Abstract
Purpose: Little is known about the factors that influence the place of inpatient care for teenage and young adult (TYA) cancer patients. Recent guidelines have recommended centralization of care for this group to a small number of specialized centers. This study aimed to investigate the influence of geography and travel times on the likelihood of admission to an age-specialist center in England during cancer treatment for patients aged 15–24 at the time of diagnosis.
Methods: Data for 6788 patients aged 15–24, diagnosed between 2001 and 2006 and treated as an inpatient in England between 2001 and 2009, were obtained from the National Cancer Data Repository. Eight TYA age-specialist centers were identified in England during this time period; road travel times to these centers were calculated using ArcGIS Network Analyst. Factors thought to affect the likelihood of admission, such as diagnostic group, gender, and age at diagnosis were modeled using logistic regression.
Results: Overall, 66.9% of patients never received inpatient treatment at a TYA age-specialist center during the course of their treatment. Increasing travel time significantly reduced the likelihood of admission to a TYA age-specialist center after adjustment for case mix factors.
Conclusion: Many TYA patients received little or no inpatient treatment at a TYA age-specialist center during their treatment. The variation between diagnostic groups suggests that factors other than distance to the closest center are affecting the likelihood of admission and demonstrates the potential need to consider improvements to the structured referral practice for this unique group of patients.
Keywords: : treatment, standard of care, epidemiology, supportive care
In recent years, several government and health service reports have described the principles behind optimal cancer care and advocated a shift from a generalist to a specialist cancer service in the United Kingdom.1,2 Following these reports, cancer care in England has been reorganized toward networks of specialist multidisciplinary centers with a focus on a centralization of specialist expertise.1,2 For less common cancers, such as those diagnosed in teenagers and young adults (TYAs), who account for less than 1% of all cancers diagnosed annually in the United Kingdom,3 this resulted in a widespread distribution of relatively few TYA age-specialist centers across the country.4
In the United Kingdom, services for TYA cancer patients encompass those aged 15–24.1 Since 2011, the UK National Health Service (NHS) has required that patients younger than 19 years old are seen at a TYA age-specialist center for at least part of their treatment. Those aged 19–24 should, according to NHS guidelines, be given a choice as to their preferred place of care, which may be a TYA age-specialist center or a hospital closer to their home.1 Little is known about the relationship between the distance from a TYA patient's home address and the hospital where they receive treatment. Previous studies examining the relationship between travel distance and treatment uptake in different patient groups in England have shown a relationship between travel and treatment uptake,5,6 whilst several international studies have been inconclusive.7–9
Specialist centers for TYA patients fall into two main categories: age-specific and diagnostic group-specific. These centers, classified as Principal Treatment Centres (PTCs), operate alongside high-volume cancer centers and general hospitals to provide care for this unique patient group. Certain aspects of care, such as some chemotherapy, can be provided at sites other than those reported to have a specialist focus; however, the overall choice of place of care should be offered to the patient.10
TYA age-specialist centers facilitate access to age-appropriate clinical and psychosocial care in an environment which is distinct from that of pediatric and adult units.4 Whilst little work has been undertaken to examine the potential survival benefit of treatment at these centers, a psychological benefit from treatment in this setting has been shown,11 and other studies have shown a survival benefit associated with specialist care in both older and younger cancer patients.12–15
During the 2001–2009 time period covered by this study, there were eight designated age-specialist units in England capable of treating inpatients. These were located within cancer centers, rather in cancer units located in general hospitals. Due to the centralization of tertiary care, treatment at these centers often requires patients to travel outside their local area, a finding which has been reflected in a study examining childhood cancer care.16 Specialist complex surgical treatment for bone and central nervous system tumors and soft tissue sarcoma occurs at diagnostic group-specific specialist centers across the United Kingdom; for bone tumors in particular this is the only possible location for major surgical resection.17 Due to the dispersal of TYA age-specialist centers and diagnostic group-specific centers, TYA patients who choose to be treated at a specialist center may travel significant distances to receive their cancer care.
Admission to TYA age-specialist centers is not uniform and is known to vary by diagnostic group and patient age.18 TYA patients' barriers to accessing specialist care are not fully known but are important to understand in order to improve care and ensure that the option of TYA age-specialist care is made available for all. A study examining the opinion of healthcare professionals as to what constitutes specialist care and potential reasons behind the variability of service usage in TYAs failed to identify professional opinion as the sole cause for this.19 The current study firstly aimed to investigate whether a relationship existed between travel time and the likelihood of admission to an age-specialist center. Secondly, we aimed to quantify this relationship and to determine whether the effect was equal for all diagnostic and patient groups or whether these factors also influenced admission patterns.
