Abstract
BACKGROUND:
Many patients referred for an elevated serum ferritin level <1000 μg/L are advised that they likely have iron overload and hemochromatosis.
AIMS:
To determine the prevalence of HFE mutations in the hemochromatosis gene for 11 serum ferritin concentration intervals from 200 μg/L to 1000 μg/L in Caucasian participants in a primary care, population-based study.
METHODS:
The Hemochromatosis and Iron Overload Screening study screened 99,711 participants for serum ferritin levels, transferrin saturation and genetic testing for the C282Y and H63D mutations of the HFE gene. This analysis was confined to 17,160 male and 27,465 female Caucasian participants because the HFE C282Y mutation is rare in other races. Post-test likelihood was calculated for prediction of C282Y homozygosity from a ferritin interval. A subgroup analysis was performed in participants with both an elevated serum ferritin level and transferrin saturation.
RESULTS:
There were 3359 male and 2416 female participants with an elevated serum ferritin level (200 μg/L to 1000 μg/L for women, 300 μg/L to 1000 μg/L for men). There were 69 male (2.1%) and 87 female (3.6%) C282Y homozygotes, and the probability of being a homozygote increased as the ferritin level increased. Post-test likelihood values were 0.3% to 16% in men and 0.3% to 30.4% in women.
CONCLUSIONS:
Iron loading HFE mutations are unlikely to be the most common cause of an elevated serum ferritin level in patients with mild hyperferritinemia. Patients should be advised that there are many causes of an elevated serum ferritin level including iron overload.
Keywords: Ferritin, Haemochromatosis, Hemochromatosis, Iron overload
Abstract
HISTORIQUE:
De nombreux patients aiguillés en raison d’un taux de ferritine sérique inférieur à 1 000 μg/L apprennent qu’ils ont probablement une surcharge en fer et une hémochromatose.
OBJECTIF:
Déterminer la prévalence de mutations du gène HFE dans le gène d’hémochromatose à l’égard de 11 intervalles de concentration de la ferritine sérique de 200 μg/L à 1 000 μg/L chez des participants blancs à une étude en soins de première ligne en population.
MÉTHODOLOGIE:
L’étude sur l’hématochromatose et la surcharge en fer a permis d’obtenir le taux de ferritine sérique, la saturation de transferrine et les tests génétiques des mutations C282Y et H63D du gène HFE chez 99 711 participants. Cette analyse s’est limitée à 17 160 participants blancs et 27 465 participantes blanches parce que la mutation du gène HFE C282Y est plus rare dans les autres races. La probabilité après le test était calculée pour prédire l’homozygotie du C282Y d’après un intervalle de ferritine. Les chercheurs ont procédé à une analyse de sous-groupe chez les participants ayant à la fois un taux de ferritine sérique élevé et une saturation de transferrine.
RÉSULTATS:
Parmi les participants, 3 359 hommes et 2 416 femmes présentaient un taux élevé de ferritine sérique (200 μg/L à 1 000 μg/L chez les femmes, 300 μg/L à 1 000 μg/L chez les hommes). On constatait la présence de 69 hommes (2,1 %) et 87 femmes (3,6 %) homozygotes au C282Y, et la probabilité d’être homozygote était directement proportionnelle au taux de ferritine. Les valeurs de probabilité après les tests s’établissaient entre 0,3 % et 16 % chez les hommes et entre 0,3 % et 30,4 % chez les femmes.
CONCLUSIONS:
La charge de fer des mutations du gène HFE sont peu susceptibles d’être la principale cause de taux élevé de ferritine sérique chez les patients ayant une hyperferritinémie bénigne. Il faudrait indiquer qu’il existe de nombreuses causes de taux élevé de ferritine sérique, y compris la surcharge en fer.
