Abstract
Despite the availability of highly effective therapy for hepatitis C virus (HCV) infection, few patients receive treatment. Barriers arising at multiple levels, from diagnosis to specialist referral, may impede the delivery of hepatitis C care. At the patient level, lack of awareness, fear of side effects, poor adherence, and comorbid conditions may prevent treatment. For providers, limited knowledge, lack of availability, and communication difficulties may be problematic. At the government and payer level, a lack of promotion, surveillance, and funding may interfere. Each of these barriers needs to be addressed if wider implementation of antiviral therapy is to be achieved.
Keywords: Hepatitis C/therapy, health services accessibility, delivery of health care, barriers to care
Recent advances in the treatment of hepatitis C virus (HCV) infection have produced antiviral therapies capable of higher cure rates and shorter treatment durations. For the 130 million to 170 million persons worldwide with chronic HCV infection, current therapies offer a greater than 60% likelihood of sustained virologic response (SVR), regardless of viral genotype.2-5 However, for effective therapy to be delivered, long-standing barriers to treatment need to be addressed. Furthermore, with increased costs, higher rates of adverse events, and complicated treatment algorithms, newer agents may present even greater challenges to patients and physicians. An understanding of existing barriers to HCV treatment is important to help guide initiatives aimed at improving treatment rates and, ultimately, outcomes.
Establishing Current Treatment Rates
Only a small minority of HCV-infected persons receives treatment. The proportion ofpatients treated with antiviral therapy has been estimated in academic, community, and VeteransAffairs (VA) hepatitis C cohorts (Table). Treatment rates ranged from 1.1% in a Vancouver inner-city population to30% at a university-affiliated VA.6,7 Market research from the United States suggests that lessthan 10% of persons with known infection have been treated.8 Likewise, market uptake of interferon (IFN) in Europe indicates anaverage treatment rate of 3.5%.9 Treatment rates may be higher among countries where government-sponsored surveillance and treatment programs are available, such as in France and other European countries 10,11.
Table.
HCV Treatment Rates in Clinical Practice
| Author | Year | Cohort/Setting | # Of Patients | Treatment Rate (%) |
|---|---|---|---|---|
| Grebely6 | 2009 | Community-based inner city cohort | 1,360 | 1.1 |
| Butt53 | 2010 | VA National Patient Database | 134,934 | 11.9 |
| Cawthorne17 | 2002 | St. Louis VA | 557 | 13.8 |
| Rocca37 | 2004 | Olmstead County Hepatitis C Registry | 366 | 15.0 |
| Bini54 | 2005 | 24 VA medical centers | 4,084 | 17.7 |
| Groom18 | 2008 | Minneapolis VA | 520 | 23.8 |
| Evon32 | 2007 | Academic medical center | 433 | 25.2 |
| Morrill36 | 2005 | Primary care clinic | 208 | 27.4 |
| Falck-Ytter19 | 2002 | Teaching county hospital | 293 | 28.3 |
| Butt16 | 2005 | Pittsburgh VA | 354 | 29.4 |
| Rowan7 | 2004 | Houston VA | 580 | 30.0 |
VA, Veterans Administration
Numerous barriers related to patient, provider, government, and payer factors may effectively prevent the delivery of HCV care. These barriers arise at multiple points beginning from the time of infection to the delivery of antiviral therapy (Figure).
Figure.
