IRES-EGFP) was generated using classic molecular biology techniques. The human GUSB cDNA was a gift from William Sly (University of St Louis). AscI/NotI-digested pHD-RIGIE or pHD-GFP were transfected into 5×106 DKZeo cells using 18 μl of Turbofect (Fermentas, Thermo Scientific) for 10 μg of linearized DNA/10 cm plate. The cells were infected with 100 pp of helper vector/cell. GFP+ cells were collected by flow cytometry 24 hr posttransfection, re-plated, and lysed by three freeze–thaw cycles 20 hr later. Cells were sorted after transfection until at least 2×106 of GFP+ cells were isolated. The cleared lysates were then incubated on a fresh monolayer of DKZeo cells using helper vector JBΔ5. Twenty-four hours postinfection, GFP+ cells were sorted by flow cytometry, replated, and lysed by three freeze–thaw cycles 20 hr later. The cleared lysate was used for amplification until 3×107 GFP+ cells were obtained. At each amplification step, DKZeo cells were coinfected with 100 pp/cell of helper vector. Finally, the last amplification occurred in ∼8×108 DKCre cells without adding helper vector. JBΔ5 contains a loxP-flanked packaging domain and an RSV-lacZ expression cassette (Soudais et al., 2001, 2004). When propagated in DKCre cells, an ∼900 bp fragment containing the packaging domain and part of the RSV promoter was excised (floxed), and the resulting 32.3 kb vector was rendered packaging deficient (Soudais et al., 2004). The helper vector retained a minimal part of the RSV promoter, which promoted lacZ expression. To test the level of helper contamination in HD vector preparations, β-galactosidase activity was assayed by X-gal staining. HD-RIGIE was purified by triple banding on CsCl density gradients: an initial step gradient of 1.25 and 1.45 g/ml, and then two self-forming isopycnic gradients using 1.32 g/ml CsCl as previously described (Soudais et al., 2004). The purified stock was stored at −80°C in phosphate-buffered saline (PBS)/10% glycerol. Physical particles titers were determined by OD at 260 nm and quantitative polymerase chain reaction (qPCR) and were found to be ∼1.3×1012 pp/ml. HD-RIGIE ip were determined by GFP expression. Combined, the pp/ip ratio was 60:1. Because of the relatively low level of GFP from the combination of the weak RSV promoter and IRES in DK cells, this ratio likely overestimates the pp-to-ip ratio. As assayed by X-gal staining and qPCR, helper vector contamination varied between preparations from <1% to ∼10%.
Animal studies
Intracranial injections
Mice were anesthetized by intraperitoneal injection of ketamine (10 mg/kg of body weight; Imalgene 500; Rhône-Merieux) and xylazine (1 mg/kg of body weight; Rompun; Bayer) and mounted onto a stereotactic frame (David Kopf Instruments). The skull was exposed by a small incision. A small burr hole was made 1 mm caudal and 1.5 mm lateral to bregma. Three microliters of the vector preparation was loaded into a Hamilton syringe mounted to the stereotactic frame. The tip of the needle was inserted into the striatum 3.0 mm in depth from the skull surface in heterozygous mice and 2.6 mm in mutant mice, and 2 μl of HD-RIGIE, corresponding to 2×109 pp, was delivered with an ultramicropump (World Precision Instruments) at a rate of 0.5 μl/min. The needle was slowly withdrawn after an additional 5 min. Mock-injected control animals were injected in the same coordinates with 2 μl of PBS.
Transient immunosuppression
Cyclophosphamide (CFA; Sigma) was diluted in PBS and administered intraperitoneally at 50 mg/kg of body weight every 2 days, from day −3 to day+13, considering day 0 as the intracranial injection time, as a modification of the treatment defined by Cao et al. (2011).
Behavioral tests
A standardized set of experimental procedures (abbreviated SHIRPA, Giménez-Holt et al., 2002) were used to characterize the phenotype of treated mice. Observation of undisturbed behavior in the home-cage was followed by assessment of fluorimeter tasks.
Rod tests
Motor coordination and equilibrium were assessed by the distance covered and the latency to fall off a horizontal wood rod (1.3 cm diameter) and a wire rod (1 cm diameter) on two consecutive 20 sec trials.
Hanger test
Prehensility and motor coordination were measured as the distance covered on the wire hang test, where the animals were allowed to cling (2 mm diameter, 40 cm long) with their forepaws for two trials of 5 sec and a third 60 sec trial. Muscle strength was measured as the time until falling off the wire in the 60 sec trial. All the apparatus were suspended 40 cm above a padded table.
Tertiary screen was designed tailored to neuropsychiatric-like deficits, assessing spontaneous exploratory behavior, anxiety-like behaviors, and cognition in a series of tests involving different degrees of complexity.
Corner test
Neophobia was recorded in a new home-cage by the horizontal (n of visited corners) and vertical (n and latency of rearings) activity during a period of 30 sec.
Open-field test
Exploratory activity and anxiety-like behaviors were evaluated for 5 min by means of horizontal (crossings of 5×5 cm) and vertical (rearings) locomotor activities recorded for each minute of the test.
T-maze
The spontaneous exploratory behavior was tested in a T-shaped maze (arms, length 25 cm). Animals were placed inside the vertical arm of the maze facing the end wall. The performance was evaluated by determining the time elapsed until the animal crossed (four-paw criteria) the intersection of the three arms.
Spatial learning and memory in a 2-day water maze
On day 1, animals were trained to criterion (90% escaping under 60 sec) in a series of cued visible platform trials (7 cm diameter, 1 cm above the water surface, position indicated by a visible 5×8 cm striped flag, 20 min intertrial time) in a pool (Intex Recreation; 91 cm diameter, 40 cm deep, 25°C opaque water). This required four platform trials (CUE1–CUE4). The last visible platform trial of any animal was considered to be its posthabituation baseline and was designated CUE4 (cued visible platform trial 4). Mice that failed to find the platform within 90 sec were manually guided to the platform and placed on it for 5–10 sec, the same period as successful animals. Twenty-four hours after the last cued platform trial, animals were tested in a series of four hidden platform trials (PT1–PT4, 20 min apart). In these place-learning tasks, the hidden platform (1.5 cm below the water surface) was located in a new position, opposite the one used for cue learning. Escape latencies were measured with a stopwatch.
Biochemical assays
Detection of lysosomal enzyme activities in tissue extracts
Deeply anesthetized animals were euthanized. The cerebrum was removed and sliced into 2-mm-thick slices using a mouse brain slicer (Zivic Instruments) and stored at −80°C. Tissues were homogenized in lysis buffer (25 mM Tris, 75 mM NaCl [pH 7.5]; both from Sigma) and centrifuged at 12,000×g for 10 min at 4°C. Ten micrograms of each slice was assayed in a fluorimeter Wallac 1420 Victor3 (Perkin Elmer) for βgluc or β-hexosaminidase activity using 10 mM 4-methylumbelliferyl-β-D-glucuronide (Sigma) or 0.01 mM 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide (Sigma) as substrate, respectively.
Detection of βgluc activity in tissue sections
Animals were anesthetized and perfused with 4% paraformaldehyde, and brains were removed and postfixed. After cryoprotection with 30% sucrose, tissues were embedded in O.C.T. Tissue Tek compound (Miles Scientific) and cut into 10-μm-thick sagittal or coronal sections. Sections were incubated for 4 hr at 37°C with 0.004% hexazotized pararosaniline in 0.25 mM naphtol-AS-BI-β-D-glucuronide (Sigma).
For volumetric estimation of βgluc extension, 100-μm-thick coronal sections were cut at 4°C after 5 hr postfixation with 4% paraformaldehyde using a vibratome (Leica). The whole cerebrum was sectioned, and one in every five sections was stained for βgluc activity. Transduction volume was estimated based on the number of slides positive for β-gluc.