Many patients are now referred to specialists because of mild elevations in serum ferritin level. The test may have been ordered to screen for iron deficiency in a fatigued patient or as a cause of liver disease, or may have been part of a multitest panel of blood tests in an annual examination (1). Elevations in serum ferritin levels often cause anxiety in patients and the magnitude of serum ferritin may be hundreds of μg/L above the upper end of the reference range, adding to the patients’ concern. Patients are frequently advised that they may have hemochromatosis and iron overload and, by the time of further assessment, they may have donated blood, switched to an iron-reduced diet and joined an Internet advocate society for patients with iron overload. In the present subanalysis of data from the Hemochromatosis and Iron Overload Screening (HEIRS) study (2), we illustrate the proportions of participants in serum ferritin concentration intervals who potentially have iron loading HFE mutations of the hemochromatosis gene.
METHODS
The study design and overall results of the HEIRS study have been previously reported (2,3). The HEIRS study was approved by all local institutional review boards. Participants ≥25 years of age who gave informed consent were recruited from five field centres that serve ethnically and socioeconomically diverse populations. All participants were screened for serum unsaturated iron-binding capacity, serum iron and serum ferritin levels (without intentional fasting), and genotyped to detect the common C282Y and H63D mutations of the HFE gene. Participants who reported a previous diagnosis of hemochromatosis or iron overload (treated or untreated) at recruitment were excluded.
Participants underwent postscreening clinical examinations if they had elevated transferrin saturation and ferritin levels, or were HFE C282Y homozygotes. The analyses were also limited to Caucasian participants because C282Y homozygotes were rare in other races in the HEIRS study (2). Patients who had a serum ferritin level <1000 μg/L were considered because this is a common clinical problem and there were a small number of participants with a ferritin level ≥1000 μg/L (76 men, 30 women). Data were grouped according to serum ferritin levels into seven intervals for men and eight intervals for women (200 μg/L to 1000 μg/L). The prevalences of C282Y homozygotes, compound heterozygotes (C282Y/H63D) and H63D homozygotes within each interval for men and women were determined. A subgroup analysis was also performed for participants with both an elevated serum ferritin level and transferrin saturation (>45% in women, >50% in men). Determining the cause of elevated ferritin levels in participants without HFE mutations was beyond the scope of the present study. However, the HEIRS study has previously reported on liver disease (4) and diabetes (5) as potential causes of elevated ferritin levels. A potential iron loading genotype was considered in the present study to be C282Y homozygote, compound heterozygote (C282Y/H63D) and H63D homozygote.
The post-test likelihood (PTL+) of being a C282Y homozygote was calculated for men and women. The PTL+ is the likelihood of the condition of interest given a positive test result (6). The estimated 95% CI for PTL+ was based on binomial distribution with a normal approximation applied.
RESULTS
There were 69 male and 87 female C282Y homozygotes with an elevated serum ferritin level identified in this population. There were 3359 male and 2416 female participants with an elevated serum ferritin level (200 μg/L to 1000 μg/L for women, 300 μg/L to 1000 μg/L for men) (Table 1). There were 137 (4%) male and 117 (4.8%) female compound heterozygotes (C282Y/H63D), and 130 male (3.9%) and 81 female (3.4%) H63D homozygotes (Table 1). The PTL+ for a ferritin interval to detect a C282Y homozygote are shown in Table 2. In participants with an elevated serum ferritin level, the prevalence of a potential iron-loading HFE genotype was 336 of 3359 (10%) in men and 285 of 2416 (12%) in women.
TABLE 1.
