Abstract
The interaction between mesenchymal stem cells (MSCs) and dendritic cells (DCs) affects T cell development and function. Further, the chemotactic capacity of MSCs, their interaction with the tumor microenvironment, and the intervention of immune-stimulatory molecules suggest possible exploitation of tumor necrosis factor-α (TNF-α) and CD40 ligand (CD40L) to genetically modify MSCs for enhanced cancer therapy. Both DCs and MSCs were isolated from BALB/c mice. DCs were then cocultured with MSCs transduced with TNF-α and/or CD40L [(TNF-α/CD40L)-MSCs]. Major DCs' maturation markers, DC and T cell cytokines such as interleukin-4, -6, -10, -12, TNF-α, tumor growth factor-β, as well as T cell proliferation, were assessed. Meantime, a BALB/c mouse breast tumor model was inducted by injecting 4T1 cells subcutaneously. Mice (n=10) in each well-defined test groups (n=13) were cotreated with DCs and/or (TNF-α/CD40L)-MSCs. The controls included untreated, empty vector-MSC, DC-lipopolysaccharide, and immature DC mouse groups. Eventually, cytokine levels from murine splenocytes, as well as tumor volume and survival of mice, were assessed. Compared with the corresponding controls, both in vitro and in vivo analyses showed induction of T helper 1 (Th1) as well as suppression of Th2 and Treg responses in test groups, which led to a valuable antitumor immune response. Further, the longest mouse survival was observed in mouse groups that were administered with DCs plus (TNF-α/CD40L)-MSCs. In our experimental setting, the present pioneered study demonstrates that concomitant genetic modification of MSCs with TNF-α and CD40L optimized the antitumor immunity response in the presence of DCs, meantime increasing the mouse lifespan.
Introduction
Mesenchymal stem cells (MSCs) are a heterogeneous population of self-renewing and multipotent cells isolated from the bone marrow (BM) (Staba et al., 1998; Liu et al., 2004). It has been demonstrated that all organs containing connective tissue contain MSCs (Vaananen, 2005). MCSs are known to display immunomodulatory activities, including suppression of lymphocyte proliferation (Aggarwal and Pittenger, 2005; Beyth et al., 2005), inhibition of dendritic cells (DCs), maturation in vitro and in vivo, inhibition of cytokines secretion, downregulation of molecules involved in the migration to the lymph nodes, antigen (Ag) presentation to CD4+ T cells, and cross-presentation to CD8+ T cells (Beyth et al., 2005; Jiang et al., 2005; Nauta et al., 2006; Pevsner-Fischer et al., 2007; Tomchuck et al., 2008; Spaggiari et al., 2009). More specifically, the suppressive actions of MSCs are exerted at two levels (Aggarwal and Pittenger, 2005; Jiang et al., 2005; Ren et al., 2008): (i) through cell–cell contact molecules (e.g., major histocompatibility I [MHC-I], intercellular-adhesion molecule 1/2, vascular cell adhesion molecule-1/2, and cyclooxygenases-1/2); (ii) through the intervention of soluble factors (e.g., interleukin [IL]-6, IL-8, tumor growth factor-β [TGF-β], prostaglandin E2, nitric oxide).
The chemotactic capacity of MSCs raised hope for their clinical exploitation of genetically modified MSCs for cancer therapy and other immune-mediated diseases (Studeny et al., 2004; Calzascia et al., 2007; Dazzi and Horwood, 2007; Uccelli et al., 2007; Uccelli and Prockop, 2010). The main advantage of MSCs for enhanced tumor therapy resides in their great tumor tropism, their use as a selective and highly bioavailable drug/gene delivery system, as well as their ease for tissue and immune reconstitution, compared with other available tumor therapeutic methods (Aboody et al., 2008; Rameshwar, 2009; Galderisi et al., 2010; Hu et al., 2010; Sun et al., 2011; Shah, 2012; Gao et al., 2013). Although antitumoral MSCs might cause some adverse effects, MSCs should be considered in metastatic cancer therapy (Dazzi and Horwood, 2007; Hall et al., 2007; Yagi et al., 2010).
Besides, tumor necrosis factor (TNF) superfamily members (i.e., TNF-α and CD40 ligand [CD40L]) are involved in the activation and maturation of DCs, which are considered as the most potent primary “professional” Ag-presenting cells, acting via MHC complexes to activate T and B cells in secondary lymphoid organs (Steinman, 1991; Schmidt et al., 2012; Ma et al., 2013). DCs belong to the hematopoietic system and arise from CD34+ stem cells in the BM (Schmidt et al., 2012).
Additionally, CD40L was reported to be the most potent DC inducer among the TNF superfamily, and similarly TNF-α (Pasparakis et al., 1996; van Horssen et al., 2006; Calzascia et al., 2007) and CD40/CD40L engagement is then important in tumor and/or infectious immunity (Yu et al., 2003). Importantly, TNF-α- (Staba et al., 1998; Liu et al., 2004) or CD40L-based gene therapy is considered for immune response induction in animals and humans (Elgueta et al., 2009). Hence, CD40L and TNF-α could be applied for DNA-based vaccine therapies toward DC activation (Yu et al., 2003).
So far, therapeutic genes have been incorporated into stem cells and delivered to tumors with high selectivity. These included prodrug-activating enzymes, apoptosis-promoting genes, metalloproteinases, and immune-enhancing agents such as IL-2, IL-4, IL-12, IL-23, and interferon-β (IFN-β) (Aboody et al., 2008; Bexell et al., 2010). Among the TNF superfamily members, TNF-related apoptosis-inducing ligand (TRAIL) has been delivered by MSCs because of its selective antitumor activity (Loebinger et al., 2009; Menon et al., 2009; Grisendi et al., 2010; Porada and Almeida-Porada, 2010). However, there is still a paucity of reports related to genetically modified MSCs for cancer therapy (Studeny et al., 2004; Gao et al., 2010). In fact, and to the best of our knowledge, there are no studies that explored TNF-α and/or CD40L delivery by MSCs for possible enhanced tumor immune activation.
Therefore, the present research study aimed to evaluate, for the first time, the potential CD40L- and TNF-α-engineered MSCs, in the presence or absence of DCs, to greatly activate immune tumor responses.
Materials and Methods
All the in vitro and in vivo experiments have been realized in triplicate for statistical analysis.
Construction of vector and engineered lentivirus production
TNF-α (GenBank: BC117057.1) and CD40L (GenBank: BC119225.1) mouse genes inserted into pCR4-Topo vectors were purchased (ImaGene). The genes were successfully subcloned into the p240 (pLOX-EWgfp modified vector) lentivirus (LV) transfer vector (Addgene), as confirmed by electrophoresis and sequencing.
HEK293T cells (NCBI code: C497, Cell Bank, Pasteur Institute of Iran) were then transduced by the LVs recombined with a mixture of three vectors: p240-TNFα or p240-CD40L, plox-MD2 (Addgene), and plox-PAX2 (Addgene). Eventually, the supernatant of infected HEK293T cells was collected and concentrated by ultracentrifugation. Expression of GFP (i.e., green fluorescent cells, also known as [aka] GFP+ cells) was used to monitor the transduced cells by immunofluorescence microscopy and titer LVs by flow cytometry.
Preparation of tumor cell lysate
The 4T1 cell line (NBCI code: C604), which mimics stage IV of human breast cancer, was obtained from the Cell Bank of Pasteur Institute of Iran.
4T1 cells were first cultured overnight in T25 culture flasks containing RPMI-1640 complete medium (Sigma) (i.e., Roswell Park Memorial Institute-1640 supplemented with 11 mM sodium bicarbonate, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum [FBS] [v/v] [Gibco]). Then, 1×107 cells were resuspended in 1 ml RPMI-1640 complete medium.
