Abstract
Juvenile neuronal ceroid lipofuscinosis (JNCL or CLN3 disease) is an autosomal recessive lysosomal storage disease resulting from mutations in the CLN3 gene that encodes a lysosomal membrane protein. The disease primarily affects the brain with widespread intralysosomal accumulation of autofluorescent material and fibrillary gliosis, as well as the loss of specific neuronal populations. As an experimental treatment for the CNS manifestations of JNCL, we have developed a serotype rh.10 adeno-associated virus vector expressing the human CLN3 cDNA (AAVrh.10hCLN3). We hypothesized that administration of AAVrh.10hCLN3 to the Cln3Δex7/8 knock-in mouse model of JNCL would reverse the lysosomal storage defect, as well as have a therapeutic effect on gliosis and neuron loss. Newborn Cln3Δex7/8 mice were administered 3×1010 genome copies of AAVrh.10hCLN3 to the brain, with control groups including untreated Cln3Δex7/8 mice and wild-type littermate mice. After 18 months, CLN3 transgene expression was detected in various locations throughout the brain, particularly in the hippocampus and deep anterior cortical regions. Changes in the CNS neuronal lysosomal accumulation of storage material were assessed by immunodetection of subunit C of ATP synthase, luxol fast blue staining, and periodic acid-Schiff staining. For all parameters, Cln3Δex7/8 mice exhibited abnormal lysosomal accumulation, but AAVrh.10hCLN3 administration resulted in significant reductions in storage material burden. There was also a significant decrease in gliosis in AAVrh.10hCLN3-treated Cln3Δex7/8 mice, and a trend toward improved neuron counts, compared with their untreated counterparts. These data demonstrate that AAVrh.10 delivery of a wild-type cDNA to the CNS is not harmful and instead provides a partial correction of the neurological lysosomal storage defect of a disease caused by a lysosomal membrane protein, indicating that this may be an effective therapeutic strategy for JNCL and other diseases in this category.
Introduction
Juvenile neuronal ceroid lipofuscinosis (JNCL, or CLN3 disease) is an autosomal recessive, progressive neurodegenerative disorder resulting from mutations in the CLN3 gene, which codes for the CLN3 protein, a lysosomal transmembrane protein of unknown function (The International Batten Disease Consortium, 1995; Hofman et al., 1999; Jarvela et al., 1999; Lerner, 2001; Mao et al., 2003). The disease is characterized by accumulation in CNS neurons and in many somatic cell types of autofluorescent storage materials that include subunit C of ATP synthase (ATPase), lipoproteins, and glycoproteins (Palmer et al., 1992). JNCL manifests at around 7 years, with initial symptoms of visual failure, followed by progressive neurodegeneration, including cognitive and motor defects and epilepsy (Munroe et al., 1997; Hofman et al., 1999; Lerner, 2001). There is no specific treatment for JNCL except for palliative care and the management of seizures and psychiatric symptoms (Hofman et al., 1999; Lerner, 2001). Most children survive into the second or third decade (Munroe et al., 1997; Lerner, 2001). The incidence of the disease is estimated at 1–5 in 100,000 live births, with an increased prevalence in the northern European population (Lerner, 2001).
Since the CNS manifestations of JNCL disease are widespread, an effective treatment strategy must restore CLN3 protein function to a large portion of the brain. Adeno-associated viral vector (AAV)-mediated gene transfer has been used to treat mouse models of the CNS manifestations of several lysosomal storage disorders and leukodystrophies, and has also been used in clinical trials (Janson et al., 2002; Crystal et al., 2004; Sands and Davidson, 2006; Worgall et al., 2008; Bowers et al., 2011; Gritti, 2011; Mingozzi and High, 2011; Leone et al., 2012; Rafi et al., 2012; Tomanin et al., 2012). However, JNCL differs from many of the lysosomal storage disorders in that it results from deficiency in a lysosomal membrane protein (Mao et al., 2003; Rakheja et al., 2008; Getty and Pearce, 2011), and the CLN3 protein is not secreted in a precursor form that enables uptake by the mannose 6-phosphate receptor (Michalewski et al., 1999). Thus, gene therapy approaches to the treatment of JNCL cannot benefit from cross-correction (Neufeld and Fratantoni, 1970; Sands and Davidson, 2006). In the case of JNCL, only the cells directly transduced by the AAV vector are likely to be corrected by the wild-type CLN3 protein expressed by the vector, although correction of the CLN3 defect in one cell may still lead to correction of neighboring cells by influencing secretion of other factors that may normally be regulated by CLN3.
On the basis of our experience in using the nonhuman primate-derived AAVrh.10 vector to treat the CNS manifestations of the late infantile neuronal ceroid lipofuscinosis (LINCL), a lysosomal storage disorder (Sondhi et al., 2007, 2008), we hypothesized that an AAVrh.10 vector coding for CLN3 would be capable of reducing the lysosomal storage abnormality in the CNS of Cln3Δex7/8 mice, a mouse model of JNCL that recreates the human disease-causing mutation (Cotman et al., 2002). To test this hypothesis, we administered AAVrh.10hCLN3, an AAVrh.10 vector driving the expression of the human CLN3 protein, to the CNS of newborn Cln3Δex7/8 mice, and 16–18 months later, evaluated the CNS of these mice compared with untreated Cln3Δex7/8 and wild-type mice.
Materials and Methods
Vector production
The genome of AAVrh.10hCLN3 includes the inverted terminal repeats from AAV2 surrounding the expression cassette (Sondhi et al., 2007). The expression cassette includes the CMV/β-actin hybrid promoter (CAG), the human CLN3 cDNA with an optimized Kozak translational initiation signal before the start codon, and a rabbit β-globin poly(A) sequence (Daly et al., 1999a). This vector was produced by cotransfection of forty 150 mm plates (80% confluent) of 293 cells with 500 μg of an expression cassette plasmid (pAAV2-CAG-hCLN3) and 1 mg of an adenovirus/AAVrh.10 helper plasmid (pPAK-MArh.10) using PolyFect reagent (Qiagen Sciences, Germantown, MD). The helper plasmid contained the AAVrh.10 cap gene and the AAV2 rep gene necessary for viral reproduction and capsid production. Cells were harvested (1150×g, 15 min) 72 hr posttransfection, and a crude viral lysate (CVL) was made by three cycles of freeze–thawing. Benzonase (50 U/ml; Sigma-Aldrich, St. Louis, MO) was used to remove any contaminant genomic DNA. The remaining CVL was centrifuged (3300×g, 20 min) and supernatant was applied to a discontinuous iodixanol gradient. It was then purified by Q-HP ion exchange chromatography (GE Healthcare, Port Washington, NY) and centrifugally concentrated into phosphate-buffered saline (PBS). Vector concentration in genome copies was determined by TaqMan real-time polymerase chain reaction (PCR) with absolute quantitation. To confirm functionality, 293ORF6 cells were infected with AAVrh.10hCLN3, and CLN3 protein expression was confirmed via in vitro immunofluorescence 72 hr postinfection (as described below). All vectors used in these studies were shown to be sterile by growth for 14 days on a medium supporting the growth of aerobic bacteria, anaerobic bacteria, or fungi and endotoxin free to a level of <200 U of endotoxin per milliliter by the Endosafe method (Charles River, Charleston, SC).
In vitro infection with AAVrh.10hCLN3
To test functionality of the virus, 293ORF6 cells were infected with AAVrh.10hCLN3 in vitro in 6-well plates (60% confluency) with 2×105 genome copies of virus per cell in 1.5 ml incomplete medium and incubated at 37°C with rocking every 10 min. After 1 hr, 1.5 ml complete medium with 100 nM zinc chloride was added and infected cells were incubated at 37°C for 72 hr. Cells were harvested with 5 mM EDTA, pelleted (200×g, 10 min), resuspended in PBS, and cytospin slides prepared at 160,000 cells per slide. Slides were air-dried for 1 hr, fixed with 4% paraformaldehyde, dehydrated with graded ethanol series, and stored at 20°C. The virus-derived human CLN3 protein was detected by immunofluorescence using a batp1 affinity-purified polyclonal rabbit antihuman CLN3 antibody (Cotman et al., 2002). Cytospin cell preparations were blocked with 1.5% normal donkey serum (Jackson Immunoresearch, West Grove, PA) while being permeabilized with 0.05% triton X-100 for 1 hr, and incubated in primary antibody diluted 1:400 in 1.5% normal donkey serum overnight at 4°C. The batp1 antibody preabsorbed with the antigenic peptide (20 μg/ml; New England Peptide, Gardner, MA) for 1 hr at room temperature was used to demonstrate antibody specificity. Donkey antirabbit IgG conjugated to Cy3 (1:200; Jackson Immunoresearch) was used to detect the primary antibody. Cells were counterstained with DAPI (1:2000; Invitrogen, Carlsbad, CA).
Homozygous Cln3Δex7/8 mice
Homozygous Cln3Δex7/8 mice (referred to as “Cln3Δex7/8 mice”) were previously established and characterized on a mixed, 129/CD1 outbred genetic background (Cotman et al., 2002). From this mixed background, mice were subsequently backcrossed for more than 10 generations onto the inbred C57BL/6N background and bred as heterozygous pairs with genotyping of pups at weaning by PCR, in order to produce homozygous mice for this study. Wild-type allele (primer sequences CTGGCTTTGACTCTGGGGTCTCGG and GCGACTCTGGACAACTTCATTCCTGAC) and mutant allele (primer sequences GGCTTTGACTCTGGGGTCTCGGTG and CAGTCAGTGGGGTTAAGCCTGTGTGG) were detected in separate PCRs. Phenotypes in Cln3Δex7/8 mice on the C57BL/6N genetic background have been recently described (Staropoli et al., 2012).
Administration of AAVrh.10hCLN3 to Cln3Δex7/8 mice
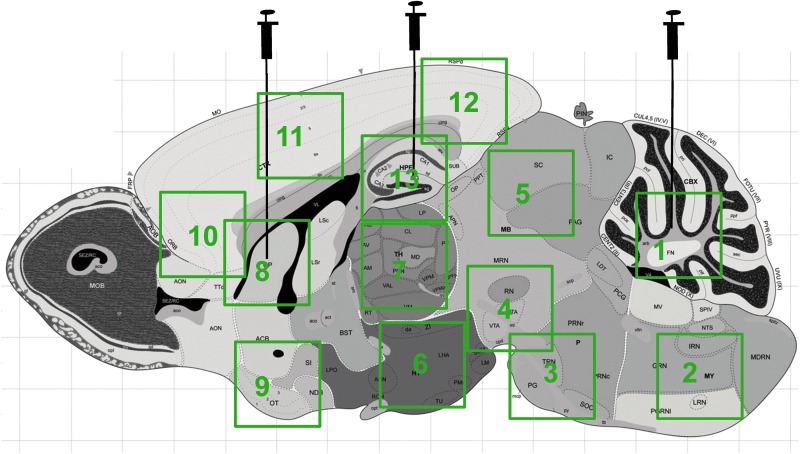
Neonatal Cln3Δex7/8 mice received direct administration of AAVrh.10hCLN3 into the brain parenchyma at 2 days of age (mice were administered without knowing their genotype; genotyping was performed at 3 weeks of age). Mice were anesthetized on ice and administered six doses of 0.5×1010 genome copies of AAVrh.10hCLN3 in 0.5 μl volume for a total of 3×1010 genome copies of AAVrh.10hCLN3 per mouse. Doses were administered bilaterally using a stereotactic frame and targeted to the striatum (−0.10 AP,±0.20 ML, −0.20 DV from bregma), hippocampus (−0.35 AP,±0.15 ML, −0.20 DV from bregma), and cerebellum (−0.60 AP,±0.10 ML, −0.20 DV from bregma) at a rate of 1 μl/min (Fig. 1). Neonatal mice were then warmed with a heat lamp and monitored for complications.
