Abstract
Objective
To compare the effects of massage therapy (moderate pressure stroking) and exercise (flexion and extension of limbs) on preterm infants’ weight gain and to explore potential underlying mechanisms for those effects.
Methods
Weight gain and parasympathetic nervous system activity were assessed in 30 preterm infants randomly assigned to a massage therapy group or to an exercise group. Infants received 10 minutes of moderate pressure massage or passive flexion and extension of the limbs 3 times per day for 5 days, and EKGs were collected during the first session to assess vagal activity.
Results
Both massage and exercise led to increased weight gain. However, while exercise was associated with increased calorie consumption, massage was related to increased vagal activity.
Conclusion
Taken together, these findings suggest that massage and exercise lead to increased preterm infant weight gain via different underlying mechanisms.
Keywords: Preterm infant, massage, exercise, vagal activity, weight gain
Prematurity is among the leading causes of infant morbidity (1). Supplementary stimulation programs in the NICU including massage therapy (moderate pressure stroking) and exercise (passively moving the limbs into flexion and extension) can improve preterm infant development, including increased weight gain (2, 3). Inasmuch as most preterm infant massage studies have combined tactile (massage) and kinesthetic (exercise) stimulation, it is unclear whether the tactile or the kinesthetic component is responsible for the increased weight gain observed in these studies.
Preterm infants receiving a combined tactile-kinesthetic stimulation protocol show increased weight gain (2, 4). Similarly, studies utilizing either only kinesthetic stimulation (3, 5) or only tactile stimulation (6) have shown increased weight gain, suggesting that both the tactile and kinesthetic components are effective by themselves in promoting preterm infant weight gain.
A potential mechanism underlying preterm infant weight gain following tactile-kinesthetic stimulation may involve the stimulation of baroreceptors and mechanoreceptors leading to the activation of vagal afferent and efferent pathways involved in the parasympathetic control of the cardiovascular and gastro-intestinal systems (7, 8). This mechanism is supported by studies revealing that a combined tactile / kinesthetic stimulation protocol elicits increases in cardiac vagal activity that are associated with increased gastric motility and preterm infant weight gain (7 – 9). Tactile and kinesthetic stimulation may promote preterm infant weight gain via different underlying mechanisms While preterm infant cardiac vagal activity has yet to be assessed independently for kinesthetic and tactile stimulation, studies with adults suggest that passive exercise, which is similar to the kinesthetic stimulation protocol used with preterm infants, inhibits cardiac vagal activity (10, 11), while moderate pressure massage therapy, which is analogous to the tactile stimulation protocol used with preterm infants, increases cardiac vagal activity (12). The aim of this study was to compare the effects of the tactile and the kinesthetic stimulation protocols on preterm infant weight gain while exploring cardiac vagal activity as a potential underlying mechanism.
Methods
Participants
Following Institutional Review Board approval and parental informed consent, medically stable preterm neonates were recruited from a Neonatal Intensive Care Unit. Preterm infants were considered eligible for recruitment if: a) their gestational age (GA) at birth was between 28 and 32 weeks; b) their birth weight was between 800 and 1,400 grams; c) their NICU stay at study entry was 15 -60 days; and d) their weight at study entry was between 1,000 and 1,500 grams. Preterm infants were excluded if: a) they had congenital malformations, chromosomal aberrations, congenital infections, genetic anomalies, congenital heart malformations, and/or central nervous system dysfunction (e.g., intraventricular hemorrhage or a history of seizure); b) they were HIV positive; c) had a history of maternal alcohol/illicit drug use (as determined by the mother’s reported history, medical records, and universal drug screen performed prior to delivery), syphilis, or hepatitis B, or d) they required surgery. All infants were bottle-fed premature infant formula. Infants were stratified based on gender, birth weight, gestational age at birth, and days on the NICU prior to group assignment. Preterm neonates meeting recruitment criteria were randomly assigned to a tactile stimulation group or a kinesthetic stimulation group. Recruitment continued until 30 preterm infants (n = 15 in each group) completed the study. Data from 5 preterm neonates (n = 3 kinesthetic, n = 2 tactile) who were discharged before completing the 5-day treatment protocol were excluded from the study. There were no systematic differences in diagnoses or treatments between groups or between infants who completed and did not complete the study.