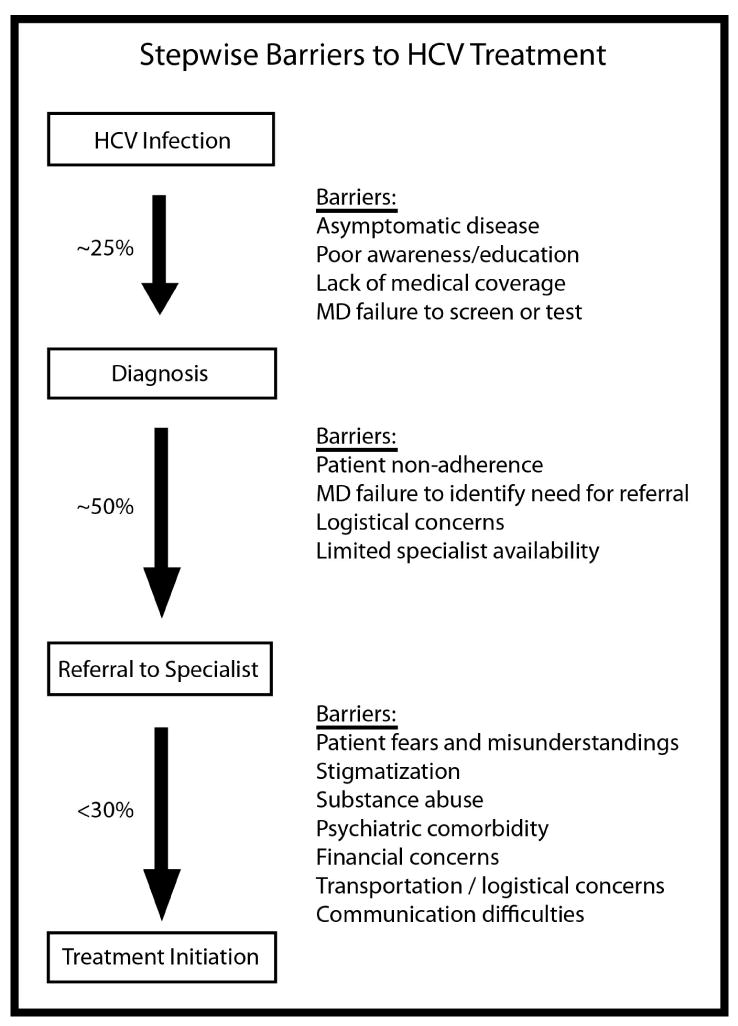
Stepwise Barriers to Hepatitis C Treatment
Patient Factors
Patient-related factors are a common source of treatment deferral and include limited awareness, poor adherence to physician recommendations, economic or social pressures, treatment fears, psychiatric disease, and injection drug use.
Knowledge and Awareness
Between 65% and 75% of patients with chronic HCV infection are unaware of their infection,12 representing the single greatest barrier to treatment. Further, among infected or at-risk persons, knowledge related to HCV is poor. Confusion regarding modes of transmission, disease complications, and interpretation of HCV screening tests is common.13-15 These deficiencies may contribute to missed treatment opportunities, continued transmission, and poorer health outcomes.12
Non-adherence
Though identification of infection may represent the largest barrier to treatment initiation, patients frequently fail to seek treatment once the diagnosis is established. Among patients referred for evaluation of HCV, between 24% and 57% will not attend their initial subspecialty evaluation.16-18 Likewise, patients may demonstrate a lack of adherence to the subsequent evaluation process, missing clinic appointments and failing to obtain recommended diagnostic testing.19 This finding is recognized by treating physicians, 80% of whom cited patient non-adherence as a barrier to high-quality service in the United Kingdom (UK).20 There are multiple reasons for this lack of adherence. Notably, patients may not recognize the urgency to treat an essentially asymptomatic disease.21 Furthermore, significant economic and social pressures may contribute.
Economic and Social Pressures
Persons with HCV are more likely to be uninsured compared to individuals without HCV. Data from the National Health and Nutrition Examination Survey (NHANES III) indicate that 29.6% of HCV-infected patients in the United States are uninsured, compared to 12.2% of persons without infection.22 Further, uninsured persons are less likely to have contact with a healthcare professional, reducing the likelihood of diagnosis and treatment. Though a lack of health insurance is a major barrier to treatment, economic pressures are not exclusive to the uninsured. A cross-sectional study of HCV patients referred to a tertiary care center found that one-half of patients cited personal financial resources as a barrier to care, despite 90% of patients possessing medical coverage.23 Fears of missed work obligations may further contribute to financial insecurity.24
In addition to financial pressures, patients cite multiple social factors as reasons for deferring therapy. These include family obligations, lack of social support, social rejection, and stigmatization.23-25 More than half of HCV patients report feeling stigmatized, primarily due to the association between HCV and HIV, promiscuity, and/or substance abuse.25,26 These patients have higher levels of anxiety, worsened quality of life, and difficulty coping.26 Furthermore, patients may feel stigmatized by their physicians,27 further reducing the likelihood of treatment adherence.
