Abstract
Seventy-five percent of rhesus macaques at national primate research centers are housed outside. Annually, 15–39% of these animals experience diarrhea and require veterinary treatment for dehydration, electrolyte imbalance, or weight loss. An estimated 21–33% of these patients will die or be euthanized. Many studies have explored the various infectious etiologies of non-human primate diarrhea. However, there is little published information on diarrhea incidence rates and risk factors in outdoor-housed rhesus macaques. Without this information, it is challenging to determine endemic and epidemic diarrhea levels, or to develop and evaluate mitigation strategies. Using electronic medical records, we conducted a retrospective cohort study to calculate diarrhea incidence rates for rhesus macaques (N = 3,181) housed in three different outdoor housing types (corrals, shelters, and temporary housing) at the Oregon National Primate Research Center between November 1, 2009 and October 31, 2010. With multiple logistic regression analysis, we determined the relative risk of housing type, sex, and age on development of diarrhea. Diarrhea incidence and mortality in our population was lower than many published ranges. Type of outdoor housing, age, and previous diarrhea episode were positively correlated with diarrhea risk. Younger animals in smaller shelters and temporary housing had a greater risk of acquiring diarrhea, with juvenile animals (0.7–3.9 years) having the highest mortality rate. Sex was not a risk factor, but adult females with diarrhea were more likely to develop life-threatening complications than adult males. We also constructed a predictive model for diarrhea-associated mortality using Classification and Regression Tree. Findings from this study will be used to develop and evaluate mitigation strategies in our outdoor-housed population and to provide a foundation for genetic susceptibility and immune function testing.
Keywords: rhesus, diarrhea, risk, morbidity, mortality, classification, tree
INTRODUCTION
Diarrhea in captive macaques has been a concern since 1950, when large numbers of rhesus and cynomolgus macaques were imported from India and the Philippines to support poliomyelitis research. By 1955, infection control plans reduced the overall mortality rate to 7.6% of the 36,248 animals imported, but did not prevent the spread of enteric disease [Schneider et al., 1960]. Sixty years later, diarrhea remains a significant source of morbidity and mortality for captive macaques [Elfenbein & McCowan, 2012; Gomez et al., 2003; Habermann & Williams, 1957; Holmberg et al., 1982; Russell et al., 1988; Schneider et al., 1960]. Diarrheal disease is particularly challenging to manage in social housing environments. Dehydrated or cachexic animals must be removed from their social group for treatment [Munoz-Zanzi et al., 1999; Wilk et al., 2008]. Removal from the social group disrupts the group hierarchy and deprives the sick animal of essential group contact. Diarrheal disease can also cause secondary pathologic changes in the cecum and colon, alterations in immune function, delayed or stunted development, and associated chronic disease such as reactive arthritis and amyloidosis that negatively impact animal health and the value of these animals for biomedical research [Blanchard et al., 1986; Desrosiers, 1997; Holmberg et al., 1982; MacGuire et al., 2009; Naumenko & Krylova, 2003; Rothschild, 2005; Sestak et al., 2003]. Finally, specialized housing, veterinary care, and the chronic nature of colitis increases operating costs for primate centers and other institutions that house rhesus macaques [Blanchard et al., 1986; George & Lerche, 1990; Wolfensohn, 1998].
Why any individual animal develops diarrhea still remains unclear, and is likely a complex interplay between pathogen, environment, and host. A number of excellent studies have characterized the association between enteric pathogens and diarrhea. Early studies noted that clinically ill animals were frequently positive on rectal fecal cultures for Shigella spp. and Salmonella spp., and the association between Shigellosis and diarