Abstract
BACKGROUND:
Traditional seven-day proton pump inhibitor triple therapy for Helicobacter pylori eradication has recently shown disappointing results outside of Canada. Prolonging therapy may be associated with poorer compliance and, hence, may not have a better outcome in a real-world setting.
OBJECTIVE:
To compare the outcomes of seven- and 14-day triple therapy for first-line treatment of H pylori infection in an effectiveness setting in Canada.
METHODS:
A total of 314 consecutive treatment-naive, adult H pylori-infected patients were allocated to either a seven- or 14-day triple therapy regimen, with a subgroup of 172 consecutive patients quasi-randomized to treatment according to date of visit. Eradication was confirmed using either urea breath test or gastric biopsies. Analysis was by intention to treat.
RESULTS:
Eradication was achieved in a higher proportion of patients who underwent 14-day versus seven-day treatment regimens (overall: 85% versus 70% [P≤0.001]; subgroup: 83% versus 64% [P≤0.01]). Although successful eradication was also associated with older age and a diagnosis of ulcer disease, multivariate analysis revealed only longer treatment duration and lack of yogurt ingestion as independent predictors of successful eradication. There was a trend toward reduced success in the latter years of the study. Side effects were similar in both groups and were not prevented by yogurt ingestion.
CONCLUSIONS:
The currently recommended duration of proton pump inhibitor triple therapy in Canada should be increased from seven to 14 days, the latter having achieved an excellent result in this particular real-world setting. Yogurt added no benefit. Further study is required to compare 10-day with 14-day treatment regimens.
Keywords: Amoxicillin, Clarithromycin, Duration, Helicobacter pylori, Proton pump inhibitor, Triple therapy, Yogurt
Abstract
HISTORIQUE :
La trithérapie classique de sept jours aux inhibiteurs de la pompe à protons pour éradiquer l’infection à Helicobacter pylori a récemment donné des résultats décevants à l’extérieur du Canada. Un traitement prolongé pourrait nuire à l’assiduité et n’aura peut-être pas de meilleurs résultats en situation réelle.
OBJECTIF :
Comparer les issues d’une trithérapie de première ligne de sept et 14 jours pour soigner une infection à H pylori dans un milieu d’efficacité au Canada.
MÉTHODOLOGIE :
Au total, 314 patients adultes consécutifs infectés par le H pylori et naïfs au traitement se sont vu allouer une trithérapie de sept ou 14 jours, tandis qu’un sous-groupe de 172 patients consécutifs a été sélectionné quasi au hasard pour subir un traitement d’après la date de leur consultation. Les chercheurs ont confirmé l’éradication au moyen d’un test respiratoire à l’urée ou de biopsies gastriques. Ils ont utilisé une analyse de l’intention de traiter.
RÉSULTATS :
Une plus forte proportion de patients qui ont suivi un traitement de 14 jours profité d’une éradication que ceux qui ont suivi un traitement de sept jours (dans l’ensemble, 85 % par rapport à 70 % [P≤0,001]; sous-groupe de 83 % par rapport à 64 % [P≤0,01]). Même si l’éradication s’associait également à un âge plus avancé et à un diagnostic de maladie ulcéreuse, l’analyse multivariée a révélé que seuls un traitement plus prolongé et la non-ingestion de yogourt étaient des prédicteurs indépendants d’éradication. On constatait une tendance vers une baisse du taux de réussite pendant les dernières années de l’étude. Les effets secondaires étaient similaires dans les deux groupes, et l’ingestion de yogourt ne permettait pas de les éviter.
CONCLUSIONS :
Au Canada, il faudrait faire passer de sept à 14 jours la durée recommandée de la trithérapie aux inhibiteurs de la pompe à protons, car la deuxième option a donné d’excellents résultats dans cette situation réelle. Le yogourt n’apportait aucun avantage. Des études plus approfondies s’imposent pour comparer le traitement de dix jours à celui de 14 jours.
Helicobacter pylori infects a large proportion of the world’s population (1). Although the majority of infected individuals will never experience clinical consequences related to this bacterium, a percentage may develop upper gastrointestinal disease including peptic ulcer disease (PUD), gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma (2). Treatment of H pylori infection has been shown to heal, as well as reduce the recurrence of duodenal and gastric ulcers (3), cause remission in MALT lymphoma (4) and there is some evidence that it may prevent gastric cancer if treated early (5). Several consensus statements have recommended H pylori eradication for individuals with PUD, MALT lymphoma, uninvestigated dyspepsia, first-degree relatives of individuals with gastric cancer and selected chronic nonsteroidal anti-inflammatory drug users (6–8).
One of the most common treatments used for H pylori eradication is standard triple therapy consisting of a proton pump inhibitor (PPI) with two antibiotics including clarithromycin and either amoxicillin or metronidazole (9). Traditional recommendations have proposed a seven-day treatment duration based on earlier studies suggesting an intention-to-treat (ITT) eradication rate >80% (6,9,10). However, some experts disagreed, believing that a prolonged duration of 10 to 14 days was required (11). Recently, H pylori eradication rates have diminished, with several studies outside of Canada reporting success rates of <80% (9) with current therapies, possibly due to increasing antibiotic resistance.