The tumor cell lysate (TL) was eventually prepared by subjecting 4T1 cells to three-to-five cycles of freezing in liquid nitrogen before thawing at 65°C. Total protein was assessed by Bradford assay. About 50 μg/ml of total protein was used as reference in all TL-loaded DC tests for specific Ag presentation and specific splenocyte stimulation.
Isolation and characterization of DCs and MSCs
BM-derived DCs
Eight- to 10-week-old female inbred BALB/c mice with an average weight of 22 g (Pasteur Institute of Iran; n=5) were obtained, and the mouse experiments were in accordance with the guidelines approved by the Ethics Committee of the Lorestan University of Medical Sciences, Iran. The procedures were performed according to the Guide for the Care and Use of Laboratory Animals.
Inbred BALB/c mice (n=5) were euthanized by cervical dislocation, and dissected. After flashing the marrow cavity of femur and tibia, cell suspension was obtained. Red blood cells were lyzed by the ammonium chloride buffer. Lysed cells were washed, and 1−1.5×106 cells/ml were cultured in a 24-well plate containing complete RPMI-1640 medium. On the first day, 20 ng/ml GM-CSF and 10 ng/ml IL-4 (R&D Systems) were added to the culture. The nonadherent cells were recultured with 10 ng/ml GM-CSF and 5 ng/ml IL-4 on day 3 till day 5. On day 5 the immature DCs (iDCs) were then harvested. Flow cytometry was performed to confirm the purity of DCs.
BM-derived MSCs
The BM cells previously isolated were incubated for 3 hr at 37°C and 5% CO2 in complete Dulbecco's modified Eagle's medium (DMEM; Sigma Chemical Co.; i.e., DMEM supplemented with 1% penicillin/streptomycin mixture and 10% FBS [v/v]; Gibco). Then, nonadherent cells were harvested and replaced in fresh DMEM. The cells were subcultured for 3 weeks in DMEM to achieve an optimal purity. Subsequently, flow cytometry was performed to confirm the purity of MSCs.
Transduction of MSCs, their coculture with DCs, and assessment of DCs' maturation markers
About 1.5×106 MSCs/ml were cultured for 24 hr in a 6-well plate containing DMEM supplemented with 10% FBS before transduction. Two hours before transduction, adhered MSCs were washed three times with phosphate buffered saline (PBS) 1× and maintained in DMEM. Then, concentrated TNF-α and CD40L LVs in multiplicity of infection (MOI=20) were added to the cells. Eventually, the cells were incubated at 37°C, and medium replacements were performed 16 hr later. The MSCs transduced with TNF-α and/or CD40L [(TNF-α/CD40L)-MSCs] represented the test groups. Control groups included empty vector-MSCs (i.e., unmodified LVs-MSCs aka untransduced MSCs) as internal control, iDCs as negative external control, and DCs-lipopolysaccharide (LPS) as positive external control.
In order to evaluate the contact effects between (TNF-α/CD40L)-MSCs and DCs 72 hr after MSC transduction, 3×105 MSCs/well of test and control groups were cultured in 1/1 ratio with TL-pulsed DCs for 24 hr in a 96-well plate containing RPMI-1640 complete medium (contact experiment) (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/hum).
To further study the involvement and molecular mechanism of soluble factors, 3×105 TL-loaded DCs/well were cultured for 24 hr with the supernatant collected from the different MSC groups in a 96-well dish containing RPMI-1640 complete medium (supernatant experiment) (Supplementary Table S1).
Eventually, after MSC-DC coculture in contact (con) and supernatant (sup) experiments, DCs were harvested and characterized for CD86 (Becton Dickinson), CD40 (Becton Dickinson), and MHC-II (Becton Dickinson) maturation markers in CD11c+ cells by a routine flow cytometry protocol adapted from Becton Dickinson and Company. The supernatants of MSC-DC cocultures were assessed for TNF-α, IL-4, IL-6, and IL-12 by enzyme-linked immune sorbent assay (ELISA) according to the manufacturer's instructions (eBiosciences).
Allostimulatory capacity of harvested DCs
In order to assess the allogenic reactions of harvested DCs, precultured with MSCs, we evaluated the allostimulatory capacity of harvested DCs (Colvin et al., 2009).
The mice were handled for experiments in accordance to guidelines approved by the Ethics Committee of the Lorestan University of Medical Sciences, Iran, and the Guide for the Care and Use of Laboratory Animals. Lymph node T cells were purified by nylon wool from inbred C57BL/6 mice.
Then, the allogenic T cells were cocultured (1:10 ratio) for 72 hr in RPMI-1640 complete medium with TL-pulsed DCs, which were harvested from “con” and “sup” experiments and pre-137Cs γ-irradiated at a dose of 3 Gy during 3 min. External negative and positive controls included iDCs (+T cells) and DCs+LPS (+T cells), respectively. Internal control was represented by empty vector-MSCs.
After incubation, T cell proliferation was assessed based on MTT reduction using cell proliferation assay kit I (Roche). Moreover, the supernatant was collected for assessing IFN-γ, TGF-β, IL-4, and IL-10 cytokines by ELISA, according to the manufacturer's instructions (eBiosciences).
Tumor model induction and in vivo treatment
4T1 cells were grown for tumor induction. When cells reached their logarithmic phase, 1×106 cells resuspended in PBS were subcutaneously injected into mouse flank. On day 7 after tumor induction, 1×106 DCs and 1×106 MSCs were coresuspended in 100 μl PBS, and intratumorally injected in different BALB/c mouse groups (n=10 mice/group). The controls included untreated, empty vector-MSC, iDC (negative), and DC-LPS (positive) mouse groups (Supplementary Table S1).
Mouse tumor volume and survival
Ten days after tumor induction (day 10) of BALB/c mice (n=5/group), the tumor volume of each BALB/c mouse was measured by digital caliper every 4 days until day 34, which allowed us to collect a 7-point measurement (i.e., day 10, day 14, day 18, day 22, day 26, day 30, day 34).
At day 10, the tumor volume was estimated according to the following formula:
V=LW2/2 (V, tumor volume; L, large diameter; W, small diameter). Tumor-induced mice were followed up until day 100. The day of their death was recorded for survival analysis using Kaplan–Meier method.
Splenocyte isolation, proliferation, and cytokine assays
On day 35, posttumor induction and treatment, half of the BALB/c mice (n=5) from each group were randomly euthanized. The splenocytes were then isolated in aseptic condition, and 4×105 cells/well were cultured in a 96-well plate containing RPMI-1640 complete medium.
For specific stimulation, 20 μg/ml TL was added to each well. About 48 hr later, the splenocyte proliferation was assessed by MTT assay kit I (Roche) according to the manufacturer's instructions.
Furthermore, the concentrations of TNF-α, TGF-β, IL-4, IL-6, IL-12, IL-10, and IFN-γ were analyzed from the splenocyte supernatant by ELISA kit (eBiosciences), according to the manufacturer's instructions.
Statistical analysis
Cytokine data were statistically analyzed by SPSS (version 17) software. Kruskal–Wallis test and Mann–Whitney U-test were used for determining within- and between-group statistical differences, respectively. Kaplan–Meier test was used for survival, and the groups were compared by Log-rank. p-Value<0.05 was considered statistical significant.
Results
Production of CD40L/TNF-α LVs
Production of recombinant (TNF-α and CD40L)-p240 vectors was confirmed by polymerase chain reaction and sequencing (Supplementary Figs. S1 and S2).
