Abstract
BACKGROUND
Methoxychlor (MXC) is specifically known to target ovarian antral follicles, increasing atresia (death via apoptosis) in them. This is of concern because females are born with a finite pool of ovarian follicles. Only limited studies have explored the phenomenon of a reduced fertility threshold for effect based on the percentage of antral follicle atresia.
METHODS
In this article, we report on adult female CD-1 mice exposed intraperitoneally to various doses of MXC for 5, 10, 20, and 30 days. In the 20-day treatment, mice were dosed with either the vehicle or MXC at 64 or 96 mg/kg/day, whereas in the 30-day treatment, mice were dosed with vehicle or MXC at 48, 64, or 96 mg/kg/day. The mice that were dosed with MXC for 30 days were also mated with untreated males for a determination of overall fertility.
RESULTS
A significantly increased percentage (50%) of atretic antral follicles was observed only after 20 and 30 days of treatment. Specifically, mice treated with MXC64 for 20 and 30 days had an increased percentage of atretic antral follicles compared with vehicle-treated mice. Interestingly, mice dosed with MXC96 had an increased percentage of atretic antral follicles after 30 days, but not after 20 days of treatment compared with vehicle-treated mice. Overall fertility of the mice was not different compared with controls.
CONCLUSIONS
The results indicate that as much as a 50% increase in atretic antral follicles does not affect the immediate fertility of the mice.
Keywords: methoxychlor, atresia, ovary, antral follicle, fertility, estrogenic
INTRODUCTION
Methoxychlor (MXC), an organochlorine pesticide, was widely used around the world to prevent crops, fruits, and livestock from being attacked by insects and pests. Although the usage of MXC has ceased in the United States, exposures may still occur via fruits and vegetables imported to U.S. markets from other countries that continue to use MXC. In the past, MXC has been well screened for its estrogenicity and for its ability to cause reproductive abnormalities in mammalian females (Eroschenko et al., 1996; Cummings, 1997; Gray et al., 1999; Gaido et al., 2000; Miller et al., 2006). Some of the adverse reproductive effects seen in female mice after treatment with MXC include premature vaginal opening, vaginal cornification, persistent estrus, ovarian atrophy, decreased ovulation, and increased atresia (death) of antral follicles (Gray et al., 1989; Eroschenko and Cooke, 1990; Borgeest et al., 2002).
Several in vivo and in vitro studies have shown that MXC specifically targets antral follicles in rodent ovaries, inhibiting growth and increasing atresia (Martinez and Swartz, 1991; Swartz and Corkern, 1992; Borgeest et al., 2002; Gupta et al., 2006). Follicle-targeted toxicity is common among ovarian toxicants, since several chemicals have been shown to target specific follicle types in the ovary (Burkl and Schiechl, 1978; Flaws et al., 1994). While chemicals that target primordial follicles may cause permanent infertility because of the depletion of a finite number of growing follicles, chemicals such as MXC that target antral follicles may either cause temporary infertility if the chemical is removed, or cause permanent infertility if the chemical is not removed (Hirshfield, 1997). MXC may also cause premature reproductive senescence as a result of increased follicle atresia. Premature reproductive senescence is a major concern in women because this may increase the risk of cardiovascular diseases, osteoporosis, and hypertension (Bagur and Mautzlen, 1992; Ashraf and Vongpatanasin, 2006; Gast et al., 2008).
In the past, studies have shown that in utero or neonatal exposure to MXC causes reproductive abnormalities, including increased atresia of antral follicles and reduced fertility that is manifested in the animal at adulthood (Swartz and Corkern, 1992; Swartz and Eroschenko, 1998). These findings suggest that exposure to MXC at critical periods of ovarian development may make the ovaries more sensitive to MXC-induced toxicity later as adults. There is limited knowledge, however, about the effects of MXC on the adult mouse ovary at various low and high doses over several exposure periods. Moreover, there is also inadequate knowledge about whether increased follicular atresia caused by MXC in the ovaries reduces the immediate fertility of the female.