GAG quantification
Twenty milligrams of each 2-mm-thick slice was homogenized in papain extraction reagent at 65°C for 3 hr. GAG content was determined using the Blyscan Sulfated Glycosaminoglycan Assay (Biocolor).
Histology and immunological assays
Ten-micrometer-thick cryosections were obtained as described above. Sections were blocked with 2% bovine serum albumin and incubated with rabbit anti-Iba1 (1:500; Wako Chemicals GmbH) or NeuN (1:200; Chemicon, Millipore) overnight at 4°C. Goat antirabbit Alexa Fluor 568 as a secondary antibody (1:200; Molecular Probes) and a Hoechst solution to stain the nuclei (Sigma) were used. To quantify cortical microglia, Iba1+ cells from different sections around the injected area were counted and normalized by the total number of cells counterstained with Hoechst.
Histopathology
About 100-μm-thick coronal brain sections were postfixed with 4% paraformaldehyde and 1% glutaraldehyde and then with 1% osmium tetroxide, and finally embedded in Epon (all reagents from Sigma). One-micrometer-thick sections were cut and stained with toluidine blue for 30 sec. Histological sections were evaluated morphologically by light microscopy. Sections were further examined, and 200 cells per section and brain structure were counted for each animal to evaluate the percentage of cells without or with very small cytoplasmic vacuoles.
Quantitative polymerase chain reaction
Genomic DNA was obtained from 2-mm-thick brain slices with 0.1 mg/ml of proteinase K (Roche Diagnostics), followed by phenol/chloroform extraction. HD-RIGIE genome copy numbers were measured by qPCR using the Bio-Rad CFX Manager (Bio-Rad Laboratories) and SYBR green PCR (Bio-Rad Laboratories). Briefly, vector sequences and mouse genomic cyclophilin (as reference gene) sequences were simultaneously amplified, and each sample was expressed in terms of its cyclophilin content. The results (vector genome copy number per cell, viral genomes [vg]/cell) were expressed as n-fold differences in the transgene sequence copy number relative to the cyclophilin gene copy number (number of vg copies for 2N genome). Samples were considered eligible for the study if the cyclophilin sequence Ct values were <26 and were scored vector-negative if the transgene sequence Ct value was >35. Thermal cycling conditions comprised an initial denaturing step at 95°C for 3 min, followed by 40 cycles at 95°C for 10 sec, 58°C for 10 sec, and 72°C for 30 sec. Each sample was analyzed in duplicate. Nucleotide sequences of primers are available on request.
Statistics
Values are represented as mean±SEM. Statistical analyses using Student's t-test or one- and two-way ANOVA with post-hoc tests were performed for each data set. Repeated-measures ANOVA with a two-factorial design T×G (T=effect of time; G=effect of group) was used for behavioral tests, followed by post-hoc Duncan's test. Differences were considered statistically significant if p<0.05.
Results and Discussion
Microglia activation prevents HD-CAV-2 expression in the mouse brain
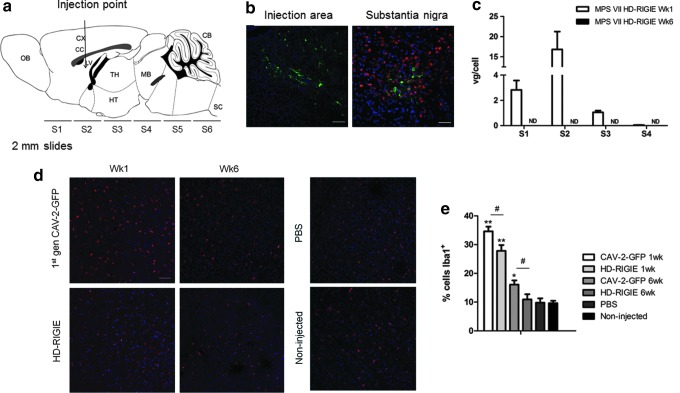
CAV-2 can be retrogradely transported to different areas of the brain after a single injection into the striatum (Soudais et al., 2004). Thus, although MPS VII causes global CNS pathology, we asked if a single injection exclusively in the striatum of 8–10-week-old MPS VII mice with 2×109 pp of HD-RIGIE could be of therapeutic efficacy. Animals were euthanized 1 and 6 weeks after the injection. Brains were sectioned into six 2-mm-thick slices, rostral to caudal, as represented in Fig. 1a. GFP expression was observed 1 week postinjection at the injection area and in more distal regions of the brain such as the substantia nigra, containing neurons from the nigrostriatal pathway and projecting their axons to the striatum (Fig. 1b), indicating that CAV-2 vectors maintain retrograde transport in MPS VII brains. Animals euthanized at 1 week posttreatment showed the presence of viral DNA in four of the six slices in the injected hemisphere, with a maximum of 16.83±4.41 vg/cell in S2, corresponding to the injection area. We also observed the presence of viral DNA in the contralateral hemisphere although at lower levels, which may be caused by retrogradely transported virus or to leakage of the vector in the cerebrospinal fluid that may lead to the infection of cells in the choroid plexus or in the ependyma around the ventricles, mainly contained in S2 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum). No DNA was detected in S5 and S6, slices containing the cerebellum and brainstem. However, no GFP expression was observed in the animals euthanized 5 weeks later, neither βgluc activity (data not shown), correlating with the disappearance of vg in these slices (Fig. 1c).
FIG. 1.
Immune response avoids long-term expression of HD-CAV-2 vectors in CNS (a) Mouse brain diagram showing the coordinates used for the administration of HD-RIGIE, and the 2-mm-thick slices analyzed (S1–S6). CB, cerebellum; CC, corpus callosum; CX, cerebral cortex; HT, hypothalamus; LV, lateral ventricle; MB, midbrain; OB, olfactory bulb; SC, spinal cord; TH, thalamus. (b) Representative pictures of MPS VII mice injected with HD-RIGIE 1 week earlier: on the left image GFP expression at the injection point (scale bar=100 μm), and at the substantia nigra on the right image (scale bar=50 μm). Nuclei counterstaining was with Hoechst (blue) and neurons with NeuN (red). (c) Viral genomes per cell were quantified by qPCR in 2-mm-thick slices of brains injected with HD-RIGIE 1 and 6 weeks later. Disappearance of viral genomes at 6 weeks was observed (ND, not detected). (d) Microglia activation is identified by Iba1 staining (red) at the injection area 1 and 6 weeks after administration (left panel) of CAVGFP (up) and HD-RIGIE (down). Nuclei were counterstained with Hoechst (blue). The right panel shows Iba1 staining in animals injected with PBS or noninjected, as a control. Scale bar=100 μm. (e) Percentage of Iba1+ cells/field in the different animals injected. Data are mean±SEM, n=2 animals euthanized at Wk1 and n=5 animals euthanized at Wk6 postinjection; *p<0.05, **p<0.01 comparing noninjected with vector-injected animals; #p<0.05 comparing CAVGFP and HD-RIGIE at the same time points. CAV-2, canine adenovirus type 2 vectors; CNS, central nervous system; HD-RIGIE, helper-dependent CAV-2 vector expressing β-glucuronidase; PBS, phosphate-buffered saline; qPCR, quantitative polymerase chain reaction; Wk, week.
