Abstract
BACKGROUND:
Sorafenib, an oral multityrosine kinase inhibitor, has been approved for treatment of unresectable hepatocellular carcinoma (HCC). British Columbia (BC) was the first province in Canada to provide drug coverage for sorafenib.
OBJECTIVE:
To review the BC experience with sorafenib to assess its effectiveness and tolerance in a ‘real-world’ clinical setting.
METHODS:
A retrospective clinic chart review identified 99 patients referred to the BC Cancer Agency from 2008 to 2010 with a diagnosis of HCC who qualified for treatment with sorafenib.
RESULTS:
Therapy with sorafenib was initiated and continued at a reduced dosage of 400 mg/day in 66 of 99 patients, with 22 patients requiring further dose reduction. Full- and reduced-dose group patients had similar baseline characteristics, except for a higher proportion of female patients (P=0.02) and individuals with alcoholic liver disease (P=0.04) in the full-dose group. The incidence of any grade of adverse effects was higher in the full-dose group (94% versus 77% in the reduced-dose group; P=0.04). Dose reduction rates were significantly higher in the full-dose group, occurring in 66% versus 24% of reduced-dose group patients (P=0.001). The overall survival rates were similar between the two groups: 7.8 months versus 7.1 months in full- versus reduced-dose groups (P=0.14), as were radiological progression rates and alpha-fetoprotein levels.
CONCLUSIONS:
In a review of 99 patients in a ‘real-world’ community setting, a sorafenib dose of 400 mg/day was better tolerated and had similar efficacy compared with a sorafenib dose of 800 mg/day with respect to survival and outcomes.
Keywords: Chemotherapy, HCC, Liver, Sorafenib, Survival
Abstract
HISTORIQUE :
Le sorafénib, un inhibiteur de la multityrosine kinase, est approuvé pour traiter les carcinomes hépatocellulaires (CHC) non résécables. La Colombie-Britannique (C.-B.) est la première province du Canada à avoir remboursé le sorafénib.
OBJECTIF :
Examiner l’expérience de la C.-B. à l’égard du sorafénib pour en évaluer l’efficacité et la tolérance dans une « véritable » clinique.
MÉTHODOLOGIE :
Dans le cadre d’une analyse rétrospective des dossiers cliniques, les chercheurs ont repéré 99 patients aiguillés à la BC Cancer Agency entre 2008 et 2010 en raison d’un diagnostic de CHC et qui étaient admissibles au traitement au sorafénib.
RÉSULTATS :
Le traitement au sorafénib était amorcé et poursuivi à une dose réduite de 400 mg/jour chez 66 des 99 patients, 22 patients ayant dû réduire leur dose davantage. Le groupe de patients ayant reçu une dose complète et celui ayant reçu une dose réduite présentaient des caractéristiques similaires en début d’étude, sauf pour une proportion plus élevée de femmes (P=0,02) et de personnes ayant une maladie hépatique d’origine alcoolique (P=0,04) dans le groupe à dose complète. L’incidence d’effets indésirables de quelque gravité que ce soit était plus élevée dans le groupe à dose complète (94 % par rapport à 77 % dans l’autre groupe; P=0,04). Le taux de diminution de la dose était considérablement plus élevé dans le groupe à dose complète, puisqu’on l’observait chez 66 % des patients, par rapport à 24 % de ceux de l’autre groupe (P=0,001). Le taux de survie globale était similaire entre les deux groupes : 7,8 mois dans le groupe à dose complète, par rapport à 7,1 mois dans l’autre groupe (P=0,14), tout comme le rythme d’évolution radiologique et le taux d’alpha-fœtoprotéine.
CONCLUSIONS :
Dans l’examen d’un groupe de 99 patients d’une « véritable » clinique communautaire, une dose de 400 mg/jour de sorafénib était mieux tolérée et avait une efficacité similaire à une dose de 800 mg/jour sur le plan de la survie et des issues.