Methods
Study period
England's National Institute for Health and Clinical Excellence issued cancer services guidelines in its 2005 document “Improving Outcomes Guidance in Children and Young People with Cancer.”1 As a result of this and the 1995 Calman-Hine report2 before it, service redesign was undertaken in the United Kingdom, leading to a centralization of services for the treatment of cancer in TYA patients. The time period covered by this study (January 2001–January 2006 for diagnosis and January 2001–January 2009 for treatment and follow-up) encompasses this change in service delivery and includes the period before, during, and after major structural reorganization. The number of TYA age-specialist centers open to TYA patients during this time period was identified using the Teenage Cancer Trust's (TCT) website (www.teenagecancertrust.org); information on the centers' opening dates was sourced before being cross-checked with the NHS Trust in question.
Data sources
The postcodes of residence were obtained from the National Cancer Data Repository (NCDR),20 which contains cancer registration data linked to hospital episode statistics (HES) data. The data were cleaned to prevent the inclusion of duplicate registrations.21 The postcodes of both each patient's address at the time of admission and of all TYA age-specialist centers in England were converted into numerical grid references for Euclidean calculations using GeoConvert, a service made available by the Census Dissemination Unit at the University of Manchester and used to derive data, such as grid reference coordinates, from the National Statistics Postcode Directory (NSPD). Postcodes in the United Kingdom refer to a single street, allowing a meaningful analysis of travel times between postcodes.
Participants and recruitment
A total of 9026 patients diagnosed with a malignancy within the specified age range were identified in the cancer registration data obtained from the NCDR; 2238 patients were excluded as they either had no admissions recorded in HES during the predefined 2001–2009 treatment and follow-up period (n=1293; 57.8%) or no address information was available for the patient (n=945; 42.2%), leaving 6788 patients included in the analysis (Table 1). There were 14,413 unique combinations of residential postcode at the time of hospital admission and hospital postcode identified for these patients in the HES data.
Table 1.
Characteristics of the Study Population
| Excluded from analysis | Analysis dataset | ||||
|---|---|---|---|---|---|
| Original dataset | n | % | n | % | |
| Age at diagnosis | |||||
| 15 | 302 | 37 | 12.3 | 265 | 87.7 |
| 16 | 674 | 100 | 14.8 | 574 | 85.2 |
| 17 | 706 | 134 | 19.0 | 572 | 81.0 |
| 18 | 709 | 146 | 20.6 | 563 | 79.4 |
| 19 | 813 | 173 | 21.3 | 640 | 78.7 |
| 20 | 932 | 232 | 24.9 | 700 | 75.1 |
| 21 | 1043 | 283 | 27.1 | 760 | 72.9 |
| 22 | 1140 | 313 | 27.5 | 827 | 72.5 |
| 23 | 1291 | 373 | 28.9 | 918 | 71.1 |
| 24 | 1416 | 447 | 31.6 | 969 | 68.4 |
| Gender | |||||
| Male | 4732 | 1045 | 22.1 | 3687 | 77.9 |
| Female | 4294 | 1193 | 27.8 | 3101 | 72.2 |
| Year of diagnosis | |||||
| 2001 | 1394 | 346 | 24.8 | 1048 | 75.2 |
| 2002 | 1454 | 372 | 25.6 | 1082 | 74.4 |
| 2003 | 1481 | 373 | 25.2 | 1108 | 74.8 |
| 2004 | 1525 | 406 | 26.6 | 1119 | 73.4 |
| 2005 | 1630 | 368 | 22.6 | 1262 | 77.4 |
| 2006 | 1542 | 373 | 24.2 | 1169 | 75.8 |
| Diagnostic group | |||||
| Leukemia | 777 | 40 | 5.1 | 737 | 94.9 |
| Lymphoma | 1,892 | 172 | 9.1 | 1720 | 90.9 |
| Central nervous system and other intracranial and intraspinal neoplasms | 932 | 99 | 10.6 | 833 | 89.4 |
| Osseous and chondromatous neoplasms | 489 | 94 | 19.2 | 395 | 80.8 |
| Soft tissue sarcoma | 375 | 15 | 4.0 | 360 | 96.0 |
| Germ cell and trophoblastic neoplasms | 1,331 | 149 | 11.2 | 1082 | 81.3 |
| Melanoma and skin carcinoma | 1,635 | 1022 | 62.5 | 613 | 37.5 |
| Carcinomas | 1,595 | 547 | 34.3 | 1048 | 65.7 |
| Total | 9026 | 2238 | 24.8 | 6788 | 75.2 |
All diagnostic groups were defined according to the Birch classification scheme for cancers in TYAs,22 which groups diagnoses based on International Disease Classification (ICD-0) codes.