Delivery of Ad vectors into the CNS induces dose-dependent innate immune responses in the form of acute inflammation, including microglial activation, macrophage recruitment, and T-cell infiltration (Thomas et al., 2001). Consequently, we detected Iba1-positive cells, a marker of microglia, in the brains of animals injected with CAV-2 vector and euthanized 1 week later, correlating with the presence of CAV-2 vg and GFP expression, as seen at the injection area (Fig. 1d) and at the substantia nigra (not shown). Quantification of this signal showed stronger Iba1 staining with first-generation CAV-2 vector than with HD vector at both times analyzed, consistent with a reduction in the immune reaction elicited by HD adenovirus (Fig. 1e). In animals euthanized 6 weeks after injection of HD-RIGIE, mild activation was present only at the injection point, nonstatistically different from brains injected with PBS or noninjected (Fig. 1e). Thus, in contrast to results seen in rats and other mouse strains (Soudais et al., 2004; Sotak et al., 2005), in our hands, E1-deleted and HD-CAV-2 vectors led to short-term transgene expression associated with Iba1 expression. Although HD-Ad vectors do not express viral antigens, innate inflammatory responses to high doses of Ad could trigger elimination of transduced cells even using HD vectors (Muhammad et al., 2012). Moreover, acute toxicity provoked by viral capsid proteins or residual helper vector could also elicit an immune response that could eliminate the transduced cells. Furthermore, in addition to viral proteins, immune responses may have occurred against GFP, used in this proof-of-principle study as a marker to identify transduced cells. Clearly, a clinical-grade vector would not have a GFP expression cassette.
Because GAG and ganglioside accumulation are associated with chronic CNS inflammation, and the response to viral vector varies between genus, species, and even strains, we transiently immunosuppressed the animals with 50 mg/kg CFA (Supplementary Fig. S2), previously used to reduce inflammation and neutralizing antibody formation (Cao et al., 2011). Iba1 immunohistochemistry in the HD-RIGIE-injected and transiently immunosuppressed mice showed no activation compared with control mice (Fig. 2a). More importantly, βgluc activity was detected in brain slices from MPS VII mice injected with HD-RIGIE 6 weeks earlier (Fig. 2b). Thus, 50 mg/kg of CFA treatment was followed for the rest of the experiments. CFA is widely used to treat autoimmune diseases, to prevent rejection after allograft organ transplantation and to suppress antibody formation (Moore et al., 2006). Probably other transitory immunosuppressants such as cyclosporine A could have also worked in our model. However, the clinical relevance of this treatable inflammatory response in mice is unknown because mice and humans have notably different reactions in many cases (Seok et al., 2013).
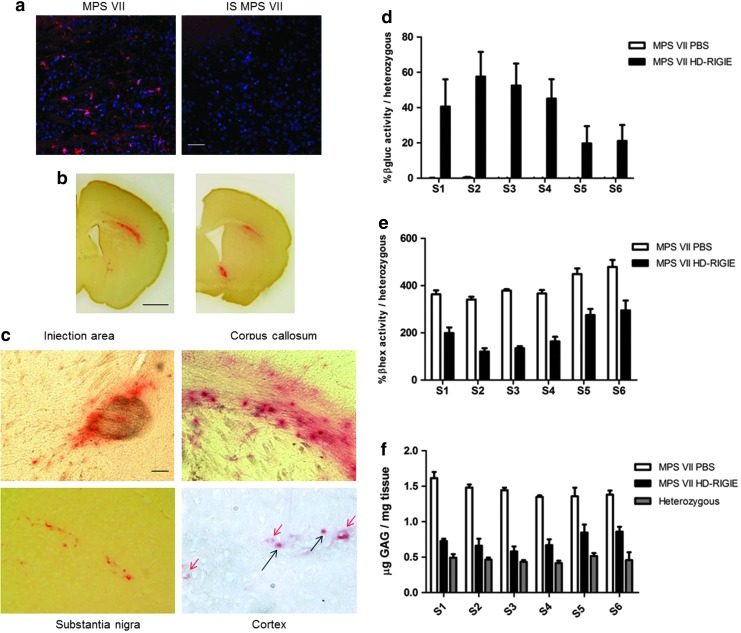
FIG. 2.
Lysosomal enzyme activity and GAG accumulation analysis 6 weeks postinjection. (a) Iba1 staining (red) in immunocompetent (left image) or transiently immunosuppressed MPS VII mice with 50 mg/kg of cyclophosphamide (right image) show lack of activated microglia after HD-RIGIE administration (scale bar=50 μm; nuclei were counterstained with Hoechst [blue]). βgluc activity (red) in (b) 100-μm-thick (scale bar=1 mm) and (c) 10-μm-thick brain slices at the injection area and other distal areas such as corpus callosum, substantia nigra, and cortex; red and black arrows indicate endothelial cells and cortical neurons, respectively, identified by morphology in cortex cryosections (scale bar=100 μm). βgluc activity (d), secondary elevation of lysosomal enzyme β-hexosaminidase (e), and GAG accumulation (f ) in 2-mm-thick slice homogenates from MPS VII mice injected with PBS or HD-RIGIE and compared with βgluc activity in heterozygous mice (p<0.01 for βgluc; p<0.01 in S5 and S6 for βhex; p<0.01 in S5 and S6 for GAG). No statistically significant differences were seen among heterozygous and HD-RIGIE-treated mice in slices spanning between S1 and S4. Data are mean±SEM; n=7 for each experimental group.
Lysosomal enzyme activities in HD-RIGIE-injected MPS VII mice
On the basis of these results, a group of 8–10-week-old animals was injected bilaterally in the striata (Fig. 1a). Control littermates (heterozygous and mutant mice) were mock injected with the same volume of PBS and treated with CFA at the same time and dose. Mice were euthanized at 6 and 16 weeks, and βgluc activity was assayed using in situ coloration by incubating slices with hexazotized pararosaniline in 0.25 mM naphtol-AS-BI-β-D-glucuronide in 100-μm-thick sections, one in every 5 sections, along the whole brain. This is an insensitive assay that stains βgluc activity in red. βgluc activity was not detected in PBS-treated or heterozygous mice, showing the low sensitivity of the assay. In HD-RIGIE-treated MPS VII mice, we detected βgluc activity in striatum, several areas of the cortex, corpus callosum, substantia nigra, and around ventricles (Fig. 2b and c), consistent with the efficient retrograde transport described for CAV-2 vectors (Soudais et al., 2001; Salinas et al., 2009). The majority of transduced cells had morphology suggesting that they were neurons. We also detected cells underlying blood vessels, consistent with endothelial cell morphology (Fig. 2c). Overall, based on this assay, we estimated a transduction area with a diameter of 4 mm around the injection area.
The brain of some of these animals (n=7 for each group) was sliced into 2-mm-thick sections, as described in Fig. 1a, and protein extracts were prepared. For each slice, we measured βgluc and β-hexosaminidase (βhex) activity using a more sensitive fluorimetric assay. In several MPS diseases, βhex activity is elevated when another lysosomal enzyme activity is missing, likely because of transcription factor EB (TFEB) (Sardiello et al., 2009). Data were plotted as the percentage of activity of each enzyme compared with heterozygous mouse levels, which have a normal phenotype (Fig. 2d and e). This MPS VII mouse model was created to be tolerant for human βgluc, by expressing a mutant inactive form of human βgluc, although protein levels are almost undetectable in most tissues (Sly et al., 2001). Notably, βgluc activity in heterozygous mice is 80% of the wild-type animals (data not shown). As seen in Fig. 2d, no βgluc activity was detected in mock-treated MPS VII mice. By contrast, HD-RIGIE-treated mice showed near 50% of the βgluc activity found in heterozygous animals from slice 1 to 4, with the maximum at the injection area (S2) (up to 57.55%±14.02%) and around 20% in S5 and S6, corresponding to cerebellum and brainstem (p<0.01). Equally relevant, we found a significant decrease in the secondary elevation of βhex in all brain areas, consistent with the biochemical correction of βgluc deficiency (Fig. 2e). βhex activity reached heterozygous levels (120.79%±14.015% and 120.228%±12.857% of heterozygous mice) in S2 and S3, respectively. We found an inverse correlation between βgluc and βhex activities in all sections. Notably, there was not a significant difference between HD-RIGIE-treated and heterozygous mice for slices S1–S4. Therefore, by administering HD-RIGIE into the striatum, we could detect CAV-2 vg and transgene activity throughout the cerebrum, which led to global protein transfer in the brain.