To produce sufficient quantity of LVs, HEK293T cells were transduced with recombinant p240 vectors before being analyzed by immunofluorescence microscopy and flow cytometry. Cells expressing GFP indicated transduced cells that produced the engineered LVs (Supplementary Fig. S3).
Eventually, MSCs were transduced successfully, with a satisfactory estimated rate of over 70% GFP+ cells.
DCs' maturation markers after coculture with MSCs
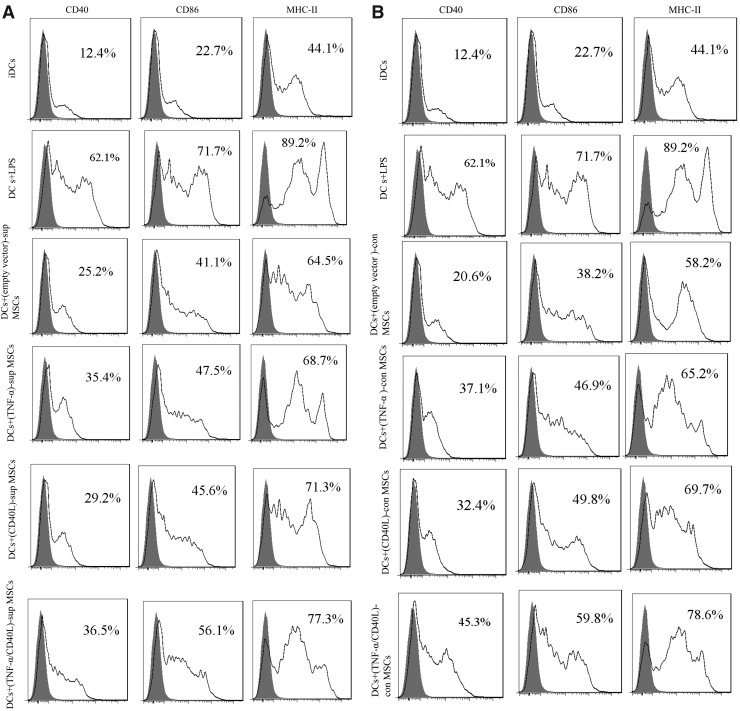
The maturation status of TL-pulsed DCs in “con” and “sup” experiments was characterized by flow cytometry for CD40, CD86, and MHC-II surface markers.
The results presented in Fig. 1 show higher percentage of CD40+/CD86+/MHC-II+-CD11c+ DCs in the sup (Fig. 1A) and con (Fig. 1B) fractions of the transduced MSC groups when compared with the sup and con fractions of untransduced MSCs. However, these differences were not statistically significant.
FIG. 1.
DCs' maturation markers. Expression levels of CD40, CD80, and CD86 on DC-MSC coculture were determined by flow cytometry. (A) Assessment in supernatant (“sup”) fraction. (B) Assessment in contact (“con”) experiment. CD11c was considered for the DC population. CD40L, CD40 ligand; DCs, dendritic cells; iDCs, immature DCs; LPS, lipopolysaccharide; MSCs, mesenchymal stem cells; TNF-α, tumor necrosis factor-α.
Cytokine assay after MSC-DC coculture
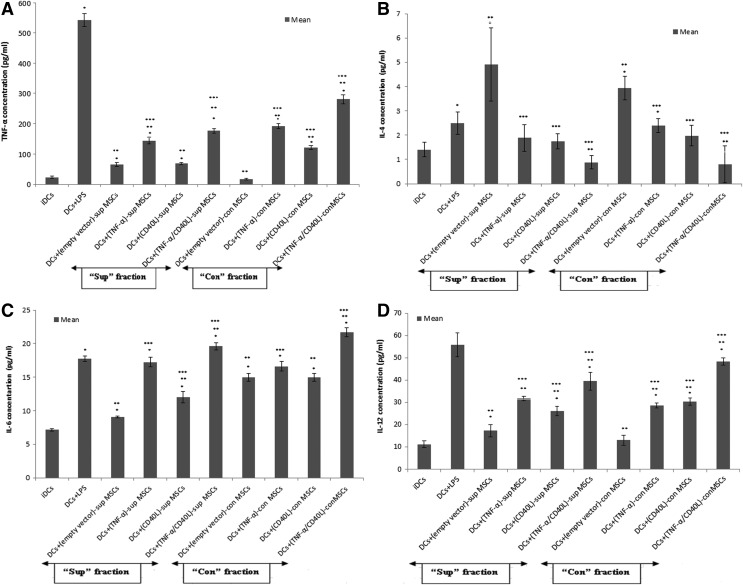
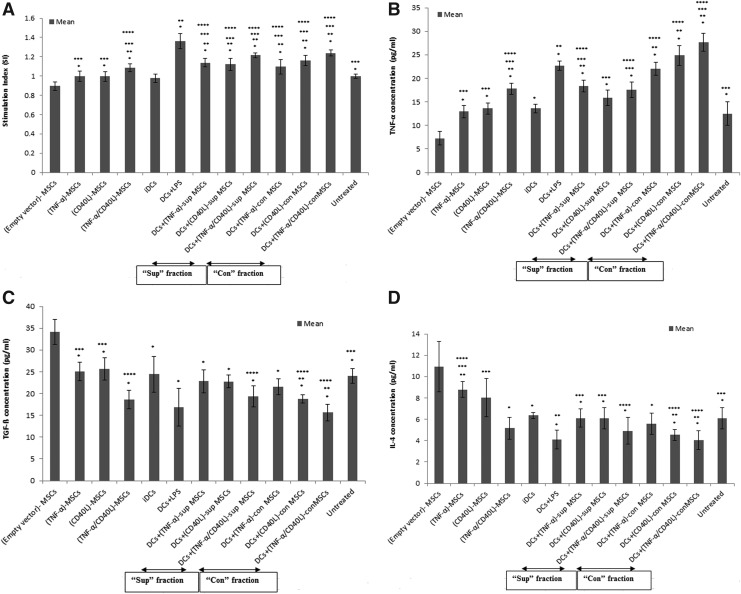
In order to evaluate the impact of soluble (sup) and contact (con) factors of genetically engineered (TNF-α/CD40L)-MSCs on TL-loaded DCs, the concentration levels of TNF-α, IL-4, IL-6, and IL-12 produced by DCs were assessed by ELISA.
As shown in Fig. 2A, TNF-α levels were significantly increased in both sup and con fractions of genetically engineered MSCs when compared with the negative (i.e., iDCs) and internal controls (i.e., empty vector-MSCs). However, these changes were significantly lower than the positive control (DCs+LPS).
FIG. 2.
Produced cytokines in DC-MSC coculture. The supernatant fraction of TNF-α- and/or CD40L-engineered MSCs was cocultured with DCs in 1/1 ratio for 24 hr. The supernatant was then collected for assessing the concentration levels of cytokines by ELISA. (A) TNF-α; (B) IL-4; (C) IL-6; (D) IL-12. Data are represented as mean±SEM. ELISA, enzyme-linked immune sorbent assay; SEM, standard error of mean. *Significant difference compared with negative control (iDCs), p<0.05. **Significant difference compared with positive control (DCs+LPS), p<0.05. ***Significant difference compared with internal control (empty vector-MSCs), p<0.05.
As presented in Fig. 2B, genetically engineered MSCs produced significantly lower IL-4 levels in both sup and con fractions of cocultured cell groups when compared with their respective internal control.
As exhibited in Fig. 2C and D, IL-6 and IL-12 levels showed a significant increase in both sup and con fractions of cocultured genetically engineered MSCs when compared with negative and internal controls.
