Abstract
Purpose
Angiogenesis inhibition has emerged as a potentially promising treatment strategy for neuroendocrine tumors. 2-Methoxyestradiol (2ME2; Panzem®) is a natural derivative of estradiol with demonstrated anti-angiogenic activity in animal models. We performed a prospective, phase II study of 2ME2, administered in combination with bevacizumab, in patients with advanced carcinoid tumors.
Methods
Thirty-one patients with advanced carcinoid tumors were treated with 2ME2, administered orally at a dose of 1,000 mg four times daily. Patients also received bevacizumab 5 mg/kg intravenously every 2 weeks. Patients were observed for evidence of toxicity, tumor response, and survival.
Results
The combination of 2ME2 and bevacizumab was relatively easily tolerated and was associated with anticipated toxicities for these two agents. No confirmed radiologic responses (by RECIST) were observed. However, 68% of the radiologically evaluable patients experienced at least some degree of tumor reduction, and the median progression-free survival (PFS) time was 11.3 months.
Conclusion
2ME2 and bevacizumab can be safely administered to patients with advanced carcinoid tumors. While major tumor regression was not observed with this regimen, the encouraging median progression-free survival time suggests that this regimen has some degree of anti-tumor activity and supports the further investigation of angiogenesis inhibitors in this disease.
Keywords: Carcinoid, Neuroendocrine Tumors, Bevacizumab, Sorafenib
Introduction
Carcinoid tumors are characterized by a clinical course that is usually more indolent than that of other malignancies but is usually fatal in patients with advanced, metastatic disease. While systemic treatment with alkylating agents has been associated with tumor regression and improved survival in patients with advanced pancreatic neuroendocrine tumors, patients with advanced carcinoid tumors have few standard systemic treatment options [1–3]. Somatostatin analogs generally control symptoms of flushing and diarrhea associated with carcinoid syndrome. Treatment with the somatostatin analog octreotide has also recently been shown to delay tumor progression, though is rarely associated with tumor regression [4]. Alpha interferon has been reported to have modest efficacy in carcinoid tumors, though its widespread use has been limited by the potential for toxicity [5]. In recent years, the development of new treatments for patients with carcinoid tumor has been an increasing focus of investigation.
The vascular nature of carcinoid tumors, together with evidence of high levels of VEGF and VEGFR expression, led to initial interest in exploring the efficacy of VEGF pathway inhibitors in this disease [6, 7]. Three such agents have been formally evaluated in prospective studies: the small molecule tyrosine kinase inhibitors sorafenib and sunitinib, and the monoclonal antibody bevacizumab. Sorafenib was evaluated in 50 patients with carcinoid and 43 patients with pancreatic neuroendocrine tumors. In a preliminary report of this study, responses were observed in 7% of the carcinoid patients and 11% of the patients with pancreatic NET [8]. Sunitinib was studied in a multi-institutional trial comprising 109 patients with advanced neuroendocrine tumors [9]. Partial responses were observed in 2% of the carcinoid cohort and 16% of the pancreatic NET cohort. Finally, bevacizumab was evaluated in a randomized phase II setting, in which 44 patients with advanced carcinoid tumors were randomly assigned to receive either bevacizumab or pegylated IFN-α-2b [10]. Four of 22 patients (18%) treated with bevacizumab were reported to have achieved confirmed radiographic partial responses, whereas none of the patients who received pegylated IFN-α-2b had a partial response.