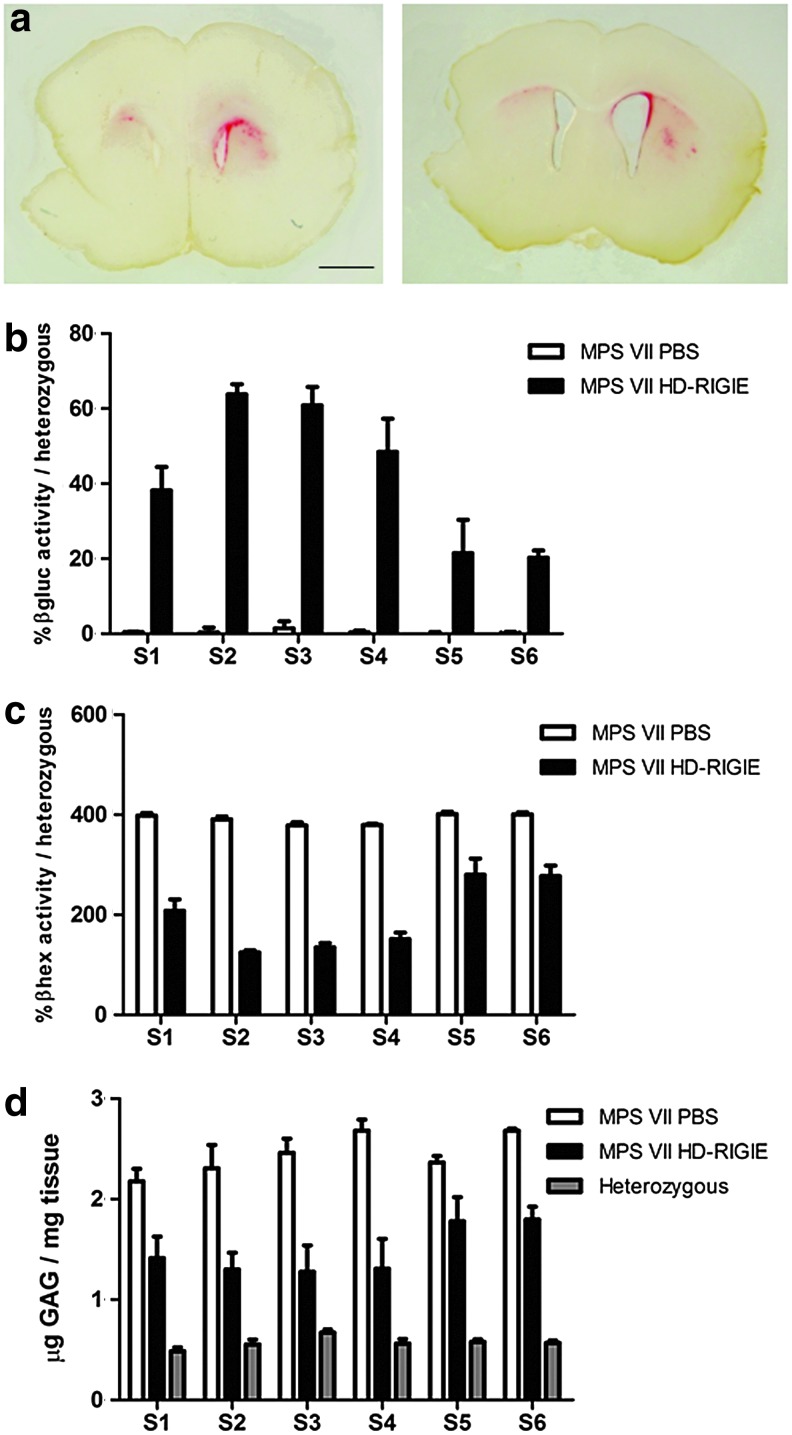
In MPS VII mice euthanized 16 weeks post-HD-RIGIE injection, we also found βgluc activity (n=4 for each group) in tissue sections of brains for a total of 4 mm (Fig. 3a). Similar to animals analyzed 6 weeks post-HD-RIGIE injection, enzyme activity detected by fluorimetry in protein homogenates of 2-mm-thick slices showed a high level of transduction, with βgluc activity found in all the slices, spanning the whole brain. HD-RIGIE-injected mice expressed 40–65% of heterozygous activity from slices S1 to S4. The maximum activity was observed near the injection point in S2 and S3, with 63.8%±2.75% and 60.86%±4.88% of the activity of heterozygous animals, respectively (Fig. 3b). Activities around 20% were also detected in the slices corresponding to cerebellum and brainstem (S5 and S6), and no enzyme activity was detected in the MPS VII animals injected with PBS (Fig. 3b).
FIG. 3.
Lysosomal enzyme activity and GAG accumulation analysis 16 weeks after the treatment. βgluc activity in (a) 100-μm-thick slices (red; scale bar=1 mm) and (b) 2-mm-thick slice homogenates, (c) secondary elevation of β-hexosaminidase activity, and (d) GAG accumulation in 2-mm-thick slice homogenate slices in MPS VII mice injected with PBS or HD-RIGIE compared with heterozygous mice (p<0.01 for βgluc; p<0.01 in S1, S5, and S6 for βhex; and p<0.01 for GAG). Data are mean±SEM; n=4 for each experimental group.
βhex activity showed similar pattern as in animals analyzed at 6 weeks postinjection and inversely correlated to the amount of βgluc observed in each slice. While MPS VII mice treated with PBS had around 400% of heterozygous activity, HD-RIGIE-injected MPS VII mice showed correction in S1, S5, and S6, and were not significantly different from heterozygous mice in S2, S3, and S4 (Fig. 3c).
GAG accumulation in MPS VII-treated mouse brain extracts
MPS VII is characterized by the inability to degrade glucuronic acid-containing GAG. GAG quantification was used to evaluate the therapeutic effect of the HD-RIGIE treatment at 6 and 16 weeks post-HD-RIGIE injection. We reduced GAG accumulation in all sections of the brain, consistent with increased βgluc and reduced βhex activities observed in Figs. 2 and 3. Mice were treated around 8–10 weeks of age and were analyzed 6 or 16 weeks later, that is, when they reached 3.5 or nearly 6 months of age. In the first experimental group, MPS VII animals had GAG levels near 1.5 μg/mg of tissue in all the slices of the brain, threefold more than heterozygous mice. Animals injected with HD-RIGIE showed no statistically significant differences in GAG levels between S1 and S4 compared with heterozygous mice and a 40% reduction in S5 and S6 compared with the same slices of MPS VII-PBS mice (p<0.01) (Fig. 2f).
MPS VII mice analyzed at 6 months showed greater GAG accumulation in all the slices, with levels reaching values of 2.7 μg/mg of tissue, fivefold higher than heterozygous mice. GAG quantification in MPS VII-HD-RIGIE mice showed a 50% decrease in S2, S3, and S4 and between 30% and 40% in S1, S5, and S6 compared with nontreated mutant mice (Fig. 3d). Although there were still statistically significant differences between HD-RIGIE-treated and heterozygous animals in S2 and S3 slices (p<0.01), this reduction was greater at 6 months of age, when the pathology of the disease was much more severe and these animals were at the end of their life expectancy.