A longer duration of therapy should theoretically be associated with an increased success rate. However, this may not necessarily be the case; antibiotic resistance may not be surmountable by prolonging therapy. In addition, prolonged therapy may be associated with poorer compliance in real-world settings compared with that of a clinical trial due to the increased days of adherence required and, possibly, the development of additional side effects. Before recommending a prolonged and more expensive treatment, a head-to-head study comparing the two alternatives is required.
The present study compared the outcomes of seven-day versus 14-day PPI triple therapy for first-line treatment of H pylori infection in a real-world (ie, effectiveness) setting.
METHODS
Patient population
Consecutive adult patients presenting to one of the authors (CAF) between January 1, 2007 and December 31, 2011, with a clinically acceptable indication for treatment of active H pylori infection documented either by histology or urea breath test (UBT) and never previously having undergone an attempt at H pylori eradication were included in the present study. Patients were from both the investigator’s university clinic and community-based offices in Montreal (Quebec) and its surrounding area. Patients with allergies to the medication used in the treatment mentioned below were excluded. The study was approved by the McGill University Health Centre Institutional Review Board (Montreal, Quebec).
Study design and therapeutic intervention
Patients underwent either a seven- or 14-day treatment regimen with lansoprazole 30 mg, amoxicillin 1000 mg and clarithromycin 500 mg, all twice per day as delivered by their local pharmacy. In 2007, the majority (86%) of patients were treated with seven-day therapy, while in 2008 the majority (91%) were treated with 14-day therapy, given the clinical suspicion of high failure rates with seven-day therapy. If seen between January 2009 and December 2011, patients were randomly assigned to treatment based on the date of visit to the gastroenterologists’ offices (ie, a quasirandomized trial). The patient was given the seven-day treatment if the visit date was an odd number or the 14-day treatment if the date was an even number. Neither the recruiting physician nor other investigators influenced the date of the visit, and the patient was unaware of which treatment would be administered on a given date. Patients were encouraged by the physician to take all medications completely and potential side effects were explained.
Outcome
Eradication was determined to be a success or a failure at least four weeks after completion of antibiotics based on the result of either a UBT or histological examination (including Giemsa stain if negative on hematoxylin and eosin) of at least two antral and two body mucosal biopsies obtained endoscopically. Those interpreting the results of either the UBT or histology were blinded to treatment group allocation. All PPIs were withheld two weeks before performance of either test. Patients were asked directly by the investigator whether they experienced side effects with the treatment; took yogurt at any time during treatment; and whether they completed the entire treatment. Demographic data and indication for treatment were documented.
Statistical analysis
Descriptive statistics for continuous variables were expressed as mean ± SD. For categorical variables, 95% CIs were calculated using the standard normal approximation of the binominal distribution. T tests and χ2 tests were used to compare baseline characteristics and to ensure comparability of both groups with equal balancing of possible confounders in each treatment group. Statistical analyses were performed using an ITT principle, in which all with missing eradication data were considered to be failures; modified ITT, in which patients who did not take any medication were excluded; per-protocol (PP) principle, in which only those for whom eradication-confirming data were available were considered; and true PP, which included only those with eradication-confirming data and took 100% of their medication. Between-group comparisons were performed using the χ2 test for categorical data or the nonparametric Wilcoxon test for continuous variables. Multivariable logistic regression models were constructed to identify predictors of successful eradication. For this analysis, the PP population was used to ensure that the patients were correctly categorized in the success versus failure groups; statistical significance was set at P<0.05.
Using 95% CIs for an expected 77% success rate with seven-day treatment (13) and a greater than 10% difference deemed as clinically significant, a sample size of 138 subjects per group was required. Based on this sample size calculation and allowing a 5% drop-out rate, more than 145 subjects were enrolled in each group.
A post hoc analysis was performed on the subgroup of patients enrolled from 2009 to 2011. Given that the treatment allocation was not truly randomized in 2007 to 2008, the patients enrolled during these years were excluded from this particular analysis. The remaining patients, who were consecutive and randomly assigned to seven versus 14 days of treatment according to the date (odd versus even) of the clinic visit, were compared in an ITT fashion using the χ2 test on rates of successful eradication.
RESULTS
A total of 314 patients met the entry criteria and were included in the present study. Fifty-eight per cent (n=182) were women and 42% (n=132) were men. The mean (± SD) age was 60.7±13.3 years. The overwhelming majority (90%) were Caucasian, but the subjects were born in 48 different countries, reflecting Montreal’s multicultural diversity and high immigrant population. Most subjects were born in Europe (n=178, with 141 from Italy reflecting the author’s clientele) or Canada (n=71). Fifty-eight per cent (n=182) of the patients were seen in the university hospital office, and the remainder in the community-based office. Indications for treatment included nonulcer dyspepsia (n=90); PUD (including duodenal ulcers or erosions) and gastric ulcers or erosions (n=62); gastroesophageal reflux disease (n=60); anemia (n=56); family history of gastric cancer (n=23); uninvestigated dyspepsia (n=7); gastric cancer (n=5); and other indications (n=11).