2-Methoxyestradiol (2ME2; Panzem®) is a natural derivative of estradiol formed by sequential hydroxylation and O-methylation of estradiol at the 2-position [11]. 2ME2 does not have a high binding affinity to estrogen receptors and accordingly does not show direct estrogenic activity in both in vitro and in vivo [11]. In tumors, 2ME2 inhibits microtubule formation in endothelial cells. Additionally, 2ME2 inhibits expression of hypoxia induced factor (HIF) 1-alpha, resulting in decreased secretion of VEGF [12, 13]. Early-stage trials of 2ME2 in solid tumors and in prostate cancer suggested modest antitumor activity [14, 15]. 2ME2 was subsequently reformulated as a NanoCrystal® dispersion (NCD) to improve its bioavailability. In subsequent phase I studies with this new formulation, the maximum tolerated dose was defined at 1,000 mg orally four times daily, and in a phase II study enrolling 18 patients with platinum-resistant ovarian cancer or primary peritoneal carcinomatosis, treatment with 2ME2 NanoCrystal dispersion was associated with clinical benefit rate of 31% [16–18].
In light of the anti-angiogenic effects of both 2ME2 and bevacizumab, we performed a prospective study to evaluate the safety and antitumor efficacy of these two agents administered together in patients with advanced carcinoid tumors. Thirty-one patients with advanced carcinoid tumor received 2ME2, administered at dose of 1,000 mg by mouth four times daily, in combination with bevacizumab 5 mg/kg intravenously every 2 weeks. Patients were followed for endpoints of toxicity, tumor response, and survival.
Methods
Patient population
All patients were required to have histologically documented, locally unresectable or metastatic carcinoid neuroendocrine tumor. Patients with small cell carcinoma or pancreatic endocrine tumors were not eligible. Measurable disease, as defined by RECIST, was required. Mandated laboratory requirements included aspartate aminotransferase (AST) and alanine aminotransferase (ALT) <2.5 times the upper limit of normal (<5 times upper limit of normal if liver metastasis was present), total bilirubin ≤2 mg/dL, serum creatinine ≤ 1.5 mg/dL, total white blood cell count >3,500/mm3, absolute neutrophil count (ANC) ≥1,500/mm3, international normalized ratio ≤ 1.5, platelet count ≥100,000/mm3. All patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status <2. Patients with history of myocardial infarction or angina pectoris in the last 12 months, clinically apparent central nervous system metastasis, concurrent treatment with therapeutic doses of any anticoagulant, history of severe bleeding, uncontrolled severe hypertension, history of nephrotic syndrome, urine protein:creatinine ratio ≥1.0, or radiotherapy or chemotherapy within the previous 4 weeks were excluded. Prior treatment with chemoembolization, cryotherapy, or radio-frequency ablation was allowed if measurable disease was not affected.
Treatment program
The study was designed as a modified phase 2, single-arm, open-label trial. Because the drugs had not previously been administered in combination, patients were enrolled sequentially into two cohorts. The first cohort (Cohort 1) comprised 3 patients who received an oral dose of 1,000 mg Panzem® NCD four times daily and a concurrent IV administration of 5 mg/kg bevacizumab every 14 days, beginning on day 1. Patients were treated for a 28-day treatment period and then observed for 7 days. If no DLT or other significant toxicity was observed, they received subsequent 28-day cycles of treatment without additional 7-day observation periods. If DLT was observed, then the cohort was to be expanded to 6 patients prior to proceeding to full enrollment. Dose-limiting toxicity was defined as ≥ grade 3 non-hematologic, or grade 4 hematologic, treatment-related toxicity that did not resolve in 2 weeks (i.e., return to baseline), or an event that made continued treatment unsafe in the opinion of the investigator. Patients were evaluated with a physical examination, blood tests, and for toxicity every other week during the treatment period. Toxicity was graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0. Tumor response was evaluated at the end of every other 28-day treatment period for the duration of therapy using multi-phasic computed tomography (CT), or magnetic resonance imaging (MRI).
Pharmacokinetics
Steady-state plasma levels of 2ME2 were measured as part of the study. Blood samples for determination of steady state plasma concentrations of 2ME2 and its metabolite, 2ME1 (2-methoxyestrone), were collected prior to the first dose of Panzem® NCD on Day 1 of the initial treatment cycle and at any time during the regular dosing schedule for subsequent cycles.