Correction of pathology in the HD-RIGIE-injected mouse brain
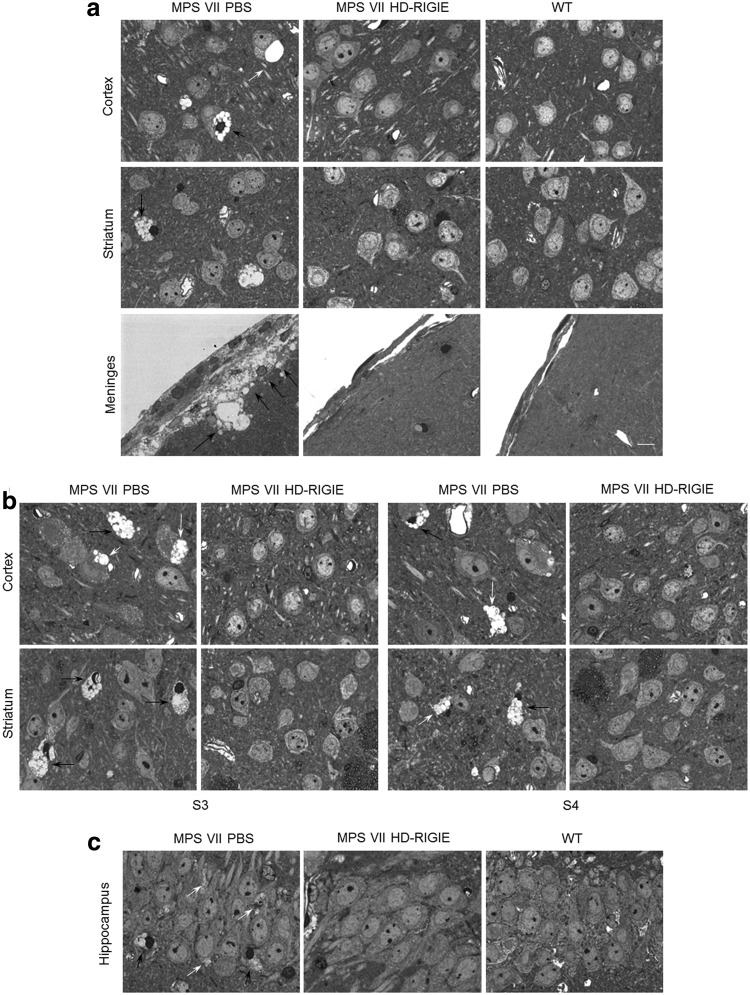
We evaluated brain pathology in treated animals 6 and 16 weeks postinjection in semi-thin sections from the cortex, striatum, and meninges around injection point (S2), cortex and striatum at S3 and S4 levels, and hippocampus.
Representative images of the different brain regions from animals euthanized 6 weeks postinjection showed a significant correction of neurons, and glial cells, which presented with greater distended lysosomal morphology in all areas analyzed (Fig. 4). More than 90% of the cells in the injection area and between 83% and 89% in more distal regions showed no, few, or minuscule vacuoles, compared with large vacuoles in nontreated MPS VII animals (Table 1). An additional group of animals received HD-RIGIE only in one hemisphere. Six weeks after treatment, contralateral hemispheres showed also correction in S2, mainly in striatum and cortex (Supplementary Fig. S3). Therefore, even when injecting in a single hemisphere, there was evidence of vg and lysosomal correction in the contralateral hemisphere (Supplementary Figs. S1 and S3). Although we cannot discard transport in the CSF and transduction of perivascular cells in this hemisphere via HD-RIGIE leakage from the injected area, the most plausible explanation would be via axonal transport of the vector and/or βgluc.
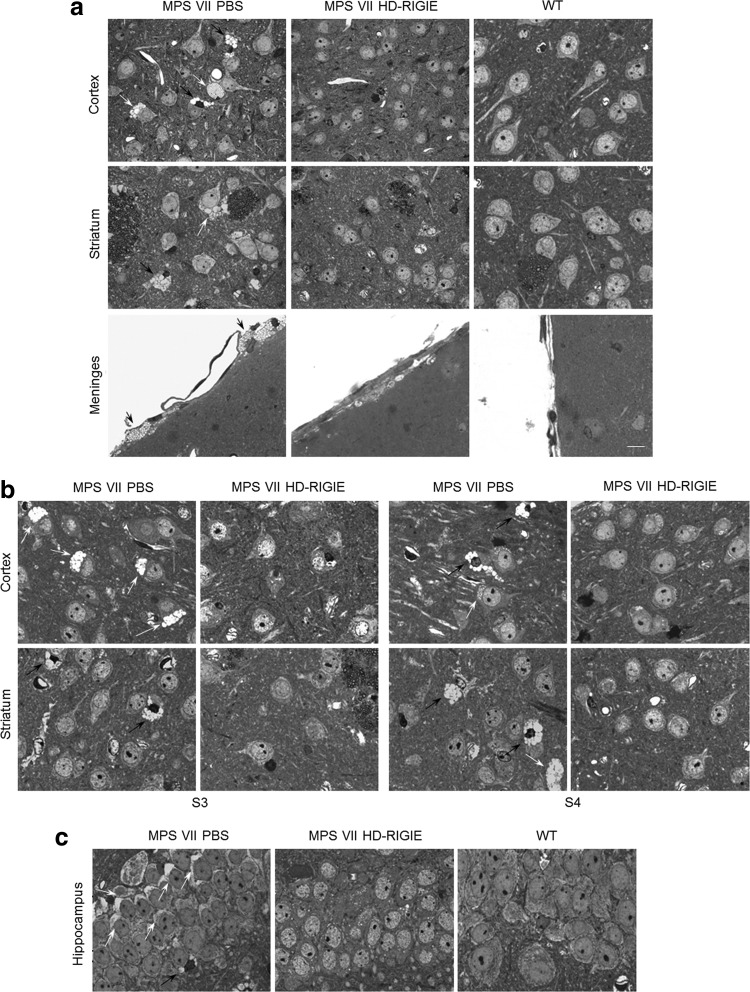
FIG. 4.
Histopathological studies 6 weeks postinjection. Semi-thin sections of different brain areas surrounding the injection point were stained with toluidine blue to highlight the enlargement of vacuoles containing lysosomal storage material as seen in PBS-treated MPS VII mice. Representative images from (a) cortex, striatum, and meninges in MPS VII PBS (left), MPS VII HD-RIGIE (middle), and WT mice (right) at injection area, and at more distal areas (b), slices in S3 (left panel) and S4 (right panel), and (c) hippocampus. HD-RIGIE-treated mice show a pattern similar to WT animals in all tissues analyzed. Quantification of corrected cells is described in Table 1. Scale bar=20 μm. Black and white arrows indicate vesicle accumulation in glial cells and neurons, respectively.
Table 1.
Percentage of Corrected Cells Present in Central Nervous System Structures Quantified in Histopathological Images from Animals Treated with HD-RIGIE and Analyzed After 6 or 16 Weeks
| Injection area | Distal regions | ||||||
|---|---|---|---|---|---|---|---|
| Cortex (%) | Striatum (%) | Cortex (S3) (%) | Striatum (S3) (%) | Cortex (S4) (%) | Striatum (S4) (%) | Hippocampus (%) | |
| HD-RIGIE 6wk | 90±5 | 95±3 | 86±5 | 89±4 | 83±9 | 85±10 | 88±6 |
| HD-RIGIE 16wk | 94±3 | 96±2 | 90±6 | 91±5 | 88±7 | 90±5 | 85±9 |
HD-RIGIE, helper-dependent CAV-2 vector expressing β-glucuronidase; wk, week.
