Abstract
This Short Review critically evaluates three hypotheses about the effects of emotion on memory: First, emotion usually enhances memory. Second, when emotion does not enhance memory, this can be understood by the magnitude of physiological arousal elicited, with arousal benefiting memory to a point but then having a detrimental influence. Third, when emotion facilitates the processing of information, this also facilitates the retention of that same information. For each of these hypotheses, we summarize the evidence consistent with it, present counter-evidence suggesting boundary conditions for the effect, and discuss the implications for future research.
Keywords: affect, episodic memory, limbic system, long-term memory, recall, recognition, stress
“Emotional memory” is a shorthand phrase to refer to a memory for an event that elicits emotional reactions. These events and reactions can vary. The events may be rewarding or aversive; they may vary in intensity and time-course. These features can influence the nature of the emotional reactions. For instance, reactions to a public or personal event that unfolds over minutes or hours may include physiological responses, changes in cognitive processes, the conscious feeling of a change in affective state, and the labeling of that feeling. Reactions to an item that is presented briefly within the context of a laboratory experiment are likely to include fleeting physiological and cognitive responses, but the participant may not be aware of them.
The effects of these emotional reactions on memory are complex, yet they are often distilled to three tenets. First, the experience of emotion enhances memory. Second, when emotion does not enhance memory, this is usually because of the impairing effects of high levels of arousal. Third, when emotion facilitates an early stage of processing, this conveys benefits at a later stage. These views are pervasive because there is evidence, and often a long history, in their support. But as this review highlights, there are boundary conditions whose existence can shed light on the multifaceted nature of the effects of emotion on memory.
Emotional Enhancement of Memory: Underlying Mechanisms and Limitations
It is commonly believed that an emotional event will be remembered better than an event lacking emotion (reviewed by Buchanan, 2007 and Hamann, 2001). William James (1890) described the effect of an emotional event as “a scar upon the cerebral tissues” (p. 670), and the term ‘flashbulb memories’ was used to describe the purportedly permanent representation created for an exceptionally emotional event (Brown & Kulik, 1977). Several studies have shown that emotional public events are remembered better than everyday events with a similar retention interval (e.g., Conway et al., 1994; Paradis, Solomon, Florer, & Thompson, 2004), and laboratory studies have shown emotional enhancement in memory for words (e.g., Kleinsmith & Kaplan, 1963; Sharot & Phelps, 2004), images (e.g., Bradley, Greenwald, Petry, & Lang, 1992), narratives (e.g., Cahill & McGaugh, 1995), and personal events (e.g., D’Argembeau, Comblain, & Van der Linden, 2003).
In explaining flashbulb memory, Brown and Kulik proposed the role of a special emotional memory mechanism based on Robert Livingston’s “Now Print” theory (1967). This theory (1967) suggests that when the brain recognizes an event as both novel and significant, the limbic system releases a command that permanently “prints” all recent brain events, leading to facilitated retrieval of all event details at a later time. Select aspects of this theory have been supported. There is increased limbic activity, and a strengthened relation between the amygdala and other medial temporal lobe and cortical regions during emotional relative to neutral event encoding (reviewed by LaBar & Cabeza, 2006). Item-by-item fluctuations in connectivity relate to the durability of an emotional memory (Ritchey, Dolcos, & Cabeza, 2008), with items associated with greater connectivity remembered over longer delays. State-based differences in connectivity also may influence how well emotional events are retained; for instance, functional coupling between the amygdala and medial prefrontal cortex during rest may relate to the ability to retain emotionally positive memories, at least among older adults (Sakaki, Nga, & Mather, in press). Thus, there is evidence that amygdala engagement – through its interactions with other regions – can lead to a strong, long-lasting memory.
Critical aspects of the “Now Print” theory, however, have not been supported. Amygdala activation does not preserve memory for all attended event details, and amygdala engagement during an emotional event does not circumvent the medial temporal lobe processes that typically enable memory consolidation (Kensinger, 2009). Thus, there is no ‘special’ memory mechanism in the strongest sense (see McCloskey, Wible, & Cohen, 1988; Weaver, 1993). Moreover, even though people retain high confidence in “flashbulb” memories (e.g., Talarico & Rubin, 2003; 2007), their accuracy decreases over time (e.g., Christianson, 1989; 1992; Rubin & Kozin, 1984). This disconnect between accuracy and confidence is consistent with research showing that emotion enhances the sense of recollection experienced during memory retrieval (reviewed by Phelps & Sharot, 2008) and may lead to a shift in participants’ response biases: Emotional words (new and old) are more likely to receive an “old” response than neutral words (See Table 1). Although emotion can sometimes enhance the accuracy of a memory representation (e.g., Choi, Kensinger, & Rajaram, 2013; Kensinger, Garoff-Eaton, & Schacter, 2007), or at least the accuracy with which some details of an event are remembered (see next section), emotion may change the qualitative characteristics of how an event is remembered even when it does not affect the likelihood that the event is remembered.
