Abstract
Hexavalent chromium [Cr(VI)] compounds are highly redox active and have long been recognized as potent cytotoxins and carcinogens. The intracellular reduction of Cr(VI) generates reactive Cr intermediates, which are themselves strong oxidants, as well as superoxide, hydrogen peroxide, and hydroxyl radical. These probably contribute to the oxidative damage and effects on redox-sensitive transcription factors that have been reported. However, the identification of events that initiate these signaling changes has been elusive. More recent studies show that Cr(VI) causes irreversible inhibition of thioredoxin reductase (TrxR) and oxidation of thioredoxin (Trx) and peroxiredoxin (Prx). Mitochondrial Trx2/Prx3 are more sensitive to Cr(VI) treatment than cytosolic Trx1/Prx1, although both compartments show thiol oxidation with higher doses or longer treatments. Thiol redox proteomics demonstrate that Trx2, Prx3, and Trx1 are among the most sensitive proteins in cells to Cr(VI) treatment. Their oxidation could therefore represent initiating events that have widespread implications for protein thiol redox control and for multiple aspects of redox signaling. This review summarizes the effects of Cr(VI) on the TrxR/Trx system and how these events could influence a number of downstream redox signaling systems that are influenced by Cr(VI) exposure. Some of the signaling events discussed include the activation of apoptosis signal regulating kinase and MAP kinases (p38 and JNK) and the modulation of a number of redox-sensitive transcription factors including AP-1, NF-κB, p53, and Nrf2.
Keywords: Chromium(VI), Thioredoxin reductase, Thioredoxin, Peroxiredoxin, Thiol oxidation, Redox signaling, Free radicals
Introduction to hexavalent chromium
Hexavalent chromium [Cr(VI)] compounds have long been recognized as potent toxins and carcinogens. Inhalation of Cr(VI)-containing particles, dusts, mists, and fumes is a common form of exposure and is associated with several respiratory diseases, including lung cancer, pulmonary fibrosis, asthma, chronic bronchitis, and others [1–9]. The greatest exposures typically occur in workers in Cr-related industries (e.g., chromate pigments and dyes, chromate-based corrosion inhibitors, stainless steel machining and welding, chrome plating, offset printing). The bronchial epithelium is directly exposed to inhaled Cr(VI) and represents a major site of resulting cell damage and tumor initiation. For example, squamous cell lung carcinoma is observed in Cr workers, with a greater lung Cr burden in those with tumors [2]. Atypical precancerous lesions are primarily located at bronchial bifurcations where Cr is preferentially deposited [2]. The relative risk of lung cancer in Cr workers increases with cumulative Cr(VI) exposure [3,9]. Skin exposure to Cr(VI) is also very common in occupational settings, including cement, chromate pigments, chrome plating, leather tanning, welding, printing, and others [9]. The predominant adverse reactions are allergic dermatitis and skin ulcers [9–11].
Industrial uses result in the annual release of more than 1.7 × 105 t of Cr into the environment [12]. Elevated Cr(VI) levels are present in groundwaters and soils at numerous sites throughout the United States, and Cr is a contaminant at more than 600 Superfund sites [13,14]. In addition to its heavy industrial use and release, fossil fuel burning and steel production are the two largest contributors to atmospheric Cr(VI) [13]. Exposure to Cr(VI) is a concern of both the OSHA [9] and the EPA, and the EPA has designated that Cr(VI) compounds are 1 of 17 chemicals that pose the greatest threat to human health [13]. While there are comparatively few data on environmental exposure, individuals living and working in areas where the soil was contaminated decades earlier with Cr slag had elevated urine Cr levels [15], which represent recent exposure. Those individuals with elevated Cr also had substantially higher DNA–protein crosslinks in their lymphocytes [14]. These crosslinks are considered an indicator of biologically active doses of Cr(VI) and were similarly observed in Bulgarian residents living in Cr-contaminated regions [14]. A fourfold increase in childhood leukemia in one community was attributed to elevated Cr(VI) in the drinking water [16]. It is difficult to assess the potential effects of long-term low-level exposure to toxins, and Cr(VI) is no exception. However, the much smaller doses from environmental sources indicate that the environmental risk is probably much less than that from occupational exposures.
Chromate (CrO42−) and dichromate (Cr2O72−) are two common forms of Cr(VI). Dichromate is the predominant form under acidic conditions when Cr(VI) concentrations exceed 10 mM [17]. Chromate predominates under physiologic conditions (i.e., when the pH is greater than 6 and Cr is less than 10 mM [17–19]). Chromate closely resembles sulfate (SO42−) and enters cells via an anion carrier [20]. Cr(VI) compounds also readily cross the skin [6]. Some chromates (e.g., Na2CrO4) are highly water soluble, whereas others are particulate in nature and have limited solubility (e.g., ZnCrO4) or very poor solubility (e.g., PbCrO4) [21]. However, particulate chromates are also taken up by cells, which can concentrate Cr intracellularly > 180-fold [22,23]. Chromate particulates are toxic and carcinogenic [24] and are solubilized by cells over time providing for prolonged continuous doses of Cr(VI) [21–23,25–27]. The genotoxicity and cell-transforming activity of Cr(VI) compounds have been extensively studied. For example, insoluble lead chromate induces cytotoxicity and morphological and neoplastic transformation of mouse embryo cells [28], and both soluble and insoluble Cr(VI) compounds induce anchorage-independent transformation of cultured human fibroblasts [29,30]. Several genetic lesions are associated with Cr(VI), including Cr–DNA adducts, oxidized bases, DNA–protein crosslinks, and DNA strand breaks [31]. This damage can impair DNA replication and can promote genomic instability [31].
Inhaled particulates, such as ZnCrO4, result in nonhomogeneous exposure of the respiratory epithelium [2]. Cells in direct contact with Cr(VI) particulates can have relatively high continuous doses, whereas those free of particles could have little to no exposure, unless there is transfer of solubilized Cr between cells. In vivo, Cr particulates may redistribute over time given the actions of various natural airway processes (e.g., mucociliary transport). Hence, Cr particulates will lead to variable cell responses, depending on the Cr dose for each cell. The overall responses measured will therefore be the blend of responses in all cells sampled. For some indicators of cytotoxicity, the effects in Cr(VI)-exposed cells could be difficult to discern in tissue samples that contain significant levels of unexposed cells. Even though Cr(VI) particulates result in heterogeneous exposure, they are forms to which humans are exposed and are an important part of testing.
Generation of reactive species and oxidants from Cr(VI)
Although many studies have implicated exposure to Cr(VI) as the predisposing factor to toxicity [32–36], Cr(VI) is not itself the toxic species. Inside cells, Cr(VI) is quickly reduced by a variety of enzymatic and chemical reductants [37–43], eventually to Cr(III), the next stable oxidation state. Cr(III) species are generally insoluble and do not easily cross cell membranes [44]. With no known single-step three-electron donors in biological systems, the reduction of Cr(VI) to Cr(III) must proceed through Cr(V) and/ or Cr(IV). One-electron reductants initially generate Cr(V), and possibly subsequently Cr(IV), whereas two-electron reductants can generate Cr(IV) in a single step. Cr(V) species have a characteristic EPR signal (g = 1.98–1.99) that has facilitated Cr(V) detection in vitro, ex vivo, and in vivo [42,43,45–51]. Cr(IV) generation has been inferred indirectly [42,52,53]. Both Cr(V) and Cr(IV) are reactive intermediates that can cause cellular damage [33,54,55], and they can act as direct oxidants [56,57]. Dismutation reactions between Cr redox states are possible [54], such as
| (1) |
It is unknown to what extent such dismutation reactions occur within cells.
Cr(V) and Cr(IV) are also recognized as proficient Fenton-like metals in their ability to generate hydroxyl radical (HO•) from H2O2 [38,41,55,58–60]:
| (2) |
| (3) |
The redox cycling of Cr by such reactions can generate a stoichiometric excess of HO• relative to the net amount of Cr(VI) reduced [41]. Although Cr(III) can similarly generate HO• [61], the reaction rate is much slower. Other reactive oxygen species (ROS) such as superoxide can be simultaneously generated during Cr(VI) reduction [41,62–66]. would be expected to be quickly converted to H2O2 through the actions of superoxide dismutase (SOD) in the cytosol (CuZnSOD) and mitochondria (MnSOD). Cr(VI) treatment of keratinocytes and prostate cancer cells has been shown to increase H2O2 generation [67,68]. The generation of ROS could be especially prominent in airway epithelial cells, in which the O2 tensions are consistently high. Cr(VI) can also enhance peroxynitrite generation in cells [66].
Overall, several reactive and pro-oxidant species can be generated by intracellular Cr(VI) reduction, and pro-oxidant effects can contribute to Cr(VI) toxicity [26,33,54–56,64,69–80] and to its ability to promote mitochondrial-dependent apoptosis [81–83]. The redox cycling of Cr could increase the generation of ROS and thereby enhance oxidative stress [41,55,70,71,84]. Several studies imply that reactive Cr and/or ROS generation contribute to Cr(VI) toxicity. Catalase decreases Cr(VI) toxicity in both cancerous and noncancerous cells [77,85–88] and diminishes HO• generation [68,87,88], implying a role for peroxides and/or peroxide- generated HO•. Similarly, the overexpression of glutathione peroxidase (GPx) protects cells from Cr(VI) [86]. Peroxidases would alter peroxide-mediated signaling, but may also act by preventing HO• generation. HO• radical scavengers such as formate and dimethyl sulfoxide also decrease Cr(VI) toxicity [77,85,88]. Deferoxamine (DFX), which chelates Fe and Cr(V) but does not chelate Cr(VI), also protects cells from Cr(VI) [75,85,88] and diminishes Cr(V) and HO• generation [68,89]. The most direct explanation is that DFX prevents Cr(V)-mediated HO• generation and/or direct oxidant attack by Cr(V).
Other oxidant scavengers (e.g., butylhydroxytoluene and vitamin E) reduce Cr(VI) toxicity in pneumocytes [75], and vitamin E protects from Cr(VI)-induced renal damage [76,90,91]. MnTBAP [Mn(III)tetrakis(4-benzoic acid)porphyrin chloride], an efficient scavenger of peroxynitrite and an SOD mimetic [92,93], protects H460 lung cancer cells from Cr(VI), as does overexpression of CuZnSOD [86]. However, MnTBAP does not show this protective effect in normal human bronchial BEAS-2B cells [79], and SOD does not protect A549 cells from Cr(VI)-induced cell cycle arrest [94] or mouse epidermal cells from Cr(VI)-induced cell death [88]. Together, these studies imply an important role for peroxides, HO•, and reactive Cr species in toxicity. Although there may be a direct role for in some cells, its role may be largely indirect as a source for H2O2.
Various intracellular Cr(VI) reductants could result in the generation of different proportions of reactive Cr or oxygen species, each mediating particular types of damage. Therefore, the mechanisms of Cr(VI) reduction, their location in the cell, and the rates of formation of the reactive intermediates could all influence the subsequent pro-oxidant effects.
Effects of Cr(VI) on cellular thiols
The redox balance of cellular thiols (−SH) is critical for normal cell function and viability. The thioredoxins and glutathione both contribute significantly to the maintenance of cellular thiol redox balance, but they are not in redox equilibrium with each other [95–97]. A major role of the thioredoxins is to maintain intracellular proteins in their reduced state [98], and the redox status of the Trx system in some cells may be more critical to cell survival than is glutathione.
Thiolates (−S−) are much more susceptible to attack by oxidants and electrophiles than are thiols [99]. The pKa values of thiols in a typical protein (8.5) and in reduced glutathione (GSH; 8.8) [99] predict limited reactivity at pH 7.4 because of the low proportion in the −S− form. Consistent with this, the rate of Cr(VI) reduction by GSH is relatively slow at pH 7.4 [100–102], and several studies show that Cr(VI) does not typically deplete cellular GSH. In normal human bronchial BEAS-2B cells, there is no decrease in GSH or increase in glutathione disulfide (GSSG) with Cr(VI) treatments that cause marked oxidation of the thioredoxin (Trx) system [80]. Similarly, other studies show that GSH levels are maintained, or even elevated, in Cr(VI)-treated cells and animals [103–105]. Overwhelming Cr(VI) treatments (e.g., 8 mM with human erythrocytes, 1 mM with rat hepatocytes) do, however, cause pronounced declines in GSH and increases in GSSG [106,107]. In some cases, prolonged treatment with Cr(VI) may deplete GSH, but this may reflect a later consequence of cell death [108]. In dermal fibroblasts, 5µM Cr(VI) can deplete GSH by 38% after 6 h, but GSH levels at later times were not reported [109].
The pKa of the thiol in free cysteine (Cys) is 8.3, and its redox potential is more negative than that of GSH. This is reflected in the 2.8-fold faster reaction of Cr(VI) with Cys vs GSH [101]. The rate of O2 consumption stimulated by the addition of Cys to reactive Cr(V/IV) is in vast excess to the rate with GSH [110]. This implies that Cys may be especially vulnerable to oxidant attack in the presence of reactive Cr intermediates. However, free Cys levels in cells are well below GSH levels [55], and protein thiols represent a larger thiol redox pool than GSH [111]. Therefore, protein thiols could represent an important target of reactive Cr species, or of other oxidants that are generated by Cr(VI) treatment. Within proteins, nearby amino acids can significantly influence the pKa of Cys thiols (i.e., some protein thiols will largely be thiolates at physiological pH). A prime example is Trx (pKa of ca. 6.5) [99,112], whose active site should be largely ionized and reactive at pH 7.4. Selenocysteine (Sec) has an even lower pKa (estimated at 5.2) [113], and the active-site Sec of thioredoxin reductase (TrxR) is an even stronger nucleophile than Cys [114,115]. This strong nucleophilicity could be a key determinant in the enhanced reactivity of Sec relative to Cys [115]. Whereas the potential inactivation of Sec in proteins has received considerably less attention relative to thiols, the inactivation of TrxR could have widespread implications for protein thiol redox balance and redox signaling.
Irreversible inhibition of TrxR
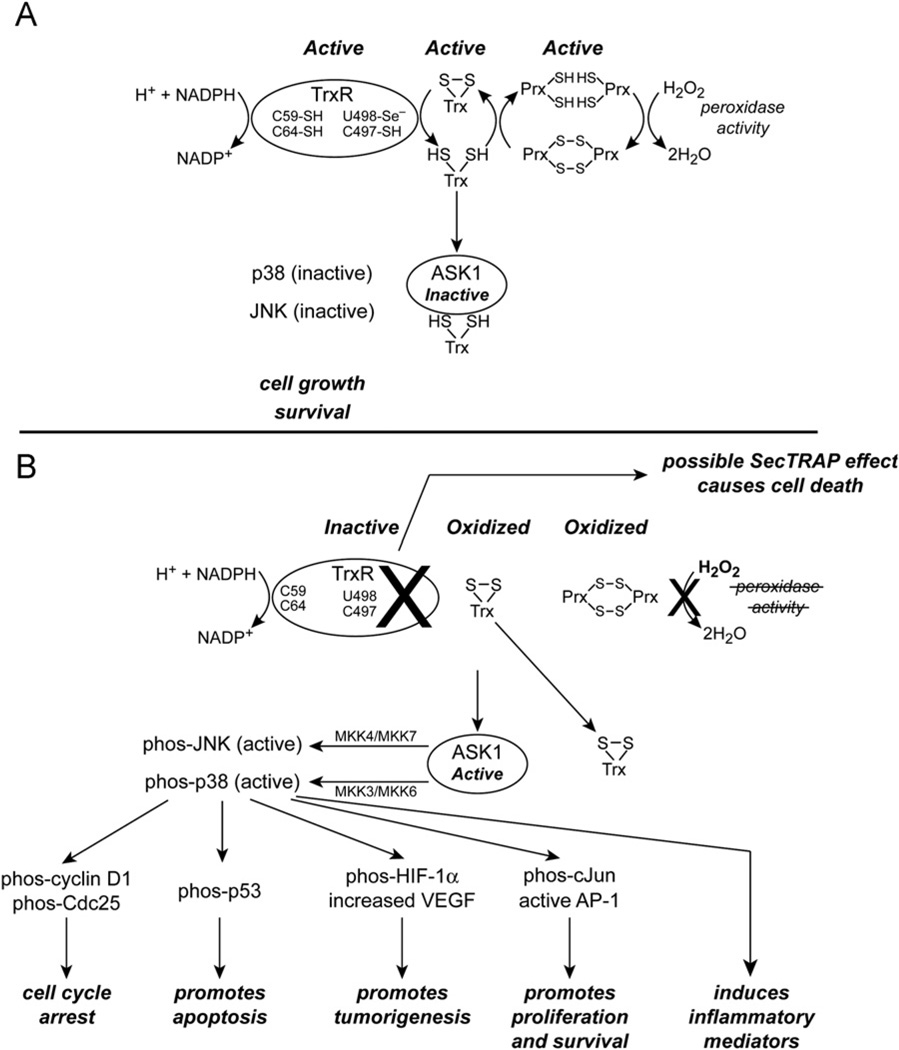
A major role of the TrxR’s is to keep their respective thioredoxins in the reduced state (Fig. 1A). The thioredoxins, in turn, keep many intracellular protein thiols reduced. TrxR activity is markedly inhibited in Cr(VI)-treated cells in a dose- and timedependent manner [78]. This decreased activity reflects TrxR inhibition, not protein degradation [78]. Activity is not restored in the cell lysates by removal of free Cr or by NADPH (the electron donor for TrxR). Even with intermittent exposures, which occur in occupational and environmental settings, the inhibition of TrxR can persist beyond the Cr(VI) exposure event itself.
