Abstract
Goals and Background
Inflammatory bowel disease (IBD) serology testing is often used in patients with indeterminate colitis (IC) to help distinguish between ulcerative colitis (UC) and Crohn’s disease (CD). We investigated the performance of serology testing in predicting future diagnosis in this setting.
Study
Observational study of individuals with IC at a single center who underwent IBD serology testing (pANCA, ASCA and anti-OmpC) and had at least 12 months follow-up from time of serology result.
Results
117 individuals with IC and 1 year follow-up data were enrolled. All IC patients had endoscopic and histologic evidence of colitis at enrollment. One year after serology testing, 58 (50%) individuals with IC were diagnosed with UC, 49 (42%) with CD, and 10 (9%) remained labeled with IC. The sensitivity/specificity of an initial positive pANCA for a subsequent diagnosis of UC was 78%/44%. For ASCA and anti-OmpC, the results were 18%/84% and 27%/75%, respectively, for a subsequent diagnosis of CD. A positive pANCA test was associated with a likelihood ratio (LR) of 1.4 (95% CI: 1.1–1.8) for a subsequent diagnosis of UC at 1 year. Neither positive ASCA (LR 1.1; 95% CI: 0.5–2.5) nor anti-OmpC (LR 1.1; 95% CI: 0.6–2.0) was associated with a subsequent diagnosis CD in patients with IC.
Conclusions
The disease phenotype in the majority of individuals initially labeled with IC evolved to be more consistent with either UC or CD on follow-up. pANCA, ASCA, and anti-OmpC, individually, were of limited utility in predicting a patient’s subsequent disease phenotype.
Keywords: Crohn’s disease, ulcerative colitis, indeterminate colitis, diagnostics
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the mucosa of the intestinal tract. The majority of individuals with IBD have disease characteristics consistent with either Crohn’s disease (CD) or ulcerative colitis (UC) based on clinical, radiological, endoscopic, and histological features1;2. A number of serum antibodies have also been associated with a diagnosis of CD or UC, including anti-Saccharomyces cerevisiae antibody (ASCA), perinuclear anti-neutrophil cytoplasmic antibody (pANCA), anti-outer membrane porin C antibody (anti-OmpC), and anti-CBir1 flagellin antibody (anti-CBir1). Published studies have reported the sensitivity and specificity of these antibody assays when distinguishing CD or UC from healthy or non-IBD diarrheal controls3.
The distinction between CD and UC at the time of initial diagnosis still has importance in patient management in this era; early intervention with biologic therapies has been advocated to reduce disease-related complications in CD, a strategy that is not the case in UC1. In addition, the extent and type of surgical management of refractory disease is different for patients with UC and CD. Approximately 10–15% of individuals with features of IBD lack definitive evidence to discriminate between UC or CD; these individuals are typically described as having “indeterminate colitis” (IC)4. Although originally a descriptive term for colectomy specimens with features of both UC and CD, in clinical practice it encompasses those patients with chronic intestinal inflammation in which clinical features, including endoscopic and biopsy findings, are inadequate to make a definitive diagnosis of either UC or CD5;6.
In 2005, a Working Party at the World Congress of Gastroenterology proposed a new term, inflammatory bowel disease unclassified (IBDU), to classify these patients, although this has yet to become widely used7. One of the outcomes of this Working Party was the recommendation that the role of serological markers be assessed in this patient population8. In the view of some experts, the classification of IC is a provisional one and, over time or with further investigations, many of these patients will be re-classified as having CD or UC9;10. However, only one published study to-date has examined the value of IBD serology assays specifically in patients with IC11.
The aim of this study was to define further the test characteristics of IBD serology in predicting a subsequent CD or UC diagnosis in a cohort of individuals with IC.