Statistical considerations
The primary endpoint of our study was tumor response rate, with the goal of evaluating whether the overall response rate was ≥10% against the null hypothesis that the overall response rate was <10%. Assuming a response rate of 10%, the type 1 error was calculated to be 4.2% and the power was 81.6%. With this goal, 2 or more radiographic responses would have been required to identify an active regimen. Secondary endpoints of the study included assessment of toxicity, overall, and progression-free survival. Progression-free and overall survival (OS) estimates were calculated using Kaplan–Meier methodology. Progression-free survival was defined as the time from date of study entry to the first documentation of objective tumor progression or death; patients who were removed from the study without evidence of disease progression or death were censored at the time they were removed from the study. Toxicity and complications of treatment were assessed based on reports of adverse events, physical examinations, and laboratory measurements.
Results
Patient demographics
A total of 31 patients were enrolled and treated in the study. The baseline characteristics of the patient population are shown in Table 1. Patients had a median age of 57 and were relatively evenly distributed by gender. Twelve (39%) patients received concurrent therapy with octreotide during the course of the study. The small bowel intestine was the most common primary disease site. Patients had received a variety of prior therapies for their disease: fourteen (45%) patients had received prior chemotherapy, 7 (23%) had undergone previous radiation therapy, and 3 (10%) had received alpha interferon. In addition, nearly half (15/31, 48%) of the patients had received other forms of anticancer therapy, including other investigational agents.
Table 1.
Baseline patient characteristics
| Characteristics | N = 31 |
|---|---|
| Age | |
| Median age (Range) | 57 (36–75) |
| Gender, n (%) | |
| Male | 17 (55%) |
| Female | 14 (45%) |
| ECOG PS | |
| 0 | 12 (39%) |
| 1 | 19 (61%) |
| Patients receiving concurrent octreotide | 12 (39%) |
| Primary disease site | |
| Lung—bronchial | 4 (13%) |
| Larynx | 1 (3%) |
| Stomach | 1 (3%) |
| Small bowel | 17 (55%) |
| Colon | 2 (6%) |
| Rectum | 2 (6%) |
| Other/Unknown | 4 (13%) |
| Prior cancer treatments | |
| Radiation | 7 (23%) |
| Cytotoxic chemotherapy | 14 (45%) |
| Alpha interferon | 3 (10%) |
| Other | 15 (48%) |
Exposure to study medication and treatment discontinuation
Of the 31 enrolled patients, 23 completed 2 or more cycles of treatment. Of the 8 patients who discontinued treatment prior to completing 2 cycles, 6 discontinued due to adverse events and 2 for other reasons. At the time of data cutoff (12 months after enrollment of the last patient), 10 patients continued to receive study therapy and 21 patients had discontinued study therapy. Seven patients discontinued due to an adverse event, of which 4 were felt to be treatment-related, 12 discontinued due to withdrawal of consent, investigator discretion, or other reasons, and 2 due to disease progression.
Pharmacokinetics
Composite plasma concentration–time profiles were generated from blood samples collected during the study. The profiles demonstrated a steady-state Cmax of 63.53 ng/mL for 2ME2 and 700.50 ng/mL for its metabolite, 2ME1. These steady-state plasma concentrations were well above the target estimated minimum effective concentration of 3.33 ng/mL [19]. The Tmax for both 2ME2 and 2ME1 was 2.00 h. The estimated AUC0–24 h was 483.43 ng h/mL for 2ME2 and 7,112.59 ng h/mL for 2ME1. The half-lives of 2ME2 and 2ME1 could not be calculated from the available data.