HFE mutations according to serum ferritin (SF) interval
|
SF interval, μg/L
|
HFE genotype
|
||||||
|---|---|---|---|---|---|---|---|
| Caucasian men | WT/WT | H63D/WT | C282Y/WT | H63D/H63D | C282Y/H63D | C282Y/C282Y | Total |
| 0.00–300 | 8659 | 3260 | 1422 | 283 | 228 | 39 | 13,801 |
| 301–400 | 900 | 411 | 190 | 53 | 39 | 4 | 1597 |
| 401–500 | 439 | 177 | 88 | 30 | 36 | 8 | 778 |
| 501–600 | 242 | 117 | 56 | 25 | 29 | 20 | 489 |
| 601–700 | 104 | 60 | 40 | 10 | 16 | 10 | 240 |
| 701–800 | 63 | 31 | 17 | 6 | 11 | 8 | 136 |
| 801–900 | 44 | 11 | 5 | 2 | 6 | 13 | 81 |
| 901–1000 | 14 | 9 | 5 | 4 | 0 | 6 | 38 |
| Total | 10,375 | 4076 | 1823 | 413 | 365 | 108 | 17,160 |
|
Caucasian women
| |||||||
| 0.00–200 | 15,309 | 6017 | 2621 | 550 | 469 | 83 | 25,049 |
| 201–300 | 845 | 373 | 152 | 51 | 67 | 17 | 1505 |
| 301–400 | 286 | 110 | 49 | 16 | 20 | 17 | 498 |
| 401–500 | 98 | 41 | 20 | 10 | 15 | 19 | 203 |
| 501–600 | 34 | 26 | 10 | 3 | 8 | 9 | 90 |
| 601–700 | 29 | 7 | 3 | 0 | 2 | 9 | 50 |
| 701–800 | 11 | 11 | 1 | 0 | 2 | 4 | 29 |
| 801–900 | 7 | 4 | 3 | 1 | 1 | 7 | 23 |
| 901–1000 | 5 | 4 | 2 | 0 | 2 | 5 | 18 |
| Total | 16,624 | 6593 | 2861 | 631 | 586 | 170 | 27,465 |
WT Wild type
TABLE 2.
Post-test likelihood of being a C282Y homozygote
| Ferritin, μg/L | Women (n=27,465) | Ferritin, μg/L | Men (n=17,160) |
|---|---|---|---|
| 0.00–200 | 0.3 (0.26–0.40)* | – | – |
| 201–300 | 1.1 (0.6–1.66) | 0.00–300 | 0.3 (0.19–0.37) |
| 301–400 | 3.4 (1.82–5.01) | 301–399 | 0.3 (0.01–0.50) |
| 401–500 | 9.4 (5.35–13.37) | 400–499 | 1 (0.32–1.74) |
| 501–600 | 10 (3.80–16.2) | 500–599 | 4.1 (2.33–5.85) |
| 601–700 | 18 (7.35–28.65) | 600–699 | 4.2 (1.64–6.69) |
| 701–800 | 13.8 (1.24–26.34) | 700–799 | 5.9 (1.93–9.84) |
| 801–900 | 30.4 (11.63–49.24) | 800–899 | 16 (8.06–24.04) |
| 901–1000 | 27.8 (7.09–48.47) | 900–999 | 15.8 (4.2–27.38) |
The estimated 95% CI for post-test likelihood was based on binomial distribution with a normal approximation applied
There were 437 of 3359 (13%) men and 311 of 2416 (12.8%) women with elevated serum ferritin level and transferrin saturation. In this subgroup, there was a higher proportion of participants who were C282Y homozygotes within each ferritin interval (Figure 1). In participants with an elevated transferrin saturation, the prevalence of a potential iron-loading HFE genotype increased to 30% in men and 42% in women.
Figure 1).