Materials and Methods
From an initial cohort of individuals who underwent IBD serology testing at a major academic medical center from 2001 to 2007, those diagnosed with IC and with at least one year follow-up after serology testing were selected. The electronic medical records (clinical notes, endoscopy, pathology and radiology reports) were reviewed by a single reviewer (SS) to confirm the established diagnosis of IC, with uncertain cases reviewed by the senior author (ACM). Only cases meeting the clinical criteria proposed by the International Organization for Inflammatory Bowel Disease for a diagnosis of IC were included; “patients who appear to have IBD colitis but who cannot be readily classified when all clinical, radiological, endoscopic, histologic, and serologic data are taken into account” 4.
All individuals underwent ASCA, pANCA, and anti-OmpC testing (IBD Serology, Prometheus Inc., San Diego, CA). Eight of 117 individuals underwent anti-CBir1 antibody testing due to its late addition to commercially available panels. Positive tests were defined as values greater than assay reference values, similar to methodology used in prior studies12;13. For ASCA, a positive IgA or IgG indicated a positive result. For pANCA, a positive ELISA test regardless of indirect immunofluorescence assay (IFA) result indicated a positive test.
The appropriate institutional IRB approval was attained prior to initiating the retrospective chart review. Data collection involved review of clinician notes, laboratory data, imaging studies, pathology reports, and serology results. At one year following IBD serology testing, patient records and results were examined to establish clinical diagnosis at the follow-up time-point (IC, UC, or CD). The follow-up diagnosis of each subject was confirmed by the authors based on accepted criteria, and blinded to the serology results (Suppl. Table 1)14;15.
Data analysis was conducted with JMP, Version 8 (SAS Institute Inc., Cary, NC). To determine associations between antibody and disease, Wilcoxon rank sum test was used for continuous outcomes and Pearson’s chi-square test for dichotomous outcomes. Two-by-two tables were used to calculate test characteristics. Finally, likelihood ratios for each antibody were also calculated to determine clinical usefulness, as performed in a prior study16. Sensitivity, specificity and likelihood ratios were based on calculations including only the inception IC cohort; healthy controls and patients with gastrointestinal illness without evidence of IBD were not included.
Results
Characteristics of IC Cohort
Of an initial cohort of individuals who underwent IBD serology testing from 2001 to 2007, 117 had a diagnosis of IC at the time of serology testing, and had at least one year of follow-up data (Table 1). Consistent with criteria for IC, all individuals had abnormal colonoscopies and evidence of chronic inflammation. Sixteen individuals also had abnormalities in the ileum noted on small bowel follow through and/or CT/MRI, in addition to continuous endoscopic colorectal involvement. Patients with prior colectomies for UC were classified as IC after developing subsequent manifestations suspicious for the alternative diagnosis (e.g. perianal fistulas or complicated pouchitis).
Table 1.
Baseline characteristics of individuals with IC at time of IBD serology testing (in %, unless otherwise indicated).
| Number of subjects (N) | 117 |
| Female | 53 |
| Age at testing (mean ± sd, in years) | 48 ± 14 |
| Symptoms | |
| Diarrhea | 89 |
| Abdominal pain | 86 |
| Rectal bleeding | 83 |
| Prior resection | 15 |
| Fistula | 10 |
| Elevated CRP/ESR | 62 |
| Abnormal colonoscopy | 100 |
| Chronic inflammation or crypt abscess on biopsy or pathology | 100 |
| Granuloma on biopsy or pathology | 9 |
| Abnormal SBFT | 15 |
| Abnormality in small bowel on | 26 |
| CT/MRI |
Of the 117 patients with a diagnosis of IC at time of enrollment, 58 (50%) were reclassified as having UC, 49 (42%) as CD, and 10 (9%) remained classified as IC after 12 months follow-up.
Test Characteristics of IBD Serology in IC
In the IC cohort at baseline, 67% of patients were pANCA positive by ELISA, 17% had elevated ASCA, and 26% had elevated OmpC levels. Mean (standard deviation) levels of pANCA IgG, ASCA IgA, ASCA IgG, and anti-OmpC IgA are noted in Table 2. The percent of individuals with elevated levels of each antibody, grouped according to their diagnosis at 12 months, is shown in Table 3. The prevalence of ASCA and anti-OmpC were similar in patients later diagnosed as UC and CD in the IC population. The presence of pANCA differentiated a subsequent diagnosis of UC from CD (p=0.008) in patients initially diagnosed with IC. None of the other serological tests distinguished a subsequent diagnosis of UC from CD, or either diagnosis from IC, in this patient population.