Toxicity
Thirty-one patients were evaluable for toxicity. The most frequently reported treatment emergent adverse events were gastrointestinal and included nausea (18/31 any grade, 58%) and diarrhea (14/31 any grade, 45%). Adverse events are summarized in Table 2. Eighteen (58%) patients experienced at least one grade-3 treatment emergent adverse event during the study. The most common grade-3 events were hypertension (5/31, 16%) and diarrhea (2/31, 6%). Bleeding is a rare but well-known complication of anti-angiogenesis therapy, and three (10%) patients in our study experienced gastrointestinal bleeding. In two of these cases, the bleeding was attributed to gastroesophageal varices; in the third case, the source of bleeding was not identified. Only three (10%) patients reported grade-4 treatment-emergent adverse events. The grade-4 events included lung infection, suicidal ideation, and hypertension. The lung infection and suicidal ideation were considered unrelated to study drug, whereas the hypertension was considered probably related.
Table 2.
Selected treatment-emergent adverse events
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total n = 31 |
|---|---|---|---|---|---|
| General | |||||
| Fatigue | 9 (29%) | 5 (16%) | 14 (45%) | ||
| Gastrointestinal disorders | |||||
| Nausea | 13 (42%) | 5 (16%) | 18 (58%) | ||
| Diarrhea | 8 (6%) | 4 (13%) | 2 (6%) | 14 (45%) | |
| Vomiting | 8 (26%) | 3 (10%) | 11 (35%) | ||
| Constipation | 6 (19%) | 2 6%) | 8 (26%) | ||
| Dyspepsia | 3 (10%) | 1 (3%) | 4 (13%) | ||
| Flatulence | 3 (10%) | 1 (3%) | 4 (13%) | ||
| Gastrointestinal hemorrhage | 3 (10%) | 3 (10%) | |||
| Diarrhea | 1 (3%) | 1 (3%) | |||
| Respiratory | |||||
| Dyspnea | 5 (16%) | 1 (3%) | 6 (19%) | ||
| Dysphonia | 5 (16%) | 5 (16%) | |||
| Cough | 4 (13%) | 4 (13%) | |||
| Hypoxia | 1 (3%) | 1 (3%) | |||
| Infections | |||||
| Urinary tract infection | 2 (16%) | 3 (10%) | 5 (16%) | ||
| Cholangitis | 1 (3%) | 1 (3%) | |||
| Lung infection | 1 (3%) | 1 (3%) | |||
| Metabolism and nutrition | |||||
| Anorexia | 3 (10%) | 4 (13%) | 7 (23%) | ||
| Dehydration | 1 (3%) | 1 (3%) | |||
| Neurologic | |||||
| Headache | 3 (10%) | 7 (23%) | 11 (35%) | 11 (35%) | |
| Vasovagal syncope | 1 (3%) | 1 (3%) | |||
| Vascular | |||||
| Hypertension | 5 (16%) | 1 (3%) | 6 (19%) | ||
| Flushing | 4 (13%) | 4 (13%) | |||
| Deep vein thrombosis | 1 (3%) | 1 (3%) | |||
| Cutaneous | |||||
| Rash | 4 (13%) | 4 (13%) | |||
| Psychiatric | |||||
| Insomnia | 2 (6%) | 1 (3%) | 1 (3%) | 4 (13%) | |
| Suicidal ideation | 1 (3%) | 1 (3%) | |||
| Hematologic | 1 (3%) | ||||
| Anemia | 1 (3%) | 1 (3%) |
The number of patients experiencing selected events is shown. Events experienced by >10% of patients or events classified as grade 3 or 4 are listed
A total of 7 patients discontinued treatment due to treatment-emergent adverse events during the course of the study. In four cases (deep vein thrombosis, hypertension, hyperbilirubinemia, and grade 2 proteinuria), the adverse event was considered probably related to treatment. Other than the single patient who developed proteinuria, no significant changes in urine protein/creatinine ratios were observed in the patient population during treatment. One death occurred during the study due to a pulmonary infection that was considered unrelated to study drug.