FIG. 1.
Schematic of a sagittal section of the brain identifying the regions of vector administration (indicated with syringes) and the various regions assessed (identified by green boxes). 1, cerebellum; 2, medulla; 3, pons; 4, ventral midbrain; 5, dorsal midbrain; 6, hypothalamus; 7, thalamus; 8, striatum; 9, olfactory tubercle; 10, anterior cortex; 11, central cortex; 12, posterior cortex; and 13, hippocampus. Color images available online at www.liebertpub.com/hum
Tissue processing and immunodetection of the CLN3 protein
Animals were euthanized by 16–18 months of age. Mice were anesthetized with a ketamine/xylazine cocktail and transcardially perfused with cold PBS followed by periodate-lysine-phosphate fixative. The brains were extracted, fixed overnight, embedded in paraffin, and cut into sagittal sections around the injection sites (Histoserv, Gaithersburg, MD) determined by comparing the neonatal and adult mouse brain size and estimating the medial-lateral location of the injection sites in adult mice. For immunostaining with the batp1 antibody, the slides were first deparaffinized and then subjected to antigen retrieval in citric acid buffer at 88°C for 20 min (Dako, Carpinteria, CA). For cell type identification, sections were costained with batp1 and mouse anti-glial fibrillary acidic protein (GFAP) GA5 mAb (#3670; Cell Signaling, Danvers, MA). The secondary antibody used to detect GFAP was goat antimouse Alexa 488 (used at 1:500; Molecular Probes, Life Technologies, Grand Island, NY). Immunostained slides were coverslipped with ProLong Gold Antifade mounting medium containing DAPI according to the manufacturer's specifications (Molecular Probes, Life Technologies). Immunostained slides were imaged on a Leica SP5 scanning laser confocal microscope using 20× and 40× oil objectives. For image comparison sets, images of like magnifications were collected using identical settings in LASAF software. Images were exported from LASAF as .tif files and subsequently loaded into Adobe Photoshop, where all brightness image settings were kept constant within each comparison set. Brains from four mice per genotype per treatment status were immunostained for CLN3 using the batp1 antibody, and for the batp1 antibody preincubated with the antigenic peptide, and results were highly comparable between individual mice. Representative images are therefore shown.
TaqMan real-time PCR
Wild-type mice (treated with AAVrh.10hCLN3 and untreated, n=3 per group) euthanized at 16 months were perfused with ice-cold PBS. The fresh brains were extracted and hemisected in an adult mouse brain slicer matrix (Zivic Instruments, Pittsburgh, PA) and then further divided into six 2 mm coronal sections per hemisphere using brain matrix razor blades (EMS, Hatfield, PA). Each section was then homogenized in 400 μl of TRIZol and the samples are stored overnight at −80°C. Total RNA was purified from these homogenized coronal brain sections and cDNA was generated using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). TaqMan relative expression assays (Applied Biosystems) using primer and probes for the human CLN3 cDNA were performed for each sample in duplicate at two dilutions with paired 18S rRNA endogenous controls. The mean background from untreated wild-type mice was used as a calibrator.
Morphological assessments
Autofluorescence
Autofluorescence was evaluated in three sections from each animal, which were deparaffinized and counterstained with DAPI (1:2000; Invitrogen). Accumulation of ATPase subunit C was detected by immunohistochemistry with an affinity-purified polyclonal rabbit antihuman ATPaseC antibody following a previously described protocol (Cotman et al., 2002). The ATPaseC antibody preabsorbed with the antigenic peptide (20 μg/ml; New England Peptide, Gardner, MA) for 1 hr at 23°C was used to demonstrate antibody specificity. Paraffin sections were stained with luxol fast blue (cresyl violet counterstain, LFB-CV) and periodic acid-Schiff (methyl green counterstain, PAS) using standard methods (Mitchison et al., 1999; Mark et al., 2007; Puntel et al., 2010). For quantification of ATPase subunit C, three randomly chosen images were analyzed from each animal with three animals being analyzed from each group for a total of nine images per group. All images were analyzed following the same protocol. The acquired ATPase images were imported into ImageJ. The image type was first adjusted to 8-bit. The image was then autobrightened and autoleveled. The image was thresholded using the “Triangle” and “B&W” settings. The images were analyzed using the analyze particles command with size parameters set at 50 to infinity, circularity set from 0 to 1.00, and the show overlay outlines option selected. The number of particles counted was averaged and the standard error was calculated for each group.
Immunohistochemical staining for glial markers
Gliosis was evaluated by immunostaining for GFAP as a marker for astrocytosis and CD68 for microglial activation. This was performed with polyclonal rabbit antimouse GFAP (1:4000; Dako, Cambridge, United Kingdom) and polyclonal rat antimouse CD68 (1:2000; AbD Serotec, Kidlington, United Kingdom). The respective primary antibodies were then detected using a VECTASTAIN Elite ABC Standard kit, using biotinylated secondary antibodies—goat antichicken IgG (1:200; Vector Laboratories, Burlingame, CA) for chicken anti-GFAP IgY (Millipore, Billerica, MA), biotinylated swine antirabbit (1:1000; Vector Laboratories, Peterborough, United Kingdom) for rabbit antimouse GFAP, and biotinylated rabbit antirat (1:1000; Vector Laboratories) for rat antimouse CD68, respectively. The sections were then developed with ImmPACT DAB (Vector Laboratories, Burlingame, CA) or 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, Dorset, United Kingdom) as required.
Staining for specific neuron populations
To assess the impact of AAVrh.10hCLN3 administration upon neuron number, mice from each treatment group (n=2–4) were analyzed histologically. To provide direct visualization of neuronal morphology, every sixth mouse brain section was stained with the Nissl dye cresyl violet as previously described (Bible et al., 2004; Pontikis et al., 2004). Briefly, sections were mounted onto gelatin-chrome alum-coated Superfrost microscope slides (VWR, Dorset, United Kingdom), air-dried overnight and incubated for 20 min at 55°C in 0.05% cresyl fast violet and 0.05% acetic acid (VWR), rinsed in distilled water and differentiated through a graded series of alcohol before clearing in xylene (VWR), and coverslipped with DPX (a xylene-based mountant for microscopy; VWR). These Nissl-stained sections were subsequently used for stereological analysis of neuron number, as described previously (Bible et al., 2004; Pontikis et al., 2004). Somatostatin was used as a marker to survey interneuron populations in the hippocampus and M1 motor cortex (Bible et al., 2004). A one-in-six series of sections from each brain was collected, and endogenous peroxidase activity was quenched in a 1% H2O2 solution in TBS (tris-buffered saline). Nonspecific protein binding was blocked in 15% normal goat serum (Vector Laboratories, Peterborough, United Kingdom) solution in TBS-T. Sections were incubated overnight at 4°C in polyclonal rabbit anti-somatostatin-14 (1:2000; Peninsula Laboratories, CA) in 10% normal goat serum (Vector Laboratories) solution in TBS-T (mixture of tris-buffered saline and tween 20). The primary antibody was detected by incubating sections with goat antirabbit IgG (H+L) (1:1000; Vector Laboratories) secondary antiserum in 10% normal goat serum (Vector Laboratories) solution in TBS-T. Sections were then treated with avidin–biotin–peroxidase complex (Vectastain Elite ABC kit, 1:1000; Vector Laboratories) and incubated in a 0.05% solution of DAB (Sigma) containing 0.001% H2O2 in TBS for 8–10 min. Sections were rinsed with TBS and then mounted on gelatin-chrome alum superfrost slides, allowed to air-dry overnight, cleared in xylene (VWR), and coverslipped with DPX (VWR).
Quantification of autofluorescence
Autofluorescence was detected in the TRITC channel on a Zeiss Axiovert 200M microscope (Carl Zeiss Ltd., Welwyn Garden City, United Kingdom) with the same image capture parameters for comparable sections. To quantify the autofluorescence, 12-bit images of 13 areas were taken for n=5 mice per group and three sections per area per mouse with matched exposure. By assessment of the fluorescent intensity distribution by pixel in selected low-intensity areas in wild-type mice, a threshold was established. All pixels with greater than this intensity in each section were counted using Metamorph imaging software (Molecular Devices, Sunnyvale, CA).
Quantification of GFAP and CD68 immunostaining
For GFAP (polyclonal rabbit antimouse GFAP) and CD68 (polyclonal rat antimouse CD68) immunostained sections, the density of immunoreactivity was measured by Image Pro Plus (Media Cybernetics, Chicago, IL), a semiautomated quantitative thresholding image analysis software package. This analysis was performed blind to genotype, as previously described (Bible et al., 2004; Pontikis et al., 2004). Thirty or forty nonoverlapping images were captured, on three or four consecutive sections for the M1 cortex, striatum, ventral posterior nucleus of the thalamus (VPM/VPL), and CA1 subfield of the hippocampus. All RGB images were captured via a live video camera (JVC, 3CCD, KY-F55B) mounted onto a Zeiss Axioplan microscope using a 40×objective, and saved as JPEGs. All parameters, including lamp intensity, video camera setup, and calibration, were maintained constant throughout image capturing. Image Pro Plus (Media Cybernetics) software was used to calculate the density of immunoreactivity in each image after setting the appropriate thresholds on each color filter (Pontikis et al., 2004, 2005).
Measurements of neuron and interneuron number
Stereoinvestigator software (Microbrightfield, Williston, VT) was used to obtain estimates of neuronal and interneuron number as previously described (Bible et al., 2004). Nissl-stained sections were used to obtain estimates of neuronal number from the dorsal-lateral geniculate nucleus of the thalamus (LGNd), lamina V of the M1 cortex, and the striatum using the unbiased optical fractionator method (West et al., 1991). This method was also used to obtain estimates of interneuron numbers from the M1 cortex. A random starting section was chosen, followed by every sixth immunostained section thereafter. The boundaries of the selected regions were defined by reference to landmarks in Paxinos and Franklin (2001). Only neurons with a clearly identifiable nucleus were sampled. Counts were carried out using a 100× oil objective (NA 1.4) for neuronal counts and a 40× objective (NA 1.3) for interneuron counts on a Axioskop2 microscope (Zeiss, Welwyn Garden City, United Kingdom), attached to a live video camera (DAGE-MTI-CCD100). The following sampling scheme was applied to each area: for neuron counts, LGNd grid size 125 μm×125 μm, frame size 70 μm×40 μm; lamina V of M1 cortex grid size 125 μm×125 μm, frame size 70 μm×40 μm; and striatum grid size 400 μm×400 μm, frame size 70 μm×40 μm. For interneuron counts, grid size and frame size were both 400 μm×200 μm.