Relevant medical history was gathered from the infant’s medical charts (measured by NICU nurses) including mean weight gain per day in grams, percent weight gain (mean weight gain per kilogram per day) and mean calories consumed per kilogram per day. Data were collected and averaged for the 2 days prior to the beginning of treatment (baseline) and the 5 days of treatment (treatment). Electrocardiograms (EKGs) were collected by researchers blind to the infant’s group assignment for a total of 30 minutes (10 minutes baseline, 10 minutes treatment, and 10 minutes post-treatment). Inasmuch as only short term, heart rate variability changes have been observed following tactile-kinesthetic stimulation (8), EKGs were collected to assess short term changes in heart rate variability on the morning of the third day of the study, as this marked the midpoint of the study treatment protocol. All infants continued to receive standard nursery care during the course of the study.
Treatment
Supplementary stimulation (tactile or kinesthetic) was provided for three 10-minute periods per day for 5 days, one hour after feeding by research associates trained in the study protocol. Training involved watching a video of the protocol followed by a series of observation and practice sessions. Reliability was assessed before the study and reevaluated throughout the study to ensure protocol compliance. Each neonate received treatment from multiple research associates to ensure that treatment effects were the result of the treatment protocol and not from any particular individual.
Neonates in the tactile stimulation group were placed in a prone position and stroked with moderate pressure (sufficient to produce a slight indentation in the skin) as in the Field et al tactile stimulation protocol (4). The tactile stimulation was applied: a) from the top of the head to the neck and back to the top of the head; b) from the neck across the shoulders and back to the neck; c) from the upper back to the waist and back to the upper back; d) from the thigh to the foot to the thigh on both legs; and e) from the shoulder to the hand to the shoulder on both arms. Each stroking motion lasted 10 seconds for a total period of 10 minutes. Hypoallergenic baby oil was applied to reduce friction.
Neonates in the kinesthetic stimulation group were placed in a supine position and their limbs were flexed and extended as in the Moyer-Mileur protocol (3). The kinesthetic stimulation was applied by: a) flexing and extending each arm at the elbow; b) flexing and extending each hand at the wrist; c) flexing and extending each leg at the knee; d) flexing and extending each foot at the ankle; and e) flexing and extending both legs together (as in a bicycling motion); Each flexion/extension motion lasted 10 seconds, for a total period of 10 minutes.
EKG
Electrocardiograms (EKGs) were collected to estimate vagal activity as a potential underlying mechanism for weight gain following tactile/ kinesthetic stimulation. EKGs were collected from each infant using a UFI Model SRS2004M-SP Electro-physiology Acquisition System, by placing three disposable, pre-wired silver chloride neonatal electrodes on the preterm infant’s chest and back. The EKG signal was filtered between 1Hz and 100Hz, amplified using a gain of 2,000 and sampled at a rate of 1000Hz. Following manual artifact correction, EKG data were converted to R-to-R-wave intervals (inter beat intervals, IBI) to the nearest millisecond and analyzed to obtain the High Frequency component of heart rate variability, defined as 0.3–1.3Hz for preterm infants (Bohrer & Porges, 1982), using data acquisition and analysis software (Acq Knowledge software V.3.5, Biopac Systems Inc.). The High Frequency component of heart rate variability, HF, provides a non-invasive assessment of cardiac vagal activity (13).
Statistical methods
Statistical analyses were performed using SPSS 18.0 for Mac (SPSS Inc., Chicago, Illinois). Analyses of variance (ANOVAs) and Chi Square (χ2) analyses were used to compare the groups on demographic and study entry characteristics. Repeated-measures ANOVAs with Group (tactile / kinesthetic) as the between subjects factor and Time (Baseline/ Post Treatment) as the within subjects factor were used to analyze study outcome variables including weight gain, calorie intake and volumetric output. A repeated measures ANOVA with Group (tactile/ kinesthetic) as the between subjects factor and Time (Pre / During /Post the first session) as the within subjects factor was also used to analyze changes in cardiac vagal activity. Significant interactions were followed by post hoc trend analyses. Pearson correlation analyses were conducted to assess the relationships between changes in calorie intake, cardiac vagal activity and mean daily weight gain. Separate correlation analyses were conducted for the Kinesthetic and Tactile stimulation groups.