Table 1.
Effect of emotion on discrimination and response bias in tests of memory recognition.
| Hit Rate | False Alarm Rate | Sensitivity | Liberal response bias | |
|---|---|---|---|---|
|
|
||||
| Brainerd et al., 2008 | Negative > Neutral Neutral > Positive |
Negative > Neutral Neutral > Positive |
Negative > Neutral Neutral > Positive |
|
| Choi, Kensinger, & Rajaram, 2013 | Negative > Neutral Positive = Negative Positive = Neutral |
Negative = Positive = Neutral | Negative > Neutral Positive > Neutral |
Negative > Neutral Negative > Positive |
| Dougal & Rotello, 2007 | Negative > Neutral Negative > Positive |
Negative > Neutral Negative > Positive |
Negative < Neutral Positive < Neutral |
Negative > Neutral Negative > Positive |
| Fernandez-Rey & Redondo, 2007 | Arousing > Neutral | Arousing > Neutral Negative > Positive |
Negative < Positive Arousing < Neutral |
Negative > Positive Arousal > Neutral (low confidence only) |
| Johansson, Mecklinger, & Treese, 2004 | Emotional = Neutral | Emotional > Neutral Negative > Positive |
||
| Maratos, Allan, & Rugg, 2000 | Negative > Neutral | Negative > Neutral | Neutral > Negative | Negative > Neutral |
| Vo et al., 2008 | Negative > Positive Positive > Neutral |
Negative > Neutral Positive > Neutral |
Negative = Positive = Neutral | Negative > Positive Positive > Neutral |
| Windmann & Kruger, 1998 | Not reported | Not reported | Neutral > Negative (control participants only) | Negative > Neutral |
| Windmann & Kutas, 2001 | Negative > Neutral | Negative > Neutral | Negative = Neutral | Negative > Neutral |
Note: Table 1 focuses on standard recognition assessments and does not include studies designed to intentionally elicit false memories. Additionally, only studies that specifically report some measure of response bias are included.
The mixed effects of emotion on memory accuracy may be explained by the frequent presence of two confounds that can exaggerate or mask the enhancing effects of emotion on memory. First, emotional stimuli are often more interrelated than neutral stimuli. This semantic relatedness can have an additive effect with arousal on memory (Buchanan, Etzel, Adolphs, & Tranel, 2006) and in some cases may entirely explain the mnemonic benefit attributed to emotion (e.g., Maratos & Rugg, 2001; Talmi, Luk, McGarry, & Moscovitch, 2007a; Talmi, Schimmack, Paterson, & Moscovitch, 2007b). This interrelatedness can also lead to enhanced conceptual priming and an increased sense of familiarity for both old and new emotional stimuli, leading to increased false memories as well as true memories (see Brainerd, Stein, Silveira, Rohenkohl, & Reyna, 2008). When interrelatedness is controlled, emotion may not enhance false memory (e.g., Choi et al., 2013). Second, the distinctiveness of an emotional stimulus among neutral items has been shown to contribute to the enhanced memory for the emotional items. Emotional stimuli typically benefit from presentation in mixed lists (containing both emotional and neutral items) but not in pure lists (Schmidt, 2012b; Talmi et al., 2007a). Controlling for distinctiveness may eliminate many of the benefits of emotion on memory, although some benefits – such as enhanced memory for taboo words – may remain, suggesting that they benefit from emotion-specific processes (reviewed by Schmidt, 2012a).
The current state of the emotional memory literature suggests that the presence of emotion often contributes to a more durable memory representation. However, this enhancement is not always present, and when it is, it may reflect the contribution of confounding processes not directly linked to the emotionality of the memoranda. By designing studies to directly control for and manipulate these parameters (see Table 2 for examples), researchers can better understand the underlying cognitive and neural mechanisms directly impacted by emotion. Such an understanding may be essential to research examining memory impairments and preservations in special populations. If emotional enhancement is held as a certainty in the memory literature, the field risks disregarding important research that does not show the effect.
Table 2.
Factors to consider when designing a study to assess emotional memory.