Previously, studies have shown that 20-day exposure of MXC at 32 and 64 mg/kg/day significantly increased the percentage of atretic antral follicles in the ovary, whereas the same doses over a 10-day exposure did not increase atresia significantly (Borgeest et al., 2002). Hence, it is not clear if increased atresia of antral follicles has associated short-term or long-term adverse effects on reproduction. To address this question, we treated mice with a wide range of MXC doses and several exposure periods to determine critical doses, and to identify windows of exposure in adult female mice that can cause ovarian toxicity. Further, we bred some of the MXC-dosed female mice to untreated males to determine whether MXC-induced toxicity results in immediate impairment of female fertility. To our knowledge, this is the first time adult cycling female mice that were exposed to MXC for 30 days were bred with untreated males to evaluate fertility. This is a novel approach to understand the dynamics of ovarian antral follicle development and atresia because limited studies have explored whether there is a reduced fertility threshold for effect based on the percentage of antral follicle atresia in adult mice. It is important to study this because humans are exposed to a mixture of endocrine disruptors, including MXC, that are known to increase atresia and deplete the antral follicle pool. However, what needs to be assessed further is whether the depletion of antral follicles in adult mammalian females can reduce or affect short-term fertility.
MATERIAL AND METHODS
Testing Facilities
This study was carried out in two parts. The first dosing regimen (Group A) was carried out for a period of 5 and 10 days at the University of Maryland, Baltimore, Maryland. The second dosing regimen (Group B) was carried out at Nucro-Technics, Scarborough, Ontario, Canada for a period of 20 and 30 days.
Chemicals
Group A—MXC (99%) was purchased from Chemservice (West Chester, PA). MXC was dissolved in sesame oil (SES) at 5, 10, 20, 40, 80, and 100 mg/ml for 8, 16, 32, 64, 128, and 160 mg/kg/day dosages. Group B—MXC (98%) was purchased from MP Biomedicals, LLC (Solon, OH) and dissolved in SES at 15, 20, and 30 mg/ml for 48, 64, 96 mg/kg/day dosages. The mice in each treatment group responded to both lots of MXC in a similar fashion.
Animals
Group A—cycling female CD-1 mice between ages 32 and 36 days were obtained from Charles River Laboratories (Wilmington, MA) and housed five animals per cage at the University of Maryland School of Medicine Central Animal Facility. Food and water were provided ad libitum and the mice were housed in a controlled animal room environment (temperatures 18–26°C, 12-hr light–dark cycles). The University of Maryland School of Medicine Institutional Animal Use and Care Committee approved all testing procedures on the mice. Group B—5- to 6-week-old female and 8-to 9-week-old male CD-1 mice were purchased from Charles River Inc. (Montreal, PQ, Canada). The mice were individually housed in rat/mouse cages at the testing facility, Nucro-Technics, and provided food and water ad libitum throughout the study period. The mice were kept in a controlled animal room environment (temperatures 18–26°C, 12-hr light–dark cycles) and monitored daily according to Nucro-Technics Standard Operating Procedures. All animals used in the study were treated in accordance with the principles described in the current “Guide for the Care and Use of Experimental Animals” as published by the Canadian Council on Animal Care.
Experimental Design
Group A—the dosing regimen was carried out for 5 or 10 days of continuous exposure periods with 3–19 female mice per treatment group. In the 5-day dosing period, each mouse received the vehicle, SES (n = 3) or 64 (n = 4), 128 (n = 4), or 160 (n = 3) mg/kg/day of MXC. In the 10-day dosing period, each mouse received either SES (n = 9) or 8 (n = 7), 16 (n = 3), 32 (n = 3), 64 (n = 4), 128 (n = 4), or 160 (n = 4) mg/kg/day of MXC. The mice were administered either SES or MXC intraperitoneally using a 1-ml syringe at 1.6-ml/kg body weight. All mice were weighed daily and the doses were adjusted based on the animal’s most recent body weight. Group B—the dosing regimen was carried out with 10 female CD-1 mice per treatment group for 20 or 30 days of continuous exposure periods. In the 20-day dosing period, each mouse received either SES (n = 10) or 64 (n = 9) or 96 (n = 10) mg/kg/day of MXC. In the 30-day dosing period, each mouse received either SES (n = 18) or 48 (n = 10), 64 (n = 9), or 96 (n = 19) mg/kg/day of MXC. The mice were administered either SES or MXC intraperitoneally using a 23 G needle attached to a 1-ml syringe at 3.2-ml/kg body weight. All mice were weighed daily, and the doses were adjusted based on the animal’s most recent body weight.