The patients received either seven (n=148 [47%]) or 14 (n=166 [53%]) days of triple therapy (Figure 1). Although there was a slight predominance of females in the seven-day group (P=0.04), the two groups were comparable in age, race, country of birth, treatment setting, indication for treatment, use of yogurt during treatment and diagnostic examination used to confirm eradication (Table 1). The majority of the patients claimed to take all of the medication prescribed (98.6% versus 97.6% in the seven- and 14-day treatments, respectively). Two patients (both in the 14-day arm) refused to commence treatment. Two patients from the seven-day and two from the 14-day arms (Figure 1) took from 7% to 79% of prescribed treatments, usually because of side effects (see below). Ten patients (three from the seven-day and seven from the 14-day arm) failed to return for confirmatory tests for various reasons (including the two who refused to start the treatments prescribed as mentioned above, one who refused UBT after having developed Clostridium difficile infection and seven who were lost to follow-up).
Figure 1).
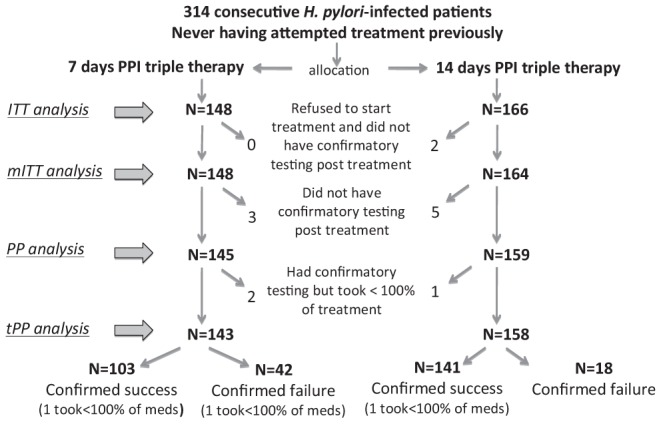
Distribution of patients according to treatment group and outcome. The group on which the specific analyses were performed is indicated. H.pylori Helicobacter pylori; Intention to treat (ITT) analysis (all those with missing eradication data were considered failures); mITT Modified ITT (two patients who took no medication were excluded); PP Per protocol (only those for whom eradication data were available were considered); tPP True PP (only those who took 100% of medication and for whom eradication data were available were included); PPI Proton pump inhibitor
TABLE 1.
Baseline characteristics of the two treatment groups
|
Triple therapy
|
||
|---|---|---|
| Seven days (n=148) | 14 days (n=166) | |
| Age, years | ||
| Mean ± SD | 60±14 | 61±13 |
| Range | 25–88 | 17–87 |
| Female sex | 95 (64, 56–72) | 87 (52, 45–60) |
| Race* | ||
| Caucasian | 135 (91, 85–95) | 146 (88, 82–92) |
| Black | 9 (6, 3–11) | 8 (5, 2–9) |
| Asian | 4 (3, 1–7) | 10 (6, 3–11) |
| Country of birth | ||
| Western Europe | 77 (52, 44–60) | 87 (52, 45–60) |
| Canada and USA | 36 (24, 18–32) | 38 (23, 17–30) |
| Central and South America | 11 (7, 4–13) | 9 (5, 3–10) |
| Eastern Europe | 9 (6, 3–11) | 5 (3, 1–7) |
| Africa | 6 (4, 2–9) | 8 (5, 2–9) |
| Asia | 3 (2, 0–6) | 10 (6, 3–11) |
| Middle East | 5 (3, 1–8) | 5 (3, 1–7) |
| Treatment setting | ||
| University hospital office | 89 (60, 52–68) | 93 (56, 48–64) |
| Community office | 59 (40, 32–48) | 73 (44, 36–52) |
| Indication | ||
| Dyspepsia | ||
| Nonulcer dyspepsia | 46 (31, 24–39) | 44 (27, 20–34) |
| Uninvestigasted dyspepsia | 4 (4, 1–7) | 3 (2, 0–5) |
| Peptic ulcer disease | ||
| Duodenal ulcer | 12 (8, 4–14) | 21(13, 8–19) |
| Gastric ulcer | 8 (5, 2–10) | 11 (7, 3–12) |
| Gastric or duodenal erosions | 4 (3, 1–7) | 6 (4, 1–8) |
| Gastroesophageal reflux disease | 26 (18, 12–25) | 34 (20, 15–27) |
| Anemia (eg, iron deficiency) | 28 (19, 13–26) | 28 (17, 11–23) |
| Family history of gastric cancer | 14 (9, 5–15) | 9 (5, 3–10) |
| Gastric cancer | 3 (2, 0–6) | 2 (1, 0–4) |
| Other† | 3 (2, 0–6) | 8 (5, 2–9) |
| Use of yogurt during treatment* | 27 (18, 12–25) | 33 (20, 14–27) |
| Confirmatory examination planned* | ||
| C-13 Urea breath test | 100 (68, 59–75) | 112 (67, 60–74) |
| C-14 Urea breath test | 36 (24, 18–32) | 41 (25, 18–32) |
| Gastroscopy and biopsy | 11 (7, 4–13) | 11 (7, 3–12) |
Data presented as n (%, 95% CI) unless otherwise indicated. P values were all nonsignificant between the two groups (ie, P>0.1) except for sex (P=0.04).