Because of the relatively fewer number of GABAergic interneurons in the hippocampus, counts for interneurons were made in each of the hippocampal subfields (hilum, CA1/2/3, stratum radiatum, and stratum oriens) under the 20× objective of the Axioplan microscope (Zeiss). Only positively stained cells with clear interneuron morphology were counted. The numbers were expressed as the mean number of interneurons per section in each of the hippocampal subfields, respectively (Bible et al., 2004).
Statistical analysis
Quantitative autofluorescence and gliosis were assessed by repeated-measures ANOVA where three individual fields on one slide represent the repeated measures. For each structure, repeated-measures ANOVA was followed by the post-hoc Fisher's PLSD test to provide p-value to compare experimental groups. For combined structures, the ANOVA was used with brain structure and experimental groups as variables. For glial thresholding image analysis and neuronal counts, a one-way ANOVA test (SPSS 11.5 Software, SPSS Inc., Chicago, IL) was used to determine the statistical significance of any differences between the groups and a post-hoc Bonferroni correction was implemented on all data, with p≤0.05 considered statistically significant.
Results
The majority of JNCL patients are homozygous for a common 1.02 kb deletion in the CLN3 gene that eliminates exons 7 and 8 and changes the reading frame to produce a premature stop codon resulting in a truncated protein product (Munroe et al., 1997; Lerner, 2001). In order to test CLN3 gene transfer as a potential treatment for JNCL, we chose the Cln3Δex7/8 knock-in mouse model. These mice accurately mimic the human 1.02 kb deletion in the endogenous mouse Cln3 gene and have been shown to exhibit a recessive JNCL-like disease on multiple genetic backgrounds (Cotman et al., 2002; Staropoli et al., 2012). At the outset of this study, we aimed to broadly assess the impact of AAVrh.10hCLN3 treatment on both pathological and motor performance parameters. However, the motor performance tasks used, which included the balance beam and grip strength apparatus, did not reveal any significant differences between wild-type mice and Cln3Δex7/8 knock-in mice up to 78 weeks of age (Sondhi et al., 2011). As such, to assess the impact of AAVrh.10hCLN3 treatment, morphological analyses were used to investigate long-term expression of the CLN3 gene, and neuropathological analyses were used to determine whether there was any treatment-dependent modulation of lysosomal storage pathology, glial activation, and neuronal loss.
CLN3 gene expression
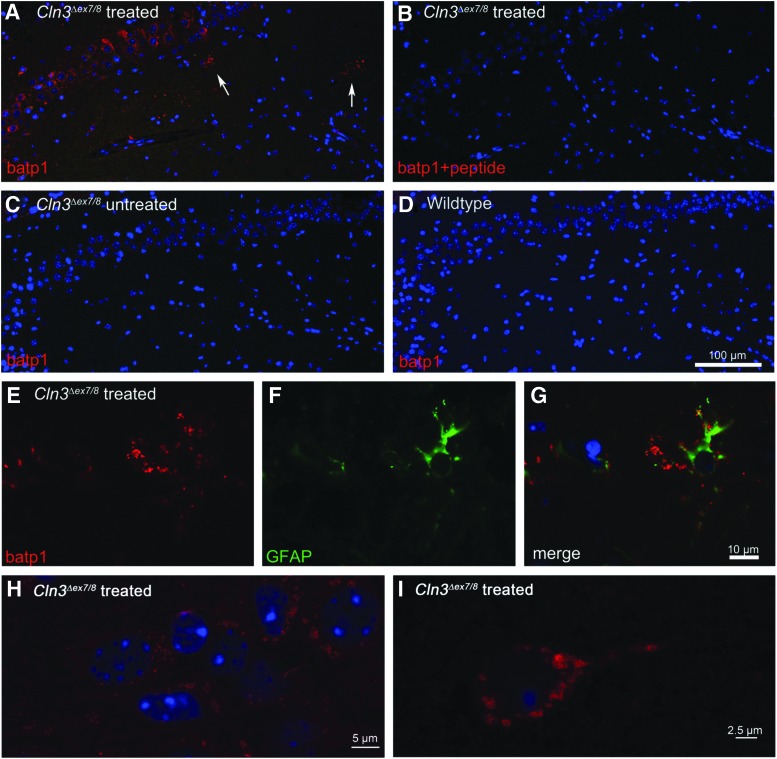
At 16–18 months of age, wild-type, untreated Cln3Δex7/8 mice and Cln3Δex7/8 mice treated with AAVrh.10hCLN3 as neonates were euthanized for analysis of transgene expression and for morphological assessment of neuropathology. Vector-derived CLN3 expression was assessed by immunofluorescent staining using the batp1 antihuman CLN3 antibody in sagittal sections of the brain (Fig. 2A, absent in wild-type controls 2D). The specificity of this immunofluorescence was demonstrated by the absence of staining in untreated Cln3Δex7/8 mice (Fig. 2C), and the absence of staining when blocking with peptide corresponding to the epitope recognized by the anti-CLN3 monoclonal antibody (Fig. 2B). In all AAVrh.10hCLN3-treated Cln3Δex7/8 mice, clusters of CLN3-expressing cells were detected in multiple brain regions, particularly near the targeted locations in the hippocampus and the anterior deep layers of the cortex (Fig. 2A and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum). The staining was vesicular in appearance and perinuclear in morphologically identified neurons, consistent with an endosomal and/or lysosomal localization, as shown in the higher magnification images in Fig. 2H and I. A high level of batp1 staining was also observed in GFAP-positive astrocytes, particularly in the deeper cortical layers and in the ventral tegmental area (Fig. 2 and Supplementary Fig. S1M). In these cells, batp1 immunoreactivity was predominantly in vesicles that extended out from the cell bodies, along astrocytic processes (Fig. 2E–G).
FIG. 2.
Long-term expression of the CLN3 cDNA after neonatal CNS gene transfer with AAVrh.10hCLN3. AAVrh.10hCLN3 (3×1010 genome copies [gc]) was administered to the CNS of Cln3Δex7/8 mice and wild-type littermates. The mice were euthanized at age 16–18 months. (A–I) Representative micrographs of the hippocampal and cortical regions in sagittal sections, immunostained using an antihuman CLN3 antibody (batp1, red). (A) Section from AAVrh.10hCLN3-treated Cln3Δex7/8 mice; batp1 immunoreactivity was evident in cells within the pyramidal cell layer that were morphologically consistent with neurons and in some glial-like cells (white arrows). (B) AAVrh.10hCLN3-treated Cln3Δex7/8 section immunostained with batp1 that had been preincubated with the antigenic peptide is shown as a control for the specificity of the batp1 immunoreactivity. (C) Cln3Δex7/8 untreated mice; note the lack of batp1 immunoreactivity. (D) Section from a wild-type littermate; as with the untreated mutant, note the lack of robust batp1 immunoreactivity, confirming the higher degree of specificity of the batp1 antibody for detection of the human CLN3 expressed by gene transfer versus endogenous mouse CLN3 protein. (E–I) Zoomed images demonstrating batp1 staining in glia-like cells, which were also positive for GFAP (E–G), and in neuron-like cells within the hippocampal pyramidal cell layer (H) and cortex (I). Nuclei were costained with DAPI (blue). Scale bars: (A–D) 100 μm; (E–G) 10 μm; (H) 5 μm; (I) 2.5 μm. AAVrh.10hCLN3, a serotype rh.10 adeno-associated virus vector expressing the human CLN3 cDNA; GFAP, glial fibrillary acidic protein.
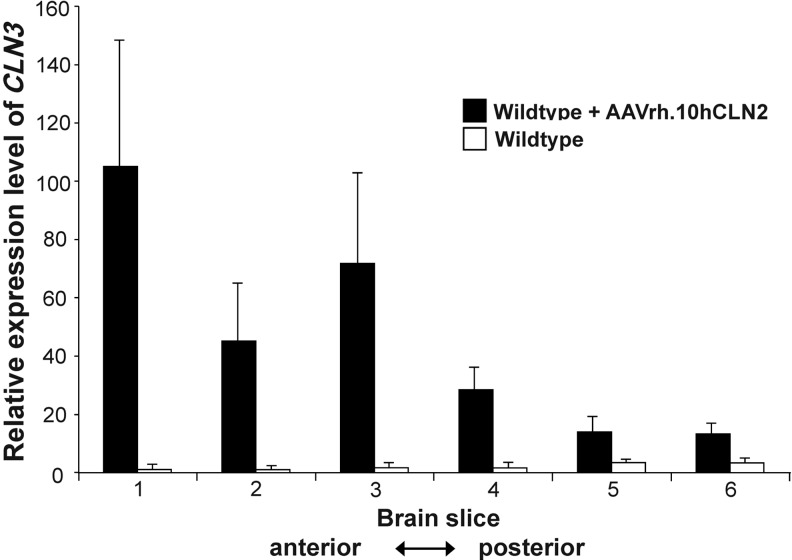
We also assessed the level of vector-dependent long-term CLN3 expression by an independent method. CLN3 mRNA levels were determined by quantitative TaqMan real-time PCR on wild-type littermate mice that had been injected as a newborns along with the Cln3Δex7/8 mice at 16 months of age, and untreated wild-type mice of the same age (16 months) were used to establish the baseline. The AAVrh.10hCLN3-treated mice had levels 12–100 times background, with higher levels of expression in the anterior part of the brain and lower levels in the posterior brain regions, consistent with our batp1 immunostaining results (Fig. 3). Thus, at both the RNA and protein levels these data suggest that long-term vector-derived expression was achieved after the neonatal administration of AAVrh.10hCLN3 to the CNS.
FIG. 3.
TaqMan assessment of CLN3 mRNA levels. Brains from untreated and treated wild-type mice (n=3 per group) were cut into 6 coronal sections (anterior to posterior) and divided into left and right hemispheres. TaqMan real-time polymerase chain reaction with relative quantitation was used to assess hCLN3 expression level. Background CLN3 levels were established using age-matched untreated wild-type mice. The data from the left and right hemispheres for each section were pooled. Data are plotted as mean expression level±standard error of measurement. The p-value was calculated using a two-way ANOVA, and the reported value for the treatment parameter is p<0.0001.