| Factor | Why consider this factor? | When is it most prevalent? | How to manipulate? | Possible to control for in data analyses? |
|---|---|---|---|---|
| semantic coherence/relatedness | Stronger semantic clustering of emotional (vs. neutral) stimuli can contribute to emotional enhancement of memory by making stimuli easier to organize. It also can boost false memories because lures are more closely related to studied items. | if not using categorized neutral items if selecting emotional stimuli from a small number of categories (e.g., vicious animals, injured people) |
use a design that fully crosses emotional content and semantic relatedness | if standardized database available, use calculated coherence of emotional and neutral stimuli as a covariate in analyses |
| attention allocation | Emotional stimuli often attract attention. This can enhance memory for the emotional stimuli but can reduce memory for neutral (or low-priority; see Mather & Sutherland, 2011) stimuli competing for processing resources. | if processing demands of task are high (e.g., limited time to process stimuli; multiple stimuli competing for resources) | manipulate task demands (e.g., divided attention and full attention) alter salience of neutral stimuli by manipulating the stimuli or the task |
measure eye gaze and use looking time as a covariate (Note: this will only co-vary overt, not covert, attention) |
| distinctiveness | Many effects of emotion may be due to the incongruent or unexpected nature of the stimulus or event, rather than to an emotional response to that stimulus or event. | if frequency (both within the study session and within an everyday context) is not matched between emotional and neutral stimuli if familiarity and frequency are not matched between emotional and neutral events if mixed lists are used rather than pure lists (this may also affect induced arousal of person; see below) |
compare performance in mixed lists to performance in pure lists compare surprising events that elicit different magnitudes of emotional reactions (e.g., garden-path sentences ending in emotional vs. semantic non sequitur) |
include ratings of frequency, familiarity, and surprise as covariates |
| arousal | Arousal can influence memory in a number of ways, depending on whether the arousal refers to the ratings given to a single stimulus within a stream of stimuli, to the state of an individual induced by the presented stimuli or event, or to the natural state of an individual that is unrelated to the stimuli or event. |
Stimulus characteristic: if stimuli are not matched for arousal; if an event is surprising; likely to be correlated with the intensity of the emotional response Induced state of person: when emotional stimuli are presented in a block (rather than intermixed with neutral stimuli), or when an event is of relatively long duration (more than a few seconds) Natural state of person: individual variations are always present but may be exaggerated when comparing different patient groups or age groups |
Stimulus characteristic: select stimuli to include multiple levels of arousal (e.g., low- and high-arousal negative stimuli) Induced state of person: compare pure to mixed lists of emotional stimuli (although this may also affect stimulus distinctiveness; see above) include intentional mood induction as part of experimental design Natural state of person: direct manipulation likely impossible, but can compare groups selected a priori to differ in baseline state (e.g., high- vs. low-anxiety group) |
Stimulus characteristic: include ratings of arousal as a covariate Induced state of person: include change in cortisol or alpha amylase as an estimate of arousal response Natural state of person: include baseline cortisol or alpha-amylase level as an estimate of natural arousal state |
Note: This table does not present an exhaustive list. Depending on the goals of the experiment, other factors to consider may include: valence of the stimuli (how positive or negative), discrete emotions elicited by the stimuli, mood of the participant, stimulus complexity, event rehearsal
Arousal and Memory: Beyond Yerkes-Dodson (1908) and Easterbrook (1959)
A second claim is that when emotion does not enhance memory, this can be understood by the magnitude of physiological arousal elicited. Yerkes and Dodson (1908) proposed that for complex tasks, performance increases with physiological or mental arousal up to a point, at which the effect of arousal becomes detrimental. This has been supported by animal and human studies on the effects of glucocorticoids and/or stress on memory, such that moderate levels during learning enhance subsequent memory, while lower or higher doses either show an impairing effect (e.g., Lupien et al., 1997) or no effect on memory (e.g., Roozendaal, Williams, & McGaugh, 1999).
A closely related explanation for why emotion does not always enhance memory is that increased arousal leads to a restriction of observed cues (Easterbrook, 1959). This narrowing of attention enables memory for salient details to be enhanced, at the cost of memory for less salient details. At high arousal, however, this restriction of cue utilization is thought to preclude processing of information crucial to event memory, such as the physical characteristics of a perpetrator (e.g., Christianson, 1992; Loftus, Loftus, & Messo, 1987).
While these hypotheses have been supported by prior literature, effects may be relevant under narrower circumstances than typically assumed. The Yerkes-Dodson law was based on a study requiring mice to discriminate between two boxes while receiving shocks of various strength, and their claim of a U-shaped curve applied only to complex tasks. In their ‘easy’ condition, there was a linear relation between shock strength and learning success. Moreover, in reanalyzing their data, Baumler and Lienert (1993) found that the dependent variable critically matters; although defining the learning criterion as ‘hits’ yields an inverted U-shaped curve for complex tasks, defining the criterion as errors results in a linear arousal-performance relation for complex tasks and no relation for the easy task (Baumler & Lienert, 1993; Hanoch & Vitouch, 2004). Thus, the U-shaped curve may exist only for complex tasks, and only when data are scored in a particular way. Similarly, the Easterbrook (1959) hypothesis was originally based upon tasks investigating drive, motivational concentration, perception, and motor skill, and focused on cue utilization during encoding-stage processes. It has since been applied more broadly to a variety of long-term memory studies and has not been reconciled with evidence that arousal often influences post-encoding processes rather than attention narrowing during encoding (e.g., Riggs, McQuiggan, Farb, Anderson, & Ryan, 2011; Mickley Steinmetz & Kensinger, 2013). Whereas the Yerkes-Dodson law and Easterbrook’s attention-narrowing account are valid explanations for arousal-enhanced memory (or the lack thereof) in some cases, the effects of arousal on memory may also depend on other factors.