The sample size was based on power calculations that were set to determine whether we had 80% power to identify differences of 10% in atresia between control and treatment groups. From an ethical standpoint, we reduced animal numbers while maintaining study integrity. Our sample size calculations indicate, given our sample size and standard deviations, that we have sufficient power to detect differences. For example, in the 10-day cohort, with our control versus 8-mg/kg dose, our calculations indicate that we need a sample size of five for both groups to observe significant differences and we used n = 9 for control and n = 7 for 8-mg/kg samples. For control versus the 64, 128, and 160 mg/kg dose, our calculations indicate that we should use a sample size = 3 for all groups to observe significant differences and we used n = 9 for controls and n = 4 for 64 and 128 mg/kg and n = 3 for 160 mg/kg. Hence, we reduced the number of mice dosed per treatment group to refrain from unnecessary handling and euthanasia of mice while maintaining the integrity of the study.
Breeding and Litter Analysis
All CD-1 females in the 30-day dosing period of Group B were mated with untreated, proven-breeder male CD-1 mice (1:1 ratio) for up to 7 days starting the day after cessation of dosing. The date of observation of the vaginal plug was recorded and considered gestational day (GD) 1. Starting on GD 1, the female mice were weighed every 3–5 days to monitor pregnancy. On GD 17–19, all pregnant dams were anesthetized by exposure to isoflurane and pups removed by Caesarian section and weighed. The uterus was examined and the dams and pups were euthanized immediately by exposure to CO2/O2. After euthanasia, the ovaries of the dams were collected weighed and fixed in Dietrick’s fixative for histological processing.
Histological Evaluation of Atresia
After fixation, the ovaries were dehydrated and embedded in Paraplast (VWR scientific, West Chester, PA), serially sectioned (8 μm) and stained for histological evaluation of atresia as previously described (Borgeest et al., 2002). Follicles were classified as antral if they contained five or more layers of granulosa cells and a clearly defined antral space. Antral follicles were classified as atretic if 10% of the granulosa cells were apoptotic (defined by the presence of pyknotic bodies in the granulosa cell layer), the granulosa cells were disorganized, or if the oocyte was fragmented or degenerating (Borgeest et al., 2002).