Results
Maternal and neonatal demographic and study entry characteristics did not differ between groups (Table 1). Group (Tactile vs Kinesthetic) × Time (Baseline/ Post Treatment) repeated measures analyses of variance conducted on study outcome variables revealed the following: a) Weight gain (grams / day): a significant main effect for time, F (1, 28) = 9.95; p < .005; η2 = .26, but no Group by Time interaction or main effect for Group, suggesting a significant increase in weight gain for both the tactile and kinesthetic stimulation groups; b) Percent Weight gain (grams / kg / day): a significant main effect for time, F (1, 28) = 6.23; p < .05; η2 = .18, but no Group by Time interaction or main effect for Group, suggesting a significant increase in the percent weight gained for both the tactile and kinesthetic stimulation groups and c) Calorie Consumption: a significant Group by Time interaction, F (1, 28) = 4.12; p = .05; η2 = .13, and significant linear trend for the kinesthetic, F (1, 14) = 6.03; p < .05; η = .30, but not the tactile stimulation group, F (1, 14) = 0.42; p = n.s.; η2 = .03, suggesting increased calorie consumption for only the kinesthetic stimulation group.
Table 1.
Study entry characteristics (Means and standard deviations in parentheses under means).
| Kinesthetic N=15 |
Tactile | N=15 | ||
|---|---|---|---|---|
| Birth weight (g) | 1156.53 (180.55) | 1232.67 (186.57) | F(1, 29) = 1.29 | p = n.s. |
| Gestational Age (wk) | 29.40 (1.59) | 29.07 (1.83) | F(1, 29) = 1.29 | p = n.s. |
| Birth Length (cm) | 38.00 (2.20) | 38.89 (1.92) | F(1, 29) = 1.34 | p = n.s. |
| Head Circumference (cm) | 26.32 (1.92) | 27.39 (1.99) | F(1, 29) = 2.19 | p = n.s. |
| Ponderal Index | 2.12 (0.24) | 2.13 (0.23) | F(1, 29) = 0.01 | p = n.s. |
| Mother's Age | 28.67 (6.45) | 29.20 (6.53) | F(1, 29) = 0.05 | p = n.s. |
| Mother's SES | 4.62 (1.04) | 4.08 (0.90) | F(1, 29) = 1.85 | p = n.s. |
| Ethnicity | ||||
| White | 0% | 0% | Χ2 (1)= .71 | p = n.s. |
| African American | 47% | 40% | ||
| Hispanic | 53% | 60% | ||
| Gender | ||||
| Male | 40% | 60% | X2(1) = .27 | p = n.s. |
| Female | 60% | 40% | ||
| Days on NICU | 36.67 (11.50) | 33.20 (14.38) | F(1, 29) = 0.53 | p = n.s. |
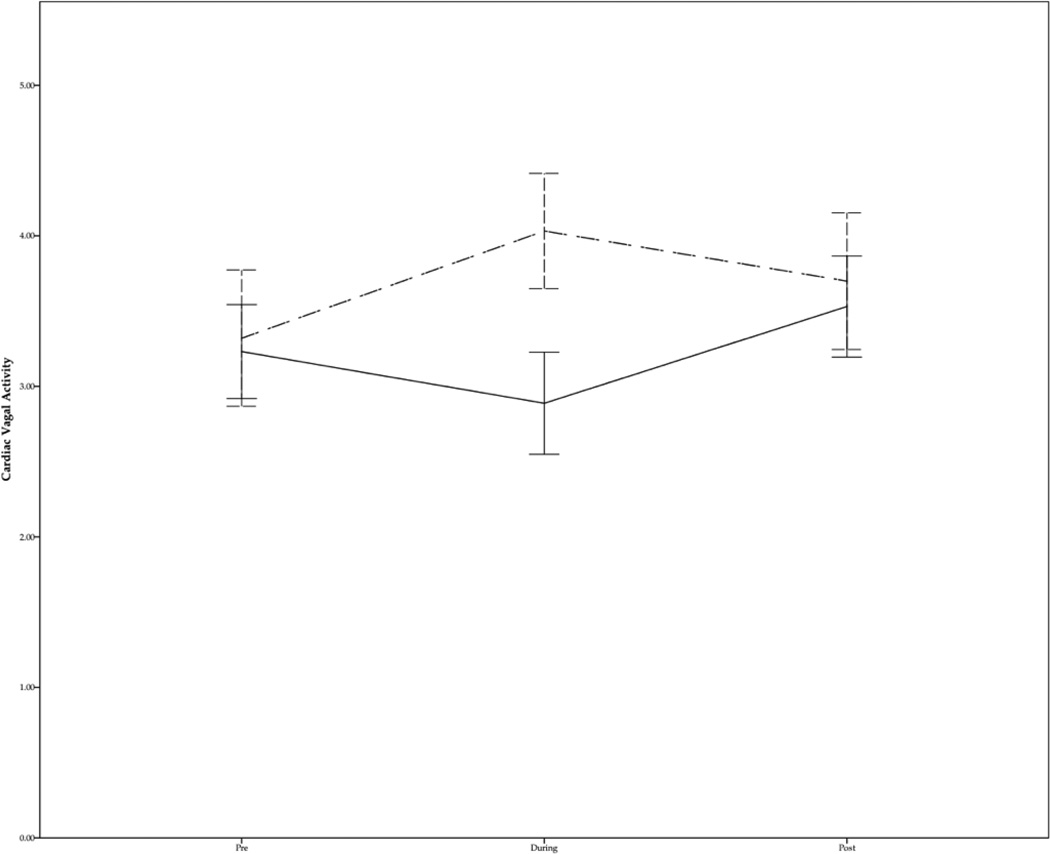
Group (Tactile vs Kinesthetic) × Time (Pre / During / Post) repeated measures analyses of variance conducted on vagal activity revealed a significant Group by Time interaction, F (2, 56) = 22.42; p < .001; η2 = .45, post hoc trend analyses further revealed a significant quadratic trend for the kinesthetic group, F (1, 14) = 30.16; p < .001; η2 = .68, and a significant quadratic trend for the tactile stimulation group, F (1, 14) = 14.04; p < .005; η2 = .50, suggesting that while vagal activity significantly increased during tactile stimulation, it significantly decreased during kinesthetic stimulation (Figure 1).
Figure 1.
Mean cardiac vagal activity for preterm infants assigned to the tactile (dashed line) and kinesthetic stimulation groups (solid line). Error bars represent +− 2 SD