Table 2.
Mean (sd) antibody levels in EU/ml at time of baseline serology testing.
| IC | |
|---|---|
| ASCA IgA | 9.3 (20) |
| ASCA IgG | 13.4 (25) |
| pANCA IgG | 39.7 (52) |
| pANCA IFA (% positive) | 50 |
| Anti-OmpC IgA | 13.1 (13) |
| Anti-CBir1 IgG | 28.6 (45) |
Table 3.
Prevalence of positive serology according to diagnosis at 12 months (in %)
| IC | UC | CD | IC vs. UC p-value* |
IC vs. CD p-value* |
|
|---|---|---|---|---|---|
| ASCA (+) | 30 | 14 | 18 | 0.20 | 0.40 |
| pANCA (+) | 70 | 78 | 53 | 0.60 | 0.33 |
| Anti-OmpC (+) | 10 | 28 | 27 | 0.24 | 0.26 |
Pearson’s chi-square test.
Test characteristics were calculated using 2×2 tables (Table 4). Test characteristics of anti-CBir1 were not determined due to the small number of individuals undergoing testing (n=8). The sensitivity and specificity of ASCA and anti-OmpC were similar for a subsequent diagnosis of either CD or UC in patients initially diagnosed with IC. pANCA demonstrated improved sensitivity and specificity for a subsequent diagnosis of UC in this population.
Table 4.
Sensitivity and specificity for a subsequent diagnosis of CD or CD at 12 months in patients initially diagnosed with IC, based on published antibody cutoff levels.
| UC | CD | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| ASCA (+) | 14 | 80 | 18 | 84 |
| pANCA (+) | 78 | 44 | 53 | 24 |
| Anti-OmpC (+) | 28 | 76 | 27 | 75 |
The positive and negative likelihood ratios of pANCA for a diagnosis of UC at one year were 1.4 (95% CI: 1.1–1.8) and 0.5 (95% CI: 0.3–0.9), respectively (Figure 1). pANCA testing also had some utility for excluding CD; the positive and negative likelihood ratios of a diagnosis of CD at one year were 0.7 (95% CI: 0.5–0.9) and 2.0 (95% CI: 1.4–3.0), respectively.
Figure 1.
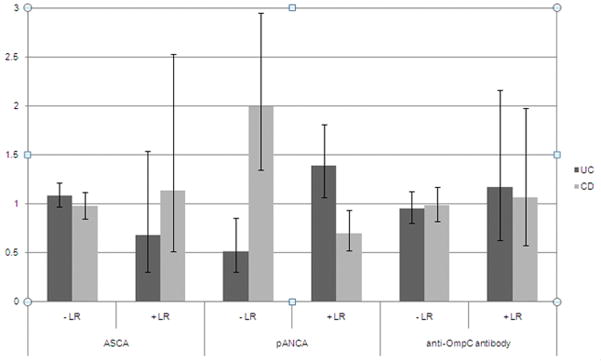
Negative (−) and positive (+) likelihood ratio (LR) for UC or CD diagnosis at 12 months following serology testing in patients with IC.
Discussion
At present, the diagnosis of IBD is based upon history and endoscopic, radiologic and/or histological data. Serologic testing for antibodies to intrinsic or extrinsic antigens has become a complementary part of this process in clinical practice. These assays have been recommended as tools to assist in diagnosis and “as predictors of an individual’s disease course”17.