Statistical Analysis
All data were analyzed using SPSS statistical software, IBM SPSS (IBM Corporation, Armonk, NY). For all comparisons, statistical significance was assigned at p ≤ 0.05. For comparisons between SES and MXC-treatment groups, analysis of variance was used followed by Tukey’s post-hoc test.
RESULTS
Atresia Caused by MXC in CD-1 Mouse Ovaries
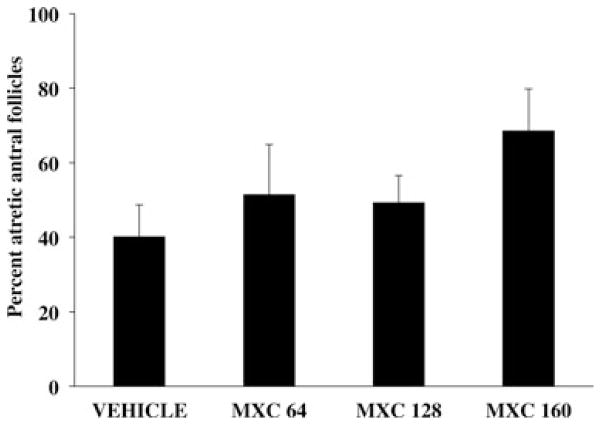
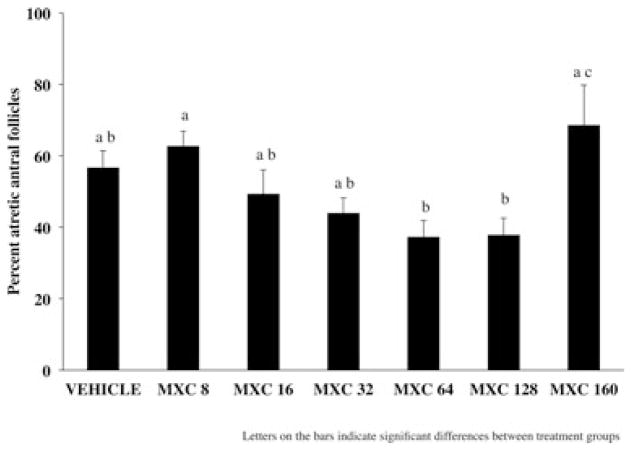
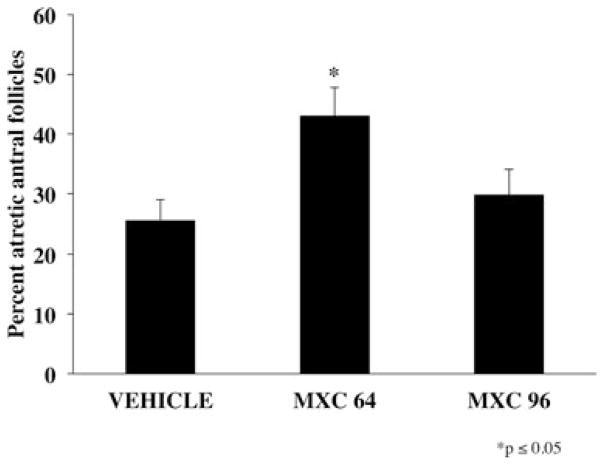
CD-1 female mice were dosed intraperitoneally with either SES or varying dosages of MXC (8–160 mg/kg/day) for 5, 10, 20, or 30 days. Five-day treatments of MXC (64, 128, and 160 mg/kg/day) did not significantly increase the percentage of atretic antral follicles compared with vehicle controls (Fig. 1). Similarly, treatment with MXC (8, 16, 32, 64, 128, and 160 mg/kg/day) for 10 days did not cause a significant difference in percentage of atretic antral follicles between vehicle-treated and MXC-treated mice (Fig. 2). However, MXC significantly decreased the percentage of atretic antral follicles at the 64 and 128 mg/kg/day dosages compared to the 8 and 160 mg/kg/day treatment groups (Fig. 2). Treatment with MXC (64 and 96 mg/kg/day) for 20 days significantly increased percentage of atretic antral follicles with 64 mg/kg/day MXC, but not with 96 mg/kg/day compared with vehicle-treated mice (Fig. 3). Treatment with MXC (48, 64, and 96 mg/kg/day) for 30 days significantly increased percentage of atretic antral follicles at 64 and 96 mg/kg/day, but not 48 mg/kg/day compared with vehicle-treated mice (Fig. 4).
Fig. 1.
Evaluation of atresia in ovaries of CD-1 mice treated with SES or MXC (64, 128, and 160 mg/kg/day) for 5 days. The percentage of atretic antral follicles were quantified and plotted. Each bar represents means ± standard error (SE; n = 3–4 mice/treatment group).
Fig. 2.
Evaluation of atresia in ovaries of CD-1 mice treated with SES or MXC (8, 16, 32, 64, 128, and 160 mg/kg/day) for 10 days. Each bar represents means ± SE (n = 3–9 mice/treatment group).
Fig. 3.
Evaluation of atresia in ovaries of CD-1 mice treated with SES or MXC (64 and 96 mg/kg/day) for 20 days. Each bar represents means ± SE (n = 9–10 mice/treatment group).
Fig. 4.
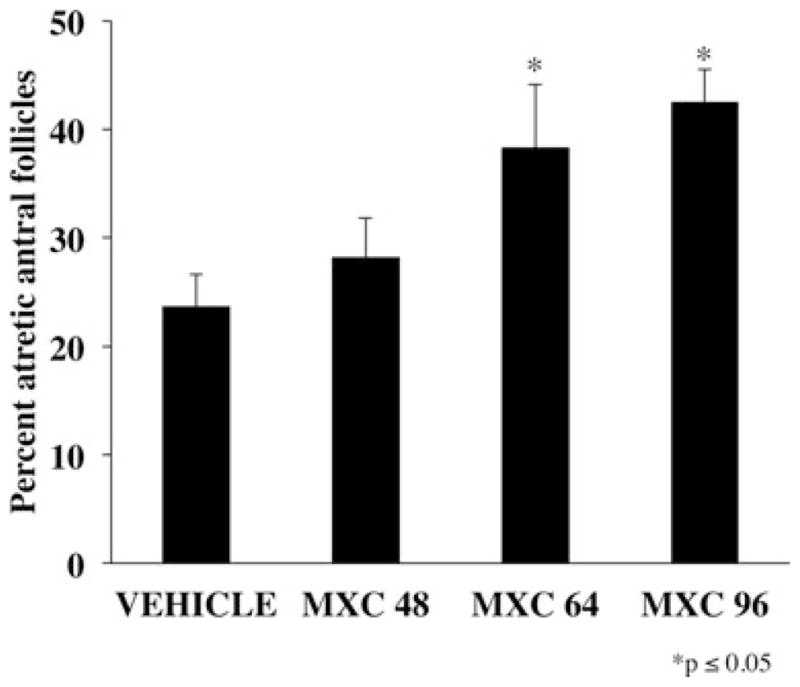
Evaluation of atresia in ovaries of CD-1 mice treated with SES or MXC (48, 64, and 96 mg/kg/day) for 30 days. Each bar represents means ± SE (n = 10–19 mice/treatment group).