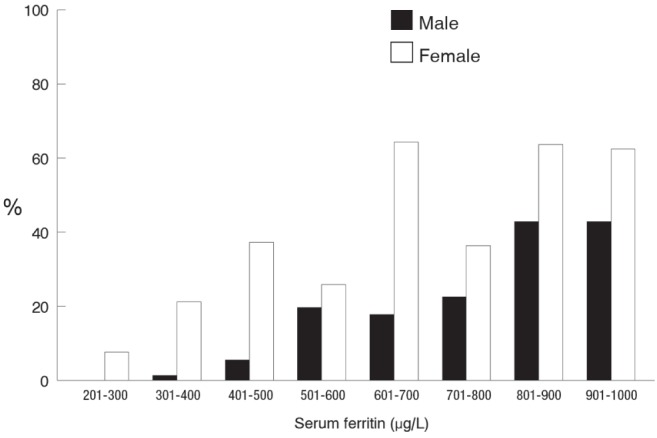
The proportion of male and female participants with an elevated ferritin level and transferrin saturation who were C282Y homozygotes
DISCUSSION
In the present analysis, we demonstrated that most Caucasian participants in the HEIRS study with an elevated serum ferritin level <1000 μg/L were not C282Y homozygotes, compound heterozygotes or H63D homozygotes. The most likely causes of elevated ferritin levels in non-C282Y homozygotes include inflammation, obesity and alcohol consumption (1). Our study cannot exclude the possibility of non-C282Y genetically linked iron overload as the cause of the ferritin elevation in non-C282Y homozygotes; however, this has been a very rare diagnosis in North America (7). In the HEIRS study, approximately 20% of all the Caucasian men had an elevated serum ferritin level (>300 μg/L). In the HEIRS study, many participants were recruited from primary care clinics and clinical phlebotomy sites; therefore, they were already being tested for other reasons, which suggests that they were not representative of a normal healthy population. This could explain the higher ranges that were apparent in the present study. A low serum ferritin level is indicative of iron deficiency, but a serum ferritin level above the current recommended reference ranges is often not an indication of total body iron overload.
Genetic testing for the C282Y mutation of the HFE gene is often used as a second-line confirmatory test for hemochromatosis in patients with an elevated ferritin level. In the present analysis, we assumed that individuals with an iron loading genotype and an elevated ferritin level had iron overload, but these patients may also have ferritin elevations for other reasons. In larger population-based studies, including the HEIRS study of compound heterozygotes and H63D homozygotes, <10% had iron overload (8). A clinician could improve the proportion of positive tests for C282Y homozygosity by the clinical interpretation of the clinical history, family history, physical examination and a careful review of other conditions causing elevated ferritin levels such as inflammation including liver disease, alcohol use and obesity. In clinical practice, it can be difficult to determine whether a patient with a mild elevation in ferritin level has iron overload. The use of invasive tests, such as liver biopsy, is not appealing to the patient. Magnetic resonance imaging has better sensitivity to detect higher levels of liver iron overload but is expensive and not widely available. A trial of phlebotomy is often performed but may lead to anemia and fatigue in patients without iron overload. A concomitant elevation in transferrin saturation has been demonstrated to increase the proportion of participants who are C282Y homozygotes. However, the biological variability of transferrin saturation is a limitation as a screening test (9). Predictors to assist in the identification of patients with iron overload in practice based on clinical conditions have been developed and validated (10). However, the restriction of HFE genotyping to highly selected cases could miss many patients with less classical presentations and often patients are referred to confirm whether hemochromatosis is the cause of the ferritin elevations. Genetic testing is less invasive than previous diagnostic tools, such as liver biopsy, and may be reassuring to patients and their families. Previous studies have suggested that only patients with a ferritin level ≥1000 μg/L should undergo further investigation for hemochromatosis and treatment (11). Previously undiagnosed C282Y homozygotes with serum ferritin values that remain <1000 μg/L are at low risk for developing hemochromatosis-related signs and symptoms at an age when the clinical manifestations would be expected to have developed (12). However, there is a fivefold increase in risk of death causally associated with iron overload in persons with hemochromatosis, C282Y homozygosity and serum ferritin level ≥1000 μg/L at diagnosis (13). This strongly suggests that it is preferable to diagnose hemochromatosis and perform phlebotomy therapy to achieve iron depletion well before seurm ferritin levels exceed 1000 μg/L. Therefore, the present study suggests that most patients with a modest elevation in ferritin level do not have C282Y-linked hemochromatosis and yet it is important to identify them. The clinical factors that increase the likelihood of being a C282Y homozygote are shown in Table 3.
TABLE 3.