Allostimulatory capacity of harvested DCs
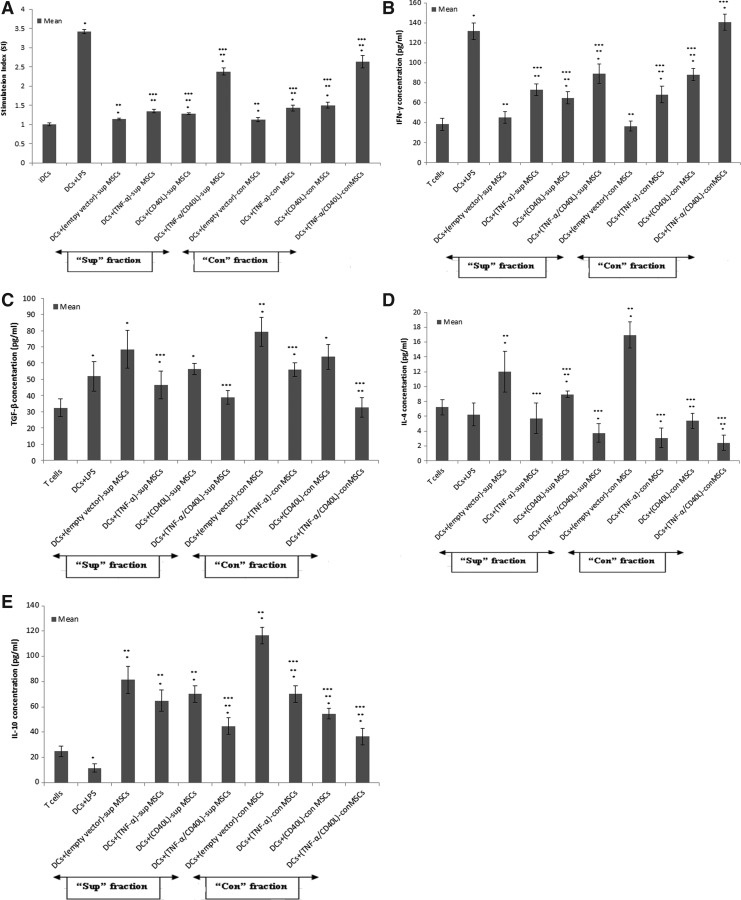
To investigate whether the cocultured DC-MSC groups in vitro (Supplementary Table S1) were able to stimulate allogeneic T cells, DCs were precultured with MSCs before being cocultured with allogenic T cells. Subsequently, T cell proliferation and IFN-γ, TGF-β, IL-4, and IL-10 levels were assessed by ELISA.
As shown in Fig. 3A, significant increased T cell proliferation (i.e., stimulation index) was noticed in all con-treated DCs (precultured with engineered MSC)-T cell groups when compared with the negative control (i.e., iDCs-T cells). This increase was also statistically significant in all sup- and con-treated DC groups cocultured with genetically engineered MSCs, when compared with the internal control (i.e., empty vector-MSCs). Nevertheless, the T cell proliferation was significantly lower when compared with the positive control (i.e., [DCs+LPS]-T cells).
FIG. 3.
Proliferation and cytokine assays in allostimulatory capacity of DCs. DCs were precultured with genetically modified MSCs before being irradiated and cocultured with allogenic T cells in 1/10 ratio for 72 hr. (A) The stimulation index (SI) was assessed by MTT. The supernatant was assessed for the following cytokines: (B) IFN-γ; (C) TGF-β; (D) IL-4; (E) IL-10. Data are represented as mean±SEM. *Significant difference compared with negative control (iDCs), p<0.05. **Significant difference compared with positive control (DCs+LPS), p<0.05. ***Significant difference compared with internal control (empty vector-MSCs), p<0.05.
As exhibited in Fig. 3B, a significant increase of IFN-γ levels was obtained in all sup- and con-treated DCs (precultured with engineered MSCs)-T cells when compared with internal controls.
As represented in Fig. 3C, TGF-β levels were significantly decreased in sup- and con-treated DCs (precultured with coengineered MSCs)-T cells or DCs (precultured with (TNF-α)-MSCs)-T cells when compared with their respective internal control.
As displayed in Fig. 3D, IL-4 levels were significantly decreased in all sup- and con-treated DCs (precultured with engineered MSCs)-T cells, when compared with their respective internal control.
Eventually, in Fig. 3E, a significant decrease of IL-10 concentration levels in sup-treated DCs (precultured with coengineered MSCs)-T cells and all con-treated DCs (precultured with engineered MSCs)-T cells was observed compared with their respective internal control.
Mouse tumor volume and survival
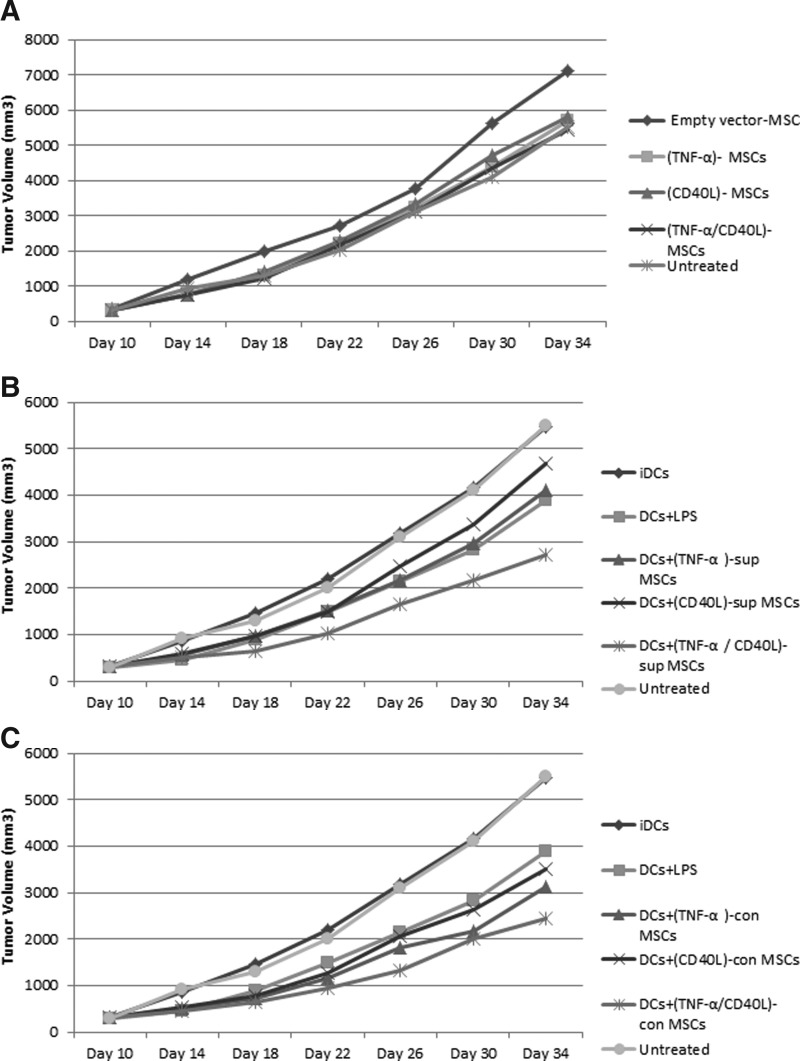
After tumor induction of BALB/c mice by 4T1 cells, the tumor volume and the survival of each mouse were monitored in a time-dependent manner (Supplementary Figs. S4 and S5).