All participants were admitted to a hospital at some point between diagnosis and the end of the treatment period and each hospitalization was recorded for each patient. Day-case admissions (elective admissions to a hospital not requiring an overnight stay) were included in this analysis. A longer time frame was assigned to those with hematological malignancies (3 years) than those with all other diagnoses (18 months) to reflect the potentially greater period of intense treatment undergone by these patients.
Data analysis
In order to assess access to healthcare and how the journey of patients was affected by the centralization of age-specialist cancer services, it was necessary to know the road travel distance and time. The Euclidean (crow-fly) distance from residence to hospital was used to simplify the assessment of whether the patient was traveling beyond the closest hospital with the potential to treat them. In order to do this, the distance between the address of the patient at the time of admission was compared to the postcode of the TYA age-specialist centers as well as to the other acute NHS trust hospitals in England.
The TYA age-specialist center in Southampton opened part way through the study period (2009). Other TYA age-specialist centers had an upper age limit that prevented some of the patients in the study cohort from being admitted to that site. These restrictions were taken into account when modeling the access to specialist centers by assigning each patient to the closest center for which they met the criteria at the time of their admission.
The ArcGIS road network analyst service23 was used to perform network-based spatial analysis to calculate the road travel time and distance between the address at admission and the hospital to which each patient was admitted. The specialist center to which each patient was admitted was compared to the closest center by both travel distance and travel time. The location of each center was mapped using the coordinates of the hospital in which it was located. For those patients seen at a center other than the one they lived closest to, the difference in travel distance and time between the hospital attended and the closest hospital was examined.
The likelihood of admission to a TYA age-specialist center was modeled for each of the eight diagnostic groups (leukemia, lymphoma, central nervous system and other intracranial and intraspinal neoplasms, osseous and chondromatous neoplasms, soft tissue sarcoma, germ cell and trophoblastic neoplasms, melanoma and skin carcinoma, and carcinomas) using logistic regression in Stata 12,24 with admission to a TYA age-specialist center during treatment as a binary outcome (yes/no), adjusting for patient characteristics alongside the distance from place of residence to the closest TYA age-specialist center. Logistic regression was used to check the data for a significant change over time in the type of center that provided the majority of treatment for each patient.
The NHS trust that provided the majority of inpatient treatment (>60%) for each patient during the treatment period was determined. The NHS trusts were then divided into those with a TYA age-specialist center, teaching hospitals (including diagnostic group-specific specialist centers), and “other” centers that were not teaching hospital trusts. The variation in the proportion of patients attending each type of center was assessed by diagnostic group and the geographical location of each patient.
Results
Characteristics of the population
Large differences were seen in the number of patients included and excluded according to their admission status in each diagnostic group (Table 1). Disease groups for which the majority of treatment can take place in an outpatient or primary care setting, such as melanoma, were admitted less frequently than those with other diagnoses. All patients with no recorded inpatient episodes were excluded from the analysis; while they may have been treated as an outpatient, it was not possible to determine this using the data available.
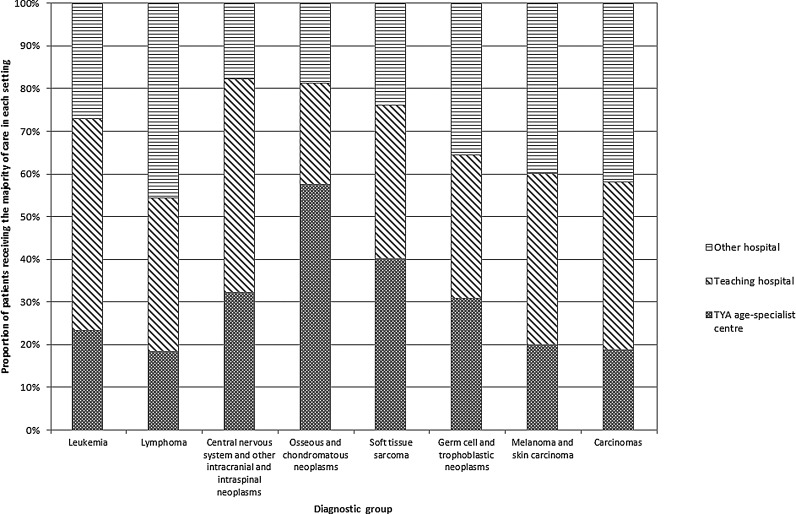
Overall, 66.9% of patients had no admission to a TYA age-specialist center during the course of their treatment. In all diagnostic groups other than bone tumors, over 50% of patients (range: 55.3–76.5%) were not admitted to a TYA age-specialist center. In the large majority of cases (40.9%), the trust providing the majority of inpatient treatment was a teaching hospital that did not have a TYA age-specialist center (Fig. 1). For the majority of patients diagnosed with a bone tumor, the trust providing the majority of inpatient treatment had a TYA age-specialist center, while for patients with lymphoma, it was an “other” type of NHS trust.