Some subject data were missing;
Other includes gastrointestinal stromal tumour, mucosa associated lymphoid tissue lymphoma, antral nodule, etc
Successful eradication was confirmed in 103 and 141 patients from the seven- and 14-day treatment groups, respectively. In the ITT analysis, in which those who did not return for UBT were considered failures, the success rate was 69.6% (103 of 148 [95% CI 62% to 77%]) in the seven-day arm versus 84.9% (141 of 166 [95% CI 79% to 90%]) in the 14-day arm (P=0.001 [Figure 2]). Similar results were obtained in the analysis that excluded the two patients who refused to commence treatment (ie, modified ITT analysis [Table 2]), with 69.6% (103 of 148) success in the seven-day treatment versus 86.0% (141 of 164) success in the 14-day treatment arm (P<0.001). The PP analysis revealed a success rate of 71% versus 89% in the seven- versus 14-day treatment arms, respectively (P<0.001) (Table 2). The post hoc ITT analysis involving only patients from 2009 to 2011 (ie, those quasirandomized according to date of visit) showed similar results. Of the patients who were randomly assigned to treatment, 49 of the 76 (64.5% [95% CI 53% to 75%]) given seven-day treatment achieved cure compared with 80 of the 96 (83.3% [95% CI 74% to 90%]) given 14-day treatment (P<0.01).
Figure 2).
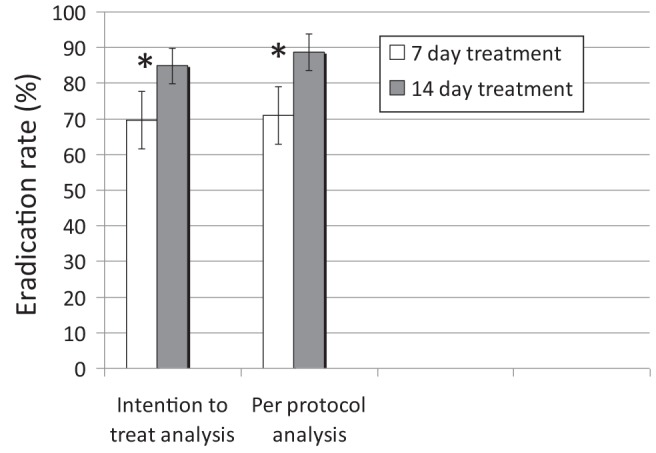
Intention to treat and per protocol eradication rates with seven versus 14 days of proton pump inhibitor triple therapy. *P≤0.001
TABLE 2.
Eradication results of the intention-to-treat (ITT) and per-protocol (PP) analyses
| Analysis |
Treatment, % (success n/total n [95% CI])
|
P | |
|---|---|---|---|
| Seven day | 14 day | ||
| ITT | 69.6 (103/148 [62–77]) | 84.9 (141/166 [79–90]) | ≤0.001 |
| mITT | 69.6 (103/148 [62–77]) | 86.0 (141/164 [81–91]) | ≤0.001 |
| PP | 71.0 (103/145 [64–79]) | 88.7 (141/159 [84–94]) | ≤0.001 |
| tPP | 71.3 (102/143 [64–79]) | 88.6 (140/158 [84–94]) | ≤0.001 |
ITT analysis (all those with missing eradication data were considered failures); Modified ITT (mITT, those two patients who took no medication were excluded); PP (only those for whom eradication data was available were considered); tPP True PP (only those who took 100% of medication and for whom eradication data were available were included)
Side effects occurred in 21.4% (95% CI 14% to 29%) of patients undergoing seven-day versus 30.8% (95% CI 23% to 39%) undergoing 14-day treatment (P=0.07). However, only four patients (two in each arm) stopped treatment because of side effects. The most common side effects included nausea, altered taste and diarrhea (Table 3). Taking yogurt was not protective against side effects: 38.6% (95% CI 26% to 52%) who took yogurt experienced side effects (eg, diarrhea 10% [95% CI 4% to 22%]), while only 21.4% (95% CI 16% to 28%) who did not take yogurt experienced side effects (eg, diarrhea 5.2% [95% CI 3% to 9%]; P=0.01 for any side effect; P>0.05 for diarrhea).
TABLE 3.