Efficacy
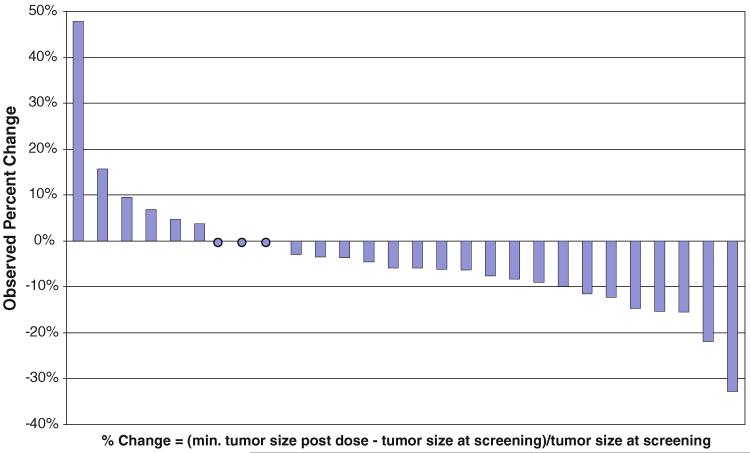
Twenty-eight patients were evaluable for radiologic response. While no patients had radiologic partial or complete responses, of the 28 evaluable patients 27 (96%) had stable disease and 1 (4%) patient had progressive disease as their best response to therapy. Confirmed tumor responses by RECIST were not observed; however, 19 of the 28 evaluable (68%) patients had some degree of reduction in the sum of tumor LDs after screening, and two patients had reductions in LD sum ≥20% (Fig. 1). Biochemical response was assessed using plasma chromogranin A levels and 24-h urine collections of 5HIAA, measured at baseline and at the initiation of every subsequent 4-week cycle. Of 24 patients with elevated chromogranin A levels at baseline, only one experienced a response, defined as a >50% decrease. Similarly, of 19 patients evaluable for 5HIAA response, none experienced a >50% decrease during the course of study treatment.
Fig. 1.
Observed percentage change in sum of tumor longest diameters from screening to minimum post-dose sum (radiologically evaluable population, n = 28)
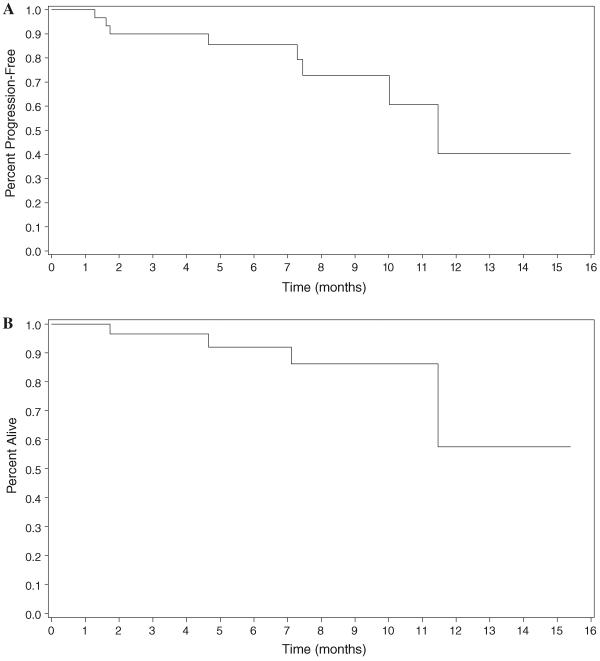
While evidence of disease progression was not a requirement for study entry, 22 (71%) patients had documented evidence of progression within the 12 months prior to study entry. The overall median progression-free survival time in our study was 11.3 months (Fig. 2a). Median overall survival could not be estimated, as overall survival was >50% at the end of the observation period (Fig. 2b).
Fig. 2.
Progression-free and overall survival times. a Progression-free survival (Intent to treat population). b Overall survival (Intent to treat population)
Discussion
We found that treatment with the combination of 2ME2 and bevacizumab was both feasible and safe in patients with advanced carcinoid tumors. The adverse events associated with this regimen were consistent with the known profiles of both agents. The efficacy observed with the combination in patients with advanced carcinoid tumors is more difficult to assess in this single arm phase II study, although our data suggest some degree of antitumor activity.