One such factor is the content of the memoranda: Although stress often enhances emotional memory (e.g., Cahill, Gorski, & Le, 2003) it typically impairs (Payne et al., 2007), or has no effect on (Buchanan & Lovallo, 2001) memory for neutral information. The effects of arousal on memory for neutral stimuli may further depend on their salience (Mather & Sutherland, 2011). Arousal may enhance memory for goal-relevant, salient neutral stimuli while having no effect on, or even impairing, memory for other neutral stimuli (e.g., Sutherland & Mather, 2012).
Even among emotional information, the effects of arousal may differ depending upon the valence of the stimuli (i.e., whether they are positive or negative). For example, free recall of negatively arousing, but not positively arousing words, is enhanced by pre-learning stress (Schwabe, Bohringer, Chatterjee, & Schachinger, 2008). Further evidence for complex interactions between arousal and valence has been shown using fMRI: High (compared to low) arousal is associated with increased amygdala connectivity to the inferior frontal gyrus and middle occipital gyrus while encoding negative stimuli, and decreased amygdala connectivity to these regions while encoding positive stimuli (Mickley Steinmetz, Addis, & Kensinger, 2010).
Another factor is the relation between the arousal experienced and the memory task. Arousal can be relevant to the task, as in the original Yerkes-Dodson experiment, or irrelevant to the task, as often occurs in studies of mood induction. Research has suggested that when the arousal is task-relevant, such as when the content of the to-be-remembered information is arousing, memory for those arousal-inducing, salient details often comes at the cost of memory for other information (the emotion-induced memory trade-off; reviewed by Reisberg & Heuer, 2004). When arousal is task-irrelevant, the effects may be more variable. Libkuman and colleagues (1999) found that sustained physiological arousal – induced by stationary running or biking – had little impact on memory for details of scenes. Sutherland and Mather (2012), however, showed that brief presentation of negative arousing sounds increased short-term memory for high-salience letters but had no benefit on memory for low-salience letters.
These studies demonstrate that when arousal does not enhance memory, this could be due not only to dose, but also to task complexity, the way performance is measured, the content of the memoranda, and the relevance of the arousal to the task. Considering only one of these factors often leads to mixed findings (See Table 3), emphasizing the need to assess multiple factors, and their potential interactions.
Table 3.
Representative examples of the mixed behavioral patterns revealed by studies examining how stimulus content or features of the arousal response influence the effect of arousal on memory.
| Manipulation Category | Specific Manipulation | Study | Key Finding for Effect of Arousal |
|---|---|---|---|
| Content of Memoranda | Emotional vs. neutral | Abercrombie et al., 2003 |
 Negative and Neutral Negative and Neutral |
| Buchanan & Lovallo, 2001 |
 Positive and Negative; No effect on Neutral Positive and Negative; No effect on Neutral |
||
| Cahill, Gorski, & Le, 2003 |
 Negative; No effect on Neutral Negative; No effect on Neutral |
||
| Payne et al., 2007 |
 Negative; Negative;
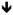 Neutral Neutral |
||
| Rimmele et al., 2003 |
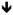 Negative; Negative;
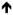 Neutral Neutral |
||
| Schwabe & Wolf, 2010 |
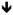 Positive, Negative, Neutral Positive, Negative, Neutral |
||
| Positive vs. negative valence | Domes et al., 2004 |
 Positive; No effect on Negative Positive; No effect on Negative |
|
| Schwabe et al., 2008 |
 Negative; No effect on Positive Negative; No effect on Positive |
||
| Memoranda related, or unrelated, to stressor | Smeets et al., 2007 | Memory for stressor-related words > Non-stressor-related words | |
| Smeets et al., 2009 | Memory for stressor-related words > Non-stressor-related words | ||
| Memoranda central or peripheral to the elicitor of arousal | Christianson, 1984 |
 Central; No effect on Peripheral Central; No effect on Peripheral |
|
| Easterbrook, 1959 | Cue-utilization:
 Central; Central;
 Peripheral Peripheral |
||
| Heuer & Reisberg, 1990 |
 Central and Peripheral Central and Peripheral |
||
| Kebeck & Lohaus, 1986 |
 Central; Central;
 Peripheral Peripheral |
||
| Loftus, Loftus, & Messo, 1987 | Weapon focus effect:
 Central; Central;
 Peripheral Peripheral |
||
| Salience of neutral stimuli | Sutherland & Mather, 2012 |
 High-salience stimuli; No effect on Low-salience stimuli High-salience stimuli; No effect on Low-salience stimuli |
|
|
| |||
| Features of Arousal | Level of Arousal | Gold & Van Buskirk, 1975 | Inverted-U; moderate (not lower or higher) doses of epinephrine enhance spatial memory |
| Lupien et al., 1997 | High stress
 memory for unrelated pairs of words memory for unrelated pairs of words |
||