Although there was an increasing trend of tumor volume in all groups in the 34th day of measurement (Fig. 4A–C), the lowest tumor volume was noticed in mouse groups treated with DCs+(TNF-α/CD40L)sup- and con-MSCs (Fig. 4B and C, respectively). In fact, the administration of engineered MSCs in the presence or absence of DCs did not cause a block of the tumor growth but led to a slower tumor progression.
FIG. 4.
Tumor volume. Ten days after tumor induction and then every 4 days until day 34, the tumor volume of BALB/c mice was measured by digital caliper (total of 7 points of measurement). (A) Mouse groups treated with empty vector-MSCs. (B) Mouse group treated with DCs plus supernatant of (TNF-α/CD40L)-MSCs. (C) Mouse group treated with DCs in contact with (TNF-α/CD40L)-MSCs.
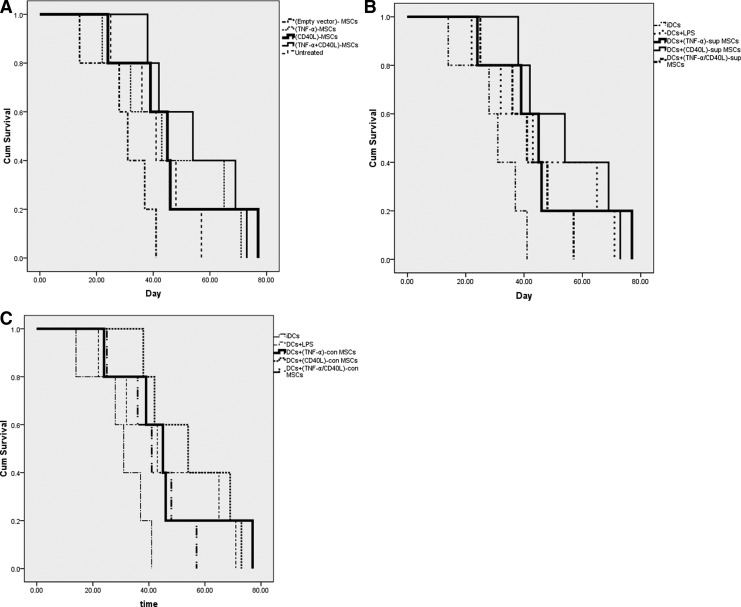
Mouse survival was assessed using mouse groups treated with (TNF-α/CD40L)-MSCs (Fig. 5A) or mouse groups treated with DCs±(TNF-α/CD40L)sup- or con-MSCs (Fig. 5B and C, respectively).
FIG. 5.
Mouse survival. The mortality rate of BALB/c mice was monitored and recorded in each studied group. (A) Mice treated with empty vector-MSCs. (B) Mice treated with DCs plus supernatant of (TNF-α/CD40L)-MSCs. (C) Mice treated with DCs in contact with (TNF-α/CD40L)-MSCs.
When compared with empty vector-MSC-treated and untreated mouse groups (mean survival of 30 and 41 days, respectively), (TNF-α/CD40L)-MSCs and (CD40L)-MSCs lasted longer (mean survival of 55 and 46 days, respectively) (Fig. 5A).
When compared with iDCs used as negative control (mean survival of 37 days), mouse groups treated with DCs+(TNF-α/CD40L)sup-MSCs, DCs+(TNF-α)sup-MSCs, and DCs+(CD40L)sup-MSCs lasted longer (respective mean survival of 56, 52, and 50 days) (Fig. 5B). However, the mouse group treated with DCs+(TNF-α/CD40L)sup-MSCs lasted shorter than the positive control-treated mouse group (i.e., DCs+LPS), which survived on average 57 days.
When compared with iDCs used as negative control (mean survival of 37 days), mouse groups treated with DCs+(TNF-α/CD40L)con-MSCs and DCs+(TNF-α)con-MSCs lasted longer (mean survival of 63 and 65, respectively) (Fig. 5C).
Splenocyte proliferation and cytokine assay
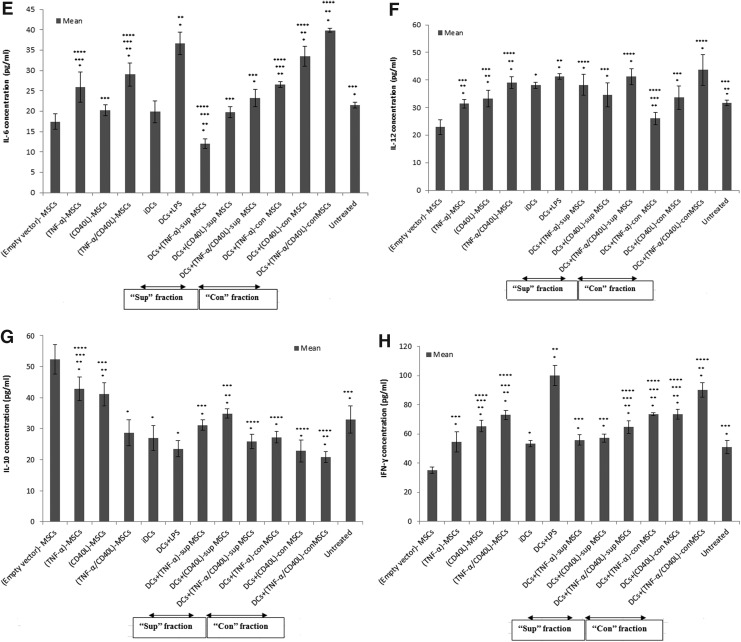
In order to identify the dividing capacity of spleen cells in the developed tumor mouse model, the splenocytes of tumorigenic BALB/c mice were specifically stimulated with the TL, before their proliferation being assessed by the MTT assay.
As shown in Fig. 6A, the results indicated that there was a significant increase of TL-stimulated splenocyte proliferation in all DC+MSC-treated mouse groups, in particular with DCs+(TNF-α/CD40L)sup- or con-MSCs, when compared with the untreated mouse group.
FIG. 6.
Splenocyte proliferation and cytokine assays. (A) Splenocytes of each group were isolated and stimulated with tumor lysate (TL). Splenocyte proliferation was assessed by MTT. The supernatant of TL-stimulated splenocytes was then collected for assessing by ELISA the concentration levels of the following cytokines: (B) TNF-α; (C) TGF-β; (D) IL-4; (E) IL-6; (F) IL-12; (G) IL-10; (H) IFN-γ. Data are represented as mean±SEM. *Significant difference compared with vector MSC, p<0.05. **Significant difference compared with negative control (iDC), p<0.05. ***Significant difference compared with positive control (DC+LPS), p<0.05. ****Significant difference compared with untreated group, p<0.05.
The supernatant of TL-stimulated splenocytes, tested in the different mouse groups, was then collected and measured for TNF-α, TGF-β, IL-4, IL-6, IL-12, IL-10, and IFN-γ using ELISA (Fig. 6B–H, respectively).
As presented in Fig. 6B, TNF-α levels were significantly increased in (TNF-α/CD40L)-MSCs as well as in all mouse groups treated with DCs+(TNF-α/CD40L)sup- or con-MSCs, when compared with either untreated and/or negative control (i.e., iDC-injected mice) groups. Interestingly, the group treated with cocultured DCs and (TNF-α/CD40L)con-MSCs showed significantly higher concentration of TNF-α than the positive control group (i.e., DCs+LPS).
As shown in Fig. 6C, TGF-β levels showed a significant decrease in mouse groups treated with DCs+(TNF-α/CD40L)sup- and con-MSCs, when compared with the negative control (i.e., iDCs) and untreated groups. Noticeably, a decrease in TGF-β levels was observed in all studied groups when compared with unmodified MSCs (i.e., empty vector-MSCs), but was significantly not the case when compared with the positive control (i.e., DCs+LPS).