Positive ASCA was previously shown to be 41–76% sensitive and 86–98% specific for a diagnosis of CD when compared to healthy controls and individuals with UC12. Several subsequent studies have supported this pattern of moderate sensitivity with good to excellent specificity for CD18–22. Antibodies to both OmpC or CBir1 have poor sensitivity for CD but are associated with subsets of complicated CD23–25. pANCA has a reported sensitivity of 50–68% and specificity of 82–94% for UC in cohorts comprised of various combinations of patients with UC, CD, or non-IBD diarrheal illness and healthy controls18;20–22;26. A meta-analysis of 60 studies concluded similar test characteristics27.
Studies assessing the combination of ASCA and pANCA assays for diagnosis of CD and UC have shown mixed results; whereas some found reduced sensitivity and improved specificity21;22;26, others demonstrated no significant change in diagnostic value18;20. As a consequence, some expert groups have advised against using serology testing in screening for IBD alone15.
In clinical practice, and based on this data (above), serology is frequently used when trying to distinguish between UC and CD in patients with IC, despite a lack of data to assess its performance 4. Joossens et al. assessed the role of ASCA and pANCA, and later anti-OmpC and anti-I2, in 97 individuals with IC11;28. At one year, sensitivity/specificity of ASCA and pANCA for a CD and UC diagnosis was 59%/75% and 57%/81%, respectively; combining the two assays reduced the sensitivity and improved the specificity modestly. Adding anti-OmpC and anti-I2 antibody serologies in a subsequent study of the same population showed a marginal improvement in positive predictive value with a significant drop in specificity. At four years from initial evaluation, the majority of individuals remained in the IC cohort, increasing suspicion for a potential separate phenotype within IBD28.
In comparison to findings by Joossens et al., other groups have reported a diagnostic change from IC to CD or UC at 1 to 2 years in up to 50% of patients 11;21;29. This is the basis for the concept that IC is a “disease in evolution”9. The majority of subjects in our study were classified with a diagnosis or CD or UC at 12 months. Of an initial cohort of 117 individuals, 58 (50%) met criteria for UC and 49 (42%) for CD at 1 year. The difference may be related to narrow diagnostic criteria used by Joossens et al.; classification for CD, for example, required “characteristic small bowel involvement, when fistula occurred, or when granulomas were found at biopsy” which would exclude many patients with colonic CD11. Regardless of whether the label of IC remains fixed or stable over time, IBD serology is frequently used to distinguish UC from CD in this patient population8.
The value of the individual antibodies for predicting diagnosis at one year differed in comparison to that reported in prior studies of populations with less obscure disease patterns. ASCA and anti-OmpC showed good specificity (84% and 75%, respectively) and poor sensitivity (18% and 27%, respectively) for CD. The specificity may be lower than previously reported given the absence of healthy controls or individuals with known non-IBD illness in our study. The sensitivity would be expected to be unchanged by individuals with non-IBD illness included in the cohort; rather, reduced sensitivity in our study may be due to a unique CD population with slowly evolving disease phenotype.
Although usually associated with CD alone, the diagnostic value of ASCA and anti-OmpC were similar for UC. This suggests potential serologic overlap in this population with atypical presentation for CD or UC, similar to their phenotypic overlap.
pANCA showed a reverse pattern from that previously reported, with sensitivity (78%) greater than specificity (44%) for UC. As stated above, the decreased specificity may be related to exclusion of healthy controls and individuals with known non-IBD gastrointestinal illness. The sensitivity of pANCA for UC in our study was slightly higher than previously reported values; at four years follow-up, only 47% of individuals with UC in the Joossens et al. study had a positive pANCA11. Some difference may be attributable to technique; whereas test characteristics in our study were based on pANCA ELISA testing, Joossens et al. used pANCA IFA28.
Amongst the IBD serologic assays studied, only pANCA provided modest diagnostic utility in this study. In patients with IC and high clinical suspicion for UC, a positive pANCA can increase the posttest probability for UC and decrease that for CD. Emphasis in this statement is given to high clinical suspicion, given that the likelihood ratios close to one correlate to a change in post-test probability of less than 15%30. More useful clinically may be pANCA testing in individuals with IC with low suspicion for UC and high suspicion for CD; in this cohort, a negative pANCA can decrease the post-test probability for UC and increase the post-test probability for CD by approximately 15%.