Data are mean±SEM. Three fields of each animal and area were counted. S3 and S4 correspond to the slides represented in Fig. 1a.
Consistent with the biochemical correction, histopathology of proximal and distal areas to the injection point showed a significant reduction of enlarged lysosomes in neurons and glial cells in mice euthanized 16 weeks post-HD-RIGIE injection (Fig. 5). We also quantified the percentage of cells with recovered phenotype in animals treated 16 weeks earlier, and we found between 90% and 96% in the injection area and higher than 85% in more distal regions (Table 1).
FIG. 5.
Histopathological studies 16 weeks postinjection. Representative images from (a) cortex, striatum, and meninges in MPS VII PBS (left), MPS VII HD-RIGIE (middle), and WT mice (right) at injection area, and at more distal areas (b), slices in S3 (left panel) and S4 (right panel), and (c) hippocampus. HD-RIGIE-treated mice show a pattern similar to WT animals in all tissues analyzed. Quantification of corrected cells is described in Table 1. Scale bar=20 μm. Black and white arrows indicate vesicle accumulation in glial cells and neurons, respectively.
Together, these data demonstrated that HD-RIGIE therapy led to stable transgene expression at least for 16 weeks throughout the mid- and the forebrain of MPS VII mice after bilateral striatal injections and that unilateral injection improved MPS VII pathology in the contralateral hemisphere.
HD-RIGIE reverses MPS VII-associated cognitive deficits
In MPS VII mice, progressive impairment in peripheral tissues and in the CNS causes behavioral alterations. Although MPS VII is a multisystem disease, our aim was to test the suitability of HD-CAV-2 in treating a global neurodegenerative disease. We analyzed the behavior of treated mice, mainly using animals treated for 6 weeks, because the poor overall physical condition of 6-month-old animals, at the end of their life, precluded interpretation of the results. This physical impairment may also contribute to the reduction in behavioral performances reported at different ages (Liu et al., 2005; Chen et al., 2012). For that reason, we chose tests with conditions and degree of difficulty to provide convergent validity (Gimenez-Llort et al., 2002, 2007).
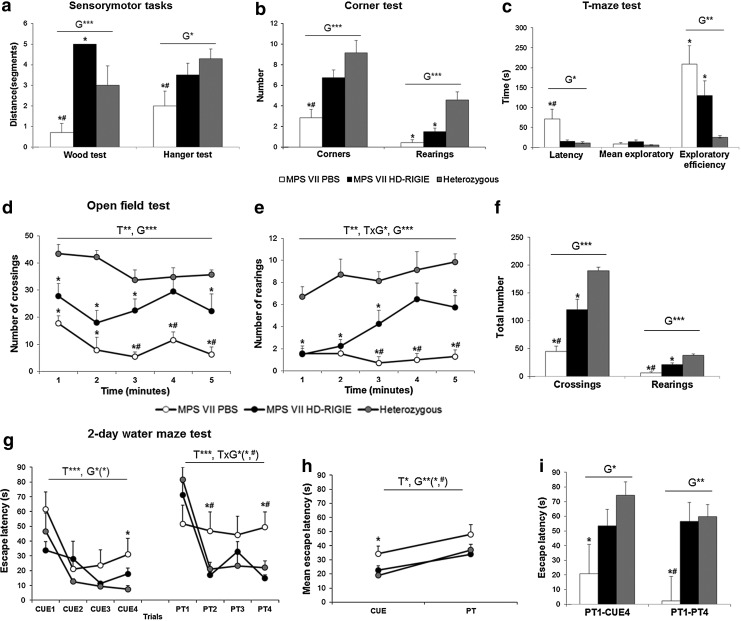
We evaluated spontaneous behavior and sensorimotor functions, behavioral and psychological symptoms (locomotor and exploratory activity, anxious-like behaviors), as well as cognition (learning and memory). Impairment of some muscle and lower motor neuron functions was found when MPS VII animals were assessed in the two-rod and hanger tests (Fig. 6a) (p<0.001). MPS VII mice showed the poorest coordination and prehensility and lower muscular strength (p<0.05), whereas equilibrium was normal. HD-RIGIE treatment restored coordination and improved prehensility (both, p<0.05) but did not modify muscular strength. Not surprisingly, this suggests that some functions depending on somatic development will require systemic or a long-term treatment from early developmental stages (O'Connor et al., 1998).
FIG. 6.
Behavioral effects of HD-RIGIE in MPS VII mice. (a) Sensorimotor tasks. HD-RIGIE treatment allowed complete restoration of coordination in the wood test, while prehensility in the hanger test, measured as the latency to fall, was improved by the treatment. (b) Corner test. MPS VII mice showed reduction of total horizontal (crossings) and vertical (rearings) activities, which were significantly improved by HD-RIGIE treatment. (c) T-maze test. HD-RIGIE treatment completely corrected the increased latency to reach the intersection of the maze and improved the overall capacity to solve the paradigm (exploratory efficiency) but did not modify the mean exploratory time in the arms. (d–f ) Open-field test. HD-RIGIE corrected the reduction of horizontal and vertical activity exhibited in the open-field test, as shown by the temporal course of the behavior (d, crossings 1–5; e, rearings 1–5) and the accumulated counts (f ). (g) Two-day water maze. Acquisition curves. Latency to find the visible (CUE1–CUE4) and hidden (PT1–PT4) platform in a 2-day water maze for mice. Decrease of escape latencies through the cue-learning task was similar between the groups, but MPS VII showed the poorest baseline performance in the last visible platform trial. In the reversal place-learning task, the MPS VII mice showed significantly worse escape latencies as compared with the good acquisition shown by HET and MPS VII-treated mice. (h) Long-term memory. Mean escape latency in the cue- and place-learning tasks. All the groups showed significantly higher mean escape latencies when the platform was hidden as compared with the previous task. However, MPS VII mice showed a worse long-term performance as compared with HET and treatment with HD-RIGIE restored this deficit. (i) Long-term and short-term memories. Long-term memory deficits observed by means of the differences in the escape latency after the 24 hr interval (PT1–CUE4) were lacking in HD-RIGIE-treated animals. Short-term memory deficits observed in the place-learning task (PT1–PT4) were reversed in HD-RIGIE-treated animals. Data are mean±SEM; *p<0.05, **p<0.01, ***p<0.001 comparing HET with the other two MPS groups; #p<0.05 comparing MPS VII PBS with MPS VII HD-RIGIE. T, time; G, group; T×G, time×group; n=7 for each group.
Classical unconditioned tests such as the corner test (Fig. 6b), the T-maze test (Fig. 6c), and open-field test (Fig. 6d–f) involving different levels of anxiogenic conditions indicated reduced horizontal and vertical locomotor activities. Severe problems to interact with the environment were also evident by reduced exploration (Fig. 6d and e) (Time, T, p<0.01; Time×Group, T×G, p<0.05; Group, G, p<0.001). HD-RIGIE treatment reversed the reduced activity in the corner test (Fig. 6b; Corners and Rearings, p<0.001), freezing episodes (p<0.001), forward locomotion (p<0.001), the delay in the onset of vertical exploratory behaviors (p<0.05), and the total vertical activity (p<0.001).
In the T-maze for spontaneous alternation, MPS VII animals showed the poorest performance with a significant delay in the consecution of the behavioral events (Fig. 6c; latency to get started and to reach the intersection, both p<0.05). Only 43% completed the test, while investing more time (exploratory efficiency, p<0.01) and committing more errors (p<0.01). HD-RIGIE treatment increased the incidence to 86%, corrected the delay to reach the intersection (p<0.05), and reduced the number of errors (p<0.05), although it did not modify the exploratory efficiency.