| Yerkes & Dodson, 1908 | Inverted-U between shock strength and learning success on complex tasks | ||
| Relevance of Arousal to Task: Reason for physiological arousal | Libkuman et al., 1999 | Task-relevant arousal
 memory for scene details Task-Irrelevant = No effect memory for scene details Task-Irrelevant = No effect |
|
Facilitated Processing of Emotional Information Does Not Guarantee Memory Accuracy
The third claim we address is that the facilitated processing of emotional information precipitates facilitated retention of that information. There is no doubt that emotional information benefits from prioritized processing. We rapidly orient our attention to emotional stimuli (e.g., Öhman, Flykt, & Esteves, 2001), and we process emotional information faster and more fluently than non-emotional information (Kityama, 1990), even in the absence of full attention (Kensinger & Corkin, 2004; Talmi et al., 2007b; Talmi, Anderson, Riggs, Caplan, & Moscovitch, 2008). This prioritized processing can be related to memory benefits, both because attended stimuli are often well-remembered (reviewed by Chun & Turk-Browne, 2007) and because the amygdala engagement triggered by emotional arousal facilitates both perceptual (e.g., Vuilleumier, Armony, Driver, & Dolan, 2001; Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004) and mnemonic processes (reviewed by LaBar & Cabeza, 2006). However, the assumptions that facilitated processing always produces enhanced memory, and that the cause of the memory enhancement is facilitated processing, are not always correct.
One demonstration of a disconnect between the effects of emotion on short-term processing and long-term retention comes from studies of working memory. Working memory efficiency can be slowed when emotional stimuli are held in mind (Kensinger & Corkin, 2003), likely because emotional reactions distract from the memory maintenance task. Emotional information may disrupt inter-item binding in working memory (e.g., remembering the relative locations of high and low arousal pictures; Mather et al., 2006) and may also disrupt dorsolateral prefrontal processes related to holding information in mind during delayed-response working memory tasks (e.g., Dolcos & McCarthy, 2006; Dolcos, Diaz-Granados, Wang, & McCarthy, 2008). Yet these same stimuli that impede working memory performance can be remembered well over the long-term (Kensinger & Corkin, 2003), revealing a distinction between the impairing effect of emotion on short-term processing and the beneficial effect on long-term retention. In these instances, the intrusive processing of the emotional content may lead to a more durable memory representation.
Emotion can also have the opposite direction of effect, benefiting short-term processing but impeding long-term retention. For instance, in Murray and Kensinger (2012), participants were faster to form a mental image combining one emotional and one neutral item into a pair, rather than two non-emotional items. However, that facilitated imagery did not lead to facilitated later memory: Individuals remembered the emotional pairs less well than the non-emotional pairs. In this case, the fluent processing of the emotional items may circumvent the effortful, deep, processing that would translate into later memory benefits. The fluent processing may even bias individuals to believe that they have spent enough time learning information, when in fact additional effort would benefit the creation of a durable memory representation. For instance, Zimmerman and Kelley (2010) demonstrated that participants were overconfident when estimating which negative word pairs they would later remember. Likely because of the fluency with which individuals processed the negative pairs, they were misled to believe they had encoded them strongly and would retain them well.
Facilitated processing of emotional cues at retrieval may also mislead individuals, but at this stage of memory, it may cause them to endorse previously unstudied emotional items as “old” (Dougal & Rotello, 2007; Fernandez-Rey & Redondo, 2007; Maratos, Allan, & Rugg, 2000). As discussed earlier, sometimes this bias may result from the increased familiarity that stems from the inherent semantic interrelatedness of emotional items. Other times it may result because emotion facilitates the processing of retrieval cues. People may misattribute that ease-of-processing for a sense of familiarity that the information was previously encountered (e.g., Windmann & Kutas, 2001).
These pieces of counter-evidence emphasize that facilitated processing of emotional information at one stage of processing does not guarantee similar facilitation at another stage. These results highlight the need to avoid the inference that if emotion has not enhanced memory retrieval, it has not facilitated earlier stages of processing. As we have reviewed, retrieval deficits can be indicative of facilitated processing at encoding that reduces post-encoding elaboration or time-on-task. More generally, these complexities provide an important reminder that memory retrieval provides only a limited window into the set of processes used to form and maintain a memory.