Impact of treatment on autofluorescence
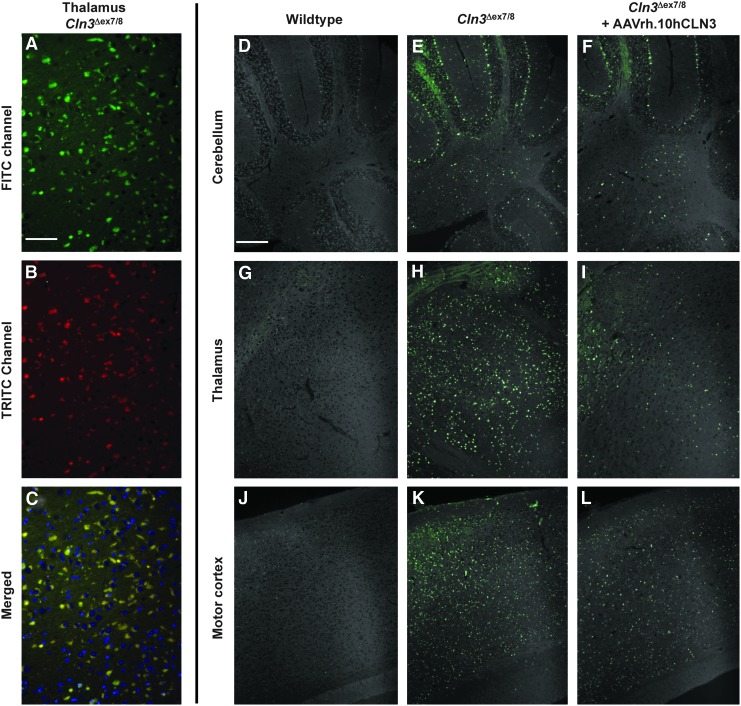
Histological assessment of storage pathology provides a measure of the impact of gene transfer on disease progression. Three groups of age-matched mice including wild-type, untreated Cln3Δex7/8 mice, and treated Cln3Δex7/8 mice (n=5 per group) were euthanized and sagittal sections of the brain were assessed for autofluorescence. The sites of administration and the various regions of the brain that were assessed were as described in Materials and Methods and are shown in Fig. 1. As expected, substantial accumulation of autofluorescent material was evident in multiple areas of the brain in untreated Cln3Δex7/8 mice compared with wild-type controls. This material was punctate, vesicular, and perinuclear in location by visualization of autofluorescence in both green and red channels (Fig. 4A–C), consistent with previously reported appearance of the autofluorescent storage material that accumulates in these mice (Cotman et al., 2002; Pontikis et al., 2005). Multiple brain structures, including cerebellum, thalamus, and cortex, showed substantial accumulation of autofluorescent material in untreated Cln3Δex7/8 mice (Fig. 4E, H, and K, respectively), compared with wild-type littermates (Fig. 4D, G, and J). Reductions in storage material were observed with AAVrh.10hCLN3 treatment of Cln3Δex7/8 mice as neonates (Fig. 4F, I, and L). In the cerebellum (Fig. 4D–F), the most prominent accumulation was in the Purkinje cells of untreated Cln3Δex7/8 mice (Fig. 4E), and this was partially reduced in AAVrh.10hCLN3-treated Cln3Δex7/8 mice (Fig. 4F). In both the thalamus (Fig. 4G–I) and frontal cortex (Fig. 4J–L), cell bodies in the gray matter were autofluorescent in untreated Cln3Δex7/8 mice (Fig. 4H and K, respectively), but their overall intensity was reduced in AAVrh.10hCLN3-treated Cln3Δex7/8 mice (Fig. 4I and L, respectively). Higher magnification views (Supplementary Fig. S2) support the conclusions drawn from Fig. 4.
FIG. 4.
Histologic assessment of lysosomal autofluorescence after AAVrh.10hCLN3 gene transfer to the CNS of Cln3Δex7/8 mice. (A–C) Accumulation of autofluorescent vesicular, perinuclear storage material in Cln3Δex7/8 mice. (A) Thalamus in Cln3Δex7/8 mice with autofluorescence detected in the FITC channel. (B) Same as in (A) with autofluorescence detected in the TRITC channel. (C) Merged image of (A) and (B) counterstained with DAPI showing accumulation of autofluorescent vesicular, perinuclear storage material. (D–L) Effects of treatment as assessed by autofluorescence in unstained sections. Each structure (cerebellum, thalamus, motor cortex) is shown as merged low-magnification images of the FITC channel (green) and TRITC channel (white). (D) Cerebellum, wild-type mouse. (E) Cerebellum, untreated Cln3Δex7/8 mice. (F) Cerebellum, homozygous Cln3Δex7/8 mice treated with AAVrh.10hCLN3. (G) Thalamus, wild-type mouse. (H) Thalamus, untreated Cln3Δex7/8 mice. (I) Thalamus, Cln3Δex7/8 mice treated with AAVrh.10hCLN3. (J) Motor cortex, wild-type mouse. (K) Motor cortex, untreated Cln3Δex7/8 mice. (L) Motor cortex, Cln3Δex7/8 mice treated with AAVrh.10hCLN3. Scale bars: (A–C) 50 μm; (D–L) 200 μm.
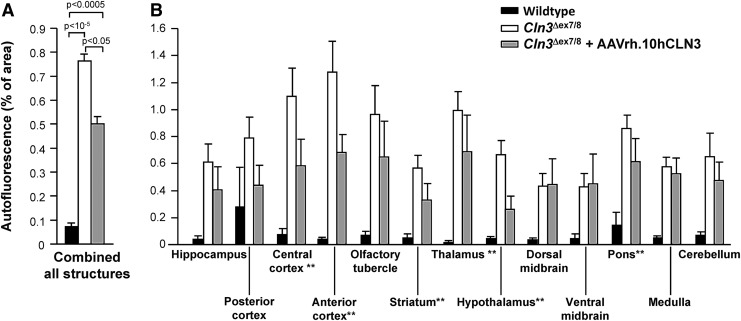
Autofluorescent storage material was quantified by counting pixels above an intensity threshold established in wild-type mice in 13 areas of the brain (Fig. 5). In wild-type mice, this threshold detected only 0.07%±0.01% of pixels in all sections, with all areas of wild-type brains displaying a similar intensity of autofluorescence (Fig. 5A). In contrast, in the untreated Cln3Δex7/8 mice, 0.76%±0.03% of pixels had intensity greater than the threshold. Neonatal treatment of Cln3Δex7/8 mice reduced this value to 0.50%±0.03%, representing a 28% correction of storage defect by this measure (Fig. 5A). The overall reduction in autofluorescence between untreated and AAVrh.10hCLN3-treated Cln3Δex7/8 mice treated with AAVrh.10hCLN3 was significant (p<0.05), but did not completely abolish autofluorescence to wild-type levels (p<0.0005). Although the neonatally treated Cln3Δex7/8 mice were administered AAVrh.10hCLN3 into three locations per hemisphere targeting the striatum, hippocampus, and cerebellum, we also assessed other brain regions (Fig. 1). The structures with most significant reduction in autofluorescence were the central and anterior cortex, the striatum, the thalamus, the hypothalamus, and the pons, corresponding to regions overlying or close to the sites of administration (Fig. 5B). In 46% (6/13) of the structures assessed, there was a significant reduction in storage burden (Fig. 5B and Supplementary Table S1). In five of the seven remaining structures, there was a trend toward decreased autofluorescence in response to treatment with AAVrh.10CLN3, although this was not statistically significant. One such region was the cerebellum, where the vector had been administered. This may be because while there is clearly some amelioration in the amount of storage material in the cerebellum (Fig. 4E and F), it was limited to the Purkinje cells. Or it could be because the levels of storage are relatively high in the cerebellum and therefore harder to reduce to wild-type levels. In the regions in the midbrain (dorsal and ventral midbrain), no impact of therapy upon storage burden was observed, likely because these regions are distant from any of the vector administration sites.
FIG. 5.
Quantitative assessment of autofluorescence after AAVrh.10hCLN3 gene transfer to Cln3Δex7/8 mice. Images were obtained for wild-type, untreated Cln3Δex7/8 mice and Cln3Δex7/8 mice treated with AAVrh.10hCLN3 as neonates. A total of n=5 mice per group were assessed for n=3 sections each. A threshold of mean intensity plus two standard deviations for representative sections of wild-type mice was established. For every section, the percentage of pixels above that threshold was calculated. (A) Mean and standard error for all sections for all structures by phenotype. (B) Mean and standard error from all sections divided by structure shown in Fig. 1. A repeated-measures ANOVA with the Fischer's PLSD post-hoc test for each pairwise comparison of groups was performed for each structure to determine structures in which the treated Cln3Δex7/8 mice had lower autofluorescence than the untreated Cln3Δex7/8 mice. These structures are indicted with the double asterisks. All the pairwise comparisons are shown in Supplementary Table S1.
Further histological assessment of storage pathology and treatment effect
To independently verify the data derived from measuring autofluorescence, three alternate histological methods were utilized to assess therapeutic impact qualitatively: ATPase subunit C immunohistochemistry, LFB stain, and PAS stain.
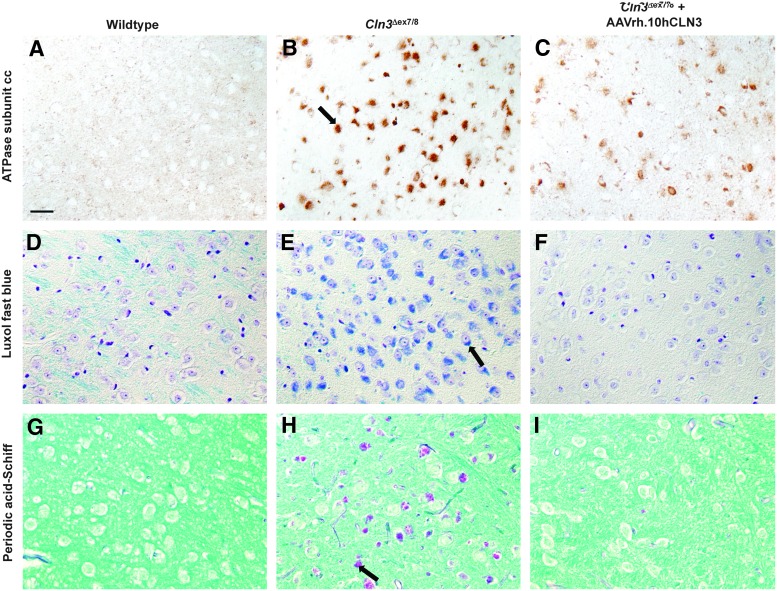
The material that accumulates in the lysosomes of Cln3Δex7/8 mice is composed largely of the ATPase subunit C, which can be detected by immunohistochemistry. As expected, ATPase subunit C was readily detected in sections of brains of Cln3Δex7/8 mice, and this staining, shown for the thalamus, appeared to be vesicular and within the cell cytoplasm (Fig. 6A–C). Consistent with the autofluorescence data, there was clear reduction of ATPase subunit C accumulation in the treated Cln3Δex7/8 (Fig. 6C and Supplementary Fig. S3) mice compared with the untreated Cln3Δex7/8 mice (Fig. 6B), although as with the autofluorescence data, subunit C immunostaining was also not reduced completely to the background levels observed in wild-type mice (Fig. 6A).
FIG. 6.
Qualitative assessment of lysosomal accumulation after AAVrh.10hCLN3 gene transfer to Cln3Δex7/8 mice. Shown in data from the thalamus. (A–C) ATPase subunit C. (A) Wild-type mice. (B) Untreated Cln3Δex7/8 mice. (C) Cln3Δex7/8 mice treated with AAVrh.10hCLN3; example of an ATPase C reactive inclusion is shown by an arrow in (B). (D–F) Periodic acid-Schiff. (D) Wild-type mice. (E) Untreated Cln3Δex7/8 mice. (F) Homozygous Cln3Δex7/8 mice treated with AAVrh.10hCLN3; example of a PAS-positive accumulation is shown by an arrow in (E). (G–I) Luxol fast blue. (G) Wild-type mice. (H) Cln3Δex7/8 mice. (I) Cln3Δex7/8 mice treated AAVrh.10hCLN3; example of an LFB-positive accumulation is shown by an arrow in (H). Deposits present in the untreated Cln3Δex7/8 mice (B, E, H) are partially alleviated by AAVrh.10hCLN3 treatment of mutant mice (C, F, I) and nearly absent in the wild-type mice (A, D, G) for all three methods of detection. (A–I) Scale bar: 25 μm.