Fears of Treatment
For those patients who present for evaluation, fears related to antiviral therapy figure prominently into their decision to pursue treatment. An important consideration among HCV patients is the risk to benefit tradeoff related to treatment.24 Though a desire to eradicate a chronic, progressive infection may seem intuitive, patients may be unable to look beyond the short-term risks of therapy, particularly side effects. Among patients deferring HCV therapy, nearly two-thirds cite a fear of side effects, coupled with the asymptomatic nature of their disease, as the primary reason for deferral.21 These findings are supported by an international study of treating physicians, who rated patients’ fear of side effects as the most important barrier to HCV treatment.28 Similar findings were noted in a survey of UK physicians.20
Fears of treatment-related side effects are not without merit. Virtually all patients will experience a treatment-related side effect, ranging from mild constitutional symptoms to significant hematologic abnormalities.2-5 Adverse effects greatly impact quality of life and may lead to dose reductions and treatment discontinuation. While these fears are valid, they may also be heightened by the availability of inaccurate or skewed information.29 Therefore, it is imperative that patients receive appropriate pre-treatment education and counseling to allay such fears. In addition to side effects, patients express concerns regarding treatment duration and effectiveness.19-21
Psychiatric Disease
Psychiatric illness represents a significant barrier to HCV treatment. Adverse psychiatric effects, including irritability, depression, and mood swings, are common complications of IFN use. Between 21% and 58% of patients will develop significant depression with IFN therapy.30 Furthermore, HCV-infected patients have a higher prevalence of preexisting psychiatric illness.31 Recognizing the risks of IFN, clinicians frequently defer therapy for patients with underlying psychiatric illness.7 Unfortunately, these patients are unlikely to obtain treatment after initial deferral.32 However, the presence of psychiatric illness is no longer considered an absolute contraindication to therapy.30 When treated with a multidisciplinary approach, including regular psychiatric monitoring, patients with psychiatric disorders can achieve comparable outcomes to those without psychiatric illness.33 In a randomized, controlled trial, participants initially deferred from therapy due to substance abuse or psychiatric illness were more likely to become eligible for subsequent antiviral therapy after enrollment in a multidisciplinary intervention compared to those receiving standard of care 34.
Injection Drug Use
Injection drug use is a frequent mode of HCV acquisition, accounting for a high proportion of new cases worldwide, and 60% of incidence cases in the United States.30,35 Active use of injection drugs is a common reason for HCV treatment deferral.18,36,37 One reason is the high prevalence of depression and psychiatric illness among intravenous drug users (IDUs).38 Additionally, concerns regarding poor patient adherence, increased risk of adverse effects, and post-treatment reinfection may lead to deferral.39 Intravenous drug users have poor awareness of current treatments, with fewer than half recognizing that HCV is a curable disease.40,41 However, between 70% and 80% of IDUs express a willingness for treatment.40-42 Furthermore, favorable treatment outcomes have been demonstrated in both active IDUs and those receiving methadone maintenance.33,43,44 Therefore, treatment for these patients should be considered in the setting of close monitoring and adjunctive psychiatric and substance use counseling.30
Provider Factors
Barriers to hepatitis C treatment may arise at the provider level, including primary care physicians and subspecialists. Key barriers to treatment include lack of knowledge and awareness, limited specialist availability and/or lack of referral, and communication issues.
Knowledge and Awareness
Healthcare professionals have demonstrated key knowledge deficits related to HCV prevalence, risk factors, prevention, and management.12 Among primary care providers, this may be attributable to a lack of experience. In a nationwide survey of primary care physicians, 73% of respondents reported seeing 5 or fewer HCV patients in the preceding year, with 44% reporting no experience with HCV treatment.45 Though most physicians correctly identified risk factors for HCV, only 59% reported regularly screening for these risk factors. Similar deficiencies in HCV testing have been noted in studies of family practitioners and obstetrics and gynecology providers.46,47 Knowledge of HCV was likewise inadequate among drug-treatment providers.48 Among patients with HCV, perceived physician incompetence is a known barrier to care.27
Specialist Referral and Availability
Primary care physicians infrequently refer HCV patients for subspecialty evaluation. Only one-half of HCV-infected patients are referred to a specialist for evaluation and management.6,49 For patients with normal liver tests, the likelihood of referral is below 30%.45 Furthermore, for those patients referred for treatment, the availability of specialists presents an additional barrier. It is estimated that 80% of chronic HCV patients are managed by 20% of gastroenterologists.8 Specialists are concentrated within academic medical centers or government-designated Centers of Excellence, limiting the availability of local treatment providers. As a result, patients may face long-distance travel, extended wait times, and a lack of scheduling flexibility.20,50 While academic hepatologists may be more adept at managing adverse effects and limiting dose reductions, the number of patients with chronic HCV may exceed their availability.8 Efforts to expand the availability of specialist expertise via telemedicine have shown promise.51
Communication Issues
Negative interactions with HCV treatment providers may serve as an additional barrier to treatment. In a cross-sectional study of 322 HCV patients treated at a tertiary care center, 41% reported communication difficulties with their physicians.27 Specifically, patients felt rushed, misled, or not listened to. Patients may question a physician’s competence, or feel stigmatized by these interactions. Healthcare workers are known to harbor negative views of injection drug users, characterizing them as manipulative and unpleasant.12 As a result of these interactions, patients may feel discouraged, less likely to listen to physician recommendations, and more inclined to defer therapy.