Assessment of spatial learning and memory in the 2-day water maze demonstrated that MPS VII mice had the poorest total cognitive capacity and deficits in the learning acquisition process as well as in both short- and long-term memories. Although all groups showed a similar acquisition curve of the simple cued-learning task (Fig. 6g; T, p<,0.001), MPS VII mice had the poorest total cognitive capacity (p<0.05) and final outcome, as shown by their baseline performance in the last visible platform trial (p<0.05), which was restored to heterozygous levels by HD-RIGIE treatment.
Place learning (PT) is a more difficult task because the platform is hidden and its location changed. Heterozygous animals spent more time in the platform's prior location, but once it was found, they efficiently remembered it. In contrast, the behavior of MPS VII during the first PT trial (PT1) was similar to their first contact with the maze (CUE1). Moreover, the overall cognitive ability of MPS VII mice to solve the tasks was lower (Fig. 6h; p<0.01), and long- and short-term memory was impaired (Fig. 6i; PT1-CUE4, p<0.05; and Fig. 6i; PT1-PT4, p<0.01, respectively) as compared with heterozygous and MPS VII HD-RIGIE mice (both, p<0.05). HD-RIGIE treatment rescued the cognitive deficits (Fig. 6g–i) and, most importantly, the total learning and memory capacities (Fig. 6h).
Our experimental design shows that reduction in behavioral performances was mostly caused by a reduced exploratory activity, has a strong influence of novelty, and is limited by the cognitive capacity of the animal to confront the situation. Such deficiencies were more clearly shown in the time course of the performances and were improved, and even corrected, by HD-RIGIE treatment. Finally, the cognitive impairment of MPS VII mice was severe, as not only short- and long-term learning and memory processes but also the strategies to solve the tasks and the cognitive capacity, itself, were compromised. Cognitive dysfunction worsened with the difficulty (hidden PT), and their cognitive plasticity did not benefit from previous experience. In contrast, heterozygous animals remembered the prior location of the platform and insisted on searching for it. Place task learning also evidenced impairment in short-term memory and deficits in the total learning capacity of MPS VII as compared with heterozygous mice. However, the major findings are that HD-RIGIE treatment completely rescued the cognitive deficits, mostly in short-term memory, and the total learning and memory capacities.
Notably, this is the first study in which elevated enzyme levels in brain, reduction of lysosomal storage, and reversal of cognitive deficits have been observed after intracranial injection of a HD-CAV-2 vector in a mouse model of disease. Previous experiments with HD adenoviral vectors had demonstrated their potential in the treatment of gliomas after direct tumor injection (Muhammad et al., 2012) and in animal models for different diseases after intravenous injection (Dimmock et al., 2011; Crane et al., 2012). Recently, HD-CAV-2 vector was injected in the CNS of a mouse model of MPS IIIA, but although discrete long-term transgene expression was obtained, only 9% reduction of storage material was achieved at the injection point and no cognitive reversion was described (Lau et al., 2012). One significant difference was the transient immunosuppression in the MPS VII animals to achieve robust, long-term transgene expression in this study. However, immune response is dose dependent. The low transgene expression obtained in MPS IIIA animals may allow the escape of the immune response, but it is not sufficient to elicit a significant therapeutic effect in the MPS IIIA model. Similarly, Dindot et al. (2011) achieved long-term expression after intrathecal administration of human HD adenovirus coding for GFP in a wild-type mouse. The authors administered 2.5×109 pp/mouse into the 40 μl volume that constitutes the mouse CSF. Here we injected a similar amount of pp but concentrated into a single injection in the brain parenchyma that may certainly lead to a higher local immune response as demonstrated by Iba1 immunohistochemistry (Fig. 1).
In summary, we have shown that intrastriatal injection of HD-RIGIE resulted in stable expression of βgluc in the brains of MPS VII mice, inducing correction in the brain of these animals. These findings are relevant to the treatment of neurological abnormalities in humans with lysosomal storage diseases and may also be possibly used in neurodegenerative disorders, although it would be necessary to assess the vector performance in large animal models of the disease.
Supplementary Material
Acknowledgments
We thank the Vector Production Unit at Center of Animal Biotechnology and Gene Therapy (CBATEG; Universitat Autònoma de Barcelona), which was supported by the Association Française contre les Myopathies, for producing CAVGFP, and Meritxell Puig, David Ramos, and Angel Vázquez (CBATEG) for technical assistance. We are indebted to Laia Acarin (Universitat Autònoma de Barcelona) for help with Iba1 immunohistochemistry. We thank the Montpellier RIO imaging and, in particular, Miriam Boyer-Clavel for help with flow cytometry.
G.P. and B.G.-L. were recipients of predoctoral fellowships (G.P. from the Generalitat de Catalunya [2009FI_B00219] and B.G.-L. from the Ministerio de Educación [EDU/3445/2011]). E.J.K. is an Inserm fellow. Funding was provided by the European Commission through European Community's 7th Framework Program (FP7/2007–2013; Grant 222992, BrainCAV to E.J.K. and A.B.), the Region Languedoc Roussillon (ARPE and CTP 115277 to E.J.K.) and AGAUR (2009 CTP 00030) to M.C., the Fondation de France (Grant #2008005416), Vaincre les Maladies Lysosomales, and the E-RARE 2009 program (project CAV-4-MPS funded by E-RARE to E.J.K. and to A.B. [Instituto de Salud Carlos III-PS0902674]).
Author Disclosure Statement
No competing financial interests exist.