As presented in Fig. 6D, Il-4 levels were significantly increased in (TNF-α)-MSCs, but significantly decreased in mouse groups treated with DCs+(TNF-α/CD40L)sup- and con-MSCs and DCs+(CD40L)con-MSCs when compared with the untreated mouse group.
As presented in Fig. 6E, IL-6 levels were significantly increased in mouse groups treated with DCs+(TNF-α/CD40L)con-MSCs, when compared with the untreated group.
As shown in Fig. 6F, IL-12 levels were significantly increased in all mouse groups treated with DCs±(TNF-α/CD40L)-MSCs when compared with the untreated group.
Eventually, as displayed in Fig. 6G and H, IL-10 levels were significantly decreased (Fig. 6G) while IFN-γ levels were significantly increased (Fig. 6H) in all mouse groups treated with DCs+(TNF-α/CD40L)sup and DCs+(TNF-α/CD40L)con-MSCs when compared with the untreated mouse group.
Discussion
MSCs can regulate immune cells, which subsequently contribute in the overall immune system control (Aggarwal and Pittenger, 2005; Beyth et al., 2005; Jiang et al., 2005; Nauta et al., 2006; Calzascia et al., 2007; Spaggiari et al., 2009).
Nevertheless, there are conflicting data regarding the effects of MSCs on tumor growth (Bexell et al., 2009; Coffelt et al., 2009; Otsu et al., 2009), exemplifying unknown aspects of MSCs. Consequently, the application of immune-suppressive property of MSCs in tumor therapy is limited. Hopefully, MSCs engineered with certain immune-stimulatory genes (e.g., TNF-α and/or CD40L) might complement their naturally occurring chemotactic ability to tumor tissues for enhancing tumor therapy.
Therefore, in this study, we investigated the immune-modulatory effects of MSCs genetically engineered with TNF-α and/or CD40L on DCs, not only in vitro but also in vivo since it is important to consider the tumor microenvironment. Thereby, the mechanisms related to cell–cell contact and secretory factors (i.e., soluble or supernatant proinflammatory molecules) were studied both in vitro and in vivo. Our in vitro data reveal the following:
1. The expression levels of DCs' maturation markers such as CD86, CD40, and MHC-II were increased when DCs were exposed to all genetically engineered/transduced MSCs (i.e., DCs+(TNF-α/CD40L)sup- or con- groups), when compared with DCs+(empty vector)-MSCs or iDCs. Despite that the data did not reach statistical significance, the obtained tendency remained promising because of suppressive effects of MSCs on costimulatory molecules such as CD80 (Ma et al., 2012). Indeed, the overexpression of DCs' maturation markers represents the increased capacity of DCs to present tumor Ag to T cells in an efficient way, which leads to maturation and effector function of the DCs-T Cells, thereby initiating immune responses (Pasparakis et al., 1996). This is confirmed in our sequential allostimulatory capacity assay of DCs, in which DCs, precultured with MSCs, were cocultured with T cells to observe the outcome in T cell responses. Indeed, although MSCs were shown to arrest the proliferation of both DCs and T cells (Glennie et al., 2005) in vitro, our present study conversely shows that the transduced MSCs led to DC and T cell activation, when compared with DCs+(empty vector)-MSCs.
2. DCs+(TNF-α/CD40L)-MSCs resulted in a significant increase of TNF-α, IL-6, and IL-12 levels, while a significant reduction of IL-4 levels was noticed, when compared with DCs+(empty vector)-MSCs. These changes were observed both in the supernatant (sup) and in the cell–cell contact (con) groups. Thus, the transduced MSCs induce a proinflammatory response in the presence of DCs.
3. The T cell proliferation observed from the allostimulatory capacity of DCs was accompanied in these cells by a significant activation of IFN-γ production and a significant reduction in TGF-β, IL-4, and IL-10 levels, in all transduced MSC (sup- or con-) groups cocultured with DCs, when compared with empty vector-MSC groups. Although CD40/CD40L complex is supposed to mainly act via contact mechanisms, our results obtained from all sup-treated groups also suggest the likelihood of soluble mechanisms involving soluble CD40L. This also suggests that there is no superiority between cell–cell contact and soluble mechanisms on the cytokine regulation. Interestingly, the increased IFN-γ (cytokine of T helper 1 [Th1]) and decreased IL-4 (cytokine of Th2) levels suggest a shift from Th2 profile toward Th1 response, which is the target of tumor immunotherapy and the consequence of pretreated DC actions. Accordingly, the declined production of TGF-β, a cytokine that plays a crucial role in regulating responses such as Treg induction, is in line with our previous antitumoral hypothesis elicited by DC+(TNF-α)-MSCs and DC+(TNF-α/CD40L)-MSCs in both sup- and con-treated groups. Thus, TNF-α was required to enhance the effectiveness of transduced MSCs with CD40L. Eventually, the decreased levels of IL-10, another important cytokine implicated in modulatory mechanisms such as Treg induction, are greatly beneficial to induce antitumor responses. Indeed, it has been reported that nitric oxide produced by MSCs induces IL-10 production in macrophages (Prockop and Oh, 2012), which was not desirable in antitumor immunity.
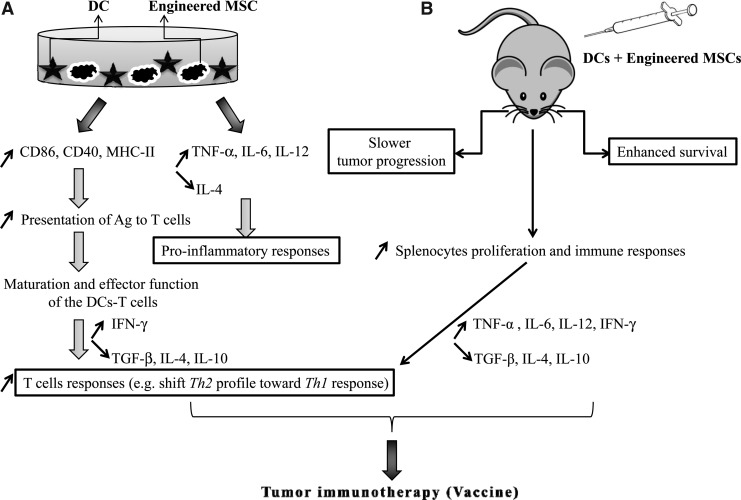
Taken together, our in vitro results showed both an induction of proinflammatory and a suppression of anti-inflammatory responses. Although increased expression levels of DCs' maturation markers were not statistically significant, the wide range of key cytokines investigated in DCs-MSCs and DCs (precultured with MSCs)-T cells supports an important role for DCs' modulation toward antitumor responses (Fig. 7A).
FIG. 7.
Putative molecular mechanisms of the combinatorial genetic and cellular approach. (A) Major in vitro molecular effects when DCs are combined with MSCs coengineered with TNF-α and CD40L. Briefly, engineered MSCs induced DC costimulatory molecules and proinflammation valuable for subsequent enhancement of T-cell responses and possible tumor immunotherapy. (B) Major in vivo effects when DCs are combined with MSCs coengineered with TNF-α and CD40L.