LFB staining can detect lipid accumulation in lysosomes (Jolly et al., 1992; Mitchison et al., 1999; Sinha et al., 2004; Anzai et al., 2006; Haltia, 2006). When applied to brain sections of untreated Cln3Δex7/8 mice, intense LFB-positive staining was observed in vesicles close to the nuclei in the majority of cells (Fig. 6E), compared with wild-type controls (Fig. 6D). Similar to the effects on autofluorescence and ATPase subunit C immunostaining, neonatal treatment of Cln3Δex7/8 mice with AAVrh.10hCLN3 considerably reduced the intensity of LFB staining (Fig. 6F).
PAS is a classic method for detecting glycogen build up in lysosomes and accumulation of PAS-positive storage material has been demonstrated in JNCL patients (Jolly et al., 1992; Sinha et al., 2004; Haltia, 2006). When applied to the brains of untreated Cln3Δex7/8 mice, this method revealed darkly PAS-positive material that was seen in large vesicles (Fig. 6H), but was virtually absent in wild-type controls (Fig. 6G). Again there was a marked reduction in PAS staining in the AAVrh.10hCLN3-treated Cln3Δex7/8 mice (Fig. 6I), although the PAS-positive storage material was not fully eliminated by this gene transfer.
Impact of AAVrh.10hCLN3 treatment on glial activation
Pronounced gliosis is a consistent feature in various forms of NCL (Bible et al., 2004; Chang et al., 2008; Kuronen et al., 2012), and has been shown to precede neuron loss in mouse models of JNCL (Pontikis et al., 2004, 2005). To assess the impact of AAVrh.10hCLN3 treatment upon gliosis and to allow an assessment of inflammatory changes associated with vector administration, we stained sections from all mice for markers of astrocytosis (GFAP) and microglial activation (CD68).
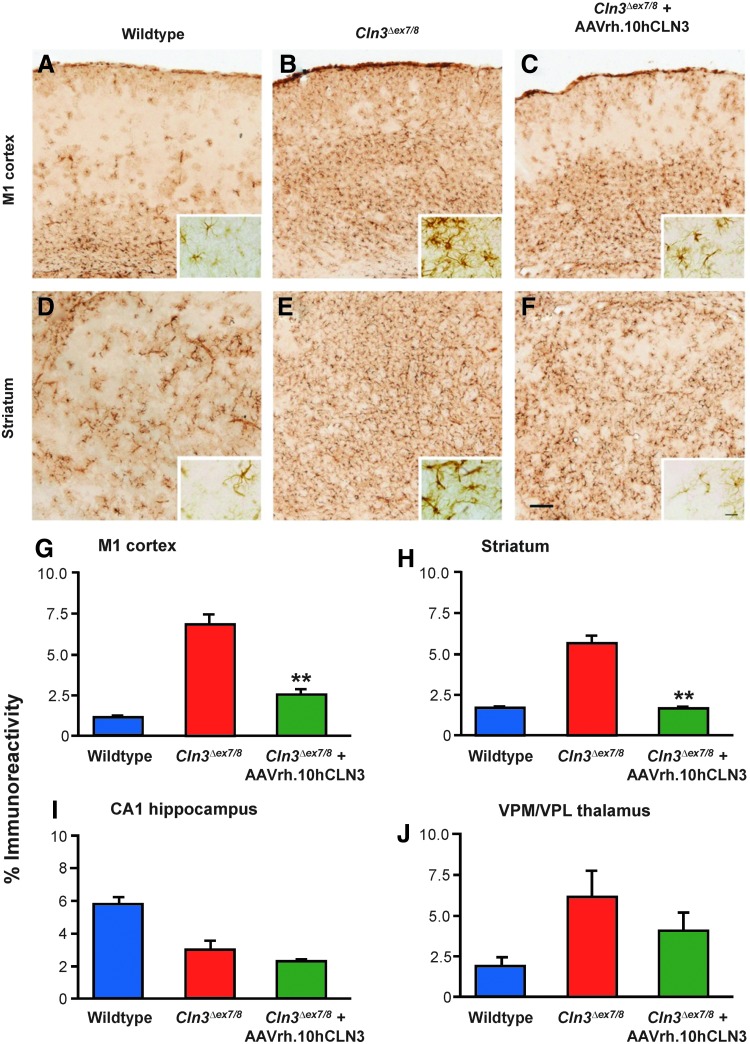
As reported previously (Pontikis et al., 2005), GFAP immunostaining revealed widespread astrocytosis in the brains of untreated Cln3Δex7/8mice. Importantly, we observed no obvious focus of intense GFAP staining at vector injection sites, above that associated with their JNCL phenotype. Qualitatively, astrocytosis in the untreated Cln3Δex7/8 mice appeared to be widespread and showed many intensely stained astrocytes (Fig. 7B and E), especially in the M1 motor cortex (Fig. 7B), compared with wild-type mice (Fig. 7A and D). In contrast, Cln3Δex7/8 mice treated with AAVrh.10hCLN3 showed much less astrocytosis than untreated mutants, especially in the more superficial cortical laminae and in patches within the striatum (Fig. 7C and F compared with Fig. 7B and E). Indeed, AAVrh.10hCLN3 treatment also appeared to impact the morphology of astrocytes in these same regions with paler staining of astrocytes in both of these regions, although it should be noted that this response varied within them with persisting patches of darker GFAP staining within the striatum and within deeper cortical laminae. Quantitative analysis of the intensity of GFAP immunoreactivity was performed by thresholding image analysis in the M1 cortex, striatum, CA1 subfield of the hippocampus, and the ventral posterior nucleus of the thalamus (Fig. 7G and H). Markedly more GFAP staining was observed in the M1 cortex, striatum, and VPM/VPL of untreated Cln3Δex7/8 mice compared with wild-type mice, but less GFAP staining in the hippocampal CA1 of untreated mutants, all consistent with our previous findings in these mice (Pontikis et al., 2005). In contrast, those Cln3Δex7/8 mice treated with the AAVrh.10hCLN3 vector showed a significant amelioration of this astrocytosis in both M1 and striatum, p<0.01 (Fig. 7G and H), and a nonsignificant reduction in GFAP staining in both CA1 and VPM/VPL. These data suggest a therapeutic effect of AAVrh.10hCLN3-mediated therapy on astrocytosis in Cln3Δex7/8 mice, although this is incomplete and some degree of astrocytosis persists.
FIG. 7.
Effects of AAVrh.10hCLN3 administration upon astrocytosis. (A–F) Immunostaining for GFAP reveals the extent of astrocytosis in the M1 cortex (A–C) and striatum (D–F) of wild-type mice (A, D), untreated Cln3Δex7/8 mice (B, E), and AAVrh.10hCLN3-treated Cln3Δex7/8 mice (C, F). Compared with the scattered, faintly stained, and mostly protoplasmic astrocytes present in wild-type mice (A, D), widespread astrocytosis with more intensely stained astrocytes with thickened processes and hypertrophied cell soma were present throughout both regions of untreated Cln3Δex7/8 mice (B, E). In contrast, AAVrh.10hCLN3-treated Cln3Δex7/8 mice displayed less widespread astrocytosis, with more pronounced effects within the more dorsal cortical laminae (C), and in localized patches within the striatum (F). Scale bar=200 μm (20 μm in inserts). (G–J) Histograms of mean area of immunoreactivity per field (%) obtained by thresholding image analysis of GFAP immunostaining in the M1 cortex (G), striatum (H), CA1 subfield of the hippocampus (I), and ventral posterior (VPM/VPL) nucleus of the thalamus (J). These data confirm the disease-associated astrocytosis in these brain regions, as previously described (Pontikis et al., 2005), being elevated in all regions except hippocampal CA1. AAVrh.10hCLN3 administration decreased the level of GFAP immunoreactivity in all regions, but this reached statistical significance only in the M1 cortex and striatum. **p<0.01, one-way ANOVA with Bonferroni correction.
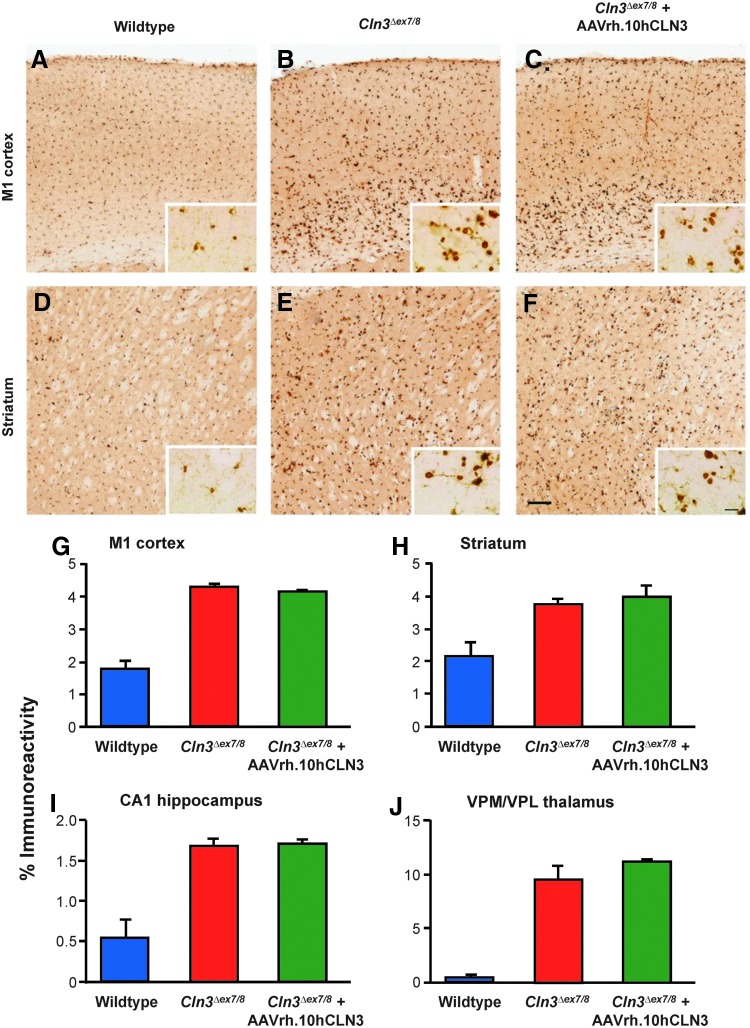
Microglial activation, as a component of gliosis, also precedes neuron loss in JNCL (Pontikis et al., 2004, 2005). Consistent with these observations, untreated Cln3Δex7/8 mice showed more pronounced CD68 immunoreactivity than either wild-type mice in the M1 motor cortex and striatum (Fig. 8A, B, D, and E). This was indistinguishable from the appearance of CD68-positive microglia in Cln3Δex7/8 mice that had been treated with AAVrh.10hCLN3, which showed little or no difference in the intensity or distribution of CD68 immunoreactivity compared with untreated Cln3Δex7/8 mice in these brain regions (Fig. 8C and F). Quantitative thresholding image analysis of CD68 immunoreactivity in M1 (Fig. 8G), striatum (Fig. 8H), hippocampal CA1 (Fig. 8I), and VPM/VPL of the thalamus (Fig. 8J) revealed markedly increased microglial activation in all regions analyzed in untreated and AAVrh.10hCLN3-treated Cln3Δex7/8 mice, when compared with wild-type mice. These data suggest that AAVrh.10hCLN3 administration had little therapeutic effect on microglial activation, but equally, it did not cause any increased inflammatory response.