Implications and Applications
Although there is support for these three hypotheses, delineating their limiting parameters is important both for basic and clinical research. First, clinical alterations in the effects of emotion on memory may reflect a re-setting of the boundaries for the effect rather than a generalized change in its presence or absence. For instance, patients with Alzheimer’s disease often show little-to-no enhancement of emotional memory within a laboratory setting. Yet when memory for a real-life experience is assessed, the patients often are more likely to remember the occurrence of that event compared to a more mundane event (Waring & Kensinger, 2009). Future research could test how the factors that set the boundary in healthy populations – including semantic relatedness, valence, arousal, and personal involvement – are modified in clinical populations.
Second, a move away from a dose-response (quantity-based) explanation for the effects of arousal may enable a focus on the quality of the arousal response. “Arousal” can incorporate multiple phenomena – mental feelings of excitation or agitation, short-lived physiological changes, and specific responses of the hypothalamic-pituitary-adrenal system. These facets of arousal may have distinct effects on memory. Thus, when trying to understand how arousal affects memory – either in healthy populations or in individuals with affective disorders – it is critical to operationalize “arousal” and to tease apart the influences of these various aspects of arousal.
Third, by realizing the complex relations between the effects of emotion on different stages of processing, we may come closer to a holistic explanation for the effects of emotion on memory in different populations. For instance, we have shown that, unlike young adults, older adults are not faster at binding emotional pairs than neutral ones. Yet when memory is tested, older adults show a mnemonic advantage for the emotional integrations (Murray & Kensinger, in press). These results can only be explained by realizing that facilitation in one aspect of processing can be disconnected from benefits in another.
As these examples highlight, although there is support for the hypotheses reviewed here, there is danger in accepting them as rules-of-thumb and much to be gained by taking the boundary conditions seriously.
Acknowledgments
Preparation of this manuscript was supported by grant MH080833 from the National Institute of Health (to E.A.K.). The authors thank Angela Gutchess and Jessica Payne for helpful conversations related to the content of this review.
Footnotes
The authors have no conflicts of interest.
References
- Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ. Cortisol variation in humans affects memory for emotionally laden and neutral information. Behavioral Neuroscience. 2003;117:505–516. doi: 10.1037/0735-7044.117.3.505. [DOI] [PubMed] [Google Scholar]
- Baumler G, Lienert GA. Reevaluation of the Yerkes-Dodson law by nonparametric-tests of trend. Studia Psychologica. 1993;35:431–436. [Google Scholar]
- Bradley MM, Greenwald MK, Petry MC, Lang PJ. Remembering pictures: Pleasure and arousal in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:379–390. doi: 10.1037//0278-7393.18.2.379. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Stein LM, Silveira RA, Rohenkohl G, Reyna VF. How does negative emotion cause false memories? Psychological Science. 2008;19:919–925. doi: 10.1111/j.1467-9280.2008.02177.x. [DOI] [PubMed] [Google Scholar]
- Brown R, Kulik J. Flashbulb memories. Cognition. 1977;5:73–99. [Google Scholar]
- Buchanan T. Retrieval of emotional memories. Psychological Bulletin. 2007;133:761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Etzel JA, Adolphs R, Tranel D. The influence of autonomic arousal and semantic relatedness on memory for emotional words. International Journal of Psychophysiology. 2006;61:26–33. doi: 10.1016/j.ijpsycho.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Consciousness and Cognition. 1995;4:410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- Choi HY, Kensinger EA, Rajaram S. Emotional content enhances true but not false memory for categorized stimuli. Memory & Cognition. 2013;41:403–415. doi: 10.3758/s13421-012-0269-2. [DOI] [PubMed] [Google Scholar]
- Christianson SA. The relationship between induced emotional arousal and amnesia. Scandinavian Journal of Psychology. 1984;25:147–160. doi: 10.1111/j.1467-9450.1984.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Flashbulb memories: Special, but not so special. Memory and Cognition. 1989;17:435–443. doi: 10.3758/bf03202615. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Emotional stress and eyewitness memory: A critical review. Psychological Bulletin. 1992;112:284–309. doi: 10.1037/0033-2909.112.2.284. [DOI] [PubMed] [Google Scholar]
- Chun MM, Turk-Browne NB. Interactions between attention and memory. Current Opinion in Neurobiology. 2007;17:177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Conway MA, Anderson SJ, Larsen SF, Donnely CM, McDaniel MA, McClelland AGR, Logie RH. The formation of flashbulb memories. Memory & Cognition. 1994;22:326–343. doi: 10.3758/bf03200860. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Comblain C, Van der Linden M. Phenomenal characteristics of autobiographical memories for positive, negative, and neutral events. Applied Cognitive Psychology. 2003;17:281–294. [Google Scholar]