Limitations to this study include the retrospective review of patients’ records, and the lack of results from a definitive IBD cohort for comparison. In addition, as shown previously, the classification of a patient with IC, versus CD, versus UC is subject to some subjective criteria and interobserver variation31. To minimize the effects of these limitations we used a comprehensive electronic medical record (EMR) to collect patient data, and a single reviewer used pre-defined criteria to classify individuals in each category for outcome measures.
We were unable to determine the characteristics of all the available serologies in this patient cohort, including CBir1 and anti-flagellin, as only ASCA, ANCA and OmpC were included in the Prometheus panel for the majority of this study. Whether a more extensive panel of antibodies would yield greater test characteristics in this patient population is unknown. The paucity of comparable studies in the literature on this topic, despite widespread use of IBD serology for patients with IC, highlights the need for further data in this field8.
In conclusion, this study suggests that many patients with a diagnosis of IC will be reclassified as having UC or CD over time, as their disease pattern evolves. In patients with IC, a positive pANCA may predict a subsequent diagnosis of UC, whereas negative pANCA may predict a subsequent diagnosis of CD. The overall ability of IBD serology to predict an individual’s subsequent disease profile (UC, or CD, or IC) is modest at best.
Supplementary Material
Acknowledgments
Sources of support requiring acknowledgment: ACM is supported by K23 DK084338 from NIDDK
Abbreviations
- anti-CBir1
anti-CBir1 flagellin antibody
- anti-OmpC
anti-outer membrane porin C antibody
- ASCA
anti-Saccharomyces cerevisiae antibody
- CD
Crohn’s disease
- IC
indeterminate colitis
- IBD
inflammatory bowel disease
- pANCA
perinuclear anti-neutrophil cytoplasmic antibody
- UC
ulcerative colitis
Footnotes
Conflict of Interest Disclosure: ASC has served on advisory boards for Prometheus Laboratories Inc.
Reference List
- 1.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–32. doi: 10.1053/j.gastro.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ. Serologic markers in inflammatory bowel disease: state of the art. Rev Gastroenterol Disord. 2004;4:167–74. [PubMed] [Google Scholar]
- 4.Geboes K, Colombel JF, Greenstein A, et al. Indeterminate colitis: a review of the concept--what’s in a name? Inflamm Bowel Dis. 2008;14:850–857. doi: 10.1002/ibd.20361. [DOI] [PubMed] [Google Scholar]
- 5.Price AB. Overlap in the spectrum of non-specific inflammatory bowel disease--’colitis indeterminate’. J Clin Pathol. 1978;31:567–77. doi: 10.1136/jcp.31.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tremaine WJ. Review article: Indeterminate colitis--definition, diagnosis and management. Aliment Pharmacol Ther. 2007;25:13–17. doi: 10.1111/j.1365-2036.2006.03159.x. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 (Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 8.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geboes K, De Hertogh G. Indeterminate colitis. Inflamm Bowel Dis. 2003;9:324–31. doi: 10.1097/00054725-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Tremaine WJ. Is indeterminate colitis determinable? Curr Gastroenterol Rep. 2012;14:162–65. doi: 10.1007/s11894-012-0244-x. [DOI] [PubMed] [Google Scholar]
- 11.Joossens S, Reinisch W, Vermeire S, et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology. 2002;122:1242–47. doi: 10.1053/gast.2002.32980. [DOI] [PubMed] [Google Scholar]
- 12.Vermeire S, Joossens S, Peeters M, et al. Comparative study of ASCA (Anti-Saccharomyces cerevisiae antibody) assays in inflammatory bowel disease. Gastroenterology. 2001;120:827–33. doi: 10.1053/gast.2001.22546. [DOI] [PubMed] [Google Scholar]