Government and Payer Barriers
Governments and payers are critical to delivering HCV services, implementing surveillance programs, disseminating information, and increasing public and provider awareness. Though patient and provider factors receive the greatest attention, obstacles arising at the government and payer level are likewise important. In an international study of HCV providers, lack of treatment promotion and insufficient funding were noted as significant government level barriers.28 Likewise, lack of insurance coverage, high out-of-pocket expenses, and excessive paperwork were cited as payer-level barriers. In a separate study of UK physicians, more than two-thirds of respondents reported inadequate funding as a barrier to quality HCV care.20 These sentiments were echoed in the recent Institute of Medicine report on the prevention and control of viral hepatitis in the United States.12 Specifically, the committee noted the poorly developed surveillance system, inadequate educational initiatives, and fragmented viral hepatitis services in this country. To address these issues, increased resource allocation and improved collaboration between government, healthcare, and educational stakeholders are needed. In the European Union, government sponsored screening and surveillance programs have greatly increased diagnosis rates of HCV infection 10,11.
Looking forward: Direct Acting Antivirals
The recent introduction of the direct acting antivirals (DAA) boceprevir and teleprevir has changed the current treatment paradigm for patients with genotype 1 infection. Among treatment-naïve patients, overall SVR rates exceeding 70% are now possible, with the added potential for abbreviated treatment duration.4,5 For patients concerned about efficacy and duration, the introduction of these agents may increase treatment appeal. However, these benefits must be balanced against increased treatment complexity, higher rates of adverse events, and the potential for drug-drug interactions.52 It remains to be seen how the availability of triple therapy will influence current treatment barriers.
Conclusions
Multiple barriers to the diagnosis, evaluation, and delivery of hepatitis C treatment serve to limit the widespread uptake of antiviral therapy. Though recent advances in HCV treatment offer the potential for high cure rates and shorter treatment durations, long-standing obstacles to treatment must be addressed. Poor awareness, misguided and exaggerated fears, relative contraindications, and insufficient funding all contribute to strikingly low treatment rates. Recognizing the global burden of infection, there is a critical need to reduce current barriers to hepatitis C treatment.
Acknowledgments
Grant Support: CEM is supported by the National Institutes of Health T32 DK07634. MWF is supported in part by Mid-Career Mentoring Award NIH K24 DK066144.
Abbreviations
- HCV
Hepatitis C virus
- VA
Veterans Administration
- UK
United Kingdom
- NHANES
National Health and Nutrition Examination Survey
- HIV
Human Immunodeficiency Virus
- IDU
injection drug user
- DAA
direct acting antiviral, IFN interferon
References
- 1.Serologic findings with hepatitis B in hemophilia. . N Engl J Med. 1978;299(16):898–900. doi: 10.1056/NEJM197810192991617. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002 Sep 26;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011 Mar 31;364(13):1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011 Jun 23;364(25):2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 6.Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009 May;16(5):352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 7.Rowan PJ, Tabasi S, Abdul-Latif M, Kunik ME, El-Serag HB. Psychosocial factors are the most common contraindications for antiviral therapy at initial evaluation in veterans with chronic hepatitis C. J Clin Gastroenterol. 2004 Jul;38(6):530–534. doi: 10.1097/01.mcg.0000123203.36471.70. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman ML. A balancing view: We cannot do it alone. Am J Gastroenterol. 2007 Sep;102(9):1841–1843. doi: 10.1111/j.1572-0241.2007.01433_4.x. [DOI] [PubMed] [Google Scholar]
- 9.Lettmeier B, Muhlberger N, Schwarzer R, et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J Hepatol. 2008 Oct;49(4):528–536. doi: 10.1016/j.jhep.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Delarocque-Astagneau E, Meffre C, Dubois F, et al. The impact of the prevention programme of hepatitis C over more than a decade: the French experience. J Viral Hepat. 2010 Jun;17(6):435–443. doi: 10.1111/j.1365-2893.2009.01196.x. [DOI] [PubMed] [Google Scholar]