Fig. 1.
Proposed effects of Cr(VI) treatment on the TrxR/Trx system and some implications for ASK1 and MAP kinase activation. (A) In untreated cells, TrxR is a major regulator of intracellular thiol redox balance through its ability to maintain Trx in the reduced state. Reduced Trx keeps ASK1 in an inactive state, supports the peroxidase activity of the Prx’s, and maintains the thiol redox balance of many proteins (not shown). (B) Cr(VI) treatment of cells causes irreversible inhibition of TrxR. This results in an inability to maintain Trx and Trx-dependent proteins such as the Prx’s in the reduced state. The oxidation of Trx and/or Prx may be accelerated by Cr(VI)-induced oxidants. Oxidized Prx’s are unable to function as peroxidases, which could increase peroxide levels and alter peroxide signaling. Oxidized Trx dissociates from ASK1 and this can promote many downstream effects through activation of the MAP kinases p38 and JNK. Some of these downstream events have been documented for Cr(VI) treatment for which p38 has a role as described in the text. Other downstream events are well known to be associated with p38/JNK activation in other systems (e.g., induced inflammatory mediators) and could well have roles in Cr(VI)-associated pulmonary diseases. There are other possible mechanisms by which MAP kinases may be activated (not shown). Overall, MAP kinase activation can promote apoptosis and cell cycle arrest, but may also enhance cell survival and tumorigenesis. Although the mechanism(s) by which TrxR is inactivated remains to be determined, this inactivation could directly promote cell death by the SecTRAP effect if the Sec of TrxR is compromised.
Because the Cr(VI)-mediated inhibition of TrxR is a fairly recent finding, the mechanism by which TrxR is inhibited is not yet known. Mammalian TrxR’s are NADPH-dependent homodimers with three essential redox centers per subunit: a flavin (FAD), an N-terminal domain dithiol (C59/C64), and a C-terminal active site containing C497/U498 (U is Sec) [116–119]. The C59/C64 dithiol is reduced by the flavin and in turn donates electrons to the C497/U498 active site [118]. Disruption of any of these redox centers could inhibit TrxR activity. The Sec in the active site could be the most susceptible to oxidant attack. It is a strong nucleophile, is exposed on the enzyme surface [114,120], and has a low pKa (ca. 5.2) [113], so it should be ionized to selenolate (−Se−) at physiological pH. Consistent with this, other agents (e.g., 2,4-dinitrochlorobenzene, curcumin, and 4-hydroxynonenal) irreversibly inhibit TrxR by covalently modifying the C497/U498 site [121–124]. In contrast, the C59/C64 dithiol is not modified by 2,4-dinitrochlorobenzene [125]. Cr(VI) does not indiscriminately target redox-sensitive enzymes in cells. Cr(VI) treatments that markedly inhibit TrxR do not inhibit the redox-sensitive enzyme GAPDH [79]. Other antioxidant enzymes (e.g. SOD, catalase, GPx) are not inhibited by massive doses (8 mM) of Cr(VI) [106]. The fact that GPx is a selenoprotein indicates that their susceptibility to Cr(VI) varies widely.
Much of TrxR1, including the C59/C64 dithiol, is similar to glutathione reductase (GR), except that GR lacks the 16-residue C-terminal Sec-containing domain found in TrxR [116,120]. GR activity in macrophages is markedly inhibited by 24 h treatment with ≥10 µM CrO3 [108], and damage to the analog of the C59/C64 dithiol in GR is one possible mechanism. GR susceptibility is not universal, however, and may depend on the cell or tissue and the form of Cr(VI). GR activity showed only a 20% decrease in the kidneys of mice injected with dichromate [126], and there is no effect on GR activity in anterior pituitary cells treated with 10 µM dichromate for 2–8 h [103]. To better understand the mechanism(s) by which TrxR is inhibited by Cr(VI), a mechanistic comparison with GR is warranted, including examination of the C59/C64 dithiol.
Several chemical species might lead to TrxR inactivation in Cr(VI)-treated cells. Although Cr(VI), Cr(III), and H2O2 do not directly inhibit purified TrxR [79,119], cell-generated oxidants could potentially inactivate TrxR [e.g., Cr(V), Cr(IV), HO•, , or peroxynitrite]. Although peroxynitrite can be generated in Cr(VI)-treated cells [66], nitration of TrxR does not increase [79]. Consistent with this, MnTBAP, an efficient scavenger of peroxynitrite [92,93], does not protect TrxR in Cr(VI)-treated cells [79]. Because commercial preparations of MnTBAP also exhibit SOD activity, it is unlikely that either peroxynitrite or directly accounts for the bulk of TrxR inhibition. The potential contribution of Cr(V), Cr(IV), and HO• to TrxR inhibition remains to be determined.
The inactivation of TrxR could have multiple adverse consequences for cells. TrxR1 knockouts are lethal at the embryonic stage [127], and TrxR inhibition can increase the susceptibility to oxidants and promote apoptosis [117]. Indeed, Cr(VI) treatments that markedly inhibit TrxR are associated with marked declines in clonogenic survival [78]. Whereas TrxR inhibition might promote cell death by facilitating Trx oxidation (see below), the selective inactivation of the Sec of TrxR can directly promote apoptosis [128,129]. The mechanisms underlying such SecTRAP (seleniumcompromised thioredoxin reductase-derived apoptotic protein) effects are not yet well understood, but they apparently do not require Trx oxidation. It remains to be determined if the Sec of TrxR is compromised by Cr(VI) treatment. In the absence of oxidant stress, the knockdown of TrxR (e.g., by siRNA) is not sufficient to promote apoptosis, whereas such knockdown concomitant with oxidant stress does promote cell death [130].
The prolonged inactivation of TrxR by Cr(VI) could be a critical event with long-term implications for the disruption of redoxsensitive signaling. It implies diminished capacity to maintain the Trx’s and Trx-dependent proteins in their reduced (active) state (Fig. 1). TrxR inactivation could compromise the function of proteins with important antioxidant roles such as the peroxiredoxins and mitochondrial glutaredoxin-2 [116]. TrxR also supports the reduction of a number of low-molecular-weight antioxidants, including vitamins C and E, flavonoids, lipoic acid, and others [116]. It is noteworthy that TrxR reduces selenite to selenide [116]. Because selenide is needed for selenoprotein synthesis, the irreversible inhibition of TrxR could compromise the ability of the cells to replenish TrxR activity. TrxR also has important roles in a number of signaling pathways that are important for the redox control of normal function and response to cell stressors [116]. Examples of signaling events that have relevance to Cr(VI) are discussed further below. A detailed review that further describes the diverse functions of TrxR can be found in [116].
Although it is clear that Cr(VI) causes marked inhibition of TrxR that is not readily reversed, additional studies are needed to determine the mechanism(s) underlying this inhibition and the full extent of its implications for cells. The prolonged inhibition of TrxR could also facilitate its use as a biomarker. Whereas the redox centers are conserved in cytosolic TrxR1 and mitochondrial TrxR2, it remains to be determined if there are differential effects on these isoforms.
Oxidation of thioredoxin
Mammalian cells have both cytosolic (Trx1) and mitochondrial (Trx2) thioredoxins [131]. Trx1 is also localized in the nucleus [96,97]. Trx1 and Trx2 are encoded by distinct genes, but they have a conserved active site (Trp-Cys-Gly-Pro-Cys-Lys) that is cycled between the reduced (dithiol) and the oxidized (disulfide) forms [132]. Trx2 has only two Cys residues, both in the active site. Trx1 has five Cys residues, two in the active site (C32/C35) and three others (C62/C69 dithiol and C73) [132]. The low pKa of the active-site thiols (above) should render Trx particularly sensitive to oxidant attack.
Cells normally maintain Trx1 and Trx2 largely in the reduced state [80,117,131–134], which is the active form. Because Trx1 and Trx2 are not in redox equilibrium with each other, they can be used to differentially assess the impacts of oxidants on the thiol redox status of the cytosolic and mitochondrial compartments [96,135].
Both soluble Na2CrO4 and particulate ZnCrO4 (poorly soluble) cause a dose- and time-dependent oxidation of Trx1 and Trx2 [80], with Na2CrO4 having more rapid effects than ZnCrO4. This is consistent with the expected slower penetration of particulate forms and with the smaller Cr(V) signals seen at early times with ZnCrO4 [26]. Trx2 is more sensitive to Cr(VI) treatment than Trx1, suggesting greater redox stress in the mitochondria. Trx2 can be completely oxidized, whereas some Trx1 remains reduced even with high Cr(VI) doses [78,80]. The reasons for the enhanced sensitivity of Trx2 are not known, but could indicate greater oxidant generation within the mitochondria or a lesser ability of the mitochondrial system to maintain Trx2 in the reduced state. Mitochondria can rapidly accumulate even 1 µM CrO42−, achieving an intramitochondrial concentration of Cr as high as 1500-fold [136]. This uptake is mediated via the dicarboxylate and phosphate transporters [136], and the intramitochondrial reduction of Cr(VI) to Cr(V/IV/III) [137] may drive continued uptake of CrO42−.
Only the partially oxidized form of Trx1 is observed in Cr(VI)-exposed cells, regardless of Cr(VI) concentration or time of exposure [80] (i.e., only one of the two dithiols of Trx1 is sensitive to Cr(VI) treatment). Given the enhanced sensitivity of the C32/C35 of Trx1 to other oxidants [132], it seems likely that this site is oxidized in Cr(VI)-treated cells, although definitive confirmation is required. Oxidation of C32/C35 renders Trx1 inactive. However, the C32/C35 disulfide is a substrate for TrxR [132] so TrxR could restore Trx1 redox status after Cr(VI) removal. There is, in fact, some redox recovery of Trx1 after Cr(VI) removal [80]. Although TrxR activity is largely inhibited by Cr(VI), the remaining amount of active TrxR could facilitate this recovery. The disulfide reductants Tris(2-carboxyethyl)phosphine hydrochloride [138] and dithiothreitol can reverse Trx1 and Trx2 oxidation in vitro [78]. Thus, Trx oxidation is consistent with the disulfide forms, or other forms that are reversible, and not with Cr–Trx adducts or other less reversible modifications to the dithiol.
There are several mechanisms by which Cr(VI) might mediate Trx oxidation in cells, including: (a) inhibition of TrxR (discussed above); (b) direct oxidation by Cr(VI), Cr(V), or Cr(IV); (c) oxidation by Cr-generated ROS or reactive nitrogen species (RNS); (d) increases in Txnip (Trx-interacting protein); or (e) nonspecific depletion/oxidation of total cellular thiols. The last possibility is inconsistent with the lack of effect on GSH levels or GSH redox state by Cr(VI) treatments that cause marked Trx oxidation [80]. Furthermore, thiol redox proteomics that examine reversible thiol oxidation show that six proteins are the most sensitive to Cr(VI) exposure; among these are Trx2 and Trx1 and the Trx2-dependent protein peroxiredoxin-3 (see more below) [79]. Therefore, Cr(VI) treatment does not cause indiscriminant thiol oxidation. Regarding possibility (d), Txnip levels do not change after Cr(VI) treatment [79], making this an unlikely explanation. Regarding possibility (c), several oxidants are generated in Cr(VI)-treated cells [66,67,80,87,88]. Even though MnTBAP can protect cells from a number of cytosolic and mitochondrial insults that promote peroxynitrite or generation [139–143], it does not protect Trx1 or Trx2 in Cr(VI)-treated cells [79]. Therefore, peroxynitrite or probably cannot account for the Trx oxidation. Spin trapping studies show that HO• is generated in Cr(VI)-treated cells [46,80,87,94,144–146]. Whereas the half-life of spin adducts is relatively short in biological systems [147–150], the fact that HO• spin adduct signals persist over time in Cr(VI)-treated cells [80] suggests a continual generation of HO•. Because many cell components could compete for trapping HO•, and because of the limited efficiency of the traps [41], the spin trap signals probably markedly underestimate the extent of HO• generation. Regarding possibility (b), Cr(VI) can oxidize purified Trx1 in vitro [80], but the extent to which this contributes to Trx oxidation in cells is not yet known. The oxidation of Trx by Cr(V) and Cr(IV) has not yet been explored.
The irreversible inhibition of TrxR [possibility (a)] is probably a major contributor to Trx oxidation in Cr(VI)-treated cells. This would compromise the ability to keep Trx reduced, especially in the presence of enhanced oxidant generation (Fig. 1). There is a significant correlation between TrxR inhibition and Trx1 oxidation in Cr(VI)-treated cells, as well as a correlation between Trx2 oxidation and TrxR inhibition [78]. Although these correlations are with total TrxR activity and not with activity in specific compartments, they imply that an inability of TrxR to maintain the thioredoxins in the reduced state contributes to Trx oxidation. Overall, then, Trx1 and Trx2 oxidation may be enhanced by ROS and/or various Cr oxidants that are generated after Cr(VI) exposure. This oxidation may be sustained or further enhanced by the inhibition of TrxR.
The sum total of data demonstrates the inherent sensitivity of the TrxR/Trx system relative to other cellular thiols. The oxidation of Trx2 and Trx1 could have a number of important consequences for cells. Because both Trx1 and Trx2 are critical to cell survival and to the function of many proteins involved in cell growth and signaling [117,131,151], the oxidation of Trx1 and/or Trx2 could have a negative impact on cell survival and could further promote oxidant stress and oxidant sensitivity [96,135,152–154]. Cr(VI) treatments that oxidize cellular thioredoxins are coincident with those that decrease cell survival [78]. Treatments that cause extensive Trx oxidation would be expected to compromise the function of Trx-dependent proteins, including peroxiredoxins, ribonucleotide reductase, protein disulfide isomerase, methionine sulfoxide reductase, and others [98,116,117,131]. Trx oxidation would also be likely to affect the function of transcription factors whose activity is mediated by Trx, as discussed below.
Oxidation of peroxiredoxin
Peroxiredoxins (Prx’s) are ubiquitous peroxidases that degrade H2O2 and organic hydroperoxides. During catalysis, their activesite Cys residues are oxidized to sulfenic acid (−SOH). In the 2-Cys Prx’s (e.g., cytosolic Prx1 and Prx2 and mitochondrial Prx3), this sulfenic acid reacts with the resolving Cys on the other subunit to form a disulfide-linked dimer [155–157]. The Prx’s are directly dependent on their respective Trx’s to reduce these disulfides to thiols, thereby regenerating active Prx [99,158]. In addition to these Prx disulfides, Prx thiols might also be compromised by adducts on the thiols, or by their overoxidation to sulfinic (Prx–SO2) or sulfonic acid (Prx–SO3) forms. Both of these possibilities would prevent disulfide linkages between the two subunits.
Prx1 (cytosolic) and Prx3 (mitochondrial) are overwhelmingly oxidized to the disulfide forms by Cr(VI) treatments that oxidize the vast majority of the respective Trx’s [78]. This is true for both short-term treatments and overnight treatments with low micromolar Cr(VI). Prx1 and Prx3 abruptly shift to the oxidized state once all, or nearly all, of their respective Trx (Trx1 or Trx2) is oxidized [78]. This implies that Prx1 and Prx3 oxidation is largely influenced by a lack of reducing equivalents from the respective thioredoxin. Prx3 is more susceptible to oxidation than Prx1, reflecting the enhanced susceptibility of Trx2 vs Trx1. Because they are Trx-dependent, the Prx’s are indirectly dependent on TrxR, and Prx oxidation occurs with Cr(VI) treatments that cause extensive inhibition of TrxR [78] (Fig. 1). These Prx results suggest that other TrxR/Trx-dependent proteins could be compromised in Cr(VI)-treated cells. Because GSH levels are maintained in these Cr(VI)-treated cells (above), it is possible that GSH might compensate for some of the functions associated with TrxR/Trx. For example, a recent report shows that GSH and TrxR1 represent complementary systems to support ribonucleotide reductase, and thus replication, in mouse hepatocytes [159]. GSH cannot, however, compensate for all functions of TrxR/Trx. In Cr(VI)-treated cells, GSH cannot keep the Prx’s in the reduced state (above) or prevent apoptosis signal regulating kinase (ASK1) activation (below). In studies with several other metals, GSH cannot protect cells from Trx oxidation, i.e., metals that oxidize Trx but not GSH promote cell death, whereas those that oxidize GSH but not Trx do not induce death [153].
The oxidized Prx dimers in Cr(VI)-treated cells are reversible in vitro by incubating the cell lysates with disulfide reductants [78]. This implies that Prx oxidation represents the formation of disulfide links between the two subunits. It is unlikely that Cr(VI) treatment results in significant levels of Cr–thiol adducts on the Prx’s, or the overoxidation of Prx thiols to the sulfinic or sulfonic forms, as these would prevent dimer formation.