- 13.Davis MK, Andres JM, Jolley CD, Novak DA, Haafiz AB, Gonzalez-Peralta RP. Antibodies to Escherichia coli outer membrane porin C in the absence of anti-Saccharomyces cerevisiae antibodies and anti-neutrophil cytoplasmic antibodies are an unreliable marker of Crohn disease and ulcerative colitis. J Pediatr Gastroenterol Nutr. 2007;45:409–13. doi: 10.1097/MPG.0b013e31812f7f6e. [DOI] [PubMed] [Google Scholar]
- 14.Lennard-Jones JE, Shivananda S. Clinical uniformity of inflammatory bowel disease a presentation and during the first year of disease in the north and south of Europe. EC-IBD Study Group. Eur J Gastroenterol Hepatol. 1997;9:353–59. doi: 10.1097/00042737-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Stange EF, Travis SP, Vermeire S, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55 (Suppl 1):i1–15. doi: 10.1136/gut.2005.081950a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermeulen N, Vermeire S, Rutgeerts P, Bossuyt X. Likelihood ratio for Crohn’s disease as a function of anti-Saccharomyces cerevisiae antibody concentration. Inflamm Bowel Dis. 2010;16:5–6. doi: 10.1002/ibd.20905. [DOI] [PubMed] [Google Scholar]
- 17.Dubinsky MC. Serologic and laboratory markers in prediction of the disease course in inflammatory bowel disease. World J Gastroenterol. 2010;16:2604–8. doi: 10.3748/wjg.v16.i21.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand V, Russell AS, Tsuyuki R, Fedorak R. Perinuclear antineutrophil cytoplasmic autoantibodies and anti-Saccharomyces cerevisiae antibodies as serological markers are not specific in the identification of Crohn’s disease and ulcerative colitis. Can J Gastroenterol. 2008;22:33–36. doi: 10.1155/2008/974540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaila B, Orr K, Bernstein CN. The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn’s disease and predicting inflammatory disease. Can J Gastroenterol. 2005;19:717–21. doi: 10.1155/2005/147681. [DOI] [PubMed] [Google Scholar]
- 20.Koutroubakis IE, Petinaki E, Mouzas IA, et al. Anti-Saccharomyces cerevisiae mannan antibodies and antineutrophil cytoplasmic autoantibodies in Greek patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96:449–54. doi: 10.1111/j.1572-0241.2001.03524.x. [DOI] [PubMed] [Google Scholar]
- 21.Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt X, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- 22.Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–91. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papp M, Altorjay I, Dotan N, et al. New serological markers for inflammatory bowel disease are associated with earlier age at onset, complicated disease behavior, risk for surgery, and NOD2/CARD15 genotype in a Hungarian IBD cohort. Am J Gastroenterol. 2008;103:665–81. doi: 10.1111/j.1572-0241.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 24.Peyrin-Biroulet L, Standaert-Vitse A, Branche J, Chamaillard M. IBD serological panels: facts and perspectives. Inflamm Bowel Dis. 2007;13:1561–66. doi: 10.1002/ibd.20226. [DOI] [PubMed] [Google Scholar]
- 25.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 26.Mokrowiecka A, Daniel P, Slomka M, Majak P, Malecka-Panas E. Clinical utility of serological markers in inflammatory bowel disease. Hepatogastroenterology. 2009;56:162–66. [PubMed] [Google Scholar]
- 27.Reese GE, Constantinides VA, Simillis C, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–2422. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 28.Joossens S, Colombel JF, Landers C, et al. Anti-outer membrane of porin C and anti-I2 antibodies in indeterminate colitis. Gut. 2006;55:1667–69. doi: 10.1136/gut.2005.089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moum B, Ekbom A, Vatn MH, et al. Inflammatory bowel disease: re-evaluation of the diagnosis in a prospective population based study in south eastern Norway. Gut. 1997;40:328–32. doi: 10.1136/gut.40.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646–49. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farmer M, Petras RE, Hunt LE, Janosky JE, Galandiuk S. The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am J Gastroenterol. 2000;95:3184–88. doi: 10.1111/j.1572-0241.2000.03199.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