Hepatocellular carcinoma (HCC) accounts for more than 80% of all liver cancers and is currently the fifth leading cause of cancer-related deaths worldwide (1). Many patients have advanced disease at the time of diagnosis, accounting for a poor five-year survival rate of only 15% (1). Sorafenib (Nexavar, Bayer HealthCare Pharmaceuticals, Germany; Onyx Pharmaceuticals, USA) is the first Food and Drug Administration (FDA)-approved systemic therapy for patients with advanced HCC not amenable to local regional therapy (2). Sorafenib is a multityrosine kinase inhibitor that targets the Raf/MEK/Erk pathway, inhibiting proliferation and angiogenesis (3). A standard dose of 800 mg/day given as 400 mg twice/day was chosen to be maximally tolerated based on phase I trials (4). This dose was subsequently tested for efficacy in phase II and phase III trials (5,6).
The landmark Sorafenib HCC Assessment Randomized Protocol Trial (SHARP) published in the New England Journal of Medicine in 2008 (6) was a multicentre, double-blinded, placebo-controlled randomized control trial (RCT) involving 602 predominantly Child-Pugh (CP) class A (ie, well-compensated cirrhosis) patients with an advanced HCC who were randomly assigned to placebo or 800 mg of sorafenib treatment. This trial was prematurely stopped at the second interim analysis due to a significant 2.8-month survival benefit in the treatment arm. The reported adverse effects (AEs) rate was 80%, necessitating dose reduction in 26% of patients; however, the full extent of AEs was difficult to extrapolate given the early trial termination. A confirmatory Asia-Pacific trial, as well as other RCTs published to date, show a great deal of variability in the incidence of AEs and dose reduction rates (7). According to the most recent systematic review by Xie et al (8), to date, seven RCTs examining the use of sorafenib in patients with advanced HCC have been published (8). The overall survival in these studies ranged from 4.2 to 15.6 months, with incidence of AEs occurring in 1% of patients in one study and 97% in another. The Global Investigation of Therapeutic DEcisions in Hepatocellular Carcinoma and Of its Treatment with SorafeNib (GIDEON) (9) is the largest study assessing safety of sorafenib in real-world clinical practice conditions in 39 countries. The first interim analysis of 479 patients followed for four months showed an incidence of AEs consistent with previous RCTs; however, it was noted that 24% of patients were started at a dose <800 mg/day. Moreover, the study reported that medical oncologists were more likely to prescribe lower doses of medications compared with hepatologists.
No consistent data regarding dose reduction rates with sorafenib exist and, to date, no study has been dedicated to assess dose-dependent outcomes. It has previously been proposed that targeted molecular agents could retain anticancer activity at reduced dosages (10). Because sorafenib therapy is costly, optimizing the dosage to balance efficacy with AEs could be of potential benefit (11,12).
British Columbia was the first province in Canada to approve and provide financial coverage for sorafenib for advanced HCC through the BC Cancer Agency (BCCA), an agency of the Ministry of Health. British Columbia has a unique patient population with high proportion of Asian descent patients and all sorafenib use in British Columbia is through the BCCA clinics. To contribute to the growing literature on sorafenib efficacy and safety, and to explore the impact of dose reduction on its effectiveness, we reviewed our clinical ‘real-world’ experience with sorafenib-treated advanced HCC outside of a clinical trial setting.
METHODS
Patients
A retrospective chart review of 99 patients referred to the clinic at BCCA with the diagnosis of advanced HCC who received sorafenib treatment for the period between January 1, 2008 to December 31, 2010, was conducted. The diagnosis of HCC was confirmed either histologically or radiologically in combination with characteristic biochemical/clinical factors according to American Association for the Study of Liver Diseases criteria (13). Patients with advanced HCC included individuals who underwent previous local therapies including resection, transarterial chemoembolization and radiofrequency ablation, as well as those with disease too advanced for the former therapeutic modalities. Demographic data, and relevant clinical, laboratory and radiological investigations, were retrospectively collected.
Treatment characteristics and outcomes
Initial sorafenib treatment dosage was determined by the treating physician based on his/her clinical judgment. Patients were either started on 800 mg/day or on a reduced dose of 400 mg/day based on their clinical assessment. AEs were graded according to the National Cancer Institute Common Terminology Criteria (CTCAE) version 3.0 grading system. Treatment response was monitored with monthly follow-up and further dose reduction to 400 mg/day, 200 mg/day or 400 mg every other day was made when grade 3/4 AEs were observed. Radiological response was recorded based on computed tomography scans and graded according to tumour burden as noted in the radiologist report. Overall survival was reported as median number of months from initiation of sorafenib treatment to death or latest follow-up date.