The peroxiredoxins are important for cell survival [160–162], so Cr(VI)-induced Prx oxidation may promote apoptosis or render cells more sensitive to apoptotic insults. For most Cr(VI) treatments, Prx3 oxidation is correlated with decreased survival [78].
Prx’s have important roles as peroxidases and are therefore important mediators of peroxide-dependent signaling [99,157, 162,163]. Mitochondria lack catalase, and they contain 30 times more Prx3 than glutathione peroxidase [160]. Prx3 may therefore be a critical regulator of mitochondrial H2O2 signaling [160]. Cr(VI) treatments that markedly oxidize Prx3 could therefore have important effects on this signaling. The resulting increases in mitochondrial peroxide levels could also further enhance oxidant damage (e.g., through Cr(V)- or Cr(IV)-mediated Fenton-like reactions). Cr(VI) has also recently been shown to inhibit mitochondrial aconitase [48]. The resulting release of iron and H2O2 from its 4Fe–4S center [164,165] can directly promote HO• generation [164], and the released iron could further stimulate the reductive activation of Cr(VI) [166].
Cultured cells can have ascorbate levels well below those observed in vivo [167]. Because ascorbate is an antioxidant and an intracellular Cr(VI) reductant, one might argue that the Cr(VI)-mediated effects on TrxR, Trx, and Prx are influenced by low ascorbate levels. However, increasing the intracellular levels of ascorbate by preloading cells with dehydroascorbate does not protect TrxR, Trx, or Prx from the effects of Cr(VI) [79]. The effects on these systems is not therefore due to diminished ascorbate levels in cultured cells.
Relationship of the effects on the TrxR/Trx/Prx system to other events
The mitochondrial oxidative stress that is reflected in the oxidation of Trx2 and Prx3 may have consequences for other core mitochondrial functions as well. For example, Cr(VI) treatments that cause Trx2 oxidation in BEAS-2B cells also cause pronounced and irreversible inhibition of aconitase [48]. Mitochondrial electron transport complexes I and II are inhibited, resulting in the appearance of EPR signals that are consistent with the disruption of electron flow through these complexes [48]. Similarly, electron transport and aconitase are inhibited in the kidneys of Cr(VI)-treated rats [91], but the effects on TrxR or Trx/Prx were not examined. The activities of complex I, complex II, and aconitase are known to be susceptible to a number of oxidants including reactive oxygen and nitrogen species [168–172], so it is possible that the disruption of mitochondrial Trx2/Prx3 by Cr(VI) facilitates damage to other mitochondrial proteins. The effects on aconitase and electron transport occur early (within 1–3 h) after Cr(VI) exposure and well before cell death. They are dose- and time-dependent and persist after Cr(VI) removal, highlighting their potential use as markers for intermittent Cr(VI) exposure [48]. The disruption of these core mitochondrial functions not only serves as an additional indicator of mitochondrial redox stress, but could contribute to Cr(VI) toxicity. As previously noted, MnTBAP does not protect Trx2, Prx3, or the electron transport complexes from Cr(VI) [79]. Interestingly, human lung cancer H460 cells that lack mitochondrial DNA (ρ0 cells) produce less ROS in response to Cr(VI), indicating the importance of mitochondria in Cr(VI)-induced ROS generation [86]. Cr(VI) induces mitochondrial ROS in prostate cancer cells [68].
The extensive TrxR inhibition and Trx/Prx oxidation precede other signs of cell stress in Cr(VI)-treated cells, including loss of mitochondrial membrane potential (C.R. Myers, unpublished). However, continued incubation in Cr(VI)-free medium after the initial Cr(VI) insult will result in cell death and detachment a day or two later [78]. Although the potential for Cr(VI)-induced Trx2/Prx3 oxidation to promote specific apoptotic events remains to be determined, Trx2 has roles in mitochondrial outer membrane permeabilization and cytochrome c release [173].
Extended exposure to Cr(VI) can lead to several types of DNA damage over time [56,57,72,102], and mitochondrial DNA can be particularly sensitive to damage from oxidant stress [141,174,175]. However, 3-h Cr(VI) treatments that elicit strong effects on TrxR, Trx2, aconitase, and electron transport do not cause detectable damage to mitochondrial or nuclear DNA [79]. This implies that the effects on the TrxR/Trx/Prx system, aconitase, and mitochondrial electron transport occur before extensive DNA damage.
Potential downstream effects of the Cr(VI)-mediated disruption of the TrxR/Trx system
The inhibition of TrxR and the oxidation of Trx by r(VI) treatment could have additional downstream effects [176], including: (a) promotion of apoptosis through activation of ASK1 and (b) effects on multiple redox-sensitive transcription factors including AP-1, NF-κB, p53, and Nrf2 [98,117,123,131].
ASK1 and MAP kinases
In unstressed cells, reduced Trx1 and Trx2 negatively regulate ASK1 by binding to an N-terminal domain, whereas Trx1 and Trx2 oxidation results in their dissociation from ASK1, facilitating ASK1 activation and promoting apoptosis [117,177–181]. Cr(VI) treatment causes the dissociation of Trx1 from ASK1 in cells [78] (Fig. 1), and this dissociation is consistent with ASK1 activation.
Activated ASK1 can phosphorylate MKK3/MKK6, which in turn activate p38 MAP kinase. Similarly, ASK1 phosphorylates MKK4/MKK7, which then activate JNK [180]. The activation of either cytosolic or mitochondrial ASK1 has been linked to MAP kinase activation [182]. The activation of p38 and JNK can contribute to the proapoptotic effects of ASK1 and to other ASK1-mediated stress responses (Fig. 1) [180,183–187]. Cr(VI) activates p38 and JNK in mouse macrophages [188], human keratinocytes [67], and various lung cells [189–193]. JNK and p38 activation are not necessarily coincident (e.g., subtoxic concentrations of Cr(VI) activate JNK, but not p38, in human lung cancer A549 cells [192]). However, A549 cells are not necessarily a good model for normal human bronchial epithelium, as they are highly oxidant-resistant [194], and p38 is typically markedly downregulated in cancer [195].
Although the initiating events have not been fully explored, the ability of Cr(VI) to cause the dissociation of ASK1 from Trx could account for the p38/JNK activation. There are, however, other possible contributors that need to be explored [e.g., the disruption of active Cys residues in protein tyrosine phosphatases that dephosphorylate (inactivate) MAP kinases can facilitate or prolong MAP kinase activation [196]]. TrxR has been shown to support protein tyrosine phosphatase activity [116], so the Cr(VI)-mediated inhibition of TrxR could facilitate p38/JNK activation through the inhibition of these phosphatases.
Although the outcome is dependent on the balance and magnitude of MAP kinase activation and on the stimulus and cell type [183,197,198], p38 and JNK activation are typically proapoptotic [199–203]. Whereas there are multiple p38 isoforms, p38α is ubiquitously expressed and has been studied the most. Mannitol, which chelates Cr(V) and scavenges HO•, reduces the Cr(VI)-induced activation of p38 and enhances the survival of CL3 cancer cells to Cr(VI) [193]. However, a chemical inhibitor of p38α did not affect Cr(VI) cytotoxicity, suggesting that p38α activation was not required for cell death [193]. Because active p38α suppresses tumor growth [195,203,204], p38 activity is markedly downregulated in many cancers [195]. Therefore, the lack of effect of p38α inhibition on Cr(VI) survival in CL3 cancer cells is perhaps not surprising. Although there are a number of ways in which p38 may promote apoptosis, one is through its ability to activate (phosphorylate) p53 (Fig. 1) [195] (see more below). Alternatively, p38 may promote Cr(VI) carcinogenesis through its ability to upregulate the transcription factor subunit HIF-1α, which then upregulates vascular endothelial growth factor (VEGF) (Fig. 1) [68]. These events are p38- and peroxide-dependent [68]. Although the mechanism by which p38 upregulates HIF-1α levels is not clear, one possibility is that HIF-1α is stabilized by p38-mediated phosphorylation [205].
The activation of p38α can promote cell cycle arrest at G1/S and G2/M through its ability to phosphorylate cyclin D1 and Cdc25 (Fig. 1), respectively, which promotes their degradation [195]. In HeLa cells, Cr(VI) treatment for 4 h induces cell cycle arrest at both the G2/M and the G1/S interfaces, and a chemical inhibitor of p38α decreases this cell cycle arrest [206]. In A549 cells, Cr(VI) induces cell cycle arrest, which is prevented by catalase but not by SOD or a HO• scavenger [94]. Together, these data indicate that peroxide-mediated events are important for inducing Cr(VI)-mediated cell cycle arrest for which p38 has a role.
In addition to the events just described, p38α activation may contribute to other respiratory diseases that are associated with Cr(VI), including pulmonary fibrosis, chronic bronchitis, and occupational asthma [1–7,207]. Inflammation and airway cell death are important contributors to these diseases. The activation of p38α, in particular, induces several inflammatory mediators that have important roles in asthma, pulmonary fibrosis, and other inflammatory respiratory diseases (Fig. 1) [195,208,209]. The activation of p38 can also promote fibroblast proliferation that is associated with pulmonary fibrosis [210].
AP-1
The transcription factor AP-1 is localized in the nucleus [211]. When activated, AP-1 (c-Jun dimers or c-Jun–c-Fos heterodimers) binds to tetradecanoyl phorbol acetate response elements in DNA, and the resulting transcriptional events tend to promote cell proliferation and suppress apoptosis (Fig. 2A), although in some cases AP-1 activation can promote apoptosis [211–213]. AP-1 activation is often mediated by p38 and/or JNK [213,214]. For example, the JNK-mediated phosphorylation of c-Jun increases AP-1 activity and promotes its binding to DNA [96,211]. Cr(VI) activates AP-1 in mouse cells in a dose- and time-dependent manner [145,188]. This activation is blunted by catalase, HO• scavengers, and deferoxamine [145], which suggests that Cr(V)-mediated generation of HO• may be involved in the activation. The initiating events may well be upstream to facilitate the activation of p38, for example. Chemical inhibition of p38 blocks Cr(VI)-induced AP-1 activation, whereas ERK inhibition does not [188]. A scavenger of HO• also blocks AP-1 activation [188], suggesting that oxidant-mediated activation of p38 may have a prominent role in Cr(VI)-induced AP-1 activation.
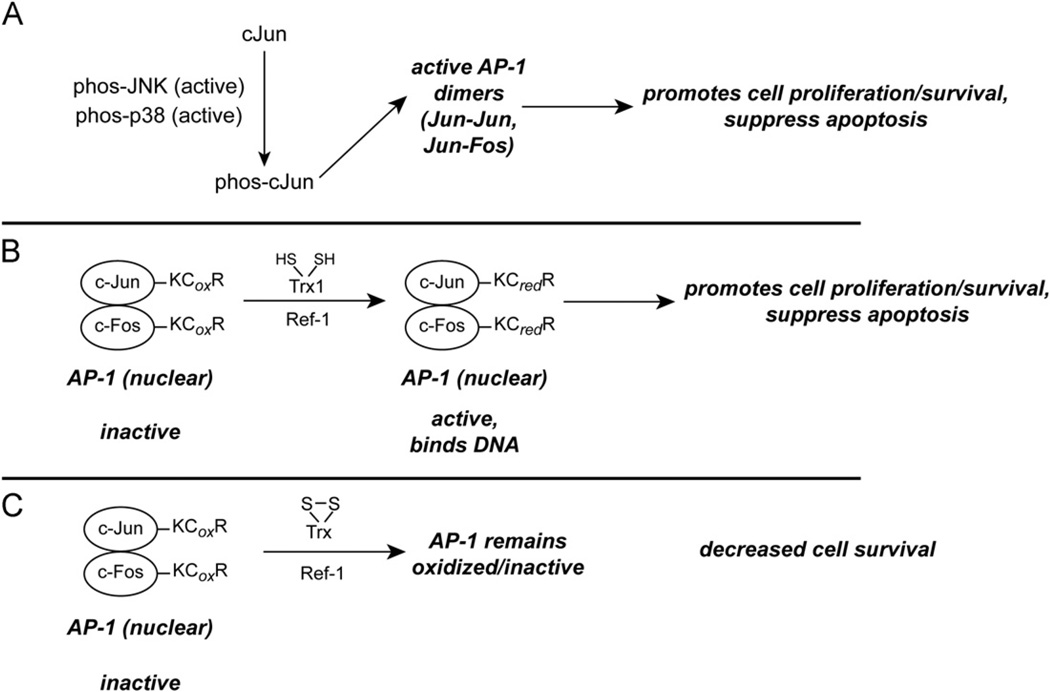
Fig. 2.
Potential implications of disruption of the TrxR/Trx system on the transcription factor AP-1. (A) The Cr(VI)-mediated activation of p38 and/or JNK as depicted in Fig. 1 can phosphorylate c-Jun, which will result in active AP-1. p38 has a prominent role in AP-1 activation after Cr(VI) exposure [188]. (B) As long as nuclear Trx1 is not oxidized, reduced AP-1 can bind DNA. This will enhance the expression of genes that promote cell proliferation and survival. (C) If nuclear Trx1 becomes oxidized, however, AP-1 could become oxidized and lose its DNA-binding ability.
The Trx redox state can affect AP-1 activity. Both c-Jun and c-Fos have a conserved Cys motif (KCR) in their DNA-binding domains, and the oxidation or elimination of this Cys prevents DNA binding [211,215]. Trx1, together with redox factor-1 (Ref-1), can reduce the critical Cys residues in AP-1 [211,216], which would promote the binding of AP-1 to DNA (Fig. 2B). Trx1 is present in both the cytosol and the nucleus, but the redox state of Trx1 can be different in these compartments [96,97,135]. Because AP-1 is primarily in the nucleus, nuclear Trx1 can control the redox state of AP-1. The oxidation of nuclear Trx1 can inhibit the binding of AP-1 to DNA [96], and this would be predicted to decrease proliferation and enhance cell death. Although Cr(VI) activates AP-1 in a dose-dependent manner, this activation falls off with high doses [188]. One possibility is that lower doses activate AP-1 as described above, whereas high doses may oxidize nuclear Trx1 and therefore inhibit DNA binding (Fig. 2C). A simultaneous examination of the Trx2 redox state, ASK1 activation, p38 activation, nuclear Trx1 redox state, and AP-1 activity is needed to better define these relationships in response to Cr(VI).
NF-κB
NF-κB can be activated by oxidative cellular insults. Cr(VI) treatment can either enhance [67,69,144,188,217–220] or hinder [145,221,222] the DNA binding or activity of NF-κB. Cr(VI)-induced NF-κB activation can be inhibited by catalase, by scavengers of HO•, by metal chelation, or by Mn2+, which depletes Cr(IV) [69,144,145,188,218]. Thus, the generation of HO• from reactive Cr intermediates and peroxides seems to be a significant facilitator of NF-κB activation. For other insults, peroxides are important for NF-κB activation [223].
NF-κB activation first requires its translocation to the nucleus. The cytosolic NF-κB dimer (p50/p65) is bound to IκB, keeping it inactive [211]. Oxidant stress and other insults can promote IκB phosphorylation, followed by the release of NF-κB and its nuclear translocation (Fig. 3A) [211]. Consistent with this, Cr(VI) treatment promotes the phosphorylation of IκBα [67], and a dominant-negative IκB kinase inhibits NF-κB activation [188].
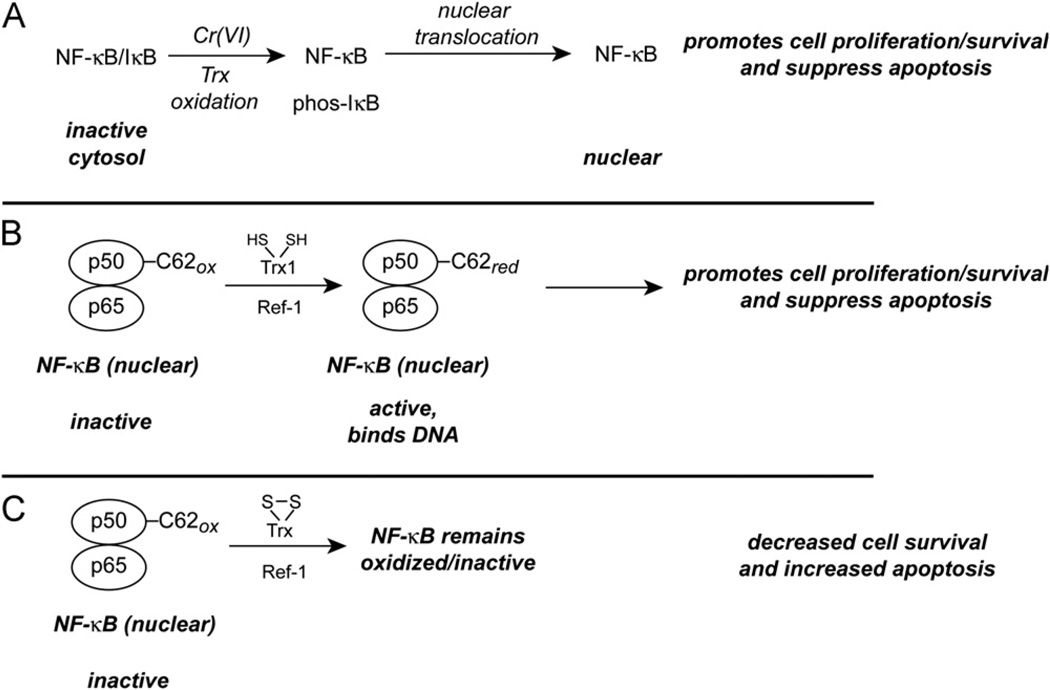
Fig. 3.