Effect of MXC on Ovarian Weights and Fertility
After 30 days of treatment with SES or MXC (48 and 96 mg/kg/day), female CD-1 mice were mated with untreated sexually mature (proven-breeder) male CD-1 mice. The pregnant dams were euthanized on GD 17–19 and the fetuses were collected. The ovarian weights of the treated dams, the litter size, and individual fetal weights were recorded. The ovarian weights of the dams treated with MXC were significantly lower than those of vehicle-treated dams (Table 1). Overall, there were no differences in the number of fetuses per litter of the treated dams compared with vehicle-treated dams. However, one dead fetus and an early and late resorption were observed at the time of collection in MXC 64 group. There was one late resorption observed in MXC 96 group at the time of collection.
Table 1.
Ovarian Weights of the Treated Dams, the Litter Size, and Individual Pup Weights
| Treatment | Ovary weight (mg) (Paired; mean ± SE) | Litter size (live pups; mean ± SE) | Individual pup weight (g) (mean ± SE) |
|---|---|---|---|
| SES (n = 18) | 42.9 ± 14.1 | 11.2 ± 3.0 | 1.2 ± 0.2 |
| 48 mg/kg/day (n = 10) | 29.5 ± 6.4 | 10.2 ± 2.6 | 1.1 ± 0.2 |
| 96 mg/kg/day (n = 19) | 31.2 ± 3.6 | 10.0 ± 2.3 | 1.0 ± 0.2 |
Adult female CD-1 mice were treated with SES or MXC (48 and 96 mg/kg/day) for 30 days and bred with untreated male mice. The pregnant dams were euthanized on GD 17–19 and the pups were collected. The ovarian weights of the treated dams, the litter size, and individual pup weights were recorded as shown above.
DISCUSSION
Our present study provides a deeper insight into the dosage of MXC that is critical for eliciting a biological (i.e., reproductive effect) response. In this study, we show that the dose as well as the duration of exposure determines the ability of MXC to increase atresia of antral follicles in the ovary. Specifically, when adult female CD-1 mice were exposed to MXC for 5 days, there were no differences in percentage of atretic antral follicles in the ovaries of vehicle or MXC-treated mice. It is possible that the ovaries are able to tolerate an acute exposure of 5 days, and the toxicity of MXC is prevented by robust defense mechanisms in the body, including detoxification by the liver. Interestingly, even after 10 days of exposure to MXC at dosages ranging from 8–160 mg/kg/day, there were no differences in percentage of atretic antral follicles between the vehicle-treated and MXC-treated mice. Between treatment groups, however, it is noteworthy that the lowest dosage (8 mg/kg/day) and the highest dosage (160 mg/kg/day) significantly increased percentage of atretic antral follicles compared to 64 and 128 mg/kg/day; thus suggesting a nonmonotonic dose–response curve for this exposure.
Previous studies have shown that when adult female CD-1 mice were treated with MXC at dosages of 8–32 mg/kg/day for 20 days, only the highest dose increased the percentage of atretic antral follicles compared with vehicle-treated animals (Borgeest et al., 2002). We expanded this previous study by including 64 and 96 mg/kg/day dosages. Interestingly, we observed an increase in percentage of atretic antral follicles at the 64 mg/kg/day dosage, but not at the 96 mg/kg/day dosage, suggesting nonmonotonic dose–response at this exposure period. However, at 30 days of exposure, only the 64 and 96 mg/kg/day MXC dosing regimens, but not the 48 mg/kg/day regimen, showed an increase in percentage of atretic antral follicles compared with vehicle treatment.