Number of patients experiencing side effects with seven- and 14-day proton pump inhibitor triple therapy
| Side effect |
Seven-day therapy (n=131)
|
14-day therapy (n=146)
|
P | ||
|---|---|---|---|---|---|
| n | % (95%CI) | n | % (95%CI) | ||
| Any side effect | 28 | 21.4 (14–29) | 45 | 30.8 (23–39) | 0.07 |
| Nausea and vomiting | 7 | 5 (2–11) | 15 | 10 (6–16) | |
| Altered taste/burning tongue | 7 | 5 (2–11) | 12 | 8 (4–14) | |
| Diarrhea | 9 | 7 (3–13) | 8 | 5 (2–11) | |
| Epigastric discomfort | 2 | 2 (0–5) | 6 | 4 (2–9) | |
| Fatigue/weakness | 4 | 3 (1–8) | 3 | 2 (0–6) | |
| Headache | 3 | 2 (0–7) | 3 | 2 (0–6) | |
| Dizziness | 3 | 2 (0–7) | 1 | 1 (0–4) | |
| Rash | 0 | 0 (0–4) | 4 | 3 (1–7) | |
| Pruritis | 1 | 1 (0–4) | 1 | 1 (0–4) | |
| Constipation | 1 | 1 (0–4) | 1 | 1 (0–4) | |
| Others* | 1 | 1 (0–4) | 6 | 4 (2–9) | |
Others include one case of Clostridium difficile infection in the seven-day group and one case each of confusion, feeling faint, abnormal cramps, hemorrhoids, elevated creatinine and elevated tegretol level in the 14-day group
Individuals achieving successful eradication were not only more likely to have received the 14-day therapy, but were also older (61 versus 58 years of age [P<0.05]), more likely to have ulcer disease (23% versus 7% [P<0.01]) and less likely to have ingested yogurt during their antibiotic treatment (19% versus 34% [P<0.05]) (see univariable analysis [Table 4]). Interestingly, 71.2% of those who consumed yogurt achieved successful eradication compared with 84.9% of those did not ingest yogurt (P<0.05). Multivariable logistic regression analysis revealed only treatment duration of seven days (OR 0.42 [95% CI 0.22 to 0.81; P<0.01) and ingestion of yogurt (OR 0.43 [95% CI 0.22 to 0.85]; P<0.05) as independent significant predictors of eradication failure. As an underlying indication, PUD failed to achieve significance as a predictor of success by a narrow margin (P=0.06).
TABLE 4.
Possible predictors of successful eradication*
| Variable |
Treatment, n (% [95% CI])
|
P† | |
|---|---|---|---|
| Failure (n=60) | Success (n=244) | ||
| Age, years, mean ± SD | 57.7±12.9 | 61.4±13.3 | 0.0453 |
| Nonulcer dyspepsia | 21 (35.0 [22.6–47]) | 68 (27.9 [22.2–33.5]) | 0.2768 |
| Gastroesophageal reflux disease | 15 (25.0 [13.7–36.3]) | 44 (18.0 [13.2–22.9]) | 0.2215 |
| Anemia or iron deficiency | 7 (11.7 [3.3–20.0]) | 48 (19.7 [14.6–24.7]) | 0.1490 |
| Ulcer or erosion | 4 (6.7 [0.2–13.2]) | 56 (23.0 [17.6–28.3]) | 0.0045 |
| Family history of gastric cancer | 7 (11.7 [3.3–20.0]) | 15 (6.1 [3.1–9.2]) | 0.1393 |
| Uninvestigated dyspepsia | 3 (5.0 [0.0–10.7]) | 3 (1.2 [0.0–2.6]) | 0.0600 |
| Gastric cancer | 2 (3.3 [0.0–8.0]) | 3 (1.2 [0.0–2.6]) | 0.2510 |
| Other‡ | 1 (1.7 [0.0–5.0]) | 7 (2.9 [0.8–5.0]) | 0.6022 |
| University hospital office | 31 (51.7 [38.6–64.7]) | 146 (59.8 [53.6–66.0]) | 0.2503 |
| Community office | 29 (48.3 [35.3–47.6]) | 98 (40.2 [34.0–46.4]) | 0.2503 |
| Use of yogurt | 17 (34.0 [20.4–47.6]) | 42 (18.5 [13.4–23.6]) | 0.0154 |
| Seven-day treatment | 42 (70.0 [58.1–81.9]) | 103 (42.2 [36.0–48.5]) | 0.0001 |
| 14-day treatment | 18 (30.0 [18.1–41.9]) | 141 (57.8 [51.5–64.0]) | 0.0001 |
| 100% treatment taken | 59 (98.3 [95.0–100]) | 242 (99.2 [98.0–100.0]) | 0.5521 |
| Side effects | 16 (31.4 [18.2–44.6]) | 55 (24.6 [18.9–30.2]) | 0.3152 |
| Female sex | 41 (68.3 [56.2–80.5]) | 138 (56.6 [50.3–62.8]) | 0.0968 |
| Caucasian | 58 (96.7 [92.0–100]) | 214 (88.4 [84.4–92.5]) | 0.0562 |
| Black | 2 (3.3 [0.0–8.0]) | 15 (6.2 [3.1–9.3]) | 0.3887 |
| Asian | 0 (0.0 [0.0–5.1]) | 13 (5.4 [2.5–8.2]) | 0.0665 |
| Canada/USA | 18 (30.5 [18.4–42.6]) | 54 (22.5 [17.2–27.8]) | 0.1974 |
| Western Europe | 28 (47.5 [34.3–60.6]) | 129 (53.8 [47.4–60.1]) | 0.3859 |
| Eastern Europe | 3 (5.1 [0.0–10.9]) | 11 (4.6 [1.9–7.2]) | 0.0873 |
| Africa | 3 (5.1 [0.0–10.9]) | 11 (4.6 [1.9–7.2]) | 0.0873 |
| Central and South America | 3 (5.1 [0.0–10.9]) | 17 (7.1 [3.8–10.4]) | 0.5820 |
| Middle East | 3 (5.1 [0.0–10.9]) | 7 (2.9 [0.8–5.1]) | 0.4066 |
| Asia | 1 (1.7 [0.0–5.1]) | 11 (4.6 [1.9–7.2]) | 0.3112 |
Values indicated in bold are statistically significant (ie, P≤0.05).