Data analysis
Patients’ baseline characteristics were compared using the paired Student’s t test. Overall survival and treatment duration was analyzed using the Kaplan-Meier method; a two-tailed P<0.05 was considered to be statistically significant. All analyses were performed using SPSS version 19 (IBM Corporation, USA).
RESULTS
Sorafenib dosage
Ninety-nine patients who received soreafib treatment were identified in the review (Figure 1). Thirty-three (33%) of these patients were started on sorafenib 800 mg/day. Twenty-two patients (66%) experienced significant AEs necessitating dose reduction. Sixty-six patients (66%) were started on 400 mg/day, 26 (40%) of whom had to further reduce the dose due to grade 3/4 AEs (P=0.018).
Figure 1).
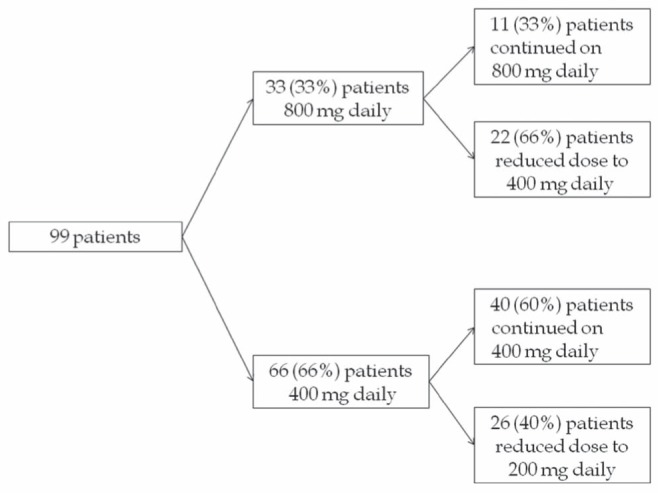
Sorafenib dose schedule in patients with advanced hepatocellular carcinoma
Patient characteristics
Differences in demographic data were analyzed in two patient groups comprising 33 patients in the full-dose and 66 patients in the one-half the recommended dose (ie, reduced-dose) groups (Table 1). There was no appreciable difference in ethnic composition between two groups; however, the reduced-dose group contained more female patients (P=0.02). Hepatitis B virus infection accounted for 44% and 45% in the full- and reduced-dose groups, respectively. Alcoholic liver damage was present more often in full-dose patients (P=0.04). There was no significant difference in Eastern Cooperative Oncology Group performance status, Barcelona Clinic Liver Cancer stage or CP scores between the two groups. There was no difference in pretreatment alpha-fetoprotein levels between the two groups. Tumour characteristics were also similar between two groups, except portal vein thrombosis, which was more prevalent in reduced-dose patients (P=0.02). Eighty-nine patients (89%) died during the follow-up period and 10 (11%) were alive at the last follow-up date (April 31, 2012).
TABLE 1.
Demographic and clinical characteristics of patients treated with sorafenib
|
Sorafenib dose/day
|
P | ||
|---|---|---|---|
| 800 mg (n=33) | 400 mg (n=66) | ||
| Age, years, mean ± SD | 58.6±10.4 | 63.4 ±11.1 | 0.1 |
| Sex | |||
| Male/female | 94/6 | 73/27 | 0.02 |
| Ethnicity | |||
| Asian | 45 | 60 | 0.2 |
| Caucasian | 55 | 40 | 0.2 |
| Etiology | |||
| Hepatitis B virus | 45 | 44 | 1.0 |
| Hepatitis C virus | 18 | 30 | 0.23 |
| Alcohol | 21 | 6 | 0.04 |
| Eastern Cooperative Oncology Group | |||
| 0 | 51 | 42 | 0.4 |
| 1 | 30 | 33 | 0.8 |
| 2 | 12 | 15 | 0.8 |
| 3 | 7 | 10 | 0.7 |
| Child-Pugh score | |||
| A | 90 | 90 | 1.0 |
| B7 | 4 | 7 | 0.7 |
| B8–9 | 6 | 3 | 0.6 |
| Barcelona Clinic Liver Cancer staging, % | |||
| B | 42 | 45 | 0.83 |
| C | 58 | 55 | 0.83 |
| AFP ng/mL, median (range) | 140 (1.5–200,000) | 158 (2–400,000) | |
| Previous treatments | |||
| TACE | 55 | 55 | 1.00 |
| Resection | 27 | 21 | 0.61 |
| Ablation* | 6 | 14 | 0.33 |
| Combination | 21 | 23 | 1.00 |
| Tumour characteristics | |||
| Multinodular | 60 | 42 | 0.09 |
| Extrahepatic spread | 30 | 30 | 1.00 |
| Portal vein thrombosis | 15 | 38 | 0.02 |
Data presented as % unless otherwise indicated. Bolded values indicate statistical significance.