Potential implications of disruption of the TrxR/Trx system on the transcription factor NF-κB. (A) Both Cr(VI) treatment and Trx oxidation are known to cause NF-κB translocation to the nucleus and the expression of NF-κB-dependent genes. NF-κB activation most often promotes proliferation and cell survival. (B) As long as nuclear Trx1 is not oxidized, NF-κB should be in the reduced state, which is needed for DNA binding. (C) If nuclear Trx1 becomes oxidized, however, NF-κB could become oxidized and lose its DNA-binding ability. This might explain why lower doses of Cr(VI) promote NF-κB activation, whereas NF-κB activity is lost with higher Cr(VI) doses.
It is interesting that mitochondrial-targeted redox stress can result in the nuclear translocation of NF-κB [224]. For example, in HeLa cells, TNFα rapidly causes the oxidation of Trx2, but not Trx1, and NF-κB translocation occurs with these treatments [224]. Overexpression of Trx2 blocks this translocation, whereas the dominant-negative C93S mutant of Trx2 does not [224]. It is therefore possible that the Cr(VI)-mediated mitochondrial redox stress, as reflected in the enhanced oxidation of Trx2/Prx3 vs Trx1/Prx1, promotes the nuclear translocation of NF-κB (Fig. 3A). Given that there is some oxidation of Trx1 by Cr(VI), it is possible that cytosolic redox stress could similarly promote NF-κB translocation.
Once in the nucleus, the DNA binding and activity of NF-κB is stimulated by reduced Trx1 in conjunction with Ref-1 (Fig. 3B) [96,211,225–227]. This interaction involves the reduction of the C62 residue on the p50 subunit of NF-κB [96,226,228]. Although Trx1 is present in both the cytosol and the nucleus, the nuclear compartment has been found to be more reducing than the cytoplasm [96,97,135]. Although it remains to be determined if Cr(VI) has differential effects on nuclear vs cytosolic Trx1, it is interesting that lower doses of Cr(VI) enhance NF-κB activity, whereas higher doses do not [144,188,219]. Such findings would be consistent with lower Cr(VI) doses causing mitochondrial and/or cytosolic redox stress to induce NF-κB translocation, whereas higher doses could oxidize nuclear Trx1, blocking the ability of NF-κB to bind to DNA (Fig. 3C). A simultaneous examination of the redox states of Trx2 and Trx1 (nuclear vs cytosolic) and the translocation and DNA binding of NF-κB is needed to better define these relationships. The inhibition of TrxR has also been shown to inhibit the binding of NF-κB to DNA [229]. Because Cr(VI) inhibits TrxR and this inhibition has a marked effect on Trx oxidation [78], the activity of nuclear TrxR could be an important regulator of nuclear NF-κB activity.
NF-κB activity is probably an important contributor to Cr(VI) carcinogenesis. NF-κB activation often promotes survival and suppresses apoptosis, whereas blocking NF-κB activity is predicted to decrease cell proliferation and enhance cell death [211,223]. The activation of NF-κB is important for decreasing Cr(VI)-induced death in human bronchial epithelial cells [219,220]. NF-κB activation enhances expression of the antiapoptotic proteins cIAP1 and cIAP2 [220] and suppresses p53 activity [219]. There is also a potential link between p38 activation (above) and the induction of some genes under the control of NF-κB. For example, p38 activation, through its downstream kinases MSK1 and MSK2, regulates histone 3 phosphorylation at the NF-κB binding sites of the promoters of some inflammatory genes [208]. Although this still requires active NF-κB, it could provide an additional level of redox control of gene expression.
p53
Under nonstressed conditions, the redox-sensitive transcription factor p53 is repressed through its binding to mdm2, which targets p53 for degradation (Fig. 4A) [230,231]. Several cell stressors, including many that cause oxidant stress, can activate p53 [230], which promotes the expression of many proteins that promote cell death [232]. There are multiple Cys residues on p53 that influence DNA binding [230], so nuclear thiol redox status can control the ability of p53 to bind to DNA (Fig. 4B and 4C). Trx (in combination with Ref-1) and TrxR have been implicated in keeping p53 in the reduced active state, which promotes DNA binding (Fig. 4B) [230]. A dominant-negative Trx1 blunts the DNA binding and function of p53 [230,233]. Although oxidant stress can induce the translocation of additional Trx1 to the nucleus [233], oxidation of nuclear Trx1 would favor the oxidized state of p53, which does not bind DNA well (Fig. 4C) [230]. Tyrosine nitration and S-glutathionylation of p53 can also hinder DNA binding [230].
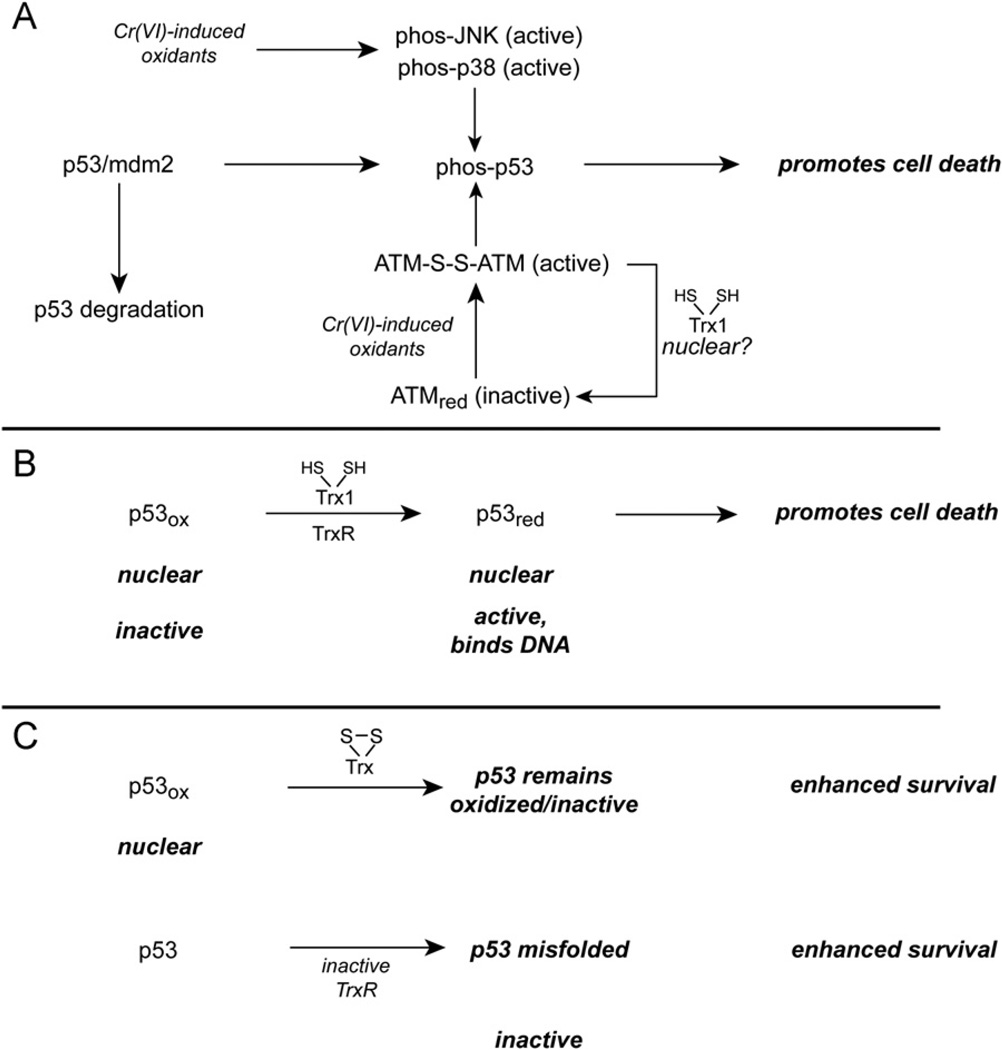
Fig. 4.
Potential implications of disruption of the TrxR/Trx system on the transcription factor p53. (A) Cr(VI) treatment causes p53 to dissociate from mdm2, which leads to elevated p53 levels and p53 activation. The phosphorylation of p53 by p38/JNK or by oxidant-activated ATM are two mechanisms by which p53 could be phosphorylated. The oxidants responsible for ATM activation in Cr(VI)-treated cells remain to be determined. Although the mechanisms that may potentially reverse ATM oxidation are not clear, it is possible that Trx1 may have some ability to reduce ATM disulfides. (B) As long as nuclear Trx1 is not oxidized, p53 should be in the reduced state, which is needed for DNA binding. (C) If nuclear Trx1 becomes oxidized, however, p53 could become oxidized and lose its DNA-binding ability. The inactivation of TrxR has the potential to cause p53 misfolding.
A number of studies have noted that Cr(VI) treatment results in the upregulation and/or activation of p53 [85,87,234–236]. Results are consistent with HO•, H2O2, and/or reactive Cr species as important drivers of this activation [85,87,88,235]. In A549 cells, Cr(VI) causes the dissociation of p53 from mdm2, which leads to elevated p53 levels and p53 activation [237]. Cr(VI) can induce apoptosis in both p53-positive and -negative lung cancer cells, although p53-dependent mechanisms can be more predominant with prolonged incubations of A549 cells [87]. The p53-regulated proteins PUMA and NOXA are induced in a time-dependent manner by Cr(VI) in p53-positive cells [234], and PUMA can have a strong role in Cr(VI)-induced apoptosis [234]. Cr(VI) also induces the migration of Bax to the mitochondria, which has been associated with p53 activation [234]. In mouse skin cells, siRNA suppression of p53 decreases p53 activation and increases survival to Cr(VI) [88]. In human fibroblasts, Cr(VI) increases p53 levels, induces p53 migration to the nucleus, and induces apoptosis that is largely p53-dependent [236].
There are p53-independent mechanisms of Cr(VI)-induced apoptosis as well. Cr(VI) induces pronounced apoptosis in normal human bronchial BEAS-2B cells [238] even though the p53 in these cells is kept inactive by the SV40 large T antigen. p53 does not seem to be involved in Cr(VI) toxicity in anterior pituitary cells [103]. Studies with human lung fibroblasts indicate that there are both p53-dependent and p53-independent pathways promoting cell death and cell cycle arrest [239].
The stability and transcriptional activity of p53 is dependent on its phosphorylation. Both p38 and JNK can phosphorylate and stabilize p53, enhancing p53-dependent apoptosis or growth arrest (Fig. 4A) [195,232,240]. For example, chemical inhibition or knockdown of p38α can attenuate p53 phosphorylation and p53-mediated apoptosis, including that caused by insults that oxidize Trx [195,241]. Thus, it is plausible that the activation of p38α and/or JNK by mechanisms discussed above could contribute to p53 activation in Cr(VI)-treated cells.
There are non-MAP kinase mechanisms that may also activate p53, one of which is the ATM kinase (Fig. 4A). ATM is typically activated by an autophosphorylation mechanism in response to double-strand DNA breaks [242]; however, Cr(VI) activates ATM even in the absence of double-strand breaks [243]. ATM activation promotes p53 activation, and ATM-null cells are more resistant to Cr(VI)-induced apoptosis [243]. ATM-null cells do not have better clonogenic survival of Cr(VI), however, which may reflect their inability to recover from Cr(VI)-induced growth arrest [243]. Whereas DNA breaks could not account for Cr(VI)-induced ATM activation, ATM oxidation is an alternative activator. Oxidants such as H2O2 can directly activate ATM by generating an ATM dimer that is linked by disulfides [242]. ATM phosphorylation is not required for this oxidative activation. ATM is primarily nuclear and its oxidative activation there could account for p53 activation [242]. Although we are unaware of studies that have examined nuclear Trx1 and ATM, it is possible that oxidation of nuclear Trx1 could promote ATM oxidation. Some cells also have considerable cytosolic levels of ATM [242]. Cr(VI) treatments that oxidize nuclear Trx1 would also be expected to interfere with the binding of p53 to DNA (Fig. 4C). As discussed above for NF-κB, the redox state of nuclear Trx1 could be a critical determinant of p53 binding that warrants further exploration in Cr(VI)-treated cells.
Whereas the mechanism by which Cr(VI) inhibits TrxR remains to be determined, one must consider that its irreversible inhibition could have additional consequences for p53 activity in the longer term. The inhibition of TrxR by electrophiles or auranofin disrupts p53 conformation and activation [230,244]. Electrophilic adducts on the Sec of TrxR1, or absence of the Sec, cause misfolding of newly synthesized p53 (Fig. 4C), whereas siRNA-mediated suppression of TrxR1 does not have this effect [123]. Overall, Cr(VI) can initially activate p53, but nuclear Trx1 oxidation or disruption of the Sec of TrxR could serve to blunt p53-promoted effects over time.
Nrf2
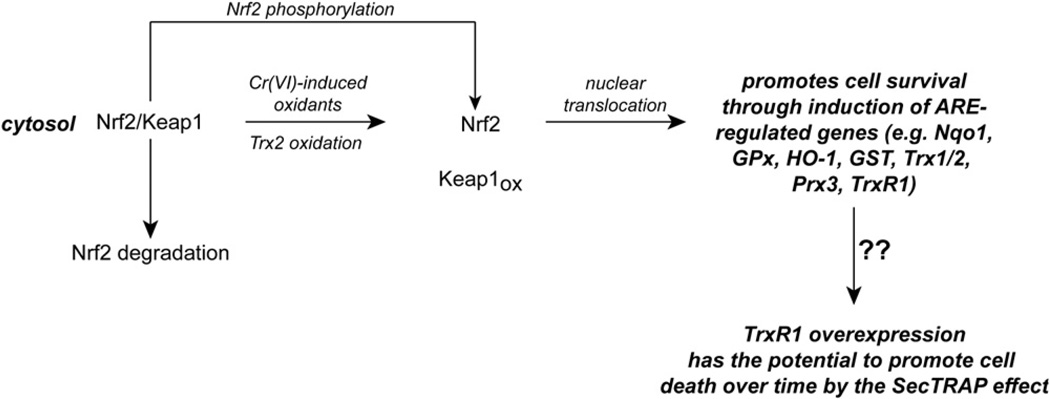
Nrf2 is a transcription factor whose activation upregulates a number of genes under the control of antioxidant-response elements (AREs), including glutathione S-transferases, NAD(P)H quinone oxidoreductase (Nqo1), γ-glutamylcysteine synthetase, heme oxygenase-1 (HO-1), UDP-glucuronyltransferases, GPx, and others [245,246]. Under nonstressed conditions, Keap1 binds Nrf2 in the cytosol, which facilitates Nrf2 degradation by proteasomal mechanisms (Fig. 5) [245,247]. Oxidative or electrophilic modification of Cys residues on Keap1 promotes the release of Nrf2, which allows for its translocation to the nucleus where it can activate ARE-controlled genes [245,248]. The Cys151 residue of Keap1 is especially important for activation by electrophiles, ROS, or RNS [245,248].
Fig. 5.
Potential implications of Trx oxidation system on the transcription factor Nrf2. Cr(VI) treatment can cause the stabilization and nuclear translocation of Nrf2. Although Keap1 oxidation is the classical mechanism by which Nrf2 is stabilized, it is unknown if Cr(VI) induces Keap1 oxidation. TrxR knockdown and Trx2 oxidation are associated with Nrf2 activation, so it is reasonable that the TrxR inhibition and Trx2 oxidation caused by Cr(VI) could facilitate Nrf2 activation. The phosphorylation of Nrf2 is an alternative mechanism that may promote its dissociation from Keap1, although this possibility has not been explored with Cr(VI) treatment. Nrf2 activation promotes survival through the induction of an array of ARE-regulated genes. Among these genes are TrxR1, whose overexpression can lead to Sec-minus protein, which could promote cell death through the SecTRAP effect. It is unknown if this occurs in Cr(VI)-treated cells.
In mouse fibroblasts, Cr(VI) induces translocation of Nrf2 to the nucleus and the expression of Nrf2-inducible genes (e.g., Nqo1 and HO-1 [249]). The induction of Nqo1 was totally Nrf2-dependent, whereas HO-1 induction was partially Nrf2-dependent [249]. Nrf2 activation can protect against Cr(VI)-induced apoptosis (i.e., Nrf2-minus mouse fibroblasts are more sensitive to Cr(VI)-induced apoptosis [249]). Although Cr(VI)-induced nuclear translocation of Nrf2 implies Keap1 oxidation, this was not specifically examined [249]. Whereas the Cr(VI)-induced oxidants that might mediate Keap1 oxidation need to be determined, it is possible that TrxR inhibition and/or Trx2 oxidation could contribute to Nrf2 activation either directly or indirectly. For example, the siRNA-mediated knockdown of TrxR can increase H2O2-mediated oxidation of Keap1 and stabilization of Nrf2 [248]. When TrxR1 is knocked out in mouse hepatocytes, Nrf2 translocates to the nucleus and a number of ARE-regulated genes are induced [250]. Trx2 oxidation has also been linked to Nrf2 activation. Two different oxidant insults that oxidize Trx2, but not Trx1 or GSH, in mouse fibroblasts or HeLa cells result in Nrf2 activation [251,252]. Importantly, this Nrf2 activation could be blocked by overexpression of Trx2, but not by overexpression of a dominant-negative C93S mutant of Trx2 [251,252]. Furthermore, siRNA knockdown of Trx2 can cause Nrf2 activation even in the absence of an oxidant insult [252]. It remains to be determined how Trx2 oxidation may signal to induce changes in the redox status of Keap1. With cytosolic oxidants, GSH seems to be more important than Trx1 as a regulator of Nrf2 activation [253].