FIG. 1.
Type of hospital trust responsible for the majority of inpatient treatment, by diagnostic group. TYA, teenage and young adult.
Diagnostic group
Between 30.7% (CNS tumors) and 58.7% (bone tumors) of patients were admitted to their closest available TYA age-specialist center. The results of the travel distance analysis (Table 2) showed that across all diagnostic groups, patients not admitted to a TYA age-specialist center traveled a shorter distance than the distance from their residence to the closest TYA age-specialist center.
Table 2.
Proportion of Patients Admitted to Their Closest TYA Age-Specialist Center, and Median Travel Distances, by Diagnostic Group
| Proportion of patients admitted to a TYA age-specialist center at any time during treatment | Median travel distance from patient's home (km) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Closest TYA age-specialist center to patient's home | Other TYA age-specialist center | No TYA age-specialist center admission | Distance to closest TYA age-specialist | Distance to admitting | |||||
| Diagnostic group | n | n | % | n | % | n | % | center | hospital |
| Leukemia | 737 | 229 | 31.1 | 22 | 3.0 | 486 | 65.9 | 31.7 | 19.8 |
| Lymphoma | 1720 | 369 | 21.5 | 36 | 2.1 | 1315 | 76.5 | 34.3 | 16.9 |
| Central nervous system and other intracranial and intraspinal neoplasms | 833 | 256 | 30.7 | 23 | 2.8 | 554 | 66.5 | 35.9 | 17.5 |
| Osseous and chondromatous neoplasms | 395 | 244 | 61.8 | 25 | 6.3 | 126 | 31.9 | 34.3 | 25.4 |
| Soft tissue sarcoma | 360 | 150 | 41.7 | 11 | 3.1 | 199 | 55.3 | 29.1 | 18.3 |
| Germ cell and trophoblastic neoplasms | 1082 | 332 | 30.7 | 48 | 4.4 | 702 | 64.9 | 33.9 | 16.0 |
| Melanoma and skin carcinoma | 613 | 167 | 27.2 | 14 | 2.3 | 432 | 70.5 | 33.1 | 10.8 |
| Carcinomas | 1048 | 295 | 28.1 | 25 | 2.4 | 728 | 69.5 | 31.0 | 15.8 |
| Overall | 6788 | 2042 | 30.1 | 204 | 3.0 | 4542 | 66.9 | 33.0 | 17.4 |
km, kilometer; TYA, teenage and young adult.
The odds of admission to a TYA age-specialist center were significantly increased in some diagnostic subgroups (Table 3). For leukemia patients, those diagnosed with “other leukemia” were significantly less likely to be admitted to a TYA age-specialist center than those diagnosed with acute lymphoid leukemia (odds ratio [OR]=0.32; 95% confidence interval [CI]: 0.12–0.81). Patients diagnosed with Hodgkin lymphoma were less likely to be admitted to a TYA age-specialist center than those with non-Hodgkin lymphoma (OR=0.71; 95% CI: 0.55–0.92). A similar result was seen when comparing patients diagnosed with Ewing sarcoma of the bone to those with osteosarcoma (OR=0.12; 95% CI: 0.05–0.28). Patients diagnosed with a non-gonadal germ cell tumor or trophoblastic neoplasm were more likely to be admitted to a TYA age-specialist center than those with gonadal germ cell or trophoblastic neoplasms (OR=4.00; 95% CI: 2.31–6.96).
Table 3.