Pearson correlation analyses conducted on the data from each group separately revealed that increased weight gain was associated with increased calorie consumption for the kinesthetic stimulation group. And, in contrast, increased weight gain was associated with increased vagal activity for the tactile stimulation group (Table 3).
Table 3.
Pearson Correlation analyses between study outcome variables.
| Group | Δ Vagal | Δ Calories | Δ Weight Gain | |
|---|---|---|---|---|
| Kinesthetic | Δ Calories | .33 | ||
| Stimulation | Δ Weight Gain | .30 | .60* | |
| Δ% Weight Gain | .26 | .61* | .99** | |
| Tactile | Δ Calories | −.01 | ||
| Stimulation | Δ Weight Gain | .57* | .07 | |
| Δ% Weight Gain | .54* | .08 | .99** |
p < .05
Discussion
Preterm infants who received three, 10-minute sessions of either tactile or kinesthetic stimulation per day, exhibited greater weight gain during the 5-day treatment period than during baseline. These findings are consistent with research documenting that both kinesthetic (3, 5) and tactile stimulation (6) are effective for promoting weight gain in preterm infants. In the present study, weight gain did not differ between infants who received tactile and kinesthetic stimulation. To our knowledge, this is the first time that the tactile versus kinesthetic stimulation effects on preterm infant weight gain have been directly compared. Since the development of the tactile-kinesthetic protocol several decades ago (4), several iterations of that protocol have been implemented in research studies, including the aforementioned variations using only kinesthetic (3, 5) or tactile stimulation (6) as well as other variations including the person administering the stimulation, the amount of pressure implemented, and the duration and frequency of the protocol (2).
Comparing the tactile and kinesthetic protocols can help identify the key components that make supplementary preterm infant stimulation effective in promoting infant growth. Recently, we showed that the pressure utilized in the tactile stimulation phase is an essential component of the tactile stimulation protocol inasmuch as only those infants who received moderate pressure stroking (compared to light pressure stroking) showed greater weight gain and increased cardiac vagal activity (7). Data also suggest that greater weight gain is achieved if oil is used during tactile stimulation (2). In terms of the frequency and duration of the protocol, greater weight gain has been consistently observed when administering the supplementary stimulation protocol for 15 minutes 3 times per day (2). In the present study the shorter 10-minute stimulation sessions 3 times a day also led to greater weight gain. However, differences in the frequency and duration of the stimulation protocol may account for inconsistent weight gain findings associated with both kinesthetic (3, 5, 14) and tactile stimulation protocols (15). Further research is needed on the frequency and duration of supplementary stimulation protocols in order to perform cost-benefit analyses on duration and frequency effects.