Although Keap1 oxidation is probably an important step, additional mechanisms that can be linked to Trx oxidation might contribute to Nrf2 activation in Cr(VI)-treated cells. The activation of ASK1 and other kinases such as p38 could have a contributing role. A number of kinases can phosphorylate Nrf2, and phosphorylation at S40 has been implicated in decreasing the binding of Nrf2 to Keap1 (Fig. 5) [245,247]. However, the contribution of p38 or other kinases to Nrf2 phosphorylation in cells and the implications for Nrf2 stability and translocation need further study.
The induction of Nrf2-regulated genes (e.g., Nqo1, glutathione S-transferase, and GPx) in the lungs of rats treated with intratracheal chromate [254] implies that Cr(VI) can activate Nrf2 in vivo. Similarly, curcumin activates Nrf2 in rat kidneys, and this protects renal mitochondrial function from Cr(VI) [91]. The upregulation of several antioxidant enzymes by curcumin (e.g., SOD, catalase, GPx, GR) [91] may contribute to this protection. The upregulation of peroxidases may be especially important given the number of studies that have implicated peroxides in Cr(VI) toxicity (above). Whereas curcumin does not chelate Cr(VI) [91], it remains to be determined if it has the ability to quench reactive Cr intermediates.
The activation of Nrf2 by Cr(VI) or other agents would be predicted to oppose the proapoptotic effects of Trx oxidation and the resulting activation of ASK1 and MAP kinases (above). Such opposing effects have been demonstrated for paraquat toxicity in neuroblastoma cells [255]. In addition, Nrf2 activation can upregulate the expression of Trx1, Trx2, Prx3, and TrxR1, but not that of TrxR2 [116,255]; such upregulation could provide additional mechanisms to counter the proapoptotic and pro-oxidant effects of Cr(VI). However, for treatments that severely compromise TrxR2, the upregulation of Trx2 and Prx3 may be of limited use given their dependence on TrxR2. Nrf2 activation may be able to overcome modest degrees of TrxR inhibition but not pronounced TrxR inhibition [256]. TrxR is required to generate selenide, which is required for selenoprotein synthesis, including TrxR [116]. So even though Nrf2 may increase TrxR1 gene expression in Cr(VI)-treated cells, the inhibition of existing TrxR may prevent the cells from efficiently supporting the synthesis of full-length TrxR1. This could lead to an accumulation of Sec-minus enzyme, which may enhance cell death through the previously described SecTRAP effects (Fig. 5) [116].
Cr(VI)-mediated Nrf2 induction may depend on the cell type, species, and other specific conditions. For example, treatment of normal human fibroblasts with 5–100 µM Cr(VI) for 2 h causes a dose-dependent increase in the expression of HO-1, whereas other Nrf2-inducible genes (e.g., GSH reductase, GPx) are not upregulated [104]. Similarly, Cr(VI) induces HO-1 and glutathione S-transferase in human dermal fibroblasts [109]. Although this may reflect Nrf2 activation, the HO-1 induction was significantly diminished by MAP kinase inhibitors [109]. Cr(VI) may be less able to induce Nrf2 in lung cells relative to other cells, however. For example, Cr(VI)-treated A549 cells do not upregulate any of these Nrf2-inducible genes, including HO-1 [104]. This may, however, reflect the relative insensitivity of A549 cells to other oxidants [194], their markedly elevated levels of antioxidants such as GSH and TrxR [104,257], and/or their endogenously high levels of Nrf2 [258]. In another study, Cr(VI) upregulated the expression of CuZnSOD and GPx in A549 cells [259], which suggests the potential for Nrf2-mediated induction. In normal human bronchial BEAS-2B cells, 5 µM Cr(VI) for 10 h (subtoxic) does not enhance HO-1 expression [260], although based on our treatments of these cells, this may not have been long enough to cause significant thiol oxidation [78,80]. Pretreatment with this subtoxic level of Cr(VI) does, however, decrease the arsenic(III)-mediated induction of HO-1 expression as well as the arsenic-induced translocation of Nrf2 to the nucleus [260]. The underlying mechanisms for these effects are not totally clear, but subtoxic intranasal doses of Cr(VI) decrease HO-1 expression in the mouse lung after a 21-day Cr-free recovery period [260]. If these studies with mice and human cells reflect an ability of inhaled Cr(VI) to suppress Nrf2 activation in human lung, then Cr(VI)-induced lung damage could well be exacerbated by the decreased ability to induce several Nrf2-regulated cytoprotective genes. In contrast to the findings with mice or human cells, intratracheal delivery of Cr(VI) does induce a number of Nrf2-dependent genes (e.g., Nqo1, GPx, and glutathione S-transferase) in rat lung [254]. It is unknown if the rat findings reflect species differences, the different indicators of Nrf2 induction, or more efficient delivery of Cr(VI) to the lungs in the rat study. Pulmonary Nrf2 is inducible in mice or human cells treated with other pro-oxidants and this induction is protective [261–263]. The human and murine results with Cr(VI) do not, therefore, reflect an overall lack of pulmonary Nrf2 response in these species.
The DNA binding of Nrf2 is dependent on its C508 being in the reduced state, and Trx1, but not GSH, seems to be important in keeping nuclear Nrf2 in the active state [97]. While not yet explored with Cr(VI), higher doses could hinder Nrf2 activity if they oxidize nuclear Trx1.
Overall, the relationship between Cr(VI) and Nrf2 induction is complex, and there is little information on its relationship to Cr(VI)-mediated disruption of thiol redox control. Additional studies are needed to better understand the potential induction and role of Nrf2 in Cr(VI)-induced cell death vs survival.
Concluding remarks
Overall, the oxidant stress generated by Cr(VI) treatment is initially quite limited to a small number of proteins, with the TrxR/Trx/Prx system being among the first affected. Given the importance of these proteins in antioxidant defense and in controlling protein thiol redox states, their disruption by Cr(VI) is likely to have widespread implications for cell survival and several aspects of redox signaling. As described herein, the effects on the TrxR/Trx/Prx system are relatively recent findings, and they could represent key early events that contribute to a number of downstream redox signaling events that had been previously associated with Cr(VI), including MAP kinase activation and control of a number of redox-sensitive transcription factors. The effects of Cr(VI) on Trx oxidation have functional consequences in cells, as reflected by ASK1 activation and the loss of ability to support Prx function. This implies that a number of other Trx-dependent functions are probably affected as well. Because the TrxR/Trx system controls many redox-sensitive proteins in cells, the downstream effects could extend beyond the examples discussed herein. The irreversible inhibition of TrxR seems to be a key event that facilitates Trx oxidation and implies that this inhibition could extend beyond the Cr(VI) exposure itself, which can be transient or intermittent.
Although the oxidants that initiate these redox changes in cells are not yet fully defined, a number of studies have indicated that reactive Cr intermediates, peroxide, and HO• are important contributors. Although the reaction of Cr(V/IV) with H2O2 can generate HO•, direct roles for peroxide and/or reactive Cr species must be considered as well. Peroxides can preferentially oxidize certain protein thiols [99], and this can initiate specific signaling events. In contrast, HO• should be very nonselective in its reactivity and is not a specific signaling molecule as such. It is therefore unlikely to explain the particular sensitivity of the TrxR/ Trx system to Cr(VI) relative to other protein thiols. However, given that HO• scavengers can blunt many of the pro-oxidant and cytotoxic effects of Cr(VI), it seems that HO• has a significant role in the overall cellular effects. Given the complexity of the multiple redox states of Cr, the ability of specific Cr species to cause differential effects on protein thiols or Sec remains to be determined, but offers some intriguing possibilities for redox signaling and thiol/selenol disruption.
As described herein, many of the redox-mediated effects initiated by Cr(VI) can have important consequences for cell survival vs cell death and for the induction of inflammatory mediators. Some of the downstream events may have synergistic effects, whereas some events would act in opposition to others. The overall outcomes probably depend on the Cr(VI) dose, duration, and chemical form of Cr(VI), as well as on the cell or tissue type examined. Studies with normal cells/tissues will better define redox and signaling events that will dictate cell death, survival, and proinflammatory events. Because cancer cells often have altered signaling, enhanced antioxidant defense, and some ability to avoid apoptosis, they are more suitable for studying how repeated Cr(VI) exposures may enhance the proliferation of preexisting cancerous cells or precancerous lesions. Although the diversity of cells and tissues that have been studied to date has helped define the potential variability in redox signaling and its implications, more thorough studies that follow a number of redox-mediated events in a given system are needed to better understand the processes from beginning to end.
Overall, the effects of Cr(VI) on the TrxR/Trx/Prx system and the various downstream signaling events in the respiratory tract could contribute to a number of conditions associated with Cr(VI) inhalation, including acute and chronic respiratory damage, asthma, fibrosis, and the initiation and progression of cancer. Because these proteins contribute to thiol redox control and oxidant defense in all cells, these effects could have relevance for Cr(VI) toxicity at other sites such as the skin.
Acknowledgments
The author’s research in this area is supported by Grant R56ES012707 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), and by the Department of Pharmacology and Toxicology at the Medical College of Wisconsin. Its contents are solely the responsibility of the author and do not necessarily represent the official views of the NIEHS, NIH. I am grateful to Dr. William Antholine of the Medical College of Wisconsin for his continued collaboration and EPR expertise over many years and to Professor Elias Arnér of the Karolinska Institute for his generous collaboration and expertise on TrxR. I am grateful to Dr. Antholine and Professor Arnér for their helpful suggestions on this article.
Abbreviations
- ARE
antioxidant response element
- ASK1
apoptosis signaling kinase-1
- Cr(VI)
hexavalent chromium
- CuZnSOD
copper–zinc superoxide dismutase
- DFX
deferoxamine
- EPR
electron paramagnetic resonance
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- HO•
hydroxyl radical
- HO-1
heme oxygenase-1
- JNK
c-Jun N-terminal kinase
- MAP
mitogen-activated protein
- MnTBAP
Mn(III)tetrakis(4-benzoic acid)porphyrin chloride
superoxide
- p38
p38 MAP kinase
- Prx
peroxiredoxin
- Ref-1
redox factor 1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- Sec
selenocysteine
- SOD
superoxide dismutase
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- Txnip
thioredoxin-interacting protein
References
- 1.Becker N, Chang-Claude J, Frentzel-Beyme R. Risk of cancer for arc welders in the Federal Republic of Germany: results of a second follow up (1983–8) Br. J. Ind. Med. 1991;48:675–683. doi: 10.1136/oem.48.10.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. Characteristics of chromate workers2019; cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br. J. Cancer. 1994;70:160–166. doi: 10.1038/bjc.1994.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakagawa K, Matsubara T, Kinoshita I, Tsuchiya E, Sugano H, Hirano T. Surveillance study of a group of chromate workers—early detection and high incidence of lung cancer. Lung Cancer. 1984;24:301–310. (in Japanese). [Google Scholar]
- 4.Franchini I, Magnani F, Mutti A. Mortality experience among chromplating workers. Scand. J. Work Environ. Health. 1983;9:247–252. doi: 10.5271/sjweh.2413. [DOI] [PubMed] [Google Scholar]
- 5.Deschamps F, Moulin JJ, Wild P, Labriffe H, Haguenoer JM. Mortality study among workers producing chromate pigments in France. Int. Arch. Occup. Environ. Health. 1995;67:147–152. doi: 10.1007/BF00626345. [DOI] [PubMed] [Google Scholar]
- 6.Baruthio F. Toxic effects of chromium and its compounds. Biol. Trace Elem. Res. 1992;32:145–153. doi: 10.1007/BF02784599. [DOI] [PubMed] [Google Scholar]
- 7.Bright P, Burge PS, O’Hickey SP, Gannon PF, Robertson AS, Boran A. Occupational asthma due to chrome and nickel electroplating. Thorax. 1997;52:28–32. doi: 10.1136/thx.52.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HS, Yu HJ, Jung KS. Occupational asthma caused by chromium. Clin. Exp. Allergy. 1994;24:676–681. doi: 10.1111/j.1365-2222.1994.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 9.Occupational Safety and Health Administration. Occupational exposure to hexavalent chromium: final rule. Fed. Regist. 2006;71:10099–10385. [PubMed] [Google Scholar]
- 10.Shelnutt SR, Goad P, Belsito DV. Dermatological toxicity of hexavalent chromium. Crit. Rev. Toxicol. 2007;37:375–387. doi: 10.1080/10408440701266582. [DOI] [PubMed] [Google Scholar]
- 11.Nethercott J, Paustenbach D, Adams R, Fowler J, Marks J, Morton C, Taylor J, Horowitz S, Finley B. A study of chromium induced allergic contact dermatitis with 54 volunteers: implications for environmental risk assessment. Occup. Environ. Med. 1994;51:371–380. doi: 10.1136/oem.51.6.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gadd GM, White C. Microbial treatment of metal pollution—a working biotechnology? TIBTECH. 1993;11 doi: 10.1016/0167-7799(93)90158-6. [DOI] [PubMed] [Google Scholar]
- 13.Chromium. Washington, DC: Office of Health and Environmental Assessment U.S. EPA; 1999. Environmental Protection Agency. [Google Scholar]
- 14.Zhitkovich A, Voitkun V, Kluz T, Costa M. Utilization of DNA–protein cross-links as a biomarker of chromium exposure. Environ. Health Perspect. 1998;106(Suppl. 4):969–974. doi: 10.1289/ehp.98106s4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagliano JA, Savrin J, Udasin I, Gochfeld M. Community exposure and medical screening near chromium waste sites in New Jersey. Regul. Toxicol. Pharmacol. 1997;26:S13–S22. doi: 10.1006/rtph.1997.1134. [DOI] [PubMed] [Google Scholar]
- 16.Costa M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997;27:431–442. doi: 10.3109/10408449709078442. [DOI] [PubMed] [Google Scholar]
- 17.Calder LM. Chromium contamination of groundwater. In: Nriagu JO, Nieboer E, editors. Chromium in the Natural and Human Environments. New York: Wiley; 1988. pp. 215–229. [Google Scholar]
- 18.Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- 19.Martin RB. Bioinorganic chemistry of metal ion toxicity. In: Sigel H, editor. Metal Ions in Biological Systems. New York: Dekker; 1986. pp. 21–65. [Google Scholar]
- 20.Buttner B, Beyersmann D. Modification of the erythrocyte anion carrier by chromate. Xenobiotica. 1985;15:735–741. doi: 10.3109/00498258509047435. [DOI] [PubMed] [Google Scholar]
- 21.Elias Z, Poirot O, Baruthio F, Daniere MC. Role of solubilized chromium in the induction of morphological transformation of Syrian hamster embryo (SHE) cells by particulate chromium(VI) compounds. Carcinogenesis. 1991;12:1811–1816. doi: 10.1093/carcin/12.10.1811. [DOI] [PubMed] [Google Scholar]
- 22.Wise SS, Holmes AL, Ketterer ME, Hartsock WJ, Fomchenko E, Katsifis S, Thompson WD, Wise JP., Sr Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. Mutat. Res. 2004;560:79–89. doi: 10.1016/j.mrgentox.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Wise JPS, Stearns DM, Wetterhahn KE, Patierno SR. Cell-enhanced dissolution of carcinogenic lead chromate particles: the role of individual dissolution products in clastogenesis. Carcinogenesis. 1994;15:2249–2254. doi: 10.1093/carcin/15.10.2249. [DOI] [PubMed] [Google Scholar]
- 24.Leonard A, Lauwerys RR. Carcinogenicity and mutagenicity of chromium. Mutat. Res. 1980;76:227–239. doi: 10.1016/0165-1110(80)90018-4. [DOI] [PubMed] [Google Scholar]