Diagnostic Subgroups Affecting the Likelihood of Admission to a TYA Age-Specialist Center During Treatment, Based on Logistic Regression Presented in Table 4
| Diagnostic group | Subgroup | Odds ratio [95% CI] | p-Valuea |
|---|---|---|---|
| Leukemia | Acute lymphoid leukemia | 1.00 | |
| Acute myeloid leukemia | 1.10 [0.75–1.60] | 0.64 | |
| Chronic myeloid leukemia | 1.06 [0.62–1.82] | 0.83 | |
| Other leukemia | 0.32 [0.12–0.81] | 0.02 | |
| Lymphoma | Non-Hodgkin lymphoma | 1.00 | |
| Hodgkin lymphoma | 0.71 [0.55–0.92] | 0.01 | |
| Central nervous system and other intracranial and intraspinal neoplasms | Astrocytoma | 1.00 | |
| Other gliomas | 0.77 [0.45–1.33] | 0.35 | |
| Ependymoma | 0.83 [0.36–1.89] | 0.65 | |
| Medulloblastoma and other PNET | 1.48 [0.76–2.89] | 0.25 | |
| Other specified and unspecified intracranial and intraspinal neoplasms | 1.06 [0.72–1.56] | 0.76 | |
| Unspecified and unspecified intracranial and intraspinal neoplasms | 1.61 [0.71–3.64] | 0.25 | |
| Osseous and chondromatous neoplasms | Osteosarcoma | 1.00 | |
| Ewing sarcoma | 0.12 [0.05–0.28] | <0.01 | |
| Other | 0.92 [0.56–1.52] | 0.74 | |
| Soft tissue sarcoma | Fibrosarcoma | 1.00 | |
| Rhabdomyosarcoma | 3.61 [1.66–7.86] | <0.01 | |
| Other | 1.70 [0.86–3.33] | 0.13 | |
| Unspecified | 3.88 [1.72–8.78] | <0.01 | |
| Germ cell and trophoblastic neoplasms | Gonadal germ cell or trophoblastic neoplasms | 1.00 | |
| Non-gonadal germ cell or trophoblastic neoplasms | 4.00 [2.31–6.96] | <0.01 | |
| Melanoma and skin carcinoma | Melanoma | 1.00 | |
| Skin carcinoma | 1.08 [0.69–1.67] | 0.74 | |
| Carcinomas | Thyroid | 1.00 | |
| Head and neck | 0.61 [0.37–1.01] | 0.05 | |
| Trachea, bronchus, lung, and pleura | 0.73 [0.33–1.61] | 0.43 | |
| Breast | 0.68 [0.35–1.31] | 0.25 | |
| Genitourinary tract | 0.53 [0.36–0.79] | <0.01 | |
| Gastrointestinal tract | 0.61 [0.41–0.92] | 0.02 | |
| Other and ill-defined sites | 0.81 [0.45–1.46] | 0.48 |
Note: Diagnostic groups were assigned in line with the Birch classification scheme.22
Values significant at p<0.05 highlighted in bold.
CI, confidence interval; PNET, primitive neuroectodermal tumor; TYA, teenage and young adult.
Gender
Gender had no effect on the likelihood of admission to a TYA age-specialist center during treatment, except in those with germ cell tumors, for which females were 95% more likely to be admitted to a TYA age-specialist center than their male equivalents (OR=1.95; 95% CI: 1.20–3.16; Table 4).
Table 4.
Factors Affecting the Likelihood of Admission to a TYA Age-Specialist Center During Treatment Based on Logistic Regression, by Diagnostic Group
| Diagnostic group | Factors | Odds ratio [95% CI] | p-Valueb |
|---|---|---|---|
| Leukemia | Gender | ||
| Male | 1.00 | ||
| Female | 0.92 [0.65–1.28] | 0.61 | |
| Age at diagnosis (single year increase) | 0.93 [0.88–0.99] | 0.02 | |
| Year of diagnosis (single year increase) | 0.97 [0.88–1.07] | 0.52 | |
| Distance to closest center (5 km increase)a | 0.91 [0.88–0.93] | <0.01 | |
| Lymphoma | Gender | ||
| Male | 1.00 | ||
| Female | 0.96 [0.75–1.22] | 0.72 | |
| Age at diagnosis (single year increase) | 0.86 [0.82–0.90] | <0.01 | |
| Year of diagnosis (single year increase) | 0.88 [0.82–0.95] | <0.01 | |
| Distance to closest center (5 km increase)a | 0.87 [0.85–0.89] | <0.01 | |
| Central nervous system and other intracranial and intraspinal neoplasms | Gender | ||
| Male | 1.00 | ||
| Female | 1.25 [0.91–1.73] | 0.17 | |
| Age at diagnosis (single year increase) | 1.02 [0.96–1.07] | 0.59 | |
| Year of diagnosis (single year increase) | 0.98 [0.89–1.08] | 0.66 | |
| Distance to closest center (5 km increase)a | 0.86 [0.83–0.89] | <0.01 | |
| Osseous and chondromatous neoplasms | Gender | ||
| Male | 1.00 | ||
| Female | 0.70 [0.69–1.76] | 0.24 | |
| Age at diagnosis (single year increase) | 1.02 [0.90–1.06] | 0.62 | |
| Year of diagnosis (single year increase) | 1.07 [0.94–1.17] | 0.29 | |
| Distance to closest center (5 km increase)a | 0.92 [0.90–0.95] | <0.01 | |
| Soft tissue sarcoma | Gender | ||
| Male | 1.00 | ||
| Female | 0.79 [0.50–1.24] | 0.31 | |
| Age at diagnosis (single year increase) | 0.95 [0.88–1.03] | 0.23 | |
| Year of diagnosis (single year increase) | 0.96 [0.84–1.10] | 0.57 | |
| Distance to closest center (5 km increase)a | 0.91 [0.88–0.94] | <0.01 | |
| Germ cell and trophoblastic neoplasms | Gender | ||
| Male | 1.00 | ||
| Female | 1.95 [1.20–3.16] | 0.01 | |
| Age at diagnosis (single year increase) | 0.94 [0.88–0.99] | 0.03 | |
| Year of diagnosis (single year increase) | 0.97 [0.89–1.06] | 0.53 | |
| Distance to closest center (5 km increase)a | 0.81 [0.79–0.84] | <0.01 | |
| Melanoma and skin carcinoma | Gender | ||
| Male | 1.00 | ||
| Female | 1.02 [0.70–1.49] | 0.93 | |
| Age at diagnosis (single year increase) | 0.98 [0.91–1.06] | 0.63 | |