Although the reasons for the nonmonotonic dose–response as seen with exposure to MXC are unknown, it is thought that U-shaped curves arise when normal levels of the endogenous ligand of the nuclear receptor are insufficient to saturate the binding sites (Kohn and Melnick, 2002). Subsequently, xenobiotic compounds bind to hormone receptors, but the resulting DNA-receptor–xenobiotic ligand complex is less stable than when the receptor is bound to the endogenous ligand, giving rise to nonmonotonic dose–response curves. Nonmonotonic dose–response curves are a common occurrence with estrogens and estrogenic compounds, and have been a topic of interest among researchers over the past several years. Several studies have shown variable tissue responses with low versus high doses of estrogenic compounds in different tissues (Alworth et al., 2002; Newbold et al., 2004). Variable responses with low versus high doses of MXC have also been reported in a few studies. One such study has shown complete reversal of effects with low and high doses of MXC. In adult female rats that were exposed to MXC neonatally, estrous cyclicity started earlier in animals exposed to 25 mg/kg/day, occurred at a normal age in animals exposed to 50–100 mg/kg/day, and was delayed in animals dosed with 200 mg/kg/day (Gray et al., 1989). Similarly, in another study, lower doses of MXC in mice caused an increase in ovarian weight and numbers of corpora lutea, while higher doses caused ovarian atrophy and depletion of corpora lutea. Furthermore, follicular cysts were recorded only with the intermediate dose of MXC (Eroschenko et al., 1995). Our data suggest that at 20-day exposure, MXC causes an inverted U-shaped dose response in percentage of atretic antral follicles.
The decrease in ovarian weights that we observed in this study is consistent with previous studies showing the adverse effects of MXC in the female reproductive tract (Martinez and Swartz, 1991; Eroschenko et al., 1995). However, after breeding female mice dosed with 48 and 96 mg/kg/day MXC for 30 days with untreated fertile males, we observed no differences in pups/litter compared with vehicle treatment. These data concur with previous observations that MXC targets antral follicles specifically (Martinez and Swartz, 1991; Borgeest et al., 2002). Hence, it is possible that once MXC is removed, immature follicles are able to grow into antral follicles and recruit them for ovulation to replace the cohort of antral follicles that became atretic with MXC treatment. Alternatively, it is possible that some preovulatory antral follicles escaped the toxic effects of MXC and were able to ovulate. In previous studies that have shown decreased fertility of mice after MXC treatment, the animals were treated either in utero or neonatally (Swartz and Corkern, 1992; Swartz and Eroschenko, 1998). This suggests that during developmental stages, MXC may be altering the blueprint of follicle development by causing a defect in the compensatory repair system of the cells because it is well established that when cells and tissues undergo injury or insult by toxicants, compensatory mechanisms occur such as DNA damage repair (Friedberg, 2003). However, neonates have underdeveloped cell/DNA repair mechanisms making them vulnerable to toxic insults to a greater extent than adults. It further suggests that developing ovaries may be more sensitive to toxicity induced by MXC, resulting in decreased fertility manifested only at adulthood at the time of reproduction. Interestingly, in our study, adult cycling CD-1 female mice that were dosed for 30 days and immediately mated with untreated adult males did not manifest decreased fertility compared to controls. Our present findings suggest that mice that have never been exposed to MXC during prenatal or neonatal development, but are exposed as adults, have an increased percentage (about 50%) of atretic antral follicles. However, these mice do not manifest an immediate decrease in fertility as a result of such increases, suggesting the possibility of a threshold in the percentage of atretic antral follicles that can cause infertility, and with indications that such a threshold has yet to be reached. Potentially, we are observing parity with the male reproductive system, in terms of the degree to which sperm counts in wild rodents need to be reduced (through animal exposures at chemically contaminated sites) before population effects become evident. The patented Rodent Sperm Analysis method identifies a 60% sperm count reduction threshold (for rodents from a contaminated site relative to rodents of a nearby, habitat-matched pristine environment), in order for fertility to be compromised (Tannenbaum et al. 2007). Although in our study we have shown that a 50% antral follicle loss does not critically reduce fertility, the possibility of premature reproductive senescence is not ruled out and should be examined in future studies.
CONCLUSION
This study has evaluated the dose–response curves of MXC over several time points using varying doses of the chemical to broaden our knowledge of the effects of MXC in the ovary. An interesting future study would be to evaluate the levels of gene expression of apoptotic factors or cell-cycle regulators at these doses and treatment regimens to further investigate the nonmonotonic effects of MXC.