- 25.Xie H, Holmes AL, Wise SS, Gordon N, Wise JP., Sr Lead chromate-induced chromosome damage requires extracellular dissolution to liberate chromium ions but does not require particle internalization or intracellular dissolution. Chem. Res. Toxicol. 2004;17:1362–1367. doi: 10.1021/tx0498509. [DOI] [PubMed] [Google Scholar]
- 26.Borthiry GR, Antholine WE, Myers JM, Myers CR. Reductive activation of hexavalent chromium by human lung epithelial cells: generation of Cr(V) and Cr(V)–thiol species. J. Inorg. Biochem. 2008;102:1449–1462. doi: 10.1016/j.jinorgbio.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie H, Holmes AL, Young JL, Qin Q, Joyce K, Pelsue SC, Peng C, Wise SS, Jeevarajan AS, Wallace WT, Hammond D, Wise JP., Sr Zinc chromate induces chromosome instability and DNA double strand breaks in human lung cells. Toxicol. Appl. Pharmacol. 2009;234:293–299. doi: 10.1016/j.taap.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patierno SR, Banh D, Landolph JR. Transformation of C3H/10T1/2 mouse embryo cells to focus formation and anchorage independence by insoluble lead chromate but not soluble calcium chromate: relationship to mutagenesis and internalization of lead chromate particles. Cancer Res. 1988;48:5280–5288. [PubMed] [Google Scholar]
- 29.Biedermann KA, Landolph JR. Induction of anchorage independence in human diploid foreskin fibroblasts by carcinogenic metal salts. Cancer Res. 1987;47:3815–3823. [PubMed] [Google Scholar]
- 30.Biedermann KA, Landolph JR. Role of valence state and solubility of chromium compounds on induction of cytotoxicity, mutagenesis, and anchorage independence in diploid human fibroblasts. Cancer Res. 1990;50:7835–7842. [PubMed] [Google Scholar]
- 31.Nickens KP, Patierno SR, Ceryak S. Chromium genotoxicity: a double-edged sword. Chem.-Biol. Interact. 2010;188:276–288. doi: 10.1016/j.cbi.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiting RF, Stich HF, Koropatnick DJ. DNA damage and DNA repair in cultured human cells exposed to chromate. Chem.–Biol. Interact. 1979;26:267–280. doi: 10.1016/0009-2797(79)90030-9. [DOI] [PubMed] [Google Scholar]
- 33.Tsapakos MJ, Hampton TH, Wetterhahn KE. Chromium(VI)-induced DNA lesions and chromium distribution on rat kidney, liver and lung. Cancer Res. 1983;43:5662–5667. [PubMed] [Google Scholar]
- 34.Levy LS, Venitt S. Carcinogenicity and mutagenicity of chromium compounds: the association between bronchial metaplasia and neoplasia. Carcinogenesis. 1986;7:831–835. doi: 10.1093/carcin/7.5.831. [DOI] [PubMed] [Google Scholar]
- 35.Christie NT, Cantoni O, Evans RM, Meyn RE, Costa M. Use of mammalian DNA repair-deficient mutants to assess the effects of toxic metal compounds on DNA. Biochem. Pharmacol. 1984;33:1661–1670. doi: 10.1016/0006-2952(84)90289-2. [DOI] [PubMed] [Google Scholar]
- 36.Cantoni O, Costa M. Analysis of the induction of alkali sensitive sites in the DNA by chromate and other agents that induce single strand breaks. Carcinogenesis. 1984;5:1207–1209. doi: 10.1093/carcin/5.9.1207. [DOI] [PubMed] [Google Scholar]
- 37.Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis. 1992;13:1319–1324. doi: 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- 38.Shi X, Dong Z, Dalal NS, Gannett PM. Chromate-mediated free radical generation from cysteine, penicillamine, hydrogen peroxide, and lipid hydroperoxides. Biochim. Biophys. Acta. 1994;1226:65–72. doi: 10.1016/0925-4439(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 39.Shi XL, Dalal NS. One-electron reduction of chromate by NADPH-dependent glutathione reductase. J. Inorg. Biochem. 1990;40:1–12. doi: 10.1016/0162-0134(90)80034-u. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki Y, Fukuda K. Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch. Toxicol. 1990;64:169–176. doi: 10.1007/BF02010721. [DOI] [PubMed] [Google Scholar]
- 41.Borthiry GR, Antholine WE, Kalyanaraman B, Myers JM, Myers CR. Reduction of hexavalent chromium by human cytochrome b5: generation of hydroxyl radical and superoxide. Free Radic. Biol. Med. 2007;42:738–755. doi: 10.1016/j.freeradbiomed.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers CR, Myers JM, Carstens BP, Antholine WE. Reduction of chromium(VI) to chromium(V) by human microsomal enzymes: effects of iron and quinones. Toxic Subst. Mech. 2000;19:25–51. [Google Scholar]
- 43.Jannetto PJ, Antholine WE, Myers CR. Cytochrome b5 plays a key role in human microsomal chromium(VI) reduction. Toxicology. 2001;159:119–133. doi: 10.1016/s0300-483x(00)00378-4. [DOI] [PubMed] [Google Scholar]
- 44.Kortenkamp A, Beyersmann D, O’Brien P. Uptake of chromium(III) complexes by erythrocytes. Toxicol. Environ. Chem. 1987;14:23–32. [Google Scholar]
- 45.Jennette KW. Microsomal reduction of the carcinogen chromate produces chromium(V) J. Am. Chem. Soc. 1982;104:874–875. [Google Scholar]
- 46.Liu KJ, Shi X. In vivo reduction of chromium (VI) and its related free radical generation. Mol. Cell. Biochem. 2001;222:41–47. [PubMed] [Google Scholar]
- 47.Liebross RH, Wetterhahn KE. In vivo formation of chromium(V) in chick embryo liver and red blood cells. Carcinogenesis. 1992;13:2113–2120. doi: 10.1093/carcin/13.11.2113. [DOI] [PubMed] [Google Scholar]
- 48.Myers CR, Antholine WE, Myers JM. The pro-oxidant chromium(VI) inhibits mitochondrial complex I, complex II, and aconitase in the bronchial epithelium: EPR markers for Fe-S proteins. Free Radic. Biol. Med. 2010;49:1903–1915. doi: 10.1016/j.freeradbiomed.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu KJ, Jiang J, Swartz HM, Shi X. Low-frequency, E. P. R. detection of chromium(V) formation by chromium(VI) reduction in whole live mice. Arch. Biochem. Biophys. 1994;313:248–252. doi: 10.1006/abbi.1994.1384. [DOI] [PubMed] [Google Scholar]
- 50.Ueno S, Kashimoto T, Susa N, Furukawa Y, Ishii M, Yokoi K, Yasuno M, Sasaki YF, Ueda J, Nishimura Y, Sugiyama M. Detection of dichromate (VI)-induced DNA strand breaks and formation of paramagnetic chromium in multiple mouse organs. Toxicol. Appl. Pharmacol. 2001;170:56–62. doi: 10.1006/taap.2000.9081. [DOI] [PubMed] [Google Scholar]
- 51.Sugiyama M, Tsuzuki K, Haramaki N. Influence of o-phenanthroline on DNA single-strand breaks, alkali-labile sites, glutathione reductase, and formation of chromium(V) in Chinese hamster V-79 cells treated with sodium chromate(VI) Arch. Biochem. Biophys. 1993;305:261–266. doi: 10.1006/abbi.1993.1420. [DOI] [PubMed] [Google Scholar]
- 52.Stearns DM, Kennedy LJ, Courtney KD, Giangrande PH, Phieffer LS, Wetterhahn KE. Reduction of chromium(VI) by ascorbate leads to chromium-DNA binding and DNA strand breaks in vitro. Biochemistry. 1995;34:910–919. doi: 10.1021/bi00003a025. [DOI] [PubMed] [Google Scholar]
- 53.Stearns DM, Wetterhahn KE. Reaction of chromium(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem. Res. Toxicol. 1994;7:219–230. doi: 10.1021/tx00038a016. [DOI] [PubMed] [Google Scholar]
- 54.Dillon CT, Lay PA, Bonin AM, Cholewa M, Legge GJF, Collins TJ, Kostka KL. Permeability, cytotoxicity, and genotoxicity of chromium(V) and chromium(VI) complexes in V79 Chinese hamster lung cells. Chem. Res. Toxicol. 1998;11:119–129. doi: 10.1021/tx9701541. [DOI] [PubMed] [Google Scholar]
- 55.Standeven AM, Wetterhahn KE. Is there a role for reactive oxygen species in the mechanism of chromium(VI) carcinogenesis? Chem. Res. Toxicol. 1991;4:616–625. doi: 10.1021/tx00024a003. [DOI] [PubMed] [Google Scholar]
- 56.Sugden KD, Campo CK, Martin BD. Direct oxidation of guanine and 7,8-dihydro-8-oxoguanine in DNA by a high-valent chromium complex: a possible mechanism for chromate genotoxicity. Chem. Res. Toxicol. 2001;14:1315–1322. doi: 10.1021/tx010088+. [DOI] [PubMed] [Google Scholar]
- 57.Sugden KD. Formation of modified cleavage termini from the reaction of chromium(V) with DNA. J. Inorg. Biochem. 1999;77:177–183. doi: 10.1016/s0162-0134(99)00189-0. [DOI] [PubMed] [Google Scholar]
- 58.Shi X, Dalal NS. NADPH-dependent flavoenzymes catalyze one electron reduction of metal ions and molecular oxygen and generate hydroxyl radicals. FEBS Lett. 1990;276:189–191. doi: 10.1016/0014-5793(90)80539-u. [DOI] [PubMed] [Google Scholar]
- 59.Shi X, Mao Y, Knapton AD, Ding M, Rojanasakul Y, Gannett PM, Dalal N, Liu K. Reaction of Cr(VI) with ascorbate and hydrogen peroxide generates hydroxyl radicals and causes DNA damage: role of a Cr(IV)-mediated Fenton-like reaction. Carcinogenesis. 1994;15:2475–2478. doi: 10.1093/carcin/15.11.2475. [DOI] [PubMed] [Google Scholar]
- 60.Luo H, Lu Y, Shi X, Mao Y, Dalal NS. Chromium(IV)-mediated Fenton-like reaction causes DNA damage: implication to genotoxicity of chromate. Ann. Clin. Lab. Sci. 1996;26:185–191. [PubMed] [Google Scholar]
- 61.Tsou T-C, Yang J-L. Formation of reactive oxygen species and DNA strand breakage during interaction of chromium(III) and hydrogen peroxide in vitro: evidence for a chromium(III)-mediated Fenton-like reaction. Chem.-Biol. Interact. 1996;102:133–153. doi: 10.1016/s0009-2797(96)03740-4. [DOI] [PubMed] [Google Scholar]
- 62.Chen F, Ye J, Zhang X, Rojanasakul Y, Shi X. One-electron reduction of chromium(VI) by α-lipoic acid and related hydroxyl radical generation, dG hydroxylation and nuclear transcription factor-κB activation. Arch. Biochem. Biophys. 1997;338:165–172. doi: 10.1006/abbi.1996.9849. [DOI] [PubMed] [Google Scholar]
- 63.Leonard S, Wang S, Zang L, Castranova V, Vallyathan V, Shi X. Role of molecular oxygen in the generation of hydroxyl and superoxide anion radicals during enzymatic Cr(VI) reduction and its implication to Cr(VI)-induced carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2000;19:49–60. [PubMed] [Google Scholar]
- 64.Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium(VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Health B. 1999;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- 65.Porter R, Jachymova M, Martasek P, Kalyanaraman B, Vasquez-Vivar J. Reductive activation of Cr(VI) by nitric oxide synthase. Chem. Res. Toxicol. 2005;18:834–843. doi: 10.1021/tx049778e. [DOI] [PubMed] [Google Scholar]
- 66.Pritchard KA, Jr, Ackerman A, Kalyanaraman B. Chromium(VI) increases endothelial cell expression of ICAM-1 and decreases nitric oxide activity. J. Environ. Pathol. Toxicol. Oncol. 2000;19:251–260. [PubMed] [Google Scholar]
- 67.Wang BJ, Sheu HM, Guo YL, Lee YH, Lai CS, Pan MH, Wang YJ. Hexavalent chromium induced ROS formation, Akt, NF-κB, and MAPK activation, and TNF-α and IL-1α production in keratinocytes. Toxicol. Lett. 2010;198:216–224. doi: 10.1016/j.toxlet.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 68.Gao N, Jiang BH, Leonard SS, Corum L, Zhang Z, Roberts JR, Antonini J, Zheng JZ, Flynn DC, Castranova V, Shi X. p38 Signaling-mediated hypoxia-inducible factor 1α and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J. Biol. Chem. 2002;277:45041–45048. doi: 10.1074/jbc.M202775200. [DOI] [PubMed] [Google Scholar]
- 69.Shi X, Ding M, Ye J, Wang S, Leonard SS, Zang L, Castranova V, Vallyathan V, Chiu A, Dalal N, Liu K. Cr(IV) causes activation of nuclear transcription factor-κB, DNA strand breaks and dG hydroxylation via free radical reactions. J. Inorg. Biochem. 1999;75:37–44. doi: 10.1016/S0162-0134(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 70.Sugiyama M. Effects of vitamins on chromium(VI)-induced damage. Environ. Health Perspect. 1991;92:63–70. doi: 10.1289/ehp.919263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molyneux MJ, Davies MJ. Direct evidence for hydroxyl radical-induced damage to nucleic acids by chromium(VI)-derived species. Carcinogenesis. 1995;16:875–882. doi: 10.1093/carcin/16.4.875. [DOI] [PubMed] [Google Scholar]
- 72.Gao M, Binks SP, Chipman JK, Levy LS, Braithwaite RA, Brown SS. Induction of DNA strand breaks in peripheral lymphocytes by soluble chromium compounds. Hum. Exp. Toxicol. 1992;11:77–82. doi: 10.1177/096032719201100203. [DOI] [PubMed] [Google Scholar]
- 73.Taioli E, Zhitkovich A, Kinney P, Udasin I, Toniolo P, Costa M. Increased DNA-protein crosslinks in lymphocytes of residents living in chromium-contaminated areas. Biol. Trace Elem. Res. 1995;50:175–180. doi: 10.1007/BF02785408. [DOI] [PubMed] [Google Scholar]
- 74.Kadiiska MB, Morrow JD, Awad JA, Roberts LJ, II, Mason RP. Identification of free radical formation and F2-isoprostanes in vivo by acute Cr(VI) poisoning. Chem. Res. Toxicol. 1998;11:1516–1520. doi: 10.1021/tx980169e. [DOI] [PubMed] [Google Scholar]
- 75.Popper HH, Woldrich A. Oxygen radical formation a probable mechanism for chromate toxicity. In: Graumann W, Drukker J, editors. Histo- and Cytochemistry as a Tool in Environmental Toxicology. New York: Fischer; 1991. pp. 220–226. [DOI] [PubMed] [Google Scholar]
- 76.Arreola-Mendoza L, Reyes JL, Melendez E, Martin D, Namorado MC, Sanchez E, Del Razo LM. α-Tocopherol protects against the renal damage caused by potassium dichromate. Toxicology. 2006;218:237–246. doi: 10.1016/j.tox.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 77.Pourahmad J, Rabiei M, Jokar F, O’Brien PJ. A comparison of hepatocyte cytotoxic mechanisms for chromate and arsenite. Toxicology. 2005;206:449–460. doi: 10.1016/j.tox.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Myers JM, Myers CR. The effects of hexavalent chromium on thioredoxin reductase and peroxiredoxins in human bronchial epithelial cells. Free Radic. Biol. Med. 2009;47:1477–1485. doi: 10.1016/j.freeradbiomed.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Myers JM, Antholine WE, Myers CR. The intracellular redox stress caused by hexavalent chromium is selective for proteins that have key roles in cell survival and thiol redox control. Toxicology. 2011;281:37–47. doi: 10.1016/j.tox.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myers JM, Antholine WE, Myers CR. Hexavalent chromium causes the oxidation of thioredoxin in human bronchial epithelial cells. Toxicology. 2008;246:222–233. doi: 10.1016/j.tox.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pritchard DE, Ceryak S, Ha L, Fornsaglio JL, Hartman SK, O’Brien TJ, Patierno SR. Mechanism of apoptosis and determination of cellular fate in chromium(VI)-exposed populations of telomerase-immortalized human fibroblasts. Cell Growth Differ. 2001;12:487–496. [PubMed] [Google Scholar]
- 82.Pritchard DE, Singh J, Carlisle DL, Patierno SR. Cyclosporin A inhibits chromium(VI)-induced apoptosis and mitochondrial cytochrome c release and restores clonogenic survival in CHO cells. Carcinogenesis. 2000;21:2027–2033. doi: 10.1093/carcin/21.11.2027. [DOI] [PubMed] [Google Scholar]
- 83.Rudolf E, Cervinka M, Cerman J, Schroterova L. Hexavalent chromium disrupts the actin cytoskeleton and induces mitochondria-dependent apoptosis in human dermal fibroblasts. Toxicol. in Vitro. 2005;19:713–723. doi: 10.1016/j.tiv.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Aiyar J, Berkovits HJ, Floyd RA, Wetterhahn KE. Reaction of chromium(VI) with hydrogen peroxide in the presence of glutathione: reactive intermediates and resulting DNA damage. Chem. Res. Toxicol. 1990;3:595–603. doi: 10.1021/tx00018a016. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, Leonard SS, Ye J, Gao N, Wang L, Shi X. Role of reactive oxygen species and Cr(VI) in Ras-mediated signal transduction. Mol. Cell. Biochem. 2004;255:119–127. doi: 10.1023/b:mcbi.0000007268.42733.53. [DOI] [PubMed] [Google Scholar]
- 86.Azad N, Iyer AK, Manosroi A, Wang L, Rojanasakul Y. Superoxide-mediated proteasomal degradation of Bcl-2 determines cell susceptibility to Cr(VI)-induced apoptosis. Carcinogenesis. 2008;29:1538–1545. doi: 10.1093/carcin/bgn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ye J, Wang S, Leonard SS, Sun Y, Butterworth L, Antonini J, Ding M, Rojanasakul Y, Vallyathan V, Castranova V, Shi X. Role of reactive oxygen species and p53 in chromium(VI)-induced apoptosis. J. Biol. Chem. 1999;274:34974–34980. doi: 10.1074/jbc.274.49.34974. [DOI] [PubMed] [Google Scholar]
- 88.Son YO, Hitron JA, Wang X, Chang Q, Pan J, Zhang Z, Liu J, Wang S, Lee JC, Shi X. Cr(VI) induces mitochondrial-mediated and caspase-dependent apoptosis through reactive oxygen species-mediated p53 activation in JB6 Cl41 cells. Toxicol. Appl. Pharmacol. 2010;245:226–235. doi: 10.1016/j.taap.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shi X, Sun X, Gannett PM, Dalal NS. Deferoxamine inhibition of Cr(V)-mediated radical generation and deoxyguanine hydroxylation: ESR and HPLC evidence. Arch. Biochem. Biophys. 1992;293:281–286. doi: 10.1016/0003-9861(92)90396-e. [DOI] [PubMed] [Google Scholar]