| Year of diagnosis (single year increase) | 1.00 [0.90–1.11] | 0.95 | |
| Distance to closest center (5 km increase)a | 0.83 [0.79–0.87] | <0.01 | |
| Carcinomas | Gender | ||
| Male | 1.00 | ||
| Female | 1.09 [0.80–1.49] | 0.58 | |
| Age at diagnosis (single year increase) | 0.93 [0.88–0.98] | 0.01 | |
| Year of diagnosis (single year increase) | 0.97 [0.89–1.05] | 0.46 | |
| Distance to closest center (5 km increase)a | 0.84 [0.82–0.87] | <0.01 |
Note: Each diagnostic group was modeled separately (diagnostic groups were assigned in line with the Birch classification scheme22).
Distance to the closest TYA age-specialist center from patient's home address.
Values significant at p<0.05 highlighted in bold.
CI, confidence interval; km, kilometer; TYA, teenage and young adult.
Age at diagnosis
Increasing age at diagnosis significantly reduced the likelihood of admission for those diagnosed with leukemia, lymphoma, germ cell neoplasms, and carcinomas (Table 3). For the other diagnostic groups, the effect was not statistically significant.
Travel distance
Distance between a patient's home and their closest TYA age-specialist center was a significant factor in the likelihood of admission to a TYA age-specialist center during treatment for all diagnostic groups, although the size of the effect varied between groups (Table 3). A 5 km increase in distance from the TYA age-specialist center had the greatest effect for patients diagnosed with germ cell tumors, decreasing the likelihood of admission by 19% (OR=0.81; 95% CI: 0.79–0.84). The smallest effect was seen in those diagnosed with bone tumors, for whom each 5 km increase reduced the likelihood of admission by 8% (OR=0.92; 95% CI: 0.90–0.95). The remaining diagnostic groups fell between these two extremes. In all cases, these results were statistically significant (p<0.01).
Of those patients admitted to a TYA age-specialist center during treatment, very few were admitted to a center other than the one nearest their home address (9.1%; Table 2).
Year of diagnosis
Despite substantial changes in service organization over the period of this study, no significant association between the year of diagnosis and the likelihood of admission to each type of center was found, other than for those diagnosed with lymphoma (OR=0.88; 95% CI: 0.82–0.95; Table 3).
Discussion
Some patients in all diagnostic groups were admitted to hospitals other than their closest TYA age-specialist center, including alternative TYA age-specialist centers, teaching hospitals, and other NHS centers. In the majority of cases, these patients were admitted to a hospital closer to their place of residence. In most cases, the majority of time spent as an inpatient occurred in a teaching hospital that did not have a TYA age-specialist center at the time of the patient's admission. Significant variation was seen between diagnostic groups when examining the proportion of patients with any admission to a TYA age-specialist center and the type of center providing inpatient treatment for the largest proportion of inpatient stays during treatment.
Older TYAs were less likely to be admitted to a TYA age-specialist center than younger TYAs; a decrease of 7% was seen for each single year increase in age. No significant effect was seen when assessing change over time, despite major service alterations—including the centralization of services and the introduction of an age-specific multi-disciplinary team—during the time period covered by this study.
Gender was found to be non-significant in all diagnostic groups except for germ cell tumors, for which females were significantly more likely than males to be admitted to a TYA age-specialist center. Gonadal germ cell tumors in females occur in the ovaries, whereas in males they occur in the testicles. The two require different surgeries, which may have influenced the increase in specialist admissions for females in this group.
The admissions to TYA age-specialist centers, teaching hospitals, and other centers could not entirely be attributed to the proximity to a TYA age-specialist center or to the distribution of the population over the country. Irrespective of age, diagnosis, and year of diagnosis, patients were significantly less likely to be admitted to a TYA age-specialist center, with increasing distance between home and the closest center. However, the variation between diagnostic groups suggests that geography is not the only cause. If it were the only cause of the variation, the effect would be expected to be similar between all diagnostic groups. The question as to the entire cause for the variation in service usage in this age group remains and warrants further investigation when additional information—such as the reason for non-attendance—becomes available.