- Dolcos F, Diaz-Granados P, Wang L, McCarthy G. Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia. 2008;46:326–335. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Rimmele U, Reichwald U, Hautzinger M. Acute stress impairs recognition for positive words – Association with stress-induced cortisol secretion. Stress. 2004;7:173–181. doi: 10.1080/10253890412331273213. [DOI] [PubMed] [Google Scholar]
- Dougal S, Rotello CM. “Remembering” emotional words is based on response bias, not recollection. Psychonomic Bulletin & Review. 2007;14(3):423–429. doi: 10.3758/bf03194083. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effect of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Fernandez-Rey J, Redondo J. Recognition memory for pictorial stimuli: Biasing effects of stimulus emotionality. Psicothema. 2007;19:375–380. [PubMed] [Google Scholar]
- Gold PE, Van Buskirk RB. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behavioral Biology. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. TRENDS in Cognitive Sciences. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hanoch Y, Vitouch O. When less is more: Information, emotional arousal, and the ecological reframing of the Yerkes-Dodson law. Theory & Psychology. 2004;14:427–452. [Google Scholar]
- Heuer F, Reisberg D. Vivid memories of emotional events: The accuracy of remembered minutiae. Memory & Cognition. 1990;18:496–506. doi: 10.3758/bf03198482. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York: Henry Holt; 1890. [Google Scholar]
- Johansson M, Mecklinger A, Treese AC. Recognition memory for emotional and neutral faces: An event-related potential study. Journal of Cognitive Neuroscience. 2004;16:1840–1853. doi: 10.1162/0898929042947883. [DOI] [PubMed] [Google Scholar]
- Kebeck G, Lohaus A. Effect of emotional arousal on free recall of complex material. Perceptual & Motor Skills. 1986;63:461–462. [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. 2009;1:99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Effect of negative emotional content on working memory and long-term memory. Emotion. 2003;3:378–393. doi: 10.1037/1528-3542.3.4.378. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences U S A. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of Memory and Language. 2007;56:575–591. [Google Scholar]
- Kityama S. Interaction between affect and cognition in word perception. Journal of Personality and Social Psychology. 1990;58:209–217. doi: 10.1037//0022-3514.58.2.209. [DOI] [PubMed] [Google Scholar]
- Kleinsmith LJ, Kaplan S. Paired associates learning as a function of arousal and interpolated interval. Journal of Experimental Psychology. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Neuroscience Reviews. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Libkuman TM, Nichols-Whitehead P, Griffith J, Thomas R. Source of arousal and memory for detail. Memory & Cognition. 1999;27:166–190. doi: 10.3758/bf03201222. [DOI] [PubMed] [Google Scholar]
- Livingston RB. Reinforcement. In: Quarton GC, McInechuck T, Schmitt FO, editors. The Neurosciences: A Study Program. New York: Rockefeller University Press; 1967. pp. 568–576. [Google Scholar]
- Loftus EF, Loftus GR, Messo J. Some facts about “weapon focus. Law and Human Behavior. 1987;11:55–62. [Google Scholar]
- Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NPV, Meaney MJ. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. Journal of Clinical Endocrinology and Metabolism. 1997;82:2070–2075. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Allan K, Rugg MD. Recognition memory for emotionally negative and neutral words: An ERP study. Neuropsychologia. 2000;38:1452–1465. doi: 10.1016/s0028-3932(00)00061-0. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Rugg MD. Electrophysiological correlates of the retrieval of emotional and non-emotional context. Journal of Cognitive Neuroscience. 2001;13:877–891. doi: 10.1162/089892901753165809. [DOI] [PubMed] [Google Scholar]
- Mather M, Mitchell KJ, Raye CL, Novak DL, Green EJ, Johnson MK. Emotional arousal can impair feature binding in working memory. Journal of Cognitive Neuroscience. 2006;18:614–625. doi: 10.1162/jocn.2006.18.4.614. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey M, Wible CG, Cohen NJ. Is there a special flashbulb-memory mechanism? Journal of Experimental Psychology: General. 1988;117:171–181. [Google Scholar]
- Mickley Steinmetz KR, Addis DR, Kensinger EA. The effect of arousal on the emotional memory network depends on valence. Neuroimage. 2010;53:318–324. doi: 10.1016/j.neuroimage.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley Steinmetz KR, Kensinger EA. The emotion-induced memory trade-off: More than an effect of overt attention? Memory and Cognition. 2013;41:69–81. doi: 10.3758/s13421-012-0247-8. [DOI] [PubMed] [Google Scholar]
- Murray BD, Kensinger EA. The effects of emotion and encoding strategy on associative memory. Memory and Cognition. 2012;40:1056–1069. doi: 10.3758/s13421-012-0215-3. [DOI] [PubMed] [Google Scholar]