- 90.Arreola-Mendoza L, Del Razo LM, Mendoza-Garrido ME, Martin D, Namorado MC, Calderon-Salinas JV, Reyes JL. The protective effect of α-tocopherol against dichromate-induced renal tight junction damage is mediated via ERK1/2. Toxicol. Lett. 2009;191:279–288. doi: 10.1016/j.toxlet.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Molina-Jijón E, Tapia E, Zazueta C, El Hafidi M, Zatarain-Barrón ZL, Hernández-Pando R, Medina-Campos ON, Zarco-Márquez G, Torres I, Pedraza-Chaverri J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011;51:1543–1557. doi: 10.1016/j.freeradbiomed.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 92.Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 93.Batinic-Haberle I, Cuzzocrea S, Reboucas JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic. Biol. Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Z, Leonard SS, Wang S, Vallyathan V, Castranova V, Shi X. Cr(VI) induces cell growth arrest through hydrogen peroxide-mediated reactions. Mol. Cell. Biochem. 2001;222:77–83. [PubMed] [Google Scholar]
- 95.Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1352–G1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 96.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu. Rev. Pharmacol. Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 97.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 99.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Wiegand HJ, Ottenwälder H, Bolt HM. The reduction of chromium(VI) to chromium(III) by glutathione: an intracellular redox pathway in the metabolism of the carcinogen chromate. Toxicology. 1984;33:341–348. doi: 10.1016/0300-483x(84)90050-7. [DOI] [PubMed] [Google Scholar]
- 101.Connett PH, Wetterhahn KE. In vitro reaction of the carcinogen chromate with cellular thiols and carboxylic acids. J. Am. Chem. Soc. 1985;107:4282–4288. [Google Scholar]
- 102.O’Brien T, Xu J, Patierno SR. Effects of glutathione on chromium-induced DNA crosslinking and DNA polymerase arrest. Mol. Cell. Biochem. 2001;222:173–182. [PubMed] [Google Scholar]
- 103.Quinteros FA, Machiavelli LI, Miler EA, Cabilla JP, Duvilanski BH. Mechanisms of chromium (VI)-induced apoptosis in anterior pituitary cells. Toxicology. 2008;249:109–115. doi: 10.1016/j.tox.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 104.Dubrovskaya VA, Wetterhahn KE. Effects of Cr(VI) on the expression of the oxidative stress genes in human lung cells. Carcinogenesis. 1998;19:1401–1407. doi: 10.1093/carcin/19.8.1401. [DOI] [PubMed] [Google Scholar]
- 105.Standeven AM, Wetterhahn KE. Possible role of glutathione in chromium(VI) metabolism and toxicity in rats. Pharmacol. Toxicol. 1991;68:469–476. doi: 10.1111/j.1600-0773.1991.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 106.Fernandes MA, Mota IM, Silva MT, Oliveira CR, Geraldes CF, Alpoim MC. Human erythrocytes are protected against chromate-induced peroxidation. Ecotoxicol. Environ. Saf. 1999;43:38–46. doi: 10.1006/eesa.1998.1755. [DOI] [PubMed] [Google Scholar]
- 107.Pourahmad J, O’Brien PJ. Biological reactive intermediates that mediate chromium(VI) toxicity. Adv. Exp. Med. Biol. 2001;500:203–207. doi: 10.1007/978-1-4615-0667-6_27. [DOI] [PubMed] [Google Scholar]
- 108.Lalaouni A, Henderson C, Kupper C, Grant MH. The interaction of chromium(VI) with macrophages: depletion of glutathione and inhibition of glutathione reductase. Toxicology. 2007;236:76–81. doi: 10.1016/j.tox.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 109.Joseph P, He Q, Umbright C. Heme-oxygenase 1 gene expression is a marker for hexavalent chromium-induced stress and toxicity in human dermal fibroblasts. Toxicol. Sci. 2008;103:325–334. doi: 10.1093/toxsci/kfn048. [DOI] [PubMed] [Google Scholar]
- 110.Lay PA, Levina A. Activation of molecular oxygen during the reactions of chromium(VI/V/IV) with biological reductants: implications for chromium-induced genotoxicities. J. Am. Chem. Soc. 1998;120:6704–6714. [Google Scholar]
- 111.Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc. Natl. Acad. Sci. USA. 2009;106:422–427. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carvalho AT, Fernandes PA, Ramos MJ. Determination of the ΔpKa between the active site cysteines of thioredoxin and DsbA. J. Comput. Chem. 2006;27:966–975. doi: 10.1002/jcc.20404. [DOI] [PubMed] [Google Scholar]
- 113.Jacob C, Giles GI, Giles NM, Sies H. Sulfur and selenium: the role of oxidation state in protein structure and function. Angew. Chem. Int. Ed. Engl. 2003;42:4742–4758. doi: 10.1002/anie.200300573. [DOI] [PubMed] [Google Scholar]
- 114.Johansson L, Gafvelin G, Arnér ES. Selenocysteine in proteins—properties and biotechnological use. Biochim. Biophys. Acta. 2005;1726:1–13. doi: 10.1016/j.bbagen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 115.Arnér ES. Selenoproteins—what unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010;316:1296–1303. doi: 10.1016/j.yexcr.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 116.Arnér ES. Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim. Biophys. Acta. 1790;2009:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 117.Nordberg J, Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 118.Cheng Q, Sandalova T, Lindqvist Y, Arnér ES. Crystal structure and catalysis of the selenoprotein thioredoxin reductase 1. J. Biol. Chem. 2009;284:3998–4008. doi: 10.1074/jbc.M807068200. [DOI] [PubMed] [Google Scholar]
- 119.Cheng Q, Antholine WE, Myers JM, Kalyanaraman B, Arnér ES, Myers CR. The selenium-independent inherent pro-oxidant NADPH oxidase activity of mammalian thioredoxin reductase and its selenium-dependent direct peroxidase activities. J. Biol. Chem. 2010;285:21708–21723. doi: 10.1074/jbc.M110.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sandalova T, Zhong L, Lindqvist Y, Holmgren A, Schneider G. Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme. Proc. Natl. Acad. Sci. USA. 2001;98:9533–9538. doi: 10.1073/pnas.171178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arnér ES, Bjornstedt M, Holmgren A. 1-Chloro-2,4-dinitrobenzene is an irreversible inhibitor of human thioredoxin reductase: loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. J. Biol. Chem. 1995;270:3479–3482. doi: 10.1074/jbc.270.8.3479. [DOI] [PubMed] [Google Scholar]
- 122.Fang J, Holmgren A. Inhibition of thioredoxin and thioredoxin reductase by 4-hydroxy-2-nonenal in vitro and in vivo. J. Am. Chem. Soc. 2006;128:1879–1885. doi: 10.1021/ja057358l. [DOI] [PubMed] [Google Scholar]
- 123.Cassidy PB, Edes K, Nelson CC, Parsawar K, Fitzpatrick FA, Moos PJ. Thioredoxin reductase is required for the inactivation of tumor suppressor p53 and for apoptosis induced by endogenous electrophiles. Carcinogenesis. 2006;27:2538–2549. doi: 10.1093/carcin/bgl111. [DOI] [PubMed] [Google Scholar]
- 124.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 125.Nordberg J, Zhong L, Holmgren A, Arnér ES. Mammalian thioredoxin reductase is irreversibly inhibited by dinitrohalobenzenes by alkylation of both the redox active selenocysteine and its neighboring cysteine residue. J. Biol. Chem. 1998;273:10835–10842. doi: 10.1074/jbc.273.18.10835. [DOI] [PubMed] [Google Scholar]
- 126.Hojo Y, Satomi Y. In vivo nephrotoxicity induced in mice by chromium(VI): involvement of glutathione and chromium(V) Biol. Trace Elem. Res. 1991;31:21–31. doi: 10.1007/BF02990356. [DOI] [PubMed] [Google Scholar]
- 127.Bondareva AA, Capecchi MR, Iverson SV, Li Y, Lopez NI, Lucas O, Merrill GF, Prigge JR, Siders AM, Wakamiya M, Wallin SL, Schmidt EE. Effects of thioredoxin reductase-1 deletion on embryogenesis and transcriptome. Free Radic. Biol. Med. 2007;43:911–923. doi: 10.1016/j.freeradbiomed.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anestål K, Arner ES. Rapid induction of cell death by selenium-compromised thioredoxin reductase 1 but not by the fully active enzyme containing selenocysteine. J. Biol. Chem. 2003;278:15966–15972. doi: 10.1074/jbc.M210733200. [DOI] [PubMed] [Google Scholar]
- 129.Anestål K, Prast-Nielsen S, Cenas N, Arner ES. Cell death by SecTRAPs: thioredoxin reductase as a prooxidant killer of cells. PLoS One. 2008;3:e1846. doi: 10.1371/journal.pone.0001846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watson WH, Heilman JM, Hughes LL, Spielberger JC. Thioredoxin reductase-1 knock down does not result in thioredoxin-1 oxidation. Biochem. Biophys. Res. Commun. 2008;368:832–836. doi: 10.1016/j.bbrc.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu. Rev. Biophys. Biomol. Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 132.Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, Jones DP. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J. Biol. Chem. 2003;278:33408–33415. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- 133.Myers CR, Myers JM. The effects of acrolein on peroxiredoxins, thioredoxins, and thioredoxin reductase in human bronchial epithelial cells. Toxicology. 2009;257:95–104. doi: 10.1016/j.tox.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Szadkowski A, Myers CR. Acrolein oxidizes the cytosolic and mitochondrial thioredoxins in human endothelial cells. Toxicology. 2008;243:164–176. doi: 10.1016/j.tox.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem. J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alexander J, Aaseth J, Norseth T. Uptake of chromium by rat liver mitochondria. Toxicology. 1982;24:115–122. doi: 10.1016/0300-483x(82)90050-6. [DOI] [PubMed] [Google Scholar]
- 137.Rossi SC, Gorman N, Wetterhahn KE. Mitochondrial reduction of the carcinogen chromate: formation of chromium(V) Chem. Res. Toxicol. 1988;1:101–107. doi: 10.1021/tx00002a003. [DOI] [PubMed] [Google Scholar]
- 138.Getz EB, Xiao M, Chakrabarty T, Cooke R, Selvin PR. A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal. Biochem. 1999;273:73–80. doi: 10.1006/abio.1999.4203. [DOI] [PubMed] [Google Scholar]
- 139.Day BJ, Shawen S, Liochev SI, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J. Pharmacol. Exp. Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- 140.Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101:563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 141.Milano J, Day BJ. A catalytic antioxidant metalloporphyrin blocks hydrogen peroxide-induced mitochondrial DNA damage. Nucleic Acids Res. 2000;28:968–973. doi: 10.1093/nar/28.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, Crapo JD, Wallace DC. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 1998;18:159–163. doi: 10.1038/ng0298-159. [DOI] [PubMed] [Google Scholar]
- 143.Malassagne B, Ferret PJ, Hammoud R, Tulliez M, Bedda S, Trebeden H, Jaffray P, Calmus Y, Weill B, Batteux F. The superoxide dismutase mimetic MnTBAP prevents Fas-induced acute liver failure in the mouse. Gastroenterology. 2001;121:1451–1459. doi: 10.1053/gast.2001.29590. [DOI] [PubMed] [Google Scholar]
- 144.Ye J, Zhang X, Young HA, Mao Y, Shi X. Chromium(VI)-induced nuclear factor-κB activation in intact cells via free radical reactions. Carcinogenesis. 1995;16:2401–2405. doi: 10.1093/carcin/16.10.2401. [DOI] [PubMed] [Google Scholar]
- 145.Leonard SS, Roberts JR, Antonini JM, Castranova V, Shi X. PbCrO4 mediates cellular responses via reactive oxygen species. Mol. Cell. Biochem. 2004;255:171–179. doi: 10.1023/b:mcbi.0000007273.23747.67. [DOI] [PubMed] [Google Scholar]
- 146.Qian Y, Jiang BH, Flynn DC, Leonard SS, Wang S, Zhang Z, Ye J, Chen F, Wang L, Shi X. Cr(VI) increases tyrosine phosphorylation through reactive oxygen species-mediated reactions. Mol. Cell. Biochem. 2001;222:199–204. [PubMed] [Google Scholar]
- 147.Kosaka H, Katsuki Y, Shiga T. Spin trapping study on the kinetics of Fe2+ autoxidation: formation of spin adducts and their destruction by super-oxide. Arch. Biochem. Biophys. 1992;293:401–408. doi: 10.1016/0003-9861(92)90412-p. [DOI] [PubMed] [Google Scholar]
- 148.Reinke LA, Moore DR, McCay PB. Degradation of DMPO adducts from hydroxyl and 1-hydroxyethyl radicals by rat liver microsomes. Free Radic. Res. 1996;25:467–474. doi: 10.3109/10715769609149069. [DOI] [PubMed] [Google Scholar]
- 149.Shi H, Timmins G, Monske M, Burdick A, Kalyanaraman B, Liu Y, Clement JL, Burchiel S, Liu KJ. Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species. Arch. Biochem. Biophys. 2005;437:59–68. doi: 10.1016/j.abb.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 150.Khan N, Wilmot CM, Rosen GM, Demidenko E, Sun J, Joseph J, O’Hara J, Kalyanaraman B, Swartz HM. Spin traps: in vitro toxicity and stability of radical adducts. Free Radic. Biol. Med. 2003;34:1473–1481. doi: 10.1016/s0891-5849(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 151.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen Y, Cai J, Jones DP. Mitochondrial thioredoxin in regulation of oxidant-induced cell death. FEBS Lett. 2006;580:6596–6602. doi: 10.1016/j.febslet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic. Biol. Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 154.Sun Y, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–8277. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 156.Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 157.Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 158.Kim JA, Park S, Kim K, Rhee SG, Kang SW. Activity assay of mammalian 2-Cys peroxiredoxins using yeast thioredoxin reductase system. Anal. Biochem. 2005;338:216–223. doi: 10.1016/j.ab.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 159.Prigge JR, Eriksson S, Iverson SV, Meade TA, Capecchi MR, Arner ES, Schmidt EE. Hepatocyte DNA replication in growing liver requires either glutathione or a single allele of txnrd1. Free Radic. Biol. Med. 2012;52:803–810. doi: 10.1016/j.freeradbiomed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J. Biol. Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 161.Cox AG, Pullar JM, Hughes G, Ledgerwood EC, Hampton MB. Oxidation of mitochondrial peroxiredoxin 3 during the initiation of receptor-mediated apoptosis. Free Radic. Biol. Med. 2008;44:1001–1009. doi: 10.1016/j.freeradbiomed.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 162.Cox AG, Brown KK, Arnér ES, Hampton MB. The thioredoxin reductase inhibitor auranofin triggers apoptosis through a Bax/Bak-dependent process that involves peroxiredoxin 3 oxidation. Biochem. Pharmacol. 2008;76:1097–1109. doi: 10.1016/j.bcp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 163.Baty JW, Hampton MB, Winterbourn CC. Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochem. J. 2005;389:785–795. doi: 10.1042/BJ20050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vásquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical: an electron spin resonance investigation. J. Biol. Chem. 2000;275:14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- 165.Gardner PR. Superoxide-driven aconitase Fe-S center cycling. Biosci. Rep. 1997;17:33–42. doi: 10.1023/a:1027383100936. [DOI] [PubMed] [Google Scholar]
- 166.Myers CR, Myers JM. Iron stimulates the rate of reduction of hexavalent chromium by human microsomes. Carcinogenesis. 1998;19:1029–1038. doi: 10.1093/carcin/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 167.Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J. Biol. Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]
- 169.Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc. Natl. Acad. Sci. USA. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 171.Powell CS, Jackson RM. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L189–L198. doi: 10.1152/ajplung.00253.2002. [DOI] [PubMed] [Google Scholar]
- 172.Pearce LL, Epperly MW, Greenberger JS, Pitt BR, Peterson J. Identification of respiratory complexes I and III as mitochondrial sites of damage following exposure to ionizing radiation and nitric oxide. Nitric Oxide. 2001;5:128–136. doi: 10.1006/niox.2001.0338. [DOI] [PubMed] [Google Scholar]
- 173.Wang D, Masutani H, Oka S, Tanaka T, Yamaguchi-Iwai Y, Nakamura H, Yodoi J. Control of mitochondrial outer membrane permeabilization and Bcl-xL levels by thioredoxin 2 in DT40 cells. J. Biol. Chem. 2006;281:7384–7391. doi: 10.1074/jbc.M509876200. [DOI] [PubMed] [Google Scholar]