Including radiofrequency ablation and lipiodol ablation. AFP Alpha-fetoprotein; TACE Transarterial chemoembolization
AEs
The overall incidence of any grade of AE was statistically higher in the full-dose group (94% versus 77%; P=0.04) (Table 2). However, the incidence of organ system-specific side effects did not differ between the two groups, indicating that the type of AEs were similar in the two groups but occurred more often in full-dose patients. Three per cent of patients in both groups developed gastrointestinal bleeding that led to discontinuation of treatment.
TABLE 2.
Incidence of adverse effects in patients treated with sorafenib
|
Sorafenib dose/day
|
P | ||
|---|---|---|---|
| 800 mg (n=33) | 400 mg (n=66) | ||
| Overall | 31 (94) | 51 (77) | 0.04 |
| Dermatological | 16 (48) | 21 (32) | 0.12 |
| Rash | 6 (18) | 11 (17) | 1.00 |
| Hand-foot syndrome | 10 (30) | 10 (15) | 0.11 |
| Constitutional | 13 (40) | 15 (23) | 0.10 |
| Fatigue | 13 (40) | 15 (23) | 0.10 |
| Weight loss | 5 (5) | 8 (12) | 0.75 |
| Gastrointestinal | 9 (27) | 20 (31) | 0.80 |
| Nausea/vomiting | 4 (12) | 9 (14) | 1.00 |
| Diarrhea | 7 (21) | 11 (17) | 0.59 |
| Elevated liver function tests | 2 (6) | 7 (11) | 0.71 |
| Hypertension | 2 (6) | 7 (11) | 0.70 |
| Gastrointestinal bleed | 1 (3) | 2 (3) | 1.00 |
| Other | 6 (18) | 14 (21) | 0.80 |
Data presented as n (%) unless otherwise indicated. Bolded value indicates statistical significance
In 26 patients started on a reduced dose of sorafenib who required further dose reduction, the main causes were hand-foot syndrome (35%), diarrhea (15%), increased liver biochemistry (15%), weight loss or fatigue (12%). The main reasons for dose reduction in full-dose patients were hand-foot syndrome (36%), diarrhea (27%) and constitutional symptoms (18%) (data not shown).
Treatment characteristics and outcomes
Dose reduction rates were significantly higher in the full-dose group, occurring in 66% compared with 40% of reduced-dose group patients (P=0.018) (Table 3). Data for time to dose reduction were available only in the full-dose group (median 1.4 months, range 0.3 to 8.4 months). The mean duration of treatment was six months in the full-dose group versus 5.4 months in the reduced-dosed group. Permanent discontinuation of treatment occurred in 85% of full-dose patients compared with 70% in reduced-dosed patients. However, AEs were the main reason for permanent interruption of treatment in only 27% of the full-dose and in 22% of the reduced-dosed group, followed by disease progression in 43% in the former and 29% in the latter groups. Five patients (15%) in the full-dose group died while on sorafenib treatment compared with 20 patients (30%) in the reduced-dose group.
TABLE 3.