- 11.Hatzakis A, Wait S, Bruix J, et al. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference*. J Viral Hepat. 2011 Sep;18(Suppl 1):1–16. doi: 10.1111/j.1365-2893.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010 Mar;51(3):729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 13.Dhopesh VP, Taylor KR, Burke WM. Survey of hepatitis B and C in addiction treatment unit. The American journal of drug and alcohol abuse. 2000 Nov;26(4):703–707. doi: 10.1081/ada-100101903. [DOI] [PubMed] [Google Scholar]
- 14.Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Depend. 2001 Feb 1;61(3):211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien S, Day C, Black E, Dolan K. Injecting drug users’ understanding of hepatitis C. Addict Behav. 2008 Dec;33(12):1602–1605. doi: 10.1016/j.addbeh.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Butt AA, Wagener M, Shakil AO, Ahmad J. Reasons for non-treatment of hepatitis C in veterans in care. J Viral Hepat. 2005 Jan;12(1):81–85. doi: 10.1111/j.1365-2893.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 17.Cawthorne CH, Rudat KR, Burton MS, et al. Limited success of HCV antiviral therapy in United States veterans. The American journal of gastroenterology. 2002 Jan;97(1):149–155. doi: 10.1111/j.1572-0241.2002.05439.x. [DOI] [PubMed] [Google Scholar]
- 18.Groom H, Dieperink E, Nelson DB, et al. Outcomes of a Hepatitis C screening program at a large urban VA medical center. J Clin Gastroenterol. 2008 Jan;42(1):97–106. doi: 10.1097/MCG.0b013e31802dc56f. [DOI] [PubMed] [Google Scholar]
- 19.Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med. 2002;136(4):288–292. doi: 10.7326/0003-4819-136-4-200202190-00008. [DOI] [PubMed] [Google Scholar]
- 20.Parkes J, Roderick P, Bennett-Lloyd B, Rosenberg W. Variation in hepatitis C services may lead to inequity of heath-care provision: a survey of the organisation and delivery of services in the United Kingdom. BMC Public Health. 2006;6:3. doi: 10.1186/1471-2458-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time? Dig Dis Sci. 2007 May;52(5):1168–1176. doi: 10.1007/s10620-006-9579-1. [DOI] [PubMed] [Google Scholar]
- 22.Ong JP, Collantes R, Pitts A, Martin L, Sheridan M, Younossi ZM. High rates of uninsured among HCV-positive individuals. J Clin Gastroenterol. 2005 Oct;39(9):826–830. doi: 10.1097/01.mcg.0000177258.95562.43. [DOI] [PubMed] [Google Scholar]
- 23.Evon DM, Simpson KM, Esserman D, Verma A, Smith S, Fried MW. Barriers to accessing care in patients with chronic hepatitis C: the impact of depression. Aliment Pharmacol Ther. 2010 Nov;32(9):1163–1173. doi: 10.1111/j.1365-2036.2010.04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraenkel L, McGraw S, Wongcharatrawee S, Garcia-Tsao G. What do patients consider when making decisions about treatment for hepatitis C? Am J Med. 2005 Dec;118(12):1387–1391. doi: 10.1016/j.amjmed.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Zacks S, Beavers K, Theodore D, et al. Social stigmatization and hepatitis C virus infection. J Clin Gastroenterol. 2006 Mar;40(3):220–224. doi: 10.1097/00004836-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Zickmund S, Ho EY, Masuda M, Ippolito L, LaBrecque DR. “They treated me like a leper”. Stigmatization and the quality of life of patients with hepatitis C. J Gen Intern Med. 2003 Oct;18(10):835–844. doi: 10.1046/j.1525-1497.2003.20826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zickmund S, Hillis SL, Barnett MJ, Ippolito L, LaBrecque DR. Hepatitis C virus-infected patients report communication problems with physicians. Hepatology. 2004 Apr;39(4):999–1007. doi: 10.1002/hep.20132. [DOI] [PubMed] [Google Scholar]