FIG. 8.
Effects of AAVrh.10hCLN3 administration upon microglial activation. (A–F) Immunostaining for CD68 reveals the extent of microglial activation in the (A–C) M1 cortex and (D–F) striatum of (A, D) wild-type mice, (B, E) untreated Cln3Δex7/8 mice, and (C, F) AAVrh.10hCLN3-treated Cln3Δex7/8 mice. Compared with the relatively small CD68-positive cell soma present in both regions of (A, D) wild-type mice, (E) untreated Cln3Δex7/8 mice display more intense CD68-stained microglia with a larger cell body distributed evenly throughout the striatum, (B) but more predominantly within the deeper layers of the M1 cortex. AAVrh.10hCLN3 administration did not overtly affect this distribution or intensity of CD68 immunoreactivity in either the (C) M1 cortex or (F) striatum. Scale bar=200 μm (20 μm in inserts). (G–J) Histograms of mean area of immunoreactivity per field (%) obtained by thresholding image analysis of CD68 immunostaining in the (G) M1 cortex, (H) striatum, (I) CA1 subfield of the hippocampus, and (J) ventral posterior (VPM/VPL) nucleus of the thalamus. These data confirmed the disease-associated microglial in all these brain regions, as previously described (Pontikis et al., 2005). However, AAVrh.10hCLN3 administration had no significant effect upon CD68 immunoreactivity in any of these regions. One-way ANOVA with Bonferroni correction.
Impact of treatment on neuron and interneuron numbers
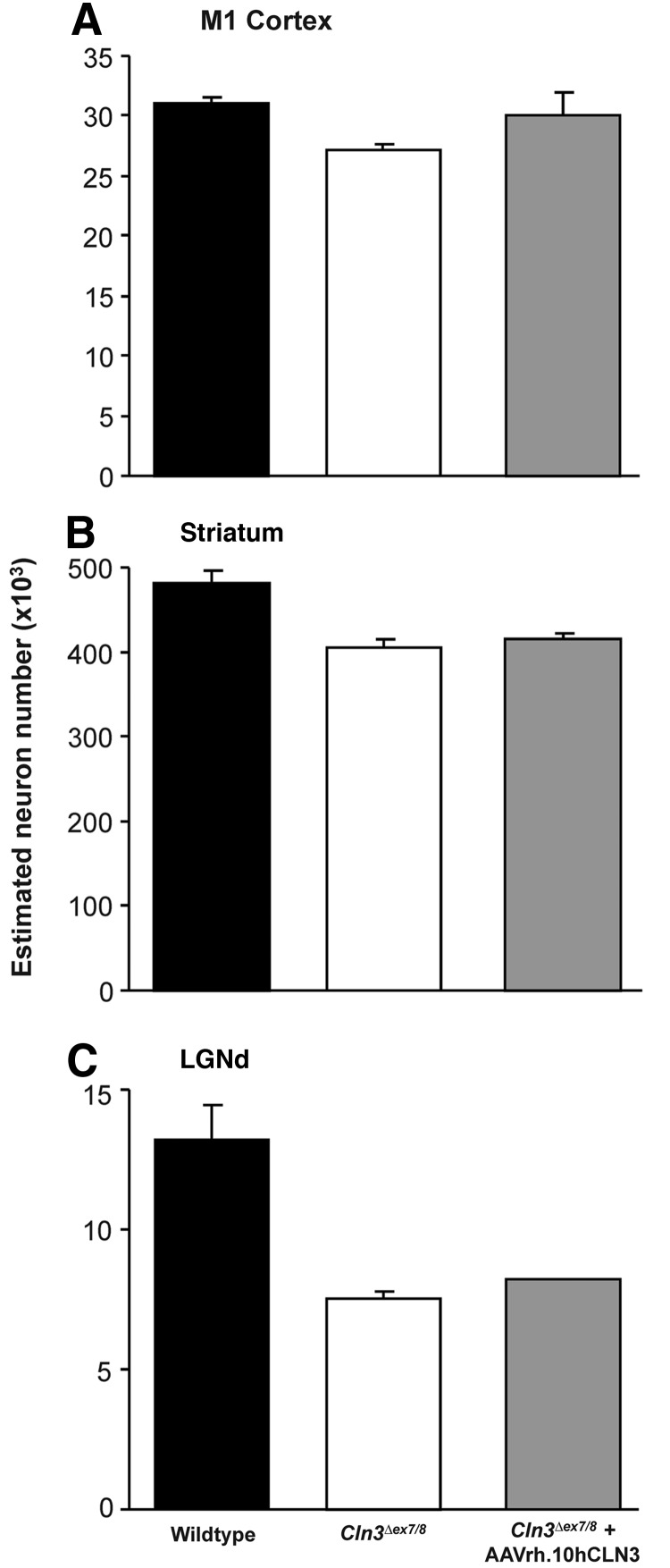
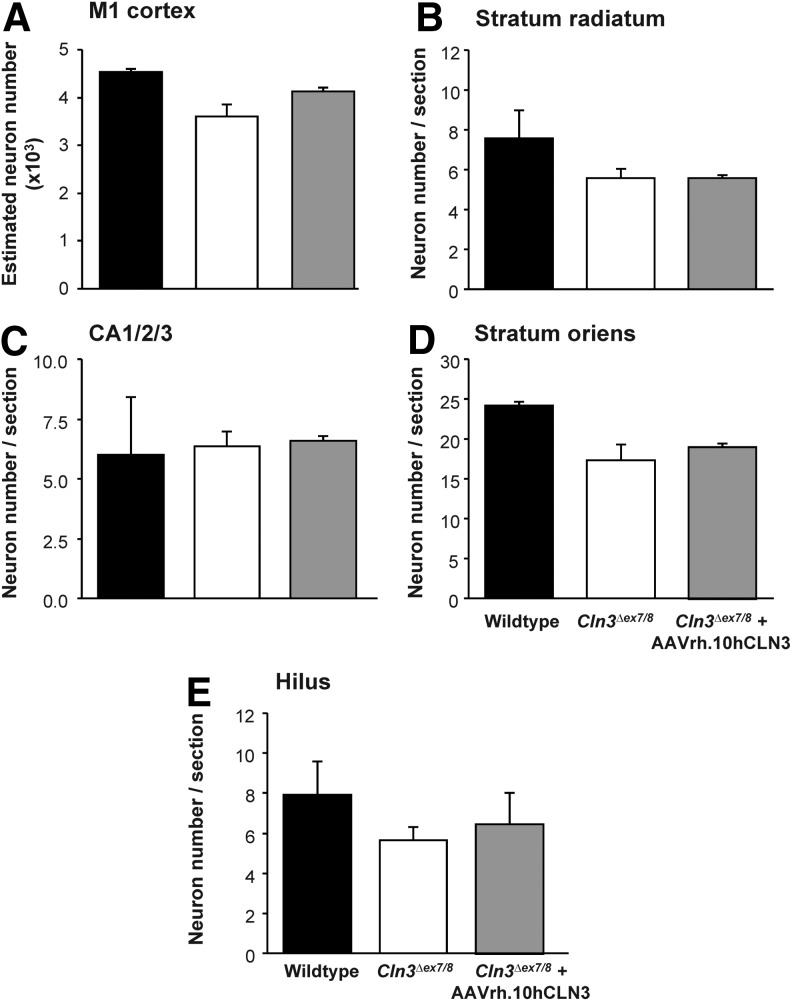
As with the other forms of NCL, neuron and interneuron loss are important features of both human and murine JNCL (Bible et al., 2004; Pontikis et al., 2004; Tyynela et al., 2004; Weimer et al., 2006, 2009). To determine whether AAVrh.10hCLN3 administration had any impact upon neuron survival, optical fractionator counts of Nissl-stained neuron number were made in lamina V of the M1 motor cortex, the LGNd, and the striatum as well as interneuron counts from the M1 cortex of mice from all treatment groups. In all regions there was a trend toward reduced neuronal number in untreated Cln3Δex7/8 mice when compared with wild-type mice. In contrast, Cln3Δex7/8 mice treated with AAVrh.10hCLN3 showed a trend toward increased neuron number in the M1 motor cortex and striatum, but not in the LGNd (Fig. 9). Similarly, optical fractionator estimates of interneuron number from the M1 cortex revealed decreased interneuron counts in untreated Cln3Δex7/8 mice when compared with wild-type mice and improved interneuron survival in AAVrh.10hCLN3-treated Cln3Δex7/8 mice, but this was not statistically significant (Fig. 10A). Direct counts of interneuron populations in the four hippocampal subfields (hilus, CA1/2/3, stratum oriens, and stratum radiatum) revealed a similar trend toward decreased interneuron number in all subfields in Cln3Δex7/8 mice, when compared with wild-type mice. Cln3Δex7/8 mice treated with AAVrh.10hCLN3 showed a general trend toward improved interneuron counts when compared with their untreated counterparts, but these values did not reach significance in any of hippocampal subfields. (Fig. 10B–E). Taken together, there was no overt loss of interneurons in any of the regions to suggest neurotoxicity associated with vector administration. Moreover, these data suggest that the AAVrh.10hCLN3 therapy had a mild neuroprotective effect in these regions.
FIG. 9.
Histograms of optical fractionator counts of the number of Nissl-stained neurons across all treatment groups. (A) In the M1 motor cortex, untreated Cln3Δex7/8 mice show a trend toward reduced lamina V neuron numbers, compared with wild-type mice, with Cln3Δex7/8 mice treated with AAVrh.10hCLN3 showing a nonsignificant trend toward an increased number of lamina V neurons. (B) In the striatum, untreated Cln3Δex7/8 mutants showed fewer neurons, compared with wild-type mice, with AAVrh.10hCLN3-treated Cln3Δex7/8 mice showing a nonsignificant trend toward improved striatal neuron number. (C) Untreated Cln3Δex7/8 mutants show a reduction of visual relay neurons in the lateral geniculate body (LGNd), compared with wild-type mice, but no improvement was observed in Cln3Δex7/8 mice treated with AAVrh.10hCLN3.
FIG. 10.
Histogram of stereological optical fractionator interneuron counts. (A) M1 motor cortex of somatostatin-stained mouse brain sections showing a trend toward fewer interneurons in untreated Cln3Δex7/8 mutants, compared with wild-type mice, with Cln3Δex7/8 mice treated with AAVrh.10hCLN3 showing a trend toward improved cortical interneuron number. (B–E) Histograms of the number of somatostatin-stained interneurons in the four individual hippocampal subfields. (B) Stratum radiatum; (C) CA1/2/3; (D) stratum oriens; and (E) hilus; the data show an overall trend to decreased interneuron counts in untreated Cln3Δex7/8 mice compared with wild-type and heterozygous mice, with some nonsignificant improvement observed in Cln3Δex7/8 mice treated with AAVrh.10hCLN3.