Clinical factors that increase the likelihood of a patient with a modest elevation in ferritin level having C282Y-linked hemochromatosis
| Caucasian |
| Elevated transferrin saturation |
| Normal AST and ALT levels |
| Absence of daily alcohol consumption |
| Absence of fatty liver disease |
| Family history of iron overload |
ALT Alanine aminotransferase; AST Aspartate aminotransferase
An important clinical observation from the present study is that C282Y-linked hemochromatosis is not the most common cause of elevated serum ferritin levels in Caucasians, suggesting that patients should not be told that they have genetic hemochromatosis and iron overload before further assessment is performed.
Acknowledgments
The authors acknowledge the statistical support of Wen-Pin Chen MS. The HEIRS study was sponsored by the National Heart Lung and Blood Institute and the National Human Genome Research Institute of the National Institute of Health (Bethesda, Maryland, USA). The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Wong K, Adams PC. The diversity of liver diseases associated with an elevated serum ferritin. Can J Gastro. 2007;20:467–70. doi: 10.1155/2006/357340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams PC, Reboussin DM, Barton JC, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–78. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 3.McLaren CE, Barton JC, Adams PC, et al. Hemochromatosis and Iron Overload Screening (HEIRS) study design for an evaluation of 100,000 primary care-based adults. Am J Med Sci. 2003;325:53–62. doi: 10.1097/00000441-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Adams PC, Passmore L, Chakrabarti S, et al. Liver diseases in the Hemochromatosis and Iron Overload Screening Study. Clin Gastroenterol Hepatol. 2006;4:918–23. doi: 10.1016/j.cgh.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Acton RT, Barton JC, Passmore LV, et al. Relationships of serum ferritin, transferrin saturation, and HFE mutations and self-reported diabetes in the Hemochromatosis and Iron Overload Screening (HEIRS) study. Diabetes Care. 2006;29:2084–9. doi: 10.2337/dc05-1592. [DOI] [PubMed] [Google Scholar]
- 6.Haynes RB, Sackett DL, Guyatt GH, Tugwell P. Clinical Epidemiology. 3rd edn. Philadelphia: Lippincott Williams and Wilkins; 2006. p. 279. [Google Scholar]
- 7.Barton JC, Lafreniere SA, Leiendecker-Foster C, et al. HFE, SLC40A1, HAMP, HJV, TFR2, and FTL mutations detected by denaturing high-performance liquid chromatography after iron phenotyping and HFE C282Y and H63D genotyping in 785 HEIRS study participants. Am J Hematol. 2009;84:710–4. doi: 10.1002/ajh.21524. [DOI] [PubMed] [Google Scholar]
- 8.Neghina AM, Anghel A. Hemochromatosis genotypes and risk of iron overload – a meta-analysis. Ann Epidemiol. 2011;21:1–14. doi: 10.1016/j.annepidem.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Adams PC, Reboussin DM, Press RD, et al. Biological variability of transferrin saturation and unsaturated iron binding capacity. Am J Med. 2007;120:999, e1–e7. doi: 10.1016/j.amjmed.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainous AG, III, Diaz VA, Everett CJ, et al. IRon Overload screeNing tool (IRON): Development of a tool to guide screening in primary care. Am J Hematol. 2011;86:733–7. doi: 10.1002/ajh.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waalen J, Felitti VJ, Gelbart T, et al. Screening for hemochromatosis by measuring ferritin levels: A more effective approach. Blood. 2008;111:3373–6. doi: 10.1182/blood-2007-07-102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen KJ, Bertalli NA, Osborne NJ, et al. HFE Cys282Tyr homozygotes with serum ferritin concentrations below 1000 microg/L are at low risk of hemochromatosis. Hepatology. 2010;52:925–33. doi: 10.1002/hep.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton JC, Barton JC, Acton RT, So J, Chan S, Adams PC. Increased risk of death from iron overload among 422 treated probands with HFE hemochromatosis and serum levels of ferritin greater than 1000 μg/L at diagnosis. Clin Gastroenterol Hepatol. 2012;10:412–6. doi: 10.1016/j.cgh.2011.11.032. [DOI] [PubMed] [Google Scholar]