Models of care for TYAs with cancer in the United Kingdom have been developed in response to the perceived lack of improvement in survival and the unique collection of requirements within this group.1,18,25 Patients are generally more satisfied with their treatment in TYA age-specialist centers.26,27 This increased satisfaction, and in some cases location of care facility, have been associated with increased quality of life.28,29 Studies have also shown a correlation between patient satisfaction and improved clinical outcomes in TYAs with cancer.30
This is the first study to focus on this age group and to cover the whole of England; previously studies focused only on older or younger patients. Our findings demonstrate that there is significant variation in service usage for TYAs with cancer across England that cannot be entirely explained by diagnostic group or patient characteristics. Although travel time influences the likelihood of admission to a TYA age-specialist center, it is unlikely to be the sole cause of the differences observed in this study. While there is little work showing a survival benefit from treatment at a TYA age-specialist center compared to other hospitals, it is unlikely that the complex psychosocial needs of this group can be met in any other setting.11
The implementation of the National Institute for Health and Clinical Excellence's “Improving Outcomes Guidance in Children and Young People with Cancer” guidance1 is continuing. Indeed, since this study was undertaken, additional TYA age-specialist centers have opened. However, the lack of a significant change in patterns of admission over time suggests that variation in admission to TYA age-specialist centers will remain, reinforcing the need for more structured service usage plans.
The primary limitation of this study was its inability to determine what proportions of the variation were due to patient preference or to differences in referral practice from practitioners outside the service into TYA age-specialist centers. Work currently being undertaken to assess the choices that this age group is making in relation to place of care will go some way to addressing this.
It was not possible to determine what proportion of patients were entered into clinical trials in each care setting, though this is often a key element of specialist care. However, this work could be undertaken in the future should trial recruitment data become available.
Finally, it was not possible to assess the place of care for outpatient appointments and treatment; as such, only inpatient and day-case (elective admissions to a hospital not requiring an overnight stay) admissions were included in the analysis. This meant that large proportions of diagnostic groups who received their care largely outside of the inpatient setting were excluded from the analysis. For some diagnostic groups, such as melanoma, this may have introduced bias into the case mix, as the patients who were admitted were more likely to have disease requiring more complex treatment. Again, should data on outpatient appointments become available further analyses could be undertaken.
Due to coding issues and statistical power, the reasons for each hospital admission were not analyzed. This presents a problem, in that patients who were acutely unwell may be more likely to be admitted to a hospital close to home than those admitted for treatment (chemotherapy, radiotherapy, etc.). However, the analyses undertaken in this project examined the factors affecting the likelihood of any admission to a TYA age-specialist center during the course of treatment, rather than the number of admissions to each type of center, meaning that there will be minimal bias in relation to the place of admission for acute cases.
The 2001–2009 time period covered by this study encompasses the centralization of specialist services for TYA patients. Whilst this occurred relatively early in the study period (2005), the possibility remains that the reorganization of services may have continued throughout. In order to address this, further study would need to be undertaken should more timely data become available.
Conclusion
Over half (66.9%) of all patients diagnosed during the study period had no admission to a TYA age-specialist center during the course of their treatment and were instead treated elsewhere as an inpatient. Only 24% of patients received the majority of their inpatient treatment at a hospital with a TYA age-specialist center. In the majority of cases, patients admitted to non-TYA age-specialist centers were admitted to a hospital closer to their place of residence. As the effect differs between diagnostic groups, it can be hypothesized that geography is not the sole cause of the variation in admission to TYA age-specialist centers. Further work is needed to determine which additional factors influence these differences.