Discussion
There are over 70 lysosomal storage diseases with primarily neurological phenotypes, including all forms of neuronal ceroid lipofuscinosis. In the case of LINCL, AAV gene transfer with AAV serotype 2 and, more recently, serotype AAVrh.10 have been used as an experimental therapy for human subjects (Crystal et al., 2004; Worgall et al., 2008; ClinicalTrials.gov, 2011). As a first step toward potentially treating human subjects with the juvenile form of neuronal ceroid lipofuscinosis, the present study sought to show an impact of treatment in a genetically precise mouse model of JNCL. The CLN3 gene presents a greater challenge than LINCL with CLN2 mutations, since the CLN3 gene product is a hydrophobic, intracellular protein that is not secreted, and the CLN3 protein can treat only vector-transduced cells. The data show that treatment of the homozygous Cln3Δex7/8 mouse model by neonatal administration to the CNS of AAV serotype rh.10 vector expressing the human CLN3 cDNA leads to long-term expression of the CLN3 gene. Importantly, gene transfer of the human CLN3 gene reduced the storage pathology and astrocytosis that is characteristic of JNCL, and did not reveal any evidence for neurotoxic or inflammatory responses associated with vector-driven CLN3 expression.
Longevity of transgene expression
AAV vectors can provide expression of transgenes in rodent brain for greater than 1 year and are therefore well suited to treat genetic diseases (Lo et al., 1999; Sondhi et al., 2005). Neonatal administration of therapeutic vectors takes advantage of the maximum plasticity of the brain and of the immature immune system, giving the maximum impact on phenotype; it also provides the opportunity for an early therapeutic intervention before the onset of overt pathology (Daly et al., 1999b; Waddington et al., 2004; Sondhi et al., 2008). These data demonstrate that expression of a human-derived transgene from an AAVrh.10 vector administered to the brain of neonatal mice is readily detected at 16–18 months of age. By immunofluorescence detection, areas of CLN3 staining are observed in brain regions of Cln3Δex7/8 mice corresponding to the vector administration sites. Detection at the mRNA level suggested an even wider distribution of the transgene expression, which may have been below the threshold for detection by immunohistochemical methods.
Histological impact of gene transfer
All forms of neuronal ceroid lipofuscinosis, including JNCL, are characterized by accumulation of autofluorescent material in the cytoplasm of neurons (Hofman et al., 1999). These autofluorescent cytosomes are LFB positive and PAS positive in paraffin sections, and are immunoreactive for subunit C of the mitochondrial ATPase (Palmer et al., 1992; Hofman et al., 1999; Haltia, 2006). NCLs are also characterized by neuron loss and gliosis (Autti et al., 1997; Hofman et al., 1999). To show a definitive impact of treatment on phenotype, we have used these neuropathological parameters to compare wild-type, untreated Cln3Δex7/8 mice and neonatal Cln3Δex7/8 mice treated with AAVrh.10hCLN3. Given the progressive nature of JNCL storage pathology and neuron loss, we focused on older mice, >16 months of age. A consistent pattern was observed with a treatment-dependent reduction of storage material, as measured by five different methods, in multiple regions of the brain.
Autofluorescence
The pathological hallmark of JNCL is autofluorescent ceroid lipofuscin deposits within the lysosomes that are enriched in subunit C of the ATPase (Palmer et al., 1992; Hofman et al., 1999). These deposits are present in the CNS neurons and also in somatic cells outside of the nervous system. The relationship of these deposits to the JNCL disease process, and the selective nature of neuron loss are not understood, but assessing the level of storage burden is nevertheless a useful measure of therapeutic efficacy. The data demonstrated that there was a noticeable reduction in the accumulation of autofluorescent storage material in the brains of AAVrh.10hCLN3-treated Cln3Δex7/8 mice compared with the untreated mutant mice, suggesting that vector-mediated expression of the CLN3 gene may have led to clearance of this material in the CNS.
ATPase subunit C
In JNCL subjects and Cln3-deficient mice, mitochondrial ATPase subunit C protein accumulates, suggesting that autophagy, a pathway that regulates mitochondrial turnover, may be disrupted in this disease (Fossale et al., 2004; Cao et al., 2006; Haltia, 2006; Herrmann et al., 2008). This pathway is typically initiated at times of stress or at times when there is a lack of sufficient nutrients to generate the necessary metabolites for cellular survival (Cuervo, 2004; Cao et al., 2006). Autophagy is a pathway involved in a number of neurodegenerative diseases, including Alzheimer, Parkinson, and Huntington diseases (Petersen et al., 2001; Stefanis et al., 2001; Cuervo, 2004; Yu et al., 2005). This has also been observed for all NCLs, including JNCL, where there is lysosomal accumulation of subunit C of the mitochondrial ATPase, suggesting defects in the turnover of this protein via the autophagic pathway (Fossale et al., 2004; Cao et al., 2006). Indeed, a direct role for the CLN3 protein in normal autophagy has been proposed (Cao et al., 2006). In the present study we demonstrated that there was an appreciable decline in subunit C accumulation in AAVrh.10hCLN3-treated Cln3Δex7/8 mice compared with untreated mutants, suggesting that vector-mediated expression of the CLN3 gene may have an impact on the autophagic pathway.
LFB and PAS staining
LFB is a lipid stain often used to demonstrate neuronal accumulation of proteolipid storage materials (Bronson et al., 1998). PAS stain is used to detect glycogen accumulation (McManus, 1948; Mark et al., 2007). Although the NCLs are primarily defects in protein turnover, a general failure of lysosomal function leads to accumulation of diverse substrates that are normally recycled through the lysosome (Hofman et al., 1999; Haltia, 2006). Consistent with the other histological approaches, both LFB and PAS staining demonstrated lysosomal dysfunction in the JNCL mouse model that was relieved by neonatal gene transfer of the AAVrh.10 vector.
Gliosis
Reactive gliosis is a hallmark of brain injury in which astrocytes undergo proliferation and morphological change, put out processes, and express higher levels of GFAP (Norton et al., 1992). This has previously been observed in several brain regions of Cln3Δex7/8 mice, in other mouse models of JNCL, and in the cortex of mice with mucopolysaccharidosis II and IIIB (Cotman et al., 2002; Pontikis et al., 2004; Herrmann et al., 2008). In the case of JNCL mice, it may be an early marker of disease preceding neurodegeneration (Savas et al., 2004; Polito et al., 2010). We found gliosis at an advanced stage of the disease in all structures with the exception of the hippocampus and cerebellum in untreated Cln3Δex7/8 mice, consistent with earlier reports (Pontikis et al., 2005). Quantitative assessment of gliosis in various regions of the brain of the treated and untreated Cln3Δex7/8 mice showed that neonatal gene transfer of AAVrh.10 vector led to an overall decrease in the level of astrocytosis, although there was little effect on microglial activation. Although AAVrh.10 mainly transduces neurons and not glial cells (Cearley and Wolfe, 2006), our data demonstrate that transduction of astrocytes with AAVrh.10hCLN3 clearly occurs. As such, the decrease in astrocytosis may be because of their successful transduction, although effects upon cytokines that influence astrocytosis may also occur. Intriguingly, the effects of AAVrh.10hCLN3 administration upon microglia were minimal, suggesting either selective effect upon cytokines that influence their activation, or that these microglia were not transduced to any great extent.
Neuronal counts
The loss of thalamocortical neurons, and the loss and hypertrophy of interneurons are a consistent feature of JNCL and are pathognomonic of a neurodegenerative disease (Bible et al., 2004; Pontikis et al., 2004; Tyynela et al., 2004; Weimer et al., 2006, 2009). Our findings of decreased neuron and interneuron numbers in Cln3Δex7/8 mice are in accordance with previous studies (Pontikis et al., 2004). In the AAVrh.10hCLN3-treated Cln3Δex7/8 mice, there was a trend toward improved neuron counts in the striatum and lamina V of the primary motor cortex, as well as a trend toward improved interneuron numbers in the hippocampus and primary motor cortex, consistent with the possibility that the AAVrh.10hCLN3-mediated gene transfer had a mild neuroprotective effect. There was also no significant decrease in neuron or interneuron number in the treated mice, as compared with their untreated counterparts, indicating that the in vivo expression of the human CLN3 protein was not at a level that would be toxic to these cells.
In summary, these diverse histological assessments show an impact of neonatal treatment by AAV serotype rh.10 gene transfer on the lysosomal storage phenotype, as well as having a significant effect on astrocytosis and a mild neuroprotective effect. In the absence of evidence for the predicted toxicity or local inflammation because of CLN3 protein overexpression, there is sufficient evidence to consider refining AAVrh.10-mediated CNS delivery of the wild-type CLN3 cDNA to treat the CNS manifestations of JNCL.