Previous studies have suggested that combining angiogenesis inhibitors in patients with cancer has the potential for both significant efficacy and toxicity. The combination of sorafenib and bevacizumab was associated with impressive clinical activity in a phase I study in patients with renal cell carcinoma, but was also associated with a high incidence of hypertension and the development of microangiopathic hemolytic uremia [20]. High rates of grade 3 or 4 hypertension, proteinuria, and bleeding were also observed in a phase I trial of sunitinib and bevacizumab in patients with renal cell carcinoma, precluding further evaluation of the combination at standard doses of both drugs [21]. In contrast, the combination of 2ME2 and bevacizumab in our study appeared to be relatively well tolerated. Grade 3 or 4 hypertension developed in 6 patients, and 3 patients developed evidence of gastrointestinal bleeding. However, hypertension led to treatment discontinuation in only one patient; and 2 of the patients with gastrointestinal bleeding had a pre-existing condition (esophageal varices) that may have led to the bleed. Only a single patient in our study discontinued treatment due to proteinuria.
The naturally indolent nature of neuroendocrine tumors and the absence of observed major tumor responses in our single-arm phase II study make it difficult to definitively assess the antitumor activity of bevacizumab and 2ME2 in advanced carcinoid disease. Our observation that no patient treated with 2ME2 and bevacizumab experienced a partial or complete response by RECIST differs from a prior phase II study of bevacizumab and octreotide, in which a response rate of 18% was reported [10]. It is possible that our use of a different bevacizumab dosing regimen (5 mg/kg every 2 weeks rather than 15 mg/kg every 3 weeks) contributed to this difference. Two patients in our study experienced reductions of ≥20% in the sum of longest tumor diameters, and 19 (68%) patients experienced at least some degree of tumor shrinkage. The overall rate of PR + SD in our study was 96%, a value that is nearly identical to the PR + SD rate of 95% observed in the prior study of bevacizumab + octreotide, and superior to the PR + SD rate of 85% in the subgroup of carcinoid patients treated in a phase II study of sunitinib [9, 10].
Decreases in plasma levels of the neurosecretory protein chromogranin A have been associated with clinical improvement and improved prognosis in patients receiving somatostatin analogs, cytotoxic chemotherapy, and other anti-tumor agents [2, 22, 23]. We found that the combination of 2ME2 and bevacizumab had minimal effect on chromogranin A levels in treated patients: only a single patient experienced a significant reduction in this marker. A similar lack of correlation between chromogranin A response and clinical outcomes in patients with neuroendocrine tumor was also observed in phase II studies of bevacizumab and sunitinib [9, 10]. In both of these studies, evidence of antitumor effect was seen on radiologic imaging studies, suggesting that chromogranin A may not be a reliable surrogate marker of response for anti-angiogenic agents [9, 23].
Progression-free survival time has also been used as an endpoint to evaluate the potential efficacy of novel agents in patients with neuroendocrine tumors. The median progression-free survival time in our study was 11.3 months, a value that compares favorably to progression-free survival times reported in other, similar studies of novel agents in carcinoid tumors (Table 3). Two prior phase II studies, one evaluating everolimus in combination with octreotide and the second evaluating bevacizumab or interferon in combination with octreotide, reported median progression-free survival times of 14.4 months in patients with carcinoid tumors, a value that is superior to the progression-free survival time of 11.3 months observed in our study [10, 23]. However, both of these earlier studies required concurrent treatment with octreotide in all patients. Somatostatin analogs, including octreotide, are commonly used in patients with advanced carcinoid tumors as a means to control symptoms of hormonal hypersecretion such as flushing and diarrhea, but more recently have also been shown to be associated with improved time to tumor progression. In a placebo-controlled randomized study enrolling patients with midgut carcinoid tumors, the median time to tumor progression was 14.3 months in patients receiving octreotide, when compared to 6 months in the cohort receiving placebo [4]. The fact that only 12 (39%) of the patients treated in our study received concurrent octreotide may have contributed to the somewhat shorter PFS observed in our study.