From our in vivo data, we can state the following:
1. The reduced progression of tumor was observed when DCs+(TNF-α/CD40L)sup- or con-MSCs were injected into mice when compared with increased tumor growth observed in empty vector-MSC-treated mice. This is a promising new result that supports our in vitro data. Indeed, in order to inhibit tumor growth, delivery of immune-stimulatory genes by MSCs has been performed in various body experiments (Bexell et al., 2010) via IL-2 (Nakamura et al., 2004), IL-12 (Hong et al., 2009), IL-18 (Xu et al., 2009), CX3CL1 aka fractalkine in humans and neurotactin in mice (Xin et al., 2007), IFN-α (Sato et al., 2005), and IFN-β (Studeny et al., 2004). Increased natural killer cells' activity along with CD4+ and CD8+ T cells' tumor infiltration (Nakamura et al., 2004; Xin et al., 2007) were almost the main consequences. However, there were no reports exploiting (TNF-α/CD40L)-MSCs for cancer treatment, and so our findings constitute pioneered advances that might be valuable in oncotherapy.
2. A prolonged survival was noticed in mouse groups treated with DCs+(coengineered MSCs)con when compared with the mouse group treated with empty vector-MSCs. This observation supports our in vitro and further in vivo immune responses. Other experiments have reported that MSCs transduced with IFN-β lead to the inhibition of MDA-231 breast carcinoma cell line and A375SM melanoma cells, resulting in increased survival of mouse tumor models (Studeny et al., 2004).
3. The increased splenocyte proliferation in all transduced MSC groups in the presence or absence of DCs indicates how gene modification could affect MSCs' effects.
4. The critical cytokine levels of Th1, Th2, and Treg were altered. Admittedly, the cytokine pattern of splenocytes is a key indicative of general immune response of the mice in each group. Thereby, increased secretion of TNF-α by splenocytes was observed in all studied groups compared with empty vector-MSC groups. This evoked a negative impact of MSCs on TNF-α in the microenvironment. In general, TNF-α-stimulated gene 6 (TSG-6) is the most potent anti-inflammatory molecule released by MSCs (Prockop and Oh, 2012). Compared with untreated mice, coinjection of DCs+MSCs in all “con” groups and some “sup” groups [i.e., (TNFα)-MSCs, (TNF-α/CD40L)-MSCs] led to elevated amount of TNF-α. This implicates the effective role of coculturing DCs with genetically engineered MSCs in con-treated groups. Although TNF-α is a secretory cytokine, the results show that it could preferably act in close distance, such as seen in our “con” model. Besides, mice treated with DCs±transduced sup- or con-MSCs showed dropped quantity of TGF-β compared with empty vector-MSCs and untreated groups. This demonstrates again the positive impact of our genetic coengineering and cellular coadministration strategies to reduce this regulatory proinflammatory cytokine. Further, mouse groups treated with DCs+(TNF-α/CD40L)sup- or con-MSCs and DCs+(CD40L)con-MSCs reduced IL-4 levels compared with untreated mice. However, (TNF-α)-MSC-treated mice showed higher concentrations of IL-4 than untreated mice did. This implies that CD40L plays a critical role on reducing IL-4. Intriguingly, increased production of IL-6 was most observed in mice treated with (TNF-α/CD40L)-MSCs and DCs+(TNF-α/CD40L)con-MSCs when compared with untreated mice. This result might support the induced proinflammatory pattern in test groups. Likewise, concentration levels of IL-12, as a key cytokine inducing a proinflammatory pattern, were significantly increased in all mouse groups treated with (TNF-α/CD40L)-MSCs±DCs when compared with untreated mice. Eventually, increased amount of IFN-γ and decreased IL-10 levels were noticed in the group treated with DCs+TNF-α/CD40L)-MSCs when compared with the untreated group.
Taken together, our in vivo data suggest an stimulatory effect of all the genetically engineered MSCs on principal cytokines of Th1 (e.g., IL-12 and IFN-γ, whose levels are increased), which is accompanied by an inhibitory effect of the genetically engineered MSCs on main cytokines of Th2 and Treg development (e.g., IL-4 and IL-10, whose levels are decreased). The observed shift from Th2/Treg toward Th1 explains, at least partially, the immune tumor response efficiency (Lin et al., 2006) when transduced MSCs cocultured with DCs are injected into our BALB/c mouse breast tumor model (Fig. 7B).
Therefore, our experiments have highlighted the importance of coadministrating DCs with genetically coengineered MSCs in cancer management. Indeed, our experimental approach has allowed to find a new therapeutic option to significantly reduce tumorigenesis and increase the survival of the BALB/c mouse breast tumor model. In our experiments, we used LV-expressing TNF-α/CD40L to transduce murine MSCs. The advantage of using LVs results in stable integration of the gene into the target cell genome, resulting in higher efficiency and longer-term expression of the protein when compared with adenoviral transduction, which, however, can result in a higher transduction efficiency (Menon et al., 2009). Besides, since the engineered MSCs can release potent therapeutic molecules in a slow and continual manner (Porada and Almeida-Porada, 2010), these cells are promising vehicles for cancer treatment. Only few studies have investigated the overexpression of the immune-stimulatory gene in MSCs. These include the TRAIL gene, which contributes to the experimental therapy of glioma, one of the most aggressive and resistant cancers (Kim et al., 2008; Menon et al., 2009). However, there is still an inconsistency among studies, which might be because of differences in experimental settings such as variation in MSC sources and tumor models (Bexell et al., 2010). In this regard, our study offers new strengths and insights in the field, allowing us to hypothesize that BM-derived murine MSCs coexpressing TNF-α and CD40L represent an effective and novel approach for tumor immunotherapy and the development of new cancer vaccines. Some of the major remaining challenges with MSCs are because of the difficulty in isolating/obtaining them in a sufficient quantity from the BM of the same patient, which is crucial for autologous transplantation contributing to overcome any difficulties related to immune rejection of the transplanted cells.
In these regards, we intend to test our genetic approach in umbilical cord blood-derived MSCs, which can be obtained and cultured without any difficulty when compared with BM-derived MSCs. We also aim to use nanobiomaterials and nanotechnology means (i.e., graphene and derivatives-based scaffolds, targeted delivery nanosystems) to both favor MSC growth in vitro and provide an immune boost in vivo (Menaa, 2013).
Conclusion and Perspectives
To the best of our knowledge, this is the first report related to TNF-α/CD40L delivery by MSCs for efficient antitumor stimulation achieved through a Th1-mediated immune response. Eventually, the present findings would be helpful in the development of new cancer vaccines based on the delivery of immune-stimulatory genes by the universal donor MSCs. Future experiments shall compare engineered BM-derived MSCs with umbilical cord blood-derived MSCs combined or not with DCs, and assess the combinatorial effects of nanobiomaterials (e.g., graphene and derivatives) in modulating the antitumor responses of the genetically engineered (TNFα/CD40L)-MSCs combined or not with DCs.
Supplementary Material
Acknowledgment
This work was supported financially by Lorestan University of Medical Sciences, Iran (Grant No. 1211).