Univariate analysis;
χ2 test for association;
Other includes gastrointestinal stromal tumour, mucosa associated lymphoid tissue lymphoma, antral nodule, etc
The examination of annual eradication rates from the beginning of the study in 2007 to the end in 2011 showed a trend toward higher failure rates with time, particularly in the seven-day treatment group in the final year (Figure 3); however, these differences did not achieve statistical significance.
Figure 3).
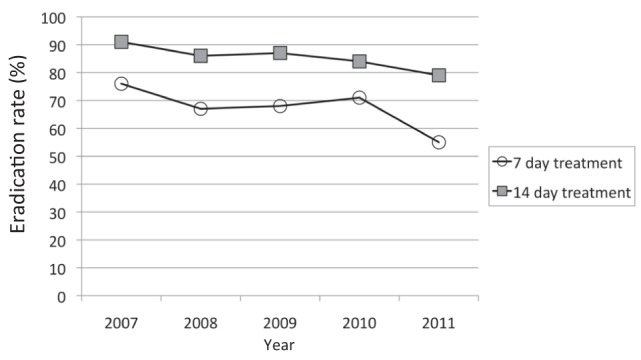
Intention-to-treat Helicobacter pylori eradication rates for seven- and 14-day proton pump inhibitor triple therapy from 2007 to 2011. A nonstatistically significant trend of decreasing eradication rates over time is apparent
DISCUSSION
In the present study of individuals with H pylori infection, significantly more achieved successful eradication with the 14-day (84.9% [141 of 166]) PPI triple therapy regimen than with the seven-day treatment (69.6% [103 of 148]; P≤0.001). Although there were slightly more side effects in the 14-day treatment group (31% versus 21%; P=0.07), compliance was not reduced (99% in the seven- versus 98% in the 14-day treatment groups).
There were some limitations to the present study. The first was the lack of physician and subject blinding, in that both parties knew the length of treatment prescribed. However, the individuals who assessed the outcome (ie, H pylori presence or absence post-treatment) were blinded to the treatment assignment. The second was the way in which subjects were allocated to treatment, which, given the lack of true randomization, may have introduced selection bias. For example, although not statistically significant, there was a slightly higher proportion of patients with underlying PUD in the 14-day arm compared with the seven-day arm, thus potentially affecting success rates; however, PUD failed to be a predictor of eradication in the multivariate analysis. In addition, the study arms were balanced on other potential confounders at baseline and the ITT analysis of the subgroup quasirandomized according to date of visit showed very similar results to the overall analysis. In the 14-day therapy group, 83.3% achieved cure compared with 64.5% in the seven-day therapy group (P<0.01). An additional study limitation may be restricted generalizability given that a large proportion of our study population was born in Italy and, hence, may not be reflective of the general Canadian population. Nevertheless, we found that the country of origin was not associated with eradication success (Table 4). It is possible that the lack of H pylori susceptibility testing was also a study limitation but, in fact, this information is rarely available to the clinician, not only on an individual patient basis but also on a community basis. Hence, the study represents results one would expect in multicultural settings within Canada and the United States where such information is not available.
Strengths of the present study include the quasirandomized subgroup; the blinding of persons assessing outcome; the large number of patients enrolled; that all consecutive eligible patients were included; that all study patients were naive to previous H pylori treatments; and that these patients were all treated by a single physician. Furthermore, the study was conducted in a ‘real-world’, Canadian setting. The patients were given a prescription and the medication was provided to them by their local pharmacist. There were no potential influences from study personnel that can occur in a more formalized research study setting.
The findings of the present study clearly suggest that seven days of PPI triple therapy for H pylori is not sufficient, demonstrated by the ITT eradication rate of 70% (95% CI 62% to 77%) and, hence, below the suggested minimal 80% threshold (6). Findings also demonstrate that 14-day triple PPI therapy is an adequate first-line therapy in a real-life North American setting, with an ITT success rate of 85% (95% CI 79% to 90%). The difference in success rates between the two treatment regimens was higher in our study than in a previously conducted meta-analysis (12), which demonstrated a 5% higher eradication rate with 14- versus seven-day triple therapy. Perhaps this is because of increasing clarithromycin resistance. Although that meta-analysis (published six years ago) also suggested an improved outcome with 10- versus seven-day treatment durations, further studies are required to determine whether 10 days would currently achieve similar eradication rates.