Table 3.
Selected phase II studies of novel therapies in patients with carcinoid tumors
| Regimen | Tumor type | N | Median PFS (or TTP) (months) | Reference | |
|---|---|---|---|---|---|
| Everolimus + Octreotide | Target | Carcinoid | 30 | 14.4 | Yao et al. [23] |
| Bevacizumab + Octreotide versus Interferon alpha + Octreotide | VEGF | Carcinoid | 44 | 14.4 | Yao et al. [10] |
| Bevacizumab + 2ME2 | VEGF, Hif-1alpha | Carcinoid | 32 | 11.3 | Current study |
| Sunitinib | VEGFR, PDGFR, C-Kit | Carcinoid | 41 | 10.2 (TTP) | Kulke et al. [9] |
| rhEndostatin | Endothelial cells | Carcinoid + Pancreatic NET | 22 | 7.6 | Kulke et al. [25] |
| Temsirolimus | mTOR | Carcinoid + Pancreatic NET | 37 | 6 | Duran et al. [26] |
| Imatinib | C-Kit | Carcinoid | 27 | 5.9 | Yao et al. [27] |
While our study suggests that treatment with 2ME2 and bevacizumab is associated with minor tumor reductions and encouraging progression-free survival durations, a study of this regimen in the randomized setting would be necessary to more definitively assess this endpoint. An international randomized phase III study to confirm the activity of sunitinib in pancreatic neuroendocrine tumors has recently been stopped early, after preliminary results demonstrated that treatment with sunitinib was associated with a median progression-free survival duration of 11.1 months, when compared with 5.5 months in the placebo arm [24]. In a second, ongoing study, led by the Southwest Oncology Group, patients with advanced carcinoid tumors are currently being randomized to receive treatment with octreotide and either IFN-α-2b or bevacizumab, with a primary end point of progression-free survival.
In conclusion, our study demonstrates the feasibility of administering two anti-angiogenic agents, 2ME2 and bevacizumab in patients with advanced carcinoid tumors. Our observations of minor decreases in tumor size and an encouraging progression-free survival duration, supports the potential for activity of angiogenesis inhibitors in this disease. The lack of confirmed RECIST-defined responses in our study also highlights the challenges of assessing the antitumor activity of antiangiogenic agents and other novel, biologic therapies in this disease. Randomized phase II designs and the development of more reliable biomarkers for response may facilitate the future evaluation and development of similar regimens for patients with neuroendocrine tumors.
Acknowledgments
Support for this study was provided by Entremed, Inc, NCI grants CA093401 (MHK) and P50 CA127003 (DF/HCC SPORE in Gastrointestinal Cancer). The authors gratefully acknowledge support from the Saul and Gitta Kurlat fund for neuroendocrine tumor research.
Footnotes
Reported in part at the American Society of Clinical Oncology 2008 Gastrointestinal Cancers Symposium.