Sorafenib dose reduction and discontinuation according to dose group
|
Sorafenib dose/day
|
P | ||
|---|---|---|---|
| 800 mg (n=33) | 400 mg (n=66) | ||
| Dose reduction | 22 (66) | 26 (40) | 0.018 |
| Time to dose reduction, months, median (range) | 1.4 (0.3–8.4) | N/A | |
| Duration of treatment, months, mean | 6 | 5.4 | |
| Discontinuation | 28 (85) | 46 (70) | 0.14 |
| Reason for discontinuation | |||
| Adverse effect | 9 (27) | 22 (33) | 0.65 |
| Disease progression | 14 (43) | 19 (29) | 0.18 |
| Other/unknown | 5 (15) | 5 (8) | 0.29 |
Data presented as n (%) unless otherwise indicated. Bolded value indicates statistical significance. N/A Not applicable
Interestingly, there was no statistical difference in overall survival rates between the two groups (7.8 months in full-dose and 7.1 months in reduced-dose groups; P=0.14) (Figure 2). Treatment effect was evaluated using radiological response rates, which were similar between the two groups, with most patients (55% in the full-dose and 45% in the reduced-dose group) showing progressive disease at three-month follow-up (Table 4). Disease control rates were also similar between the groups. Alpha-fetoprotein levels – a proposed surrogate of treatment response – did not appear to statistically differ between full- and reduced-dose groups at baseline and at three months after initiation of treatment.
Figure 2).
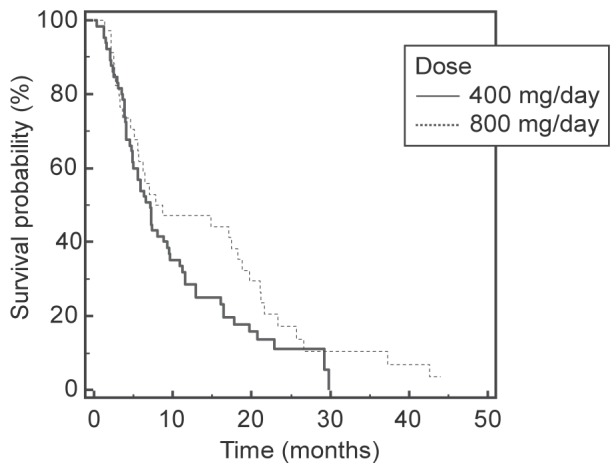
Kaplan-Meier survival curves for patients treated with sorafenib
TABLE 4.
Survival and treatment outcomes of patients treated with sorafenib
|
Sorafenib dose/day
|
P | ||
|---|---|---|---|
| 800 mg (n=33) | 400 mg (n=66) | ||
| Overall survival, months, mean | 7.8 | 7.1 | 0.14 |
| Three-month radiological response (tumour burden) | |||
| Stable | 6 (18) | 13 (20) | 1.0 |
| Increased | 18 (55) | 30 (45) | 0.4 |
| Decreased | 1 (3) | 4 (6) | 0.6 |
| Unknown | 8 (24) | 19 (29) | 0.8 |
| Disease control rate | 7 (21) | 17 (26) | 0.8 |
| Three-month alpha-fetoprotein levels | |||
| Stable | 5 (15) | 8 (12) | 0.7 |
| Increased | 12 (37) | 25 (38) | 1.0 |
| Decreased | 1 (3) | 10 (15) | 0.09 |
| Unknown | 15 (45) | 23 (35) | 0.4 |
Data presented as n (%) unless otherwise indicated
DISCUSSION
Treatment of advanced HCC with sorafenib represents a significant new development in the management of this disease. A sorafenib dose of 800 mg/day was approved by the FDA for treatment of advanced HCC and showed significant survival benefit in phase III RCTs (6,7). Significant adverse effects often necessitate reduction in dosage; however, outcome data involving these patients are limited. In the present study, we report a retrospective review of 99 patients with advanced HCC, 66 of whom received ≤0.5 the manufacturer-recommended dose of sorafenib. All of our patients were treated in a nonclinical trial, ‘real-world’, community setting. It has been previously noted that patient outcomes in community settings are often different from those reported in controlled clinical trials due to the high selectivity of patients participating in the latter. Our study provides ‘real-world’ patient outcomes but is limited by its retrospective nature. Given that sorafenib therapy is quite costly, optimizing the dosage may result in significant cost savings if there is no survival disadvantage, in addition to an improvement in patient tolerability.