- 174.Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- 175.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010;396:120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 177.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell. Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- 180.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 181.Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J. Biol. Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- 182.Zhang R, Al-Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ. Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 183.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim. Biophys. Acta. 2008:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. 1780. [DOI] [PubMed] [Google Scholar]
- 184.Machino T, Hashimoto S, Maruoka S, Gon Y, Hayashi S, Mizumura K, Nishitoh H, Ichijo H, Horie T. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates hydrogen peroxide-induced apoptosis in human pulmonary vascular endothelial cells. Crit. Care Med. 2003;31:2776–2781. doi: 10.1097/01.CCM.0000098027.49562.29. [DOI] [PubMed] [Google Scholar]
- 185.Hoeflich KP, Yeh WC, Yao Z, Mak TW, Woodgett JR. Mediation of TNF receptor-associated factor effector functions by apoptosis signal-regulating kinase-1 (ASK1) Oncogene. 1999;18:5814–5820. doi: 10.1038/sj.onc.1202975. [DOI] [PubMed] [Google Scholar]
- 186.Chen Z, Seimiya H, Naito M, Mashima T, Kizaki A, Dan S, Imaizumi M, Ichijo H, Miyazono K, Tsuruo T. ASK1 mediates apoptotic cell death induced by genotoxic stress. Oncogene. 1999;18:173–180. doi: 10.1038/sj.onc.1202276. [DOI] [PubMed] [Google Scholar]
- 187.Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–4374. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- 188.Chen F, Ding M, Lu Y, Leonard SS, Vallyathan V, Castranova V, Shi X. Participation of MAP kinase p38 and IkB kinase in chromium(VI)-induced NF-κB and AP-1 activation. J. Environ. Pathol. Toxicol. Oncol. 2000;19:231–238. [PubMed] [Google Scholar]
- 189.Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. (Lung Cell. Mol. Physiol.) 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 190.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radic. Biol. Med. 2004;37:1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 191.Tessier DM, Pascal LE. Activation of MAP kinases by hexavalent chromium, manganese and nickel in human lung epithelial cells. Toxicol. Lett. 2006;167:114–121. doi: 10.1016/j.toxlet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 192.O’Hara KA, Klei LR, Barchowsky A. Selective activation of Src family kinases and JNK by low levels of chromium(VI) Toxicol. Appl. Pharmacol. 2003;190:214–223. doi: 10.1016/s0041-008x(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 193.Chuang SM, Liou GY, Yang JL. Activation of JNK, p38 and ERK mitogen-activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis. 2000;21:1491–1500. [PubMed] [Google Scholar]
- 194.Watjen W, Beyersmann D. Cadmium-induced apoptosis in C6 glioma cells: influence of oxidative stress. BioMetals. 2004;17:65–78. doi: 10.1023/a:1024405119018. [DOI] [PubMed] [Google Scholar]
- 195.Bulavin DV, Fornace AJ., Jr p38 MAP kinase’s emerging role as a tumor suppressor. Adv. Cancer Res. 2004;92:95–118. doi: 10.1016/S0065-230X(04)92005-2. [DOI] [PubMed] [Google Scholar]
- 196.Rinna A, Forman HJ. SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. Am. J. Respir. Cell Mol. Biol. 2008;39:97–104. doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 199.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 200.Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G, Nebreda AR, Anderson CW, Kallioniemi A, Fornace AJ, Jr, Appella E. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 2002;31:210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 201.Chai W, Liu Z. p38 Mitogen-activated protein kinase mediates palmitate-induced apoptosis but not inhibitor of nuclear factor-B degradation in human coronary artery endothelial cells. Endocrinology. 2007;148:1622–1628. doi: 10.1210/en.2006-1068. [DOI] [PubMed] [Google Scholar]
- 202.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 203.Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more? Front. Biosci. 2008;13:3581–3593. doi: 10.2741/2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 205.Kwon SJ, Song JJ, Lee YJ. Signal pathway of hypoxia-inducible factor-1α phosphorylation and its interaction with von Hippel-Lindau tumor suppressor protein during ischemia in MiaPaCa-2 pancreatic cancer cells. Clin. Cancer Res. 2005;11:7607–7613. doi: 10.1158/1078-0432.CCR-05-0981. [DOI] [PubMed] [Google Scholar]
- 206.Wakeman TP, Wyczechowska D, Xu B. Involvement of the p38 MAP kinase in Cr(VI)-induced growth arrest and apoptosis. Mol. Cell. Biochem. 2005;279:69–73. doi: 10.1007/s11010-005-8216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stern RM. Assessment of risk of lung cancer for welders. Arch. Environ. Health. 1983;38:148–155. doi: 10.1080/00039896.1983.10543996. [DOI] [PubMed] [Google Scholar]
- 208.Chopra P, Kanoje V, Semwal A, Ray A. Therapeutic potential of inhaled p38 mitogen-activated protein kinase inhibitors for inflammatory pulmonary diseases. Expert Opin. Invest. Drugs. 2008;17:1411–1425. doi: 10.1517/13543784.17.10.1411. [DOI] [PubMed] [Google Scholar]
- 209.Mercer BA, D’Armiento JM. Emerging role of MAP kinase pathways as therapeutic targets in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006;1:137–150. doi: 10.2147/copd.2006.1.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-β1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J. Biol. Chem. 2005;280:43000–43009. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- 211.Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-κB and AP-1. Toxicol. Lett. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 212.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 213.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 214.Mossman BT, Lounsbury KM, Reddy SP. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am. J. Respir. Cell Mol. Biol. 2006;34:666–669. doi: 10.1165/rcmb.2006-0047SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Abate C, Patel L, Rauscher FJ, III, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 216.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen F, Ding M, Castranova V, Shi X. Carcinogenic metals and NF-κB activation. Mol. Cell. Biochem. 2001;222:159–171. [PubMed] [Google Scholar]
- 218.Kim YD, An SC, Oyama T, Kawamoto T, Kim H. Oxidative stress, hogg1 expression and NF-κB activity in cells exposed to low level chromium. J. Occup. Health. 2003;45:271–277. doi: 10.1539/joh.45.271. [DOI] [PubMed] [Google Scholar]
- 219.Wang S, Chen F, Zhang Z, Jiang BH, Jia L, Shi X. NF-κB prevents cells from undergoing Cr(VI)-induced apoptosis. Mol. Cell. Biochem. 2004;255:129–137. doi: 10.1023/b:mcbi.0000007269.74532.98. [DOI] [PubMed] [Google Scholar]
- 220.Chen F, Bower J, Leonard SS, Ding M, Lu Y, Rojanasakul Y, Kung HF, Vallyathan V, Castranova V, Shi X. Protective roles of NF-κB for chromium(VI)-induced cytotoxicity is revealed by expression of IκB kinase-β mutant. J. Biol. Chem. 2002;277:3342–3349. doi: 10.1074/jbc.M101089200. [DOI] [PubMed] [Google Scholar]
- 221.Shumilla JA, Broderick RJ, Wang Y, Barchowsky A. Chromium(VI) inhibits the transcriptional activity of nuclear factor-κB by decreasing the interaction of p65 with cAMP-responsive element-binding protein-binding protein. J. Biol. Chem. 1999;274:36207–36212. doi: 10.1074/jbc.274.51.36207. [DOI] [PubMed] [Google Scholar]
- 222.Shumilla JA, Wetterhahn KE, Barchowsky A. Inhibition of NF-κB binding to DNA by chromium, cadmium, mercury, zinc, and arsenite in vitro: evidence of a thiol mechanism. Arch. Biochem. Biophys. 1998;349:356–362. doi: 10.1006/abbi.1997.0470. [DOI] [PubMed] [Google Scholar]
- 223.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 224.Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-α-induced reactive oxygen species generation, NF-κB activation, and apoptosis. Toxicol. Sci. 2006;91:643–650. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 225.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus: a two-step mechanism of redox regulation of transcription factor NF-κB. J. Biol. Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 226.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-κB by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Das KC. c-Jun NH2-terminal kinase-mediated redox-dependent degradation of IκB: role of thioredoxin in NF-κB activation. J. Biol. Chem. 2001;276:4662–4670. doi: 10.1074/jbc.M006206200. [DOI] [PubMed] [Google Scholar]
- 228.Horton ND, Biswal SS, Corrigan LL, Bratta J, Kehrer JP. Acrolein causes inhibitor κB-independent decreases in nuclear factor κB activation in human lung adenocarcinoma (A549) cells. J. Biol. Chem. 1999;274:9200–9206. doi: 10.1074/jbc.274.14.9200. [DOI] [PubMed] [Google Scholar]
- 229.Ueno H, Kajihara H, Nakamura H, Yodoi J, Nakamuro K. Contribution of thioredoxin reductase to T-cell mitogenesis and NF-κB DNA-binding promoted by selenite. Antioxid. Redox Signaling. 2007;9:115–121. doi: 10.1089/ars.2007.9.115. [DOI] [PubMed] [Google Scholar]
- 230.Kim DH, Kundu JK, Surh YJ. Redox modulation of p53: mechanisms and functional significance. Mol. Carcinog. 2011;50:222–234. doi: 10.1002/mc.20709. [DOI] [PubMed] [Google Scholar]
- 231.Shen H, Maki CG. Pharmacologic activation of p53 by small-molecule MDM2 antagonists. Curr. Pharm. Des. 2011;17:560–568. doi: 10.2174/138161211795222603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol. Ther. 2004;3:156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 233.Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J. Biol. Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- 234.Russo P, Catassi A, Cesario A, Imperatori A, Rotolo N, Fini M, Granone P, Dominioni L. Molecular mechanisms of hexavalent chromium- induced apoptosis in human bronchoalveolar cells. Am. J. Respir. Cell Mol. Biol. 2005;33:589–600. doi: 10.1165/rcmb.2005-0213OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yao H, Guo L, Jiang BH, Luo J, Shi X. Oxidative stress and chromium(VI) carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2008;27:77–88. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- 236.Carlisle DL, Pritchard DE, Singh J, Patierno SR. Chromium(VI) induces p53-dependent apoptosis in diploid human lung and mouse dermal fibroblasts. Mol. Carcinog. 2000;28:111–118. doi: 10.1002/1098-2744(200006)28:2<111::aid-mc7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 237.Wang S, Shi X. Mechanisms of Cr(VI)-induced p53 activation: the role of phosphorylation, mdm2 and ERK. Carcinogenesis. 2001;22:757–762. doi: 10.1093/carcin/22.5.757. [DOI] [PubMed] [Google Scholar]
- 238.Gambelunghe A, Piccinini R, Abbritti G, Ambrogi M, Ugolini B, Marchetti C, Migliorati G, Balducci C, Muzi G. Chromium VI-induced apoptosis in a human bronchial epithelial cell line (BEAS-2B) and a lymphoblastic leukemia cell line (MOLT-4) J. Occup. Environ. Med. 2006;48:319–325. doi: 10.1097/01.jom.0000197859.46894.7d. [DOI] [PubMed] [Google Scholar]
- 239.Ceryak S, Zingariello C, O’Brien T, Patierno SR. Induction of pro-apoptotic and cell cycle-inhibiting genes in chromium (VI)-treated human lung fibroblasts: lack of effect of ERK. Mol. Cell. Biochem. 2004;255:139–149. doi: 10.1023/b:mcbi.0000007270.82431.3e. [DOI] [PubMed] [Google Scholar]
- 240.Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl. Acad. Sci. USA. 1998;95:10541–10546. doi: 10.1073/pnas.95.18.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Piccirillo S, Filomeni G, Brune B, Rotilio G, Ciriolo MR. Redox mechanisms involved in the selective activation of Nrf2-mediated resistance versus p53-dependent apoptosis in adenocarcinoma cells. J. Biol. Chem. 2009;284:27721–27733. doi: 10.1074/jbc.M109.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem. Sci. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ha L, Ceryak S, Patierno SR. Chromium(VI) activates ataxia telangiectasia mutated (ATM) protein: requirement of ATM for both apoptosis and recovery from terminal growth arrest. J. Biol. Chem. 2003;278:17885–17894. doi: 10.1074/jbc.M210560200. [DOI] [PubMed] [Google Scholar]
- 244.Moos PJ, Edes K, Cassidy P, Massuda E, Fitzpatrick FA. Electrophilic prostaglandins and lipid aldehydes repress redox-sensitive transcription factors p53 and hypoxia-inducible factor by impairing the selenoprotein thioredoxin reductase. J. Biol. Chem. 2003;278:745–750. doi: 10.1074/jbc.M211134200. [DOI] [PubMed] [Google Scholar]
- 245.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Keum YS. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: implications of posttranslational modifications. Ann. N. Y. Acad. Sci. 2011;1229:184–219. doi: 10.1111/j.1749-6632.2011.06092.x. [DOI] [PubMed] [Google Scholar]
- 247.Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic. Biol. Med. 2004;37:433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 248.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010;285:8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.He X, Lin GX, Chen MG, Zhang JX, Ma Q. Protection against chromium(VI)-induced oxidative stress and apoptosis by Nrf2: recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association. Toxicol. Sci. 2007;98:298–309. doi: 10.1093/toxsci/kfm081. [DOI] [PubMed] [Google Scholar]
- 250.Suvorova ES, Lucas O, Weisend CM, Rollins MF, Merrill GF, Capecchi MR, Schmidt EE. Cytoprotective Nrf2 pathway is induced in chronically txnrd 1-deficient hepatocytes. PLoS One. 2009;4:e6158. doi: 10.1371/journal.pone.0006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem. J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 252.Imhoff BR, Hansen JM. Tert-butylhydroquinone induces mitochondrial oxidative stress causing Nrf2 activation. Cell Biol. Toxicol. 2010;26:541–551. doi: 10.1007/s10565-010-9162-6. [DOI] [PubMed] [Google Scholar]
- 253.Hansen JM, Watson WH, Jones DP. Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol. Sci. 2004;82:308–317. doi: 10.1093/toxsci/kfh231. [DOI] [PubMed] [Google Scholar]
- 254.Izzotti A, Cartiglia C, Balansky R, D’Agostini F, Longobardi M, De Flora S. Selective induction of gene expression in rat lung by hexavalent chromium. Mol. Carcinog. 2002;35:75–84. doi: 10.1002/mc.10077. [DOI] [PubMed] [Google Scholar]
- 255.Niso-Santano M, Gonzalez-Polo RA, Bravo-San Pedro JM, Gomez-Sanchez R, Lastres-Becker I, Ortiz-Ortiz MA, Soler G, Moran JM, Cuadrado A, Fuentes JM. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: modulation by the Nrf2/Trx axis. Free Radic. Biol. Med. 2010;48:1370–1381. doi: 10.1016/j.freeradbiomed.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 256.Chew EH, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD, Holmgren A, Westwell AD. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention. Free Radic. Biol. Med. 2010;48:98–111. doi: 10.1016/j.freeradbiomed.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 257.Eriksson SE, Prast-Nielsen S, Flaberg E, Szekely L, Arnér ES. High levels of thioredoxin reductase 1 modulate drug-specific cytotoxic efficacy. Free Radic. Biol. Med. 2009;47:1661–1671. doi: 10.1016/j.freeradbiomed.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 258.Hu L, Miao W, Loignon M, Kandouz M, Batist G. Putative chemopreventive molecules can increase Nrf2-regulated cell defense in some human cancer cell lines, resulting in resistance to common cytotoxic therapies. Cancer Chemother. Pharmacol. 2010;66:467–474. doi: 10.1007/s00280-009-1182-7. [DOI] [PubMed] [Google Scholar]
- 259.Ye J, Shi X. Gene expression profile in response to chromium-induced cell stress in A549 cells. Mol. Cell. Biochem. 2001;222:189–197. [PubMed] [Google Scholar]
- 260.O’Hara KA, Nemec AA, Alam J, Klei LR, Mossman BT, Barchowsky A. Chromium(VI) inhibits heme oxygenase-1 expression in vivo and in arsenic-exposed human airway epithelial cells. J. Cell. Physiol. 2006;209:113–121. doi: 10.1002/jcp.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One. 2011;6:e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Reddy NM, Potteti HR, Mariani TJ, Biswal S, Reddy SP. Conditional deletion of Nrf2 in airway epithelium exacerbates acute lung injury and impairs the resolution of inflammation. Am. J. Respir. Cell Mol. Biol. 2011;45:1161–1168. doi: 10.1165/rcmb.2011-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rubio V, Zhang J, Valverde M, Rojas E, Shi ZZ. Essential role of Nrf2 in protection against hydroquinone- and benzoquinone-induced cytotoxicity. Toxicol. in Vitro. 2011;25:521–529. doi: 10.1016/j.tiv.2010.10.021. [DOI] [PubMed] [Google Scholar]