References
- 1.Moertel C, Lefkopoulo M, Lipsitz S, et al. Streptozocindoxorubicin, stretpozocin-fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 2.Kulke M, Hornick J, Frauenhoffer C, et al. O6-methyl-guanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338–345. doi: 10.1158/1078-0432.CCR-08-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 4.Arnold R, Muller H, Schade-Brittinger C, et al. Placebo-controlled, double blind, prospective, randomized study of the efect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. 2009 Gastrointestinal Cancers Symposium; 2009. [DOI] [PubMed] [Google Scholar]
- 5.Faiss S, Pape UF, Bohmig M, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors–the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Jia Z, Li Q, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer. 2007;109:1478–1486. doi: 10.1002/cncr.22554. [DOI] [PubMed] [Google Scholar]
- 7.Terris B, Scoazec J, Rubbia L. Expression of vascular endothelial growth factor in digestive neuroendocrine tumors. Histopathology. 1998;32:133–138. doi: 10.1046/j.1365-2559.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 8.Hobday TJ, Rubin J, Holen K, et al. MC044 h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): a Phase II Consortium (P2C) study (abstract) J of Clin Onc ASCO Annual Meeting Proc Part 1. 2007;25:4504. [Google Scholar]
- 9.Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–3410. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RA, Harris T, Altmann E, et al. 2-Methoxyestradiol and analogs as novel antiproliferative agents: analysis of three-dimensional quantitative structure-activity relationships for DNA synthesis inhibition and estrogen receptor binding. Mol Pharmacol. 2002;61:1053–1069. doi: 10.1124/mol.61.5.1053. [DOI] [PubMed] [Google Scholar]
- 12.Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 13.Becker CM, Rohwer N, Funakoshi T, et al. 2-methoxyestradiol inhibits hypoxia-inducible factor-1{alpha} and suppresses growth of lesions in a mouse model of endometriosis. Am J Pathol. 2008;172:534–544. doi: 10.2353/ajpath.2008.061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahut WL, Lakhani NJ, Gulley JL, et al. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006;5:22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney C, Liu G, Yiannoutsos C, et al. A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:6625–6633. doi: 10.1158/1078-0432.CCR-05-0440. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, sidor C, Feierabend C, et al. Phase Ib trial of 2ME2 administered as a nanocrystal dispersion (NCD) in patients with advanced cancer. Proceedings of EORTC/AACR/NCI.2005. p. 129. [Google Scholar]
- 17.Sweeney C, Slebe K, Li M, et al. A single center open label dose escalation safety and pharmacokinetic study of 2 methoxyestriadiol nanocrystal colloidal dispersion in patients with advanced cancer. Proceedings of EORTC/AACR/NCI.2005. p. 157. [Google Scholar]
- 18.Matei D, Schilder J, Sutton G, et al. Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: a Hoosier Oncology Group trial. Gynecol Oncol. 2009;115:90–96. doi: 10.1016/j.ygyno.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher G, Kataoka M, Roth JA, et al. Potent antitumor activity of 2-methoxyestradiol in human pancreatic cancer cell lines. Clin Cancer Res. 1999;5:493–499. [PubMed] [Google Scholar]
- 20.Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115:2368–2375. doi: 10.1002/cncr.24234. [DOI] [PubMed] [Google Scholar]
- 21.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korse CM, Bonfrer JM, Aaronson NK, et al. Chromogranin A as an alternative to 5-hydroxyindoleacetic acid in the evaluation of symptoms during treatment of patients with neuroendocrine Tumors. Neuroendocrinology. 2009;89:296–301. doi: 10.1159/000162876. [DOI] [PubMed] [Google Scholar]
- 23.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. [erratum appears in J Clin Oncol. 2008 Dec 1;26(34):5660] J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond E. Phase III randomised double blind trial of suntinib versus placebo in patients with progressive, well-differentiated pancreatic neuroendocrine tumors. World Congress on Gastrointestinal Cancers; Barcelona, Spain: 2009. [Google Scholar]
- 25.Kulke MH, Bergsland EK, Ryan DP, et al. Phase II study of recombinant human endostatin in patients with advanced neuroendocrine tumors. J Clin Oncol. 2006;24:3555–3561. doi: 10.1200/JCO.2006.05.6762. [DOI] [PubMed] [Google Scholar]
- 26.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao JC, Zhang JX, Rashid A, et al. Clinical and in vitro studies of imatinib in advanced carcinoid tumors. Clin Cancer Res. 2007;13:234–240. doi: 10.1158/1078-0432.CCR-06-1618. [DOI] [PubMed] [Google Scholar]