- Murray BD, Kensinger EA. Age-related changes in associative memory for emotional and non-emotional integrative representations. Psychology and Aging. doi: 10.1037/a0034443. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Paradis CM, Solomon LZ, Florer F, Thompson T. Flashbulb memories of personal events of 9/11 and the day after for a sample of New York City residents. Psychological Reports. 2004;95:304–310. doi: 10.2466/pr0.95.1.304-310. [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learning and Memory. 2007;14:861–868. doi: 10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Sharot T. How (and why) emotion enhances the subjective sense of recollection. Current Directions in Psychological Science. 2008;17:147–152. doi: 10.1111/j.1467-8721.2008.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg D, Heuer F. Memory for emotional events. In: Reisberg D, Hertel P, editors. Memory and emotion. Oxford: University Press; 2004. [Google Scholar]
- Riggs L, McQuiggan DA, Farb N, Anderson A, Ryan JD. The role of overt attention in emotion-modulated memory. Emotion. 2011;11:776–785. doi: 10.1037/a0022591. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Domes G, Mathiak K, Hautzinger M. Cortisol has different effects on human memory for emotional and neutral stimuli. Neuro Report. 2003;14:2485–2488. doi: 10.1097/00001756-200312190-00038. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Cabeza R. Role of amygdala connectivity in the persistence of emotional memories over time: An event-related fMRI investigation. Cerebral Cortex. 2008;18:2494–2504. doi: 10.1093/cercor/bhm262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: Involvement of the basolateral amygdala. European Journal of Neuroscience. 1999;11:1317–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Kozin M. Vivid memories. Cognition. 1984;16:63–80. doi: 10.1016/0010-0277(84)90037-4. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Nga L, Mather M. Amygdala functional connectivity with medial prefrontal cortex at rest predicts the positivity effect in older adults. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_00392. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SR. Extraordinary memories for exceptional events. New York: Psychological Press; 2012a. [Google Scholar]
- Schmidt SR. Memory for emotional words in sentences: The importance of emotional contrast. Cognition & Emotion. 2012b;26:1015–1035. doi: 10.1080/02699931.2011.631986. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. Effects of pre-learning stress on memory for neutral, positive, and negative words: Different roles of cortisol and autonomic arousal. Neurobiology of Learning and Memory. 2008;90:44–53. doi: 10.1016/j.nlm.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Learning under stress impairs memory formation. Neurobiology of Learning and Memory. 2010;93:183–188. doi: 10.1016/j.nlm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biological Psychology. 2007;76:116–123. doi: 10.1016/j.biopsycho.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Smeets T, Wolf OT, Giesbrecht T, Sijstermans K, Telgen S, Joëls M. Stress selectively and lastingly promotes learning of context-related high arousing information. Psychoneuroendocrinology. 2009;34:1152–1161. doi: 10.1016/j.psyneuen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Sutherland MR, Mather M. Negative arousal amplifies the effects of saliency in short-term memory. Emotion. 2012;12:1367–1372. doi: 10.1037/a0027860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico JM, Rubin DC. Confidence, not consistency, characterizes flashbulb memories. Psychological Science. 2003;14:455–461. doi: 10.1111/1467-9280.02453. [DOI] [PubMed] [Google Scholar]
- Talarico JM, Rubin DC. Flashbulb memories are special after all; In phenomenology, not accuracy. Applied Cognitive Psychology. 2007;21:557–578. [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learning and Memory. 2008;15:172–182. doi: 10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Luk BTC, McGarry LM, Moscovitch M. The contribution of relatedness and distinctiveness to emotionally-enhanced memory. Journal of Memory and Language. 2007a;56:555–574. [Google Scholar]
- Talmi D, Schimmack U, Paterson T, Moscovitch M. The role of attention and relatedness in emotionally enhanced memory. Emotion. 2007b;7:89–102. doi: 10.1037/1528-3542.7.1.89. [DOI] [PubMed] [Google Scholar]
- Vo MLH, Jacobs AR, Kuchinke L, Hogmann M, Conrad M, Schacht A, Hutzler F. The coupling of emotion and cognition in the eye. Psychophysiology. 2008;45:130–140. doi: 10.1111/j.1469-8986.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Waring JD, Kensinger EA. Emotional memory in Alzheimer’s disease. In: Sun M-K, editor. Research Progress In Alzheimer’s Disease and Dementia. Vol. 4. Hauppage, NY: Nova Publishers; 2009. pp. 9–36. [Google Scholar]
- Weaver CA., III Do you need a “flash” to form a flashbulb memory? Journal of Experimental Psychology: General. 1993;122:39–46. [Google Scholar]
- Windmann S, Kruger T. Subconscious detection of threat as reflected by an enhanced response bias. Consciousness & Cognition. 1998;7:603–633. doi: 10.1006/ccog.1998.0337. [DOI] [PubMed] [Google Scholar]
- Windmann S, Kutas M. Electrophysiological correlates of emotion-induced recognition bias. Journal of Cognitive Neuroscience. 2001;13:577–592. doi: 10.1162/089892901750363172. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]
- Zimmerman CA, Kelley CM. “I’ll remember this!” Effects of emotionality on memory predictions versus memory performance. Journal of Memory and Language. 2010;62:240–253. [Google Scholar]