- 28.McGowan CEMA, Bacon BR, Mallolas J, Goncales FL, Goulis I, Poordad F, Afdhal N, Zeuzem S, Piratvisuth T, Marcellin P, Fried MW. Barriers to hepatitis C treatment: a global analysis of physician perceptions. Hepatology. 2011;54(S1):409A–410A. doi: 10.1002/hep.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124(6):1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 30.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009 Apr;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123(2):476–482. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- 32.Evon DM, Verma A, Dougherty KA, et al. High deferral rates and poorer treatment outcomes for HCV patients with psychiatric and substance use comorbidities. Dig Dis Sci. 2007 Nov;52(11):3251–3258. doi: 10.1007/s10620-006-9669-0. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in "difficult-to-treat" psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007 Oct;46(4):991–998. doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- 34.Evon DM, Simpson K, Kixmiller S, et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am J Gastroenterol. 2011 Oct;106(10):1777–1786. doi: 10.1038/ajg.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009 Aug 15;49(4):561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 36.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. Patient, provider, and system factors. J Gen Intern Med. 2005 Aug;20(8):754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocca LG, Yawn BP, Wollan P, Kim WR. Management of patients with hepatitis C in a community population: diagnosis, discussions, and decisions to treat. Ann Fam Med. 2004 Mar-Apr;2(2):116–124. doi: 10.1370/afm.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golub ET, Latka M, Hagan H, et al. Screening for depressive symptoms among HCV-infected injection drug users: examination of the utility of the CES-D and the Beck Depression Inventory. J Urban Health. 2004 Jun;81(2):278–290. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. The New England journal of medicine. 2001 Jul 19;345(3):215–217. doi: 10.1056/NEJM200107193450312. [DOI] [PubMed] [Google Scholar]
- 40.Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clin Infect Dis. 2005 Apr 15;40(Suppl 5):S313–320. doi: 10.1086/427446. [DOI] [PubMed] [Google Scholar]
- 41.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008 Jun;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strathdee SA, Latka M, Campbell J, et al. Factors associated with interest in initiating treatment for hepatitis C Virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005 Apr 15;40(Suppl 5):S304–312. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001 Jul;34(1):188–193. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- 44.Mauss S, Berger F, Goelz J, Jacob B, Schmutz G. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004 Jul;40(1):120–124. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- 45.Shehab TM, Sonnad SS, Lok AS. Management of hepatitis C patients by primary care physicians in the USA: results of a national survey. J Viral Hepat. 2001 Sep;8(5):377–383. doi: 10.1046/j.1365-2893.2001.00310.x. [DOI] [PubMed] [Google Scholar]
- 46.Ferrante JM, Winston DG, Chen PH, de la Torre AN. Family physicians’ knowledge and screening of chronic hepatitis and liver cancer. Fam Med. 2008 May;40(5):345–351. [PubMed] [Google Scholar]
- 47.Boaz K, Fiore AE, Schrag SJ, Gonik B, Schulkin J. Screening and counseling practices reported by obstetrician-gynecologists for patients with hepatitis C virus infection. Infect Dis Obstet Gynecol. 2003;11(1):39–44. doi: 10.1155/S106474490300005X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strauss SM, Astone-Twerell JM, Munoz-Plaza C, et al. Hepatitis C knowledge among staff in U.S. drug treatment programs. J Drug Educ. 2006;36(2):141–158. doi: 10.2190/3EMQ-N350-W4XN-WT1X. [DOI] [PubMed] [Google Scholar]
- 49.Shehab TM, Orrego M, Chunduri R, Lok AS. Identification and management of hepatitis C patients in primary care clinics. The American journal of gastroenterology. 2003 Mar;98(3):639–644. doi: 10.1111/j.1572-0241.2003.07331.x. [DOI] [PubMed] [Google Scholar]
- 50.Naffah F. Patients with hepatitis C are best managed by a specialist in liver diseases CON: The management of hepatitis C in a community-based practice. The American journal of gastroenterology. 2007 Sep;102(9):1839–1841. doi: 10.1111/j.1572-0241.2007.01433_3.x. [DOI] [PubMed] [Google Scholar]
- 51.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011 Jun 9;364(23):2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011 Oct;54(4):1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butt AA, McGinnis KA, Skanderson M, Justice AC. Hepatitis C treatment completion rates in routine clinical care. Liver Int. 2010 Feb;30(2):240–250. doi: 10.1111/j.1478-3231.2009.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. The American journal of gastroenterology. 2005 Aug;100(8):1772–1779. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]