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Aboody K.S., Najbauer J., and Danks M.K. (2008). Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 15, 739–752 [DOI] [PubMed] [Google Scholar]
- Aggarwal S., and Pittenger M.F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 [DOI] [PubMed] [Google Scholar]
- Bexell D., Gunnarsson S., Tormin A., et al. (2009). Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 17, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexell D., Scheding S., and Bengzon J. (2010). Toward brain tumor gene therapy using multipotent mesenchymal stromal cell vectors. Mol. Ther. 18, 1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyth S., Borovsky Z., Mevorach D., et al. (2005). Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 105, 2214–2219 [DOI] [PubMed] [Google Scholar]
- Calzascia T., Pellegrini M., Hall H., et al. (2007). TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J. Clin. Invest. 117, 3833–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt S.B., Marini F.C., Watson K., et al. (2009). The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. USA 106, 3806–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin B.L., Sumpter T.L., Tokita D., et al. (2009). Allostimulatory activity of bone marrow-derived plasmacytoid dendritic cells is independent of indoleamine dioxygenase but regulated by inducible costimulator ligand expression. Hum. Immunol. 70, 313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzi F., and Horwood N.J. (2007). Potential of mesenchymal stem cell therapy. Curr. Opin. Oncol. 19, 650–655 [DOI] [PubMed] [Google Scholar]
- Elgueta R., Benson M.J., de Vries V.C., et al. (2009). Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi U., Giordano A., and Paggi M.G. (2010). The bad and the good of mesenchymal stem cells in cancer: boosters of tumor growth and vehicles for targeted delivery of anticancer agents. World J. Stem Cells 2, 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Ding Q., Wu Z., et al. (2010). Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 290, 157–166 [DOI] [PubMed] [Google Scholar]
- Gao Z., Zhang L., Hu J., and Sun Y. (2013). Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles. Nanomedicine 9, 174–184 [DOI] [PubMed] [Google Scholar]
- Glennie S., Soeiro I., Dyson P.J., et al. (2005). Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 105, 2821–2827 [DOI] [PubMed] [Google Scholar]
- Grisendi G., Bussolari R., Cafarelli L., et al. (2010). Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 70, 3718–3729 [DOI] [PubMed] [Google Scholar]
- Hall B., Dembinski J., Sasser A.K., et al. (2007). Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int. J. Hematol. 86, 8–16 [DOI] [PubMed] [Google Scholar]
- Hong X., Miller C., Savant-Bhonsale S., and Kalkanis S.N. (2009). Antitumor treatment using interleukin-12-secreting marrow stromal cells in an invasive glioma model. Neurosurgery 64, 1139–1146; discussion 1146–1147. [DOI] [PubMed] [Google Scholar]
- Hu Y.L., Fu Y.H., Tabata Y., and Gao J.Q. (2010). Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J. Control Release 147, 154–162 [DOI] [PubMed] [Google Scholar]
- Jiang X.X., Zhang Y., Liu B., et al. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 105, 4120–4126 [DOI] [PubMed] [Google Scholar]
- Kim S.M., Lim J.Y., Park S.I., et al. (2008). Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 68, 9614–9623 [DOI] [PubMed] [Google Scholar]
- Lin K.W., Jacek T., and Jacek R. (2006). Dendritic cells heterogeneity and its role in cancer immunity. J. Cancer Res. Ther. 2, 35–40 [DOI] [PubMed] [Google Scholar]
- Liu Y., Saxena A., Zheng C., et al. (2004). Combined alpha tumor necrosis factor gene therapy and engineered dendritic cell vaccine in combating well-established tumors. J. Gene Med. 6, 857–868 [DOI] [PubMed] [Google Scholar]
- Loebinger M.R., Eddaoudi A., Davies D., and Janes S.M. (2009). Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 69, 4134–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Zhou Z., Zhang D., et al. (2012). Immunosuppressive function of mesenchymal stem cells from human umbilical cord matrix in immune thrombocytopenia patients. Thromb. Haemost. 107, 937–950 [DOI] [PubMed] [Google Scholar]
- Ma Y., Shurin G.V., Peiyuan Z., and Shurin M.R. (2013). Dendritic cells in the cancer microenvironment. J. Cancer 4, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaa F. (2013). 2-D graphene and derivatives-based scaffolds in regenerative medicine: innovative boosters mimicking 3-D cell microenvironment. J. Regen. Med. 2 DOI: 10.4172/2325-9620.1000e107 [DOI] [Google Scholar]
- Menon L.G., Kelly K., Yang H.W., et al. (2009). Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells 27, 2320–2330 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Ito Y., Kawano Y., et al. (2004). Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 11, 1155–1164 [DOI] [PubMed] [Google Scholar]
- Nauta A.J., Kruisselbrink A.B., Lurvink E., et al. (2006). Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J. Immunol. 177, 2080–2087 [DOI] [PubMed] [Google Scholar]
- Otsu K., Das S., Houser S.D., et al. (2009). Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood 113, 4197–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M., Alexopoulou L., Episkopou V., and Kollias G. (1996). Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner-Fischer M., Morad V., Cohen-Sfady M., et al. (2007). Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109, 1422–1432 [DOI] [PubMed] [Google Scholar]
- Porada C.D., and Almeida-Porada G. (2010). Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv. Drug Deliv. Rev. 62, 1156–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D.J., and Oh J.Y. (2012). Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol. Ther. 20, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P. (2009). Microenvironment at tissue injury, a key focus for efficient stem cell therapy: a discussion of mesenchymal stem cells. World J. Stem Cells 1, 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Zhang L., Zhao X., et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 [DOI] [PubMed] [Google Scholar]
- Sato H., Kuwashima N., Sakaida T., et al. (2005). Epidermal growth factor receptor-transfected bone marrow stromal cells exhibit enhanced migratory response and therapeutic potential against murine brain tumors. Cancer Gene Ther. 12, 757–768 [DOI] [PubMed] [Google Scholar]
- Schmidt S.V., Nino-Castro A.C., and Schultze J.L. (2012). Regulatory dendritic cells: there is more than just immune activation. Front. Immunol. 3, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. (2012). Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 64, 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari G.M., Abdelrazik H., Becchetti F., and Moretta L. (2009). MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 113, 6576–6583 [DOI] [PubMed] [Google Scholar]
- Staba M.J., Mauceri H.J., Kufe D.W., et al. (1998). Adenoviral TNF-alpha gene therapy and radiation damage tumor vasculature in a human malignant glioma xenograft. Gene Ther. 5, 293–300 [DOI] [PubMed] [Google Scholar]
- Steinman R.M. (1991). The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9, 271–296 [DOI] [PubMed] [Google Scholar]
- Studeny M., Marini F.C., Dembinski J.L., et al. (2004). Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 96, 1593–1603 [DOI] [PubMed] [Google Scholar]
- Sun X.Y., Nong J., Qin K., et al. (2011). Mesenchymal stem cell-mediated cancer therapy: a dual-targeted strategy of personalized medicine. World J. Stem Cells 3, 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchuck S.L., Zwezdaryk K.J., Coffelt S.B., et al. (2008). Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 26, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A., and Prockop D.J. (2010). Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr. Opin. Immunol. 22, 768–774 [DOI] [PubMed] [Google Scholar]
- Uccelli A., Pistoia V., and Moretta L. (2007). Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 28, 219–226 [DOI] [PubMed] [Google Scholar]
- Vaananen H.K. (2005). Mesenchymal stem cells. Ann. Med. 37, 469–479 [DOI] [PubMed] [Google Scholar]
- van Horssen R., Ten Hagen T.L., and Eggermont A.M. (2006). TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 11, 397–408 [DOI] [PubMed] [Google Scholar]
- Xin H., Kanehira M., Mizuguchi H., et al. (2007). Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells 25, 1618–1626 [DOI] [PubMed] [Google Scholar]
- Xu G., Jiang X.D., Xu Y., et al. (2009). Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol. Int. 33, 466–474 [DOI] [PubMed] [Google Scholar]
- Yagi H., Soto-Gutierrez A., Parekkadan B., et al. (2010). Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 19, 667–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Gu J.X., Kovacs C., et al. (2003). Cooperation of TNF family members CD40 ligand, receptor activator of NF-kappa B ligand, and TNF-alpha in the activation of dendritic cells and the expansion of viral specific CD8+ T cell memory responses in HIV-1-infected and HIV-1-uninfected individuals. J. Immunol. 170, 1797–1805 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.