References
- Birkenmeier E.H., Davisson M.T., Beamer W.G., et al. (1989). Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J. Clin. Invest. 83, 1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch A., Perret E., Desmaris N., and Heard J.M. (2000a). Long-term and significant correction of brain lesions in adult mucopolysaccharidosis type VII mice using recombinant AAV vectors. Mol. Ther. 1, 63–70 [DOI] [PubMed] [Google Scholar]
- Bosch A., Perret E., Desmaris N., et al. (2000b). Reversal of pathology in the entire brain of mucopolysaccharidosis type VII mice after lentivirus-mediated gene transfer. Hum. Gene Ther. 11, 1139–1150 [DOI] [PubMed] [Google Scholar]
- Cao H., Yang T., Li X.F., et al. (2011). Readministration of helper-dependent adenoviral vectors to mouse airway mediated via transient immunosuppression. Gene Ther. 18, 173–181 [DOI] [PubMed] [Google Scholar]
- Cardone M., Polito V.A., Pepe S., et al. (2006). Correction of Hunter syndrome in the MPSII mouse model by AAV2/8-mediated gene delivery. Hum. Mol. Genet. 15, 1225–1236 [DOI] [PubMed] [Google Scholar]
- Chen Y.H., Claflin K., Geoghegan J.C., and Davidson B.L. (2012). Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Mol. Ther. 20, 1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N., Propert K., Magosin S., et al. (1999). Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6, 1574–1583 [DOI] [PubMed] [Google Scholar]
- Ciron C., Desmaris N., Colle M.A., et al. (2006). Gene therapy of the brain in the dog model of Hurler's syndrome. Ann. Neurol. 60, 204–213 [DOI] [PubMed] [Google Scholar]
- Crane B., Luo X., Demaster A., et al. (2012). Rescue administration of a helper-dependent adenovirus vector with long-term efficacy in dogs with glycogen storage disease type Ia. Gene Ther. 19, 443–452 [DOI] [PubMed] [Google Scholar]
- Cressant A., Desmaris N., Verot L., et al. (2004). Improved behavior and neuropathology in the mouse model of Sanfilippo type IIIB disease after adeno-associated virus-mediated gene transfer in the striatum. J. Neurosci. 24, 10229–10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock D., Brunetti-Pierri N., Palmer D.J., et al. (2011). Correction of hyperbilirubinemia in gunn rats using clinically relevant low doses of helper-dependent adenoviral vectors. Hum. Gene Ther. 22, 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindot S., Piccolo P., Grove N., et al. (2011). Intrathecal injection of helper-dependent adenoviral vectors results in long-term transgene expression in neuroependymal cells and neurons. Hum. Gene Ther. 22, 745–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinwood N.M., Ausseil J., Desmaris N., et al. (2011). Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol. Ther. 19, 251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C.M., Guffon N., Wilcox W.R., et al. (2001). Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry's disease. N. Engl. J. Med. 345, 9–16 [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L., Fernandez-Teruel A., Escorihuela R.M., et al. (2002). Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur. J. Neurosci. 16, 547–550 [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L., Blazquez G., Canete T., et al. (2007). Modeling behavioral and neuronal symptoms of Alzheimer's disease in mice: a role for intraneuronal amyloid. Neurosci. Biobehav. Rev. 31, 125–147 [DOI] [PubMed] [Google Scholar]
- Grabowski G.A., Leslie N., and Wenstrup R. (1998). Enzyme therapy for Gaucher disease: the first 5 years. Blood Rev. 12, 115–133 [DOI] [PubMed] [Google Scholar]
- Harmatz P., Giugliani R., Schwartz I., et al. (2006). Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J. Pediatr. 148, 533–539 [DOI] [PubMed] [Google Scholar]
- Kakkis E.D., Muenzer J., Tiller G.E., et al. (2001). Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 344, 182–188 [DOI] [PubMed] [Google Scholar]
- Keriel A., Rene C., Galer C., et al. (2006). Canine adenovirus vectors for lung-directed gene transfer: efficacy, immune response, and duration of transgene expression using helper-dependent vectors. J. Virol. 80, 1487–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E.J., Boutin S., Chillon M., and Danos O. (2000). Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J. Virol. 74, 505–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford-Smith A., Wilkinson F.L., Langford-Smith K.J., et al. (2012). Hematopoietic stem cell and gene therapy corrects primary neuropathology and behavior in mucopolysaccharidosis IIIA mice. Mol. Ther. 20, 1610–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A.A., Rozaklis T., Ibanes S., et al. (2012). Helper-dependent canine adenovirus vector-mediated transgene expression in a neurodegenerative lysosomal storage disorder. Gene 491, 53–57 [DOI] [PubMed] [Google Scholar]
- LeBowitz J.H., Grubb J.H., Maga J.A., et al. (2004). Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc. Natl. Acad. Sci. USA 101, 3083–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B., Galvin N., Vogler C., et al. (1996). Neuropathology of murine mucopolysaccharidosis type VII. Acta Neuropathol. 92, 562–568 [DOI] [PubMed] [Google Scholar]
- Liu G., Martins I., Wemmie J.A., et al. (2005). Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J. Neurosci. 25, 9321–9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Chen Y.H., He X., et al. (2007). Adeno-associated virus type 5 reduces learning deficits and restores glutamate receptor subunit levels in MPS VII mice CNS. Mol. Ther. 15, 242–247 [DOI] [PubMed] [Google Scholar]
- Lowenstein P.R., Mandel R.J., Xiong W.D., et al. (2007). Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr. Gene Ther. 7, 347–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.J., Markmann J.F., and Deng S. (2006). Avenues for immunomodulation and graft protection by gene therapy in transplantation. Transpl. Int. 19, 435–445 [DOI] [PubMed] [Google Scholar]
- Muenzer J. (2011). Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 50Suppl. 5, v4–v12 [DOI] [PubMed] [Google Scholar]
- Muhammad A.K., Xiong W., Puntel M., et al. (2012). Safety profile of gutless adenovirus vectors delivered into the normal brain parenchyma: implications for a glioma phase 1 clinical trial. Hum. Gene Ther. Methods 23, 271–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor L.H., Erway L.C., Vogler C.A., et al. (1998). Enzyme replacement therapy for murine mucopolysaccharidosis type VII leads to improvements in behavior and auditory function. J. Clin. Invest. 101, 1394–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau M., and Kremer E.J. (2005). Frequency, proliferation, and activation of human memory T cells induced by a nonhuman adenovirus. J. Virol. 79, 14595–14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau M., Guerin M.C., Drouet C., and Kremer E.J. (2007a). Interactions between human plasma components and a xenogenic adenovirus vector: reduced immunogenicity during gene transfer. Mol. Ther. 15, 1998–2007 [DOI] [PubMed] [Google Scholar]
- Perreau M., Mennechet F., Serratrice N., et al. (2007b). Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: implications for clinical efficacy. J. Virol. 81, 3272–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas S., Bilsland L.G., Henaff D., et al. (2009). CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 5, e1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardiello M., Palmieri M., di Ronza A., et al. (2009). A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 [DOI] [PubMed] [Google Scholar]
- Schagen F.H., Rademaker H.J., Rabelink M.J., et al. (2000). Ammonium sulphate precipitation of recombinant adenovirus from culture medium: an easy method to increase the total virus yield. Gene Ther. 7, 1570–1574 [DOI] [PubMed] [Google Scholar]
- Schuldt A.J., Hampton T.J., Chu V., et al. (2004). Electrocardiographic and other cardiac anomalies in beta-glucuronidase-null mice corrected by nonablative neonatal marrow transplantation. Proc. Natl. Acad. Sci. USA 101, 603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J., Warren H.S., Cuenca A.G., et al. (2013). Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley J.M., Klinkenberg M., Wu B.M., et al. (1993). Mutational analysis of a patient with mucopolysaccharidosis type VII, and identification of pseudogenes. Am. J. Hum. Genet. 52, 517–526 [PMC free article] [PubMed] [Google Scholar]
- Sly W.S., Vogler C., Grubb J.H., et al. (2001). Active site mutant transgene confers tolerance to human beta-glucuronidase without affecting the phenotype of MPS VII mice. Proc. Natl. Acad. Sci. USA 98, 2205–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper B.W., Lessard M.D., Vogler C.A., et al. (2001). Nonablative neonatal marrow transplantation attenuates functional and physical defects of beta-glucuronidase deficiency. Blood 97, 1498–1504 [DOI] [PubMed] [Google Scholar]
- Sotak B.N., Hnasko T.S., Robinson S., et al. (2005). Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 1061, 88–96 [DOI] [PubMed] [Google Scholar]
- Soudais C., Laplace-Builhe C., Kissa K., and Kremer E.J. (2001). Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15, 2283–2285 [DOI] [PubMed] [Google Scholar]
- Soudais C., Skander N., and Kremer E.J. (2004). Long-term in vivo transduction of neurons throughout the rat CNS using novel helper-dependent CAV-2 vectors. FASEB J. 18, 391–393 [DOI] [PubMed] [Google Scholar]
- Thomas C.E., Birkett D., Anozie I., et al. (2001). Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 3, 36–46 [DOI] [PubMed] [Google Scholar]
- Thurberg B.L., Lynch Maloney C., Vaccaro C., et al. (2006). Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab. Invest. 86, 1208–1220 [DOI] [PubMed] [Google Scholar]
- Vogler C., Levy B., Kyle J.W., et al. (1994). Mucopolysaccharidosis VII: postmortem biochemical and pathological findings in a young adult with beta-glucuronidase deficiency. Mod. Pathol. 7, 132–137 [PubMed] [Google Scholar]
- Vogler C., Sands M.S., Galvin N., et al. (1998). Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease. J. Inherit. Metab. Dis. 21, 575–586 [DOI] [PubMed] [Google Scholar]
- Wilcox W.R., Banikazemi M., Guffon N., et al. (2004). Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am. J. Hum. Genet. 75, 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.