Other alternatives to the standard seven-day triple therapy have also been proposed. These include levofloxacin triple therapies (consisting of levofloxacin, amoxicillin and a PPI); however, increasing H pylori resistance to levofloxacin has limited the potential of this therapy (6,13). Sequential therapy has been suggested, consisting of a 10-day treatment with a PPI given with amoxicillin for the first five days followed by its administration with tinidazole and clarithromycin for the subsequent five days. This regimen has been studied mostly in Italy and found to be superior to seven-day PPI triple therapy (14). The superiority of this regimen is most likely due to the high rate of clarithromycin resistance in that country, and the poor outcome of seven-day clarithromycin-based PPI triple therapy in clarithromycin-resistant cases (6). In a setting of less clarithromycin resistance, superiority may not exist. Alternatively, it could simply be due to the duration of therapy, given that sequential therapy was administered for 10 days compared with seven days for the PPI triple therapy in most of these studies (15). Indeed, a large study from Latin America obtained a higher success rate with 14-day triple therapy than with a 10-day sequential treatment (16). The success rates of sequential therapy outside of Italy appear to be more disappointing, with only 76.5% eradication in the latter study (16). Perhaps this is because tinidazole, not being available in these areas, is replaced with metronidazole which may not necessarily have the same effect in this particular combination therapy. Sequential therapy may also be associated with disadvantages compared with 14-day PPI triple therapy in that the patient is exposed to more antibiotics and, hence, is at a higher risk of side effects including C difficile-associated diarrhea. In addition, failure of this first-line therapy would likely result in an organism resistant to both clarithromycin and metronidazole, whereas the PPI amoxicillin triple therapy would likely only result in an organism resistant to clarithromycin, thereby preserving the option of a second-line treatment with quadruple therapy. Another alternative is concomitant quadruple therapy, in which four agents are given twice daily and concomitantly rather than sequentially. Some of these concomitant quadruple therapies have proven to be quite successful with, for example, an ITT cure rate of 92% in one study using only five-day levofloxacin-containing therapy (17). This type of therapy, however, still has the disadvantage of the potential development of dual antibiotic resistance after a failed treatment, as mentioned above. An additional treatment option is bismuth-containing quadruple therapy using a three-in-one capsule containing bismuth, metronidazole and tetracycline along with a PPI, which, with a 10-day duration, demonstrated an 80% ITT success rate and a reduced pill burden compared with traditional quadruple therapies (18).
In 1997, clarithromycin resistance in the Montreal community was relatively low (1% [19]). One would expect this to have increased over time given the results of other studies showing increases from 9% in 1998 (20) to 17.6% in 2009 (6) in Europe. No recent data are reported from Montreal. The increasing clarithromycin resistance may be the cause of decreasing success rates of 14-day, but especially seven-day, regimens over time (Figure 3).
Treatment compliance, although not confirmed with a pill count, was excellent in the present real-life setting study, with more than 95% of patients claiming to have taken all medications. Although some patients may not have been truthful, especially because the investigator was also their physician, there is no reason to suspect this to have been any different between the two treatment groups. In addition, all but 10 of the subjects complied with confirmatory testing. This excellent compliance was achieved despite a relatively high rate of side effects, especially in the 14-day treatment group (31% versus 21%; P=0.07); however, these adverse events did not lead to increased premature treatment cessation or treatment failure (Table 4).
Although it has been suggested that ingesting yogurt containing probiotics, such as Bifidobacteria and Lactobacilli, during treatment may improve H pylori eradication rates or reduce antibiotic-induced side effects (21–23), this was not demonstrated in the present study. In fact, consumption of yogurt was associated with treatment failure, with 29% failing eradication compared with only 15% of those not taking yogurt (P<0.05). There was also no benefit of yogurt in reducing side effects: 39% of those taking yogurt versus 21% of those not taking yogurt experienced side effects (P=0.01). This is not necessarily a cause-effect relationship because those who consumed yogurt could have taken it because of the presence of side effects given that we did not control as to when in the treatment course the yogurt was taken. We also cannot confirm whether probiotics were included in the consumed yogurt. These exploratory results are, however, consistent with the conclusion of a recent large study showing that Saccharomyces boulardii was not effective in preventing antibiotic-associated diarrhea (24). Although that study involved elderly hospitalized patients, one would not suspect a different outcome in our patient population.
CONCLUSION
The duration of recommended PPI triple therapy should be increased from seven to 14 days. This longer treatment duration was associated with an excellent treatment success rate in the present real-life setting study, although the success rate appears to be decreasing over time. Whereas a nonsignificant trend toward more side effects with the longer treatment was observed, this was not associated with an increase in early treatment termination. In the present study, there appeared to be no advantage to recommending yogurt during treatment. Further study is needed to determine whether 10-day is as effective as 14-day PPI triple therapy.