Our patient population experienced a similar degree of liver damage, indicated by the CP score; when compared with phase III trials, 90% of our patients fell into the CP A category versus 95% and 98% in SHARP and Asia-Pacific trials, respectively. The etiology of liver disease was mostly related to hepatitis B virus infection, similar to the Asia-Pacific trial and explained by the high prevalence of patients of Asian descent. The proportion of patients with extrahepatic spread was 30% in our study versus 50% and 53% in phase III trials. Overall survival in our patient population was also similar to that reported in the literature (8).
The concept that sorafenib may maintain anticancer activity at reduced doses has been discussed previously. A recent RCT by the SOraFenib Italian Assessment (SOFIA) group in Italy (12) analyzed 296 patients with advanced HCC treated with sorafenib for 3.8 months, 40% of whom required dose reduction. The median survival of 77 patients treated with one-half the sorafenib dose for >70% of the time was 21.6 months compared with 9.6 months in 219 patients treated with full dose. A study by Kim et al (14) analyzed a dose-escalation method in 25 patients with high risk factors for AEs. Although the dose increase to 800 mg/day was possible in only 64% of these patients, the disease progression rates were similar between the groups. The explanation of these findings may lie in the way molecularly targeted agents are developed (10), namely, how the effective dose is titrated to tolerance in phase I trials that is then retested in phase II trials under the assumption that the higher dose produces more effects. However, because molecularly targeted agents often act on receptors specifically overexpressed in malignant tissue, they possibly maintain their antitumour activity at lower doses.
Another explanation for the similar survival outcomes in the reduced sorafenib dose patients compared with the full-dose patients, at least in our ‘real world’ study, may also lie in the nature of controlled clinical trials versus a standard outpatient clinical setting. In a controlled clinical trial, there are many study visits, more so than in a ‘real-world’ setting. Moreover, clinical trial patients are followed by professional research assistants who perform pill counts and monitor patients’ medication logs; therefore, adherence to the study protocol is typically very high. This usually does not happen in a ‘real-world’ clinical setting. Hence, when patients are taking a drug that may have significant adverse side effects at higher doses, adherence to the recommended dosing may be suboptimal; therefore, the pharmacological dose-dependent effect between full-dose and reduced-dose patients may have been partially negated.
CONCLUSION
In the present study, a sorafenib dose of 400 mg/day was better tolerated in our patient population with similar efficacy compared with a sorafenib dose of 800 mg/day in terms of survival and outcomes. Additional studies are needed to evaluate the potential use of reduced sorafenib dosages in patients with advanced HCC. Better sorafenib-like drugs with fewer AEs will also be needed.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.American Cancer Society, Cancer Facts and Figures 2012. Atlanta: American Cancer Society; 2012. < www.cancer.org/Research/CancerFactsFigures/index> (Accessed December 15, 2012) [Google Scholar]
- 2.USFDA Approval for Sorafenib Tosylate. 2007. < www.cancer.gov/cancertopics/druginfo/fda-sorafenib-tosylate> (Accessed December 15, 2012)
- 3.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 4.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double- blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 8.Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: A systematic review. Dig Dis Sci. 2012;57:1122–9. doi: 10.1007/s10620-012-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lencioni R, Kudo M, Ye SL, et al. First interim analysis of the GIDEON (Global Investigation of therapeutic Decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675–83. doi: 10.1111/j.1742-1241.2012.02940.x. [DOI] [PubMed] [Google Scholar]
- 10.Kummar S, Gutierrez M, Doroshow JH, Murgo AJ. Drug development in oncology: Classical cytotoxics and molecularly targeted agents. Br J Clin Pharmacol. 2006;62:15–26. doi: 10.1111/j.1365-2125.2006.02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wörns MA, Weinmann A, Pfingst K, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43:489–95. doi: 10.1097/MCG.0b013e31818ddfc6. [DOI] [PubMed] [Google Scholar]
- 12.Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology. 2011;54:2055–63. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Kim JE, Ryoo BY, Ryu MH, et al. Sorafenib dose escalation in the treatment of advanced hepatocellular carcinoma. Oncology. 2012;82:119–25. doi: 10.1159/000336082. [DOI] [PubMed] [Google Scholar]