Acknowledgments
Grant sponsor: National Institute of Environmental Health Sciences; Grant number: R01ES019178.
The authors thank National Institute of Environmental Health Sciences for funding the study (R01ES019178 to J.A.F.) and Liying Gao for technical help.
References
- Alworth LC, Howdeshell KL, Ruhlen RL, Day JK, Lubahn DB, Huang H-M, Besch-Williford CL, vom Saal FS. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethystilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol. 2002;183:10–22. doi: 10.1006/taap.2002.9459. [DOI] [PubMed] [Google Scholar]
- Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep. 2006;8:368–376. doi: 10.1007/s11906-006-0080-1. [DOI] [PubMed] [Google Scholar]
- Bagur AC, Mautzlen CA. Risk for developing osteoporosis in untreated premature menopause. Calcif Tiss Int. 1992;51:4–7. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–478. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- Burkl W, Schiechl H. The growth of follicles in the rat ovary under the influence of busulphan and endoxan. Cell Tiss Res. 1978;186:351–359. doi: 10.1007/BF00225543. [DOI] [PubMed] [Google Scholar]
- Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- Eroschenko VP, Abuel-Atta AA, Grober MS. Neonatal exposures to technical methoxychlor alters ovaries in adult mice. Reprod Toxicol. 1995;9:379–387. doi: 10.1016/0890-6238(95)00025-6. [DOI] [PubMed] [Google Scholar]
- Eroschenko VP, Cooke PS. Morphological and biochemical alterations in reproductive tracts of neonatal female mice treated with the pesticide methoxychlor. Biol Reprod. 1990;42:573–583. doi: 10.1095/biolreprod42.3.573. [DOI] [PubMed] [Google Scholar]
- Eroschenko VP, Rourke AW, Sims WF. Estradiol or methoxychlor stimulates estrogen receptor (ER) expression in uteri. Reprod Toxicol. 1996;10:265–271. doi: 10.1016/0890-6238(96)00055-x. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod Toxicol. 1994;8:509–514. doi: 10.1016/0890-6238(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Friedberg EC. DNA damage and repair. Nature. 2003;421(6921):436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor α and β, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–858. [PubMed] [Google Scholar]
- Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands-van Gent CJ, Samsioe GN, Nilsson PM, van der Schouw YT. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51:1492–1498. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Cooper RL, Kelce WR. The estrogenic pesticide and antiandrogenic pesticide methoxychlor alters the reproductive tract and behavior without affecting pituitary size or LH and prolactin secretion on male rats. Toxicol Ind Health. 1999;15:37–47. doi: 10.1177/074823379901500105. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989;12:92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Overview of ovarian follicular development: considerations of the toxicologist. Environ Mol Mutagen. 1997;29:10–15. [PubMed] [Google Scholar]
- Kohn MC, Melnick RL. Biochemical origins of the non-monotonic receptor-mediated dose-response. J Mol Endorinol. 2002;28:113–123. doi: 10.1677/jme.0.0290113. [DOI] [PubMed] [Google Scholar]
- Martinez EM, Swartz WJ. Effects of methoxychlor on the reproductive system of the adult female mouse. 1. Gross and histologic observations. Reprod Toxicol. 1991;5:139–147. doi: 10.1016/0890-6238(91)90042-e. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathways. Toxicol Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E, Haseman J. Developmental exposure to diethylstilbesterol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod Toxicol. 2004;18:399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Swartz WJ, Corkern M. Effects of methoxychlor treatment of pregnant mice on female offspring of the treated and subsequent pregnancies. Reprod Toxicol. 1992;6:431–437. doi: 10.1016/0890-6238(92)90006-f. [DOI] [PubMed] [Google Scholar]
- Swartz WJ, Eroschenko VP. Neonatal exposure to technical methoxychlor alters pregnancy outcome in female mice. Reprod Toxicol. 1998;12:565–573. doi: 10.1016/s0890-6238(98)00041-0. [DOI] [PubMed] [Google Scholar]
- Tannenbaum LV, Thran BH, Williams KJ. Demonstrating ecological receptor health at contaminated sites with wild rodent sperm parameters. Arch Environ Contam Toxicol. 2007;53:459–465. doi: 10.1007/s00244-006-0169-1. [DOI] [PubMed] [Google Scholar]