Calorie consumption increased during the treatment period for the kinesthetic, but not the tactile stimulation group. These findings are consistent with prior research indicating that tactile stimulation does not increase calorie consumption (6), but at odds with research indicating that kinesthetic stimulation does not increase calorie consumption (14). As was the case with weight gain, the discrepancy in caloric intake findings between the kinesthetic stimulation group in the present study and those in previous research may result from differences between these studies on the duration and frequency of the stimulation sessions. In the kinesthetic stimulation group, calorie consumption was related to greater weight gain, suggesting that preterm infant weight gain following kinesthetic stimulation may involve increased calorie consumption.
Cardiac vagal activity increased during tactile stimulation and was, in turn, associated with increased weight gain. This finding is consistent with research showing that tactile stimulation increases cardiac vagal activity in adults (12) and studies indicating that preterm infants receiving combined tactile and kinesthetic stimulation, show increased cardiac vagal activity, but only when moderate pressure tactile stimulation is applied (7–9). Taken together these findings suggest that weight gain following tactile stimulation may be mediated by increased vagal activity. Tactile stimulation may stimulate the vagal efferent fibers that innervate the digestive system by stimulating baroreceptors and mechanoreceptors within the skin. Baroreceptors and mechanoreceptors within the skin are innervated by vagal afferent fibers, which constitute the predominant source of afferent inputs to the neurons that give rise to the efferent vagal fibers that provide most of the parasympathetic control of the gastro-intestinal system (16, 17). The weight gain effects may, in turn, be mediated by increased gastric motility also reported following combined tactile/kinesthetic stimulation of preterm neonates (7) Kinesthetic stimulation, on the other hand, decreased cardiac vagal activity. This finding is consistent with research showing that passive exercise in adults inhibits cardiac vagal activity via the activation of afferent stretch mechanoreceptors in muscle called tentonoreceptors (10, 11).
The clinical implications of these data are that both the massage therapy and exercise protocols that have been used in the neonatal intensive care units can lead to preterm infant weight gain and earlier hospital discharge. It is also possible that both protocols might lead to other important clinical effects such as increased insulin and growth hormone (IgF-1) (18) and improved bone growth variables (3, 14). Given the simplicity of the two protocols, volunteers and parents could be given demonstrations and could then provide cost-effective stimulation sessions to facilitate weight gain and earlier discharge in a larger group of preterm infants. This stimulation would also be expected to have longer -term effects as it has in other similar stimulation studies, with the weight gain advantage continuing into later infancy (eight months) and mental development also being superior for the stimulated infants at that time (19). And, parents would also benefit from lower stress hormones as they provided the stimulation (20) and by having their infants discharged earlier.
Table 2.
Study outcome variables for the 2-day pre treatment period (Baseline) and 5-days of treatment (Treatment). Means and standard deviations in parentheses under means.
| Kinesthetic Stimulation | Tactile Stimulation | |||
|---|---|---|---|---|
| Baseline | Treatment | Baseline | Treatment | |
| Weight Gain (g) | 19.06 a(11.56) | 26.38b(15.55) | 17.77 a (10.52) | 29.493 b (13.24) |
| Percent Weight gain (g/kg/day) | 1.10% a (0.67%) | 1.40% b (0.72%) | 1.03% a (0.64%) | 1.57% b (0.66%) |
| Calorie Consumption | 110.12a (11.56) | 118.12b (14.13) | 113.85a (7.67) | 114.71 a(6.47) |
Different subscripts denote significant differences between means.
Acknowledgments
We thank the mothers and neonates who participated in this study and the Jackson Memorial NICU nurses and neonatologists, research assistants and massage therapists for their help with this study. This research was supported by NIH Senior Research Scientist Awards (#MH00331 and AT#001585) and an NCCAM research Grant (#AT00370) to Tiffany Field, an NCCAM research supplement (#AT00370-02S1) to Miguel Diego and funding from Johnson and Johnson Pediatric Institute to the Touch Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There was no conflict of interest, real or perceived for any of the authors and the aforementioned sponsors did not influence in any way the (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication.