Supplementary Material
Acknowledgments
We thank Benjamin Van de Graaf and Scott H. Coppel for technical assistance and N. Mohamed and D.N. McCarthy for help in preparing this article. The studies were supported, in part, by National Contest for Life-Stiftung, The Natalie Fund, and DEM-CHILD funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under Grant Agreement No. 281234. S.L.C. is supported by the National Institutes of Health (National Institute of Neurological Disorders & Stroke; R01-NS073813) and the Dubai–Harvard Foundation for Medical Research.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Anzai Y., Hayashi M., Fueki N., et al. (2006). Protracted juvenile neuronal ceroid lipofuscinosis—an autopsy report and immunohistochemical analysis. Brain Dev. 28, 462–465 [DOI] [PubMed] [Google Scholar]
- Autti T., Raininko R., Santavuori P., et al. (1997). MRI of neuronal ceroid lipofuscinosis. II. Postmortem MRI and histopathological study of the brain in 16 cases of neuronal ceroid lipofuscinosis of juvenile or late infantile type. Neuroradiology 39, 371–377 [DOI] [PubMed] [Google Scholar]
- Bible E., Gupta P., Hofmann S.L., et al. (2004). Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 16, 346–359 [DOI] [PubMed] [Google Scholar]
- Bowers W.J., Breakefield X.O., and Sena-Esteves M. (2011). Genetic therapy for the nervous system. Hum. Mol. Genet. 20, R28–R41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson R.T., Donahue L.R., Johnson K.R., et al. (1998). Neuronal ceroid lipofuscinosis (nclf), a new disorder of the mouse linked to chromosome 9. Am. J. Med. Genet. 77, 289–297 [DOI] [PubMed] [Google Scholar]
- Cao Y., Espinola J.A., Fossale E., et al. (2006). Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J. Biol. Chem. 281, 20483–20493 [DOI] [PubMed] [Google Scholar]
- Cearley C.N., and Wolfe J.H. (2006). Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 13, 528–537 [DOI] [PubMed] [Google Scholar]
- Chang M., Cooper J.D., Sleat D.E., et al. (2008). Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 16, 649–656 [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov (2011). AAVRh.10 administered to children with late infantile neuronal ceroid lipofuscinosis with uncommon genotypes or moderate/severe impairment. Available at http://clinicaltrials.gov/ct2/show/NCT01414985 (accessed September2013)
- Cotman S.L., Vrbanac V., Lebel L.A., et al. (2002). Cln3(Deltaex7/8) knock-in mice with the common JNCL mutation exhibit progressive neurologic disease that begins before birth. Hum. Mol. Genet. 11, 2709–2721 [DOI] [PubMed] [Google Scholar]
- Crystal R.G., Sondhi D., Hackett N.R., et al. (2004). Clinical protocol. Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum. Gene Ther. 15, 1131–1154 [DOI] [PubMed] [Google Scholar]
- Cuervo A.M. (2004). Autophagy: in sickness and in health. Trends Cell Biol. 14, 70–77 [DOI] [PubMed] [Google Scholar]
- Daly T.M., Okuyama T., Vogler C., et al. (1999a). Neonatal intramuscular injection with recombinant adeno-associated virus results in prolonged beta-glucuronidase expression in situ and correction of liver pathology in mucopolysaccharidosis type VII mice. Hum. Gene Ther. 10, 85–94 [DOI] [PubMed] [Google Scholar]
- Daly T.M., Vogler C., Levy B., et al. (1999b). Neonatal gene transfer leads to widespread correction of pathology in a murine model of lysosomal storage disease. Proc. Natl. Acad. Sci. USA 96, 2296–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossale E., Wolf P., Espinola J.A., et al. (2004). Membrane trafficking and mitochondrial abnormalities precede subunit c deposition in a cerebellar cell model of juvenile neuronal ceroid lipofuscinosis. BMC Neurosci. 5, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty A.L., and Pearce D.A. (2011). Interactions of the proteins of neuronal ceroid lipofuscinosis: clues to function. Cell Mol. Life Sci. 68, 453–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti A. (2011). Gene therapy for lysosomal storage disorders. Expert Opin. Biol. Ther. 11, 1153–1167 [DOI] [PubMed] [Google Scholar]
- Haltia M. (2006). The neuronal ceroid-lipofuscinoses: from past to present. Biochim. Biophys. Acta 1762, 850–856 [DOI] [PubMed] [Google Scholar]
- Herrmann P., Druckrey-Fiskaaen C., Kouznetsova E., et al. (2008). Developmental impairments of select neurotransmitter systems in brains of Cln3(Deltaex7/8) knock-in mice, an animal model of juvenile neuronal ceroid lipofuscinosis. J. Neurosci. Res. 86, 1857–1870 [DOI] [PubMed] [Google Scholar]
- Hofman I., Kohlschutter A., Santavuori P., et al. (1999). CLN3 juvenile NCL. In The Neuronal Ceroid Lipofuscinoses (Batten Disease). Goebel H.H., ed. (IOS Press, Birmingham, AL: ), pp.55–76 [Google Scholar]
- Janson C., McPhee S., Bilaniuk L., et al. (2002). Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum. Gene Ther. 13, 1391–1412 [DOI] [PubMed] [Google Scholar]
- Jarvela I., Lehtovirta M., Tikkanen R., et al. (1999). Defective intracellular transport of CLN3 is the molecular basis of Batten disease (JNCL). Hum. Mol. Genet. 8, 1091–1098 [DOI] [PubMed] [Google Scholar]
- Jolly R.D., Martinus R.D., and Palmer D.N. (1992). Sheep and other animals with ceroid-lipofuscinoses: their relevance to Batten disease. Am. J. Med. Genet. 42, 609–614 [DOI] [PubMed] [Google Scholar]
- Kuronen M., Lehesjoki A.E., Jalanko A., et al. (2012). Selective spatiotemporal patterns of glial activation and neuron loss in the sensory thalamocortical pathways of neuronal ceroid lipofuscinosis 8 mice. Neurobiol. Dis. 47, 444–457 [DOI] [PubMed] [Google Scholar]
- Leone P., Shera D., McPhee S.W., et al. (2012). Long-term follow-up after gene therapy for canavan disease. Sci. Transl. Med. 4, 165ra163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner T.J. (2001). Positional cloning of the JNCL gene, CLN3. Adv. Genet. 45, 107–121 [DOI] [PubMed] [Google Scholar]
- Lo W.D., Qu G., Sferra T.J., et al. (1999). Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum. Gene Ther. 10, 201–213 [DOI] [PubMed] [Google Scholar]
- Mao Q., Foster B.J., Xia H., et al. (2003). Membrane topology of CLN3, the protein underlying Batten disease. FEBS Lett. 541, 40–46 [DOI] [PubMed] [Google Scholar]
- Mark M., Teletin M., Antal C., et al. (2007). Histopathology in mouse metabolic investigations. Curr. Protoc. Mol. Biol. Chapter 29, Unit 29B.4. [DOI] [PubMed] [Google Scholar]
- McManus J.F. (1948). Histological and histochemical uses of periodic acid. Stain Technol. 23, 99–108 [DOI] [PubMed] [Google Scholar]
- Michalewski M.P., Kaczmarski W., Golabek A.A., et al. (1999). Posttranslational modification of CLN3 protein and its possible functional implication. Mol. Genet. Metab. 66, 272–276 [DOI] [PubMed] [Google Scholar]
- Mingozzi F., and High K.A. (2011). Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 12, 341–355 [DOI] [PubMed] [Google Scholar]
- Mitchison H.M., Bernard D.J., Greene N.D., et al. (1999). Targeted disruption of the Cln3 gene provides a mouse model for Batten disease. The Batten Mouse Model Consortium [corrected]. Neurobiol. Dis. 6, 321–334 [DOI] [PubMed] [Google Scholar]
- Munroe P.B., Mitchison H.M., O'Rawe A.M., et al. (1997). Spectrum of mutations in the Batten disease gene, CLN3. Am. J. Hum. Genet. 61, 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E.F., and Fratantoni J.C. (1970). Inborn errors of mucopolysaccharide metabolism. Science 169, 141–146 [DOI] [PubMed] [Google Scholar]
- Norton W.T., Aquino D.A., Hozumi I., et al. (1992). Quantitative aspects of reactive gliosis: a review. Neurochem. Res. 17, 877–885 [DOI] [PubMed] [Google Scholar]
- Palmer D.N., Fearnley I.M., Walker J.E., et al. (1992). Mitochondrial ATP synthase subunit c storage in the ceroid-lipofuscinoses (Batten disease). Am. J. Med. Genet. 42, 561–567 [DOI] [PubMed] [Google Scholar]
- Paxinos G., and Franklin K.B.J. (2001). The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego, CA: ) [Google Scholar]
- Petersen A., Larsen K.E., Behr G.G., et al. (2001). Expanded CAG repeats in exon 1 of the Huntington's disease gene stimulate dopamine-mediated striatal neuron autophagy and degeneration. Hum. Mol. Genet. 10, 1243–1254 [DOI] [PubMed] [Google Scholar]
- Polito V.A., Abbondante S., Polishchuk R.S., et al. (2010). Correction of CNS defects in the MPSII mouse model via systemic enzyme replacement therapy. Hum. Mol. Genet. 19, 4871–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontikis C.C., Cella C.V., Parihar N., et al. (2004). Late onset neurodegeneration in the Cln3-/- mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 1023, 231–242 [DOI] [PubMed] [Google Scholar]
- Pontikis C.C., Cotman S.L., MacDonald M.E., et al. (2005). Thalamocortical neuron loss and localized astrocytosis in the Cln3Deltaex7/8 knock-in mouse model of Batten disease. Neurobiol. Dis. 20, 823–836 [DOI] [PubMed] [Google Scholar]
- Puntel M., Kroeger K.M., Sanderson N.S., et al. (2010). Gene transfer into rat brain using adenoviral vectors. Curr. Protoc. Neurosci. Chapter 4, Unit 4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafi M.A., Rao H.Z., Luzi P., et al. (2012). Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease. Mol. Ther. 20, 2031–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakheja D., Narayan S.B., and Bennett M.J. (2008). The function of CLN3P, the Batten disease protein. Mol. Genet. Metab. 93, 269–274 [DOI] [PubMed] [Google Scholar]
- Sands M.S., and Davidson B.L. (2006). Gene therapy for lysosomal storage diseases. Mol. Ther. 13, 839–849 [DOI] [PubMed] [Google Scholar]
- Savas P.S., Hemsley K.M., and Hopwood J.J. (2004). Intracerebral injection of sulfamidase delays neuropathology in murine MPS-IIIA. Mol. Genet. Metab. 82, 273–285 [DOI] [PubMed] [Google Scholar]
- Sinha S., Satishchandra P., Santosh V., et al. (2004). Neuronal ceroid lipofuscinosis: a clinicopathological study. Seizure 13, 235–240 [DOI] [PubMed] [Google Scholar]
- Sondhi D., Peterson D.A., Giannaris E.L., et al. (2005). AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Ther. 12, 1618–1632 [DOI] [PubMed] [Google Scholar]
- Sondhi D., Hackett N.R., Peterson D.A., et al. (2007). Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 15, 481–491 [DOI] [PubMed] [Google Scholar]
- Sondhi D., Peterson D.A., Edelstein A.M., et al. (2008). Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp. Neurol. 213, 18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi D., Hackett N.R., Scott E., et al. (2011). Correction of the lysosomal storage defect in mouse model of juvenile neuronal ceroid lipofuscinosis by neonatal injection of AAV serotype rh.10 vector expressing the human CLN3 gene. Mol. Ther. 19[Supplement 1], S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staropoli J.F., Haliw L., Biswas S., et al. (2012). Large-scale phenotyping of an accurate genetic mouse model of JNCL identifies novel early pathology outside the central nervous system. PLoS One 7, e38310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L., Larsen K.E., Rideout H.J., et al. (2001). Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J. Neurosci. 21, 9549–9560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Batten Disease Consortium (1995). Isolation of a novel gene underlying Batten disease, CLN3. Cell 82, 949–957 [DOI] [PubMed] [Google Scholar]
- Tomanin R., Zanetti A., Zaccariotto E., et al. (2012). Gene therapy approaches for lysosomal storage disorders, a good model for the treatment of mendelian diseases. Acta Paediatr. 101, 692–701 [DOI] [PubMed] [Google Scholar]
- Tyynela J., Cooper J.D., Khan M.N., et al. (2004). Hippocampal pathology in the human neuronal ceroid-lipofuscinoses: distinct patterns of storage deposition, neurodegeneration and glial activation. Brain Pathol. 14, 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington S.N., Kennea N.L., Buckley S.M., et al. (2004). Fetal and neonatal gene therapy: benefits and pitfalls. Gene Ther. 11Suppl. 1, S92–S97 [DOI] [PubMed] [Google Scholar]
- Weimer J.M., Custer A.W., Benedict J.W., et al. (2006). Visual deficits in a mouse model of Batten disease are the result of optic nerve degeneration and loss of dorsal lateral geniculate thalamic neurons. Neurobiol. Dis. 22, 284–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer J.M., Yokota Y., Stanco A., et al. (2009). MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development 136, 2965–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M.J., Slomianka L., and Gundersen H.J. (1991). Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 231, 482–497 [DOI] [PubMed] [Google Scholar]
- Worgall S., Sondhi D., Hackett N.R., et al. (2008). Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 19, 463–474 [DOI] [PubMed] [Google Scholar]
- Yu W.H., Cuervo A.M., Kumar A., et al. (2005). Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J. Cell Biol. 171, 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.