Acknowledgment
This work was supported by Cancer Research UK (PI Richard G. Feltbower; grant number 473630).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.National Institute for Health and Clinical Excellence Improving outcomes in children and young people with cancer. London: National Institute for Health and Clinical Excellence; August2005 [Google Scholar]
- 2.Calman K, Hine D. A policy framework for commissioning cancer services: a report by the Expert Advisory Group on Cancer to the chief medical officers of England and Wales. London: Department of Health; 1995 [Google Scholar]
- 3.Cancer Research UK Teenage and young adult cancer: key facts. Accessed March25, 2013 from: www.cancerresearchuk.org/cancer-info/cnacerstats/keyfacts/teenage-and-young-adult-cancer/
- 4.Teenage Cancer Trust Teenage Cancer Trust—a charity devoted to improving the lives of teenagers and young adults with cancer. Accessed October1, 2012 from: www.teenagecancertrust.org
- 5.Crawford SM, Sauerzapf V, Haynes R, et al. Social and geographical factors affecting access to treatment of lung cancer. Br J Cancer. 2009;101(6):897–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones AP, Haynes R, Sauerzapf V, et al. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer. 2008;44(7):992–9 [DOI] [PubMed] [Google Scholar]
- 7.Zucca A, Boyes A, Newling G, et al. Travelling all over the countryside: travel-related burden and financial difficulties reported by cancer patients in New South Wales and Victoria. Aust J Rural Health. 2011;19(6):298–305 [DOI] [PubMed] [Google Scholar]
- 8.Russell E, Kramer MR, Cooper HL, et al. Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. J Urban Health. 2011;88(6):1117–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currow DC, Allingham S, Bird S, et al. Referral patterns and proximity to palliative care inpatient services by level of socio-economic disadvantage. A national study using spatial analysis. BMC Health Serv Res. 2012;12(1):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Peer Review—National Cancer Action Team Manual for cancer services: teenage and young adult measures (version 2.0). London: National Cancer Action Team, National Health Service; April2013 [Google Scholar]
- 11.Marris S, Morgan S, Stark D. “Listening to patients”: what is the value of age-appropriate care to teenagers and young adults with cancer? Eur J Cancer Care. 2011;20(2):145–51 [DOI] [PubMed] [Google Scholar]
- 12.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. New Engl J Med. 2001;345(3):181–8 [DOI] [PubMed] [Google Scholar]
- 13.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245(5):777–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000;18(11):2327–40 [DOI] [PubMed] [Google Scholar]
- 15.Selby P, Gillis C, Haward R. Benefits from specialised cancer care. Lancet. 1996;348(9023):313–8 [DOI] [PubMed] [Google Scholar]
- 16.CLIC Sargent A long way from home: the impact of travel on children and young people with cancer. London: CLIC Sargent; December2010 [Google Scholar]
- 17.National Health Service NHS specialised services. Accessed January31, 2012 from: www.specialisedservices.nhs.uk
- 18.Whelan J, Dolbear C, Mak V, et al. Where do teenagers and young adults receive treatment for cancer? J Public Health (Oxf). 2007;29(2):178–82 [DOI] [PubMed] [Google Scholar]
- 19.Birch RJ, Morris EJA, West RM, et al. A cross-sectional survey of healthcare professionals to determine what they believe constitutes “specialist” care for teenage and young adult patients with cancer. BMJ Open. 2013;3(5):e002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Intelligence Network National Cancer Data Repository. 2013. Accessed August3, 2013 from: http://ncin.org.uk/collecting_and_using_data/national_data_repository
- 21.Birch RJ, Morris E, Thomas J, et al. National cancer registrations in teenagers and young adults diagnosed between 2001 and 2006—a data cleaning exercise. Poster presented at the 2011 National Cancer Intelligence Network's Cancer Outcomes Conference; London, June15–17, 2011 [Google Scholar]
- 22.Birch JM, Alston RD, Kelsey AM, et al. Classification and incidence of cancers in adolescents and young adults in England 1979–1997. Br J Cancer. 2002;87(11):1267–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ArcGIS Network Analyst [computer program]. Version 10.1 Redlands, CA: Environmental Systems Research Institute; 2012 [Google Scholar]
- 24.Stata Statistical Software: Release 12 [computer program] College Station, TX: StataCorp LP; 2011 [Google Scholar]
- 25.Birch JM, Pang D, Alston RD, et al. Survival from cancer in teenagers and young adults in England, 1979–2003. Br J Cancer. 2008;99(5):830–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds BC, Windebank KP, Leonard RC, Wallace WH. A comparison of self-reported satisfaction between adolescents treated in a “teenage” unit with those treated in adult or paediatric units. Pediatr Blood Cancer. 2005;44(3):259–63 [DOI] [PubMed] [Google Scholar]
- 27.Ramphal R, D'Agostino N, Klassen A, et al. Practices and resources devoted to the care of adolescents and young adults with cancer in Canada: a survey of pediatric and adult cancer treatment centers. J Adolesc Young Adult Oncol. 2011;1(3):140–4 [DOI] [PubMed] [Google Scholar]
- 28.Muthny FA, Koch U, Stump S. Quality of life in oncology patients. Psychother Psychosom. 2010;54(2–3):145–60 [DOI] [PubMed] [Google Scholar]
- 29.Fern LA, Taylor RM, Whelan J, et al. The art of age-appropriate care: reflecting on a conceptual model of the cancer experience for teenagers and young adults. Cancer Nurs. 2013;36(5):E27–38 [DOI] [PubMed] [Google Scholar]
- 30.Butow P, Palmer S, Pai A, et al. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28(32):4800–9 [DOI] [PubMed] [Google Scholar]