Acknowledgments
The authors thank Norma Baysa for nursing care and data entry.
Footnotes
AUTHOR DISCLOSURES: C Fallone has served as a speaker, consultant or on advisory board for Takeda, AstraZeneca, Janssen-Cilag and Pendopharm. A Barkun has served as a speaker, consultant or on advisory board for Takeda, Boston Scientific, Olympus, Pendopharm and AstraZeneca and has received research funding from Boston Scientific and Cook. A Szilagyi has served as a speaker, consultant or on advisory board for Ferring, Janssen-Cilag and Abbott. K Herba, M Sewitch, M Martel and S Fallone have no financial disclosures or conflicts of interest to declare.
FINANCIAL SUPPORT: No financial support was provided for this study.
REFERENCES
- 1.Megraud F, Brassens-Rabbe MP, Denis F, et al. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27:1870–3. doi: 10.1128/jcm.27.8.1870-1873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fallone CA. Treatment of Helicobacter pylori infection. Minerva Gastroenterol Dietol. 2003;49:1–9. [PubMed] [Google Scholar]
- 3.Graham DY, Lew GW, Klein PD, et al. Effects of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705–8. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 4.Lee SK, Lee YC, Chung JB, et al. Low grade gastric mucosa associated lymphoid tissue lymphoma: Treatment strategies based on 10 year follow-up. World J Gastroenterol. 2004;10:223–6. doi: 10.3748/wjg.v10.i2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong BCY, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection – the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 7.Chey WD, Wong BCY, Practice Parameters Committee of the American College of Gastroenterology American College of Gastroenterology guide on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones N, Chiba N, Fallone C, et al. Helicobacter pylori in First Nations and recent immigrant populations in Canada. Can J Gastroenterol. 2012;26:97–103. doi: 10.1155/2012/174529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 10.Veldhuyzen van Zanten S, Chiba N, Barkun A, et al. A randomized trial comparing seven-day ranitidine bismuth citrate and clarithromycin dual therapy to seven day omeprazole, clarithromycin and amoxicillin triple therapy for the eradication of Helicobacter pylori. Can J Gastroenterol. 2003;17:533–8. doi: 10.1155/2003/425293. [DOI] [PubMed] [Google Scholar]
- 11.Anonymous The report of the Digestive Health Initiative International Update conference on Helicobacter pylori. Gastroenterology. 1997;113(Suppl):S4–8. doi: 10.1016/s0016-5085(97)80003-0. [DOI] [PubMed] [Google Scholar]
- 12.Fuccio L, Minardi ME, Zagari RM, et al. Meta-analysis: Duration of first line proton pump inhibitor-based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–62. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 13.Molina-Infante J, Perez-Gallardo B, Fernandez-Bermejo M, et al. Clinical trial: Clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2010;31:1077–84. doi: 10.1111/j.1365-2036.2010.04274.x. [DOI] [PubMed] [Google Scholar]
- 14.Gisbert JP, Calvet X, O’Connor A, et al. Sequential therapy for Helicobacter pylori eradication: A critical review. J Clin Gastroenterol. 2010;44:313–25. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]
- 15.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Sequential therapy for Helicobacter pylori eradication in children. Aliment Pharmacol Ther. 2012;36:534–541. doi: 10.1111/j.1365-2036.2012.05229.x. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: A randomized trial. Lancet. 2011;378:507–14. doi: 10.1016/S0140-6736(11)60825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federico A, Nardone G, Gravina AG, et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology. 2012;143:55–61. doi: 10.1053/j.gastro.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 18.Malfertheiner P, Bazzoli F, Delchier JC, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomized open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905–13. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 19.Loo VG, Fallone CA, De Souza E, et al. In-vitro susceptibility of Helicobacter pylori to ampicillin, clarithromycin, metronidazole and omeprazole. J Antimicrob Chemother. 1997;40:881–3. doi: 10.1093/jac/40.6.881. [DOI] [PubMed] [Google Scholar]
- 20.Glupczynski Y, Megraud F, Lopez-Brea M, Anderson LP. European Multicenter survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2001;20:820–3. doi: 10.1007/s100960100611. [DOI] [PubMed] [Google Scholar]
- 21.Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: A systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45–53. doi: 10.1097/MEG.0b013e32830d0eff. [DOI] [PubMed] [Google Scholar]
- 22.Tong JL, Ran ZH, Shen J, et al. Meta-analysis: The effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2007;25:155–68. doi: 10.1111/j.1365-2036.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 23.Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: The effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069–79. doi: 10.1111/j.1365-2036.2010.04457.x. [DOI] [PubMed] [Google Scholar]
- 24.Pozzoni P, Riva A, Bellatorre AG, et al. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: A single center, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2012;107:922–31. doi: 10.1038/ajg.2012.56. [DOI] [PubMed] [Google Scholar]