References
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, Osterman MJ. Births: final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- 2.Field T, Diego M, Hernandez-Reif M. Moderate pressure is essential for massage therapy effects. Int J Neurosci. 2010;120:381–385. doi: 10.3109/00207450903579475. [DOI] [PubMed] [Google Scholar]
- 3.Moyer-Mileur LJ, Brunstetter V, McNaught TP, Gill G, Chan GM. Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics. 2000;106:1088–1092. doi: 10.1542/peds.106.5.1088. [DOI] [PubMed] [Google Scholar]
- 4.Field TM, Schanberg SM, Scafidi F, Bauer CR, Vega-Lahr N, Garcia R, Nystrom J, Kuhn CM. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics. 1986;77:654–658. [PubMed] [Google Scholar]
- 5.Nemet D, Dolfin T, Litmanowitz I, Shainkin-Kestenbaum R, Lis M, Eliakim A. Evidence for exercise-induced bone formation in premature infants. Int J Sports Med. 2002;23:82–85. doi: 10.1055/s-2002-20134. [DOI] [PubMed] [Google Scholar]
- 6.Ferber SG, Kuint J, Weller A, Feldman R, Dollberg S, Arbel E, Kohelet D. Massage therapy by mothers and trained professionals enhances weight gain in preterm infants. Early Hum Dev. 2002;67:37–45. doi: 10.1016/s0378-3782(01)00249-3. [DOI] [PubMed] [Google Scholar]
- 7.Diego MA, Field T, Hernandez-Reif M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J Pediatr. 2005;147(1):50–55. doi: 10.1016/j.jpeds.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Begert G. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta Paediatr. 2007;96:1588–1591. doi: 10.1111/j.1651-2227.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee HK. The effect of infant massage on weight gain, physiological and behavioral responses in premature infants. Taehan Kanho Hakhoe Chi. 2005;35:1451–1460. doi: 10.4040/jkan.2005.35.8.1451. [DOI] [PubMed] [Google Scholar]
- 10.Drew RC, Bell MP, White MJ. Modulation of spontaneous baroreflex control of heart rate and indexes of vagal tone by passive calf muscle stretch during graded metaboreflex activation in humans. J Appl Physiol. 2008;104:716–723. doi: 10.1152/japplphysiol.00956.2007. [DOI] [PubMed] [Google Scholar]
- 11.Gladwell VF, Fletcher J, Patel N, Elvidge LJ, Lloyd D, Chowdhary S, Coote JH. The influence of small fibre muscle mechanoreceptors on the cardiac vagus in humans. J Physiol. 2005;567:713–721. doi: 10.1113/jphysiol.2005.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diego MA, Field T. Moderate pressure massage elicits a parasympathetic nervous system response. Int J Neurosci. 2009;119:630–638. doi: 10.1080/00207450802329605. [DOI] [PubMed] [Google Scholar]
- 13.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 14.Moyer-Mileur LJ, Ball SD, Brunstetter VL, Chan GM. Maternal-administered physical activity enhances bone mineral acquisition in premature very low birth weight infants. J Perinatol. 2008;28:432–437. doi: 10.1038/jp.2008.17. [DOI] [PubMed] [Google Scholar]
- 15.Massaro AN, Hammad TA, Jazzo B, Aly H. Massage with kinesthetic stimulation improves weight gain in preterm infants. J Perinatol. 2009;29:352–357. doi: 10.1038/jp.2008.230. [DOI] [PubMed] [Google Scholar]
- 16.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- 17.Kandel E, Schwartz JH, Jessell TM. Principles of Neural Science. 4th ed. New York: McGraw-Hill; 2000. [Google Scholar]
- 18.Field T, Diego M, Hernandez-Reif M, Dieter JN, Kumar AM, Schanberg S, Kuhn C. Insulin and insulin-like growth factor-1 increased in preterm neonates following massage therapy. J Dev Behav Pediatr. 2008;29:463–466. doi: 10.1097/DBP.0b013e3181856d3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field T, Scafidi F, Schanberg S. Massage of preterm newborns to improve growth and development. Pediatric Nursing. 1987;13:385–387. [Google Scholar]
- 20.Field T, Hernandez-Reif M, Quintino O, Schanberg S, Kuhn C. Elder retired volunteers benefit from giving massage therapy to infants. The Journal of Applied Gerontology. 1998;17:229–239. [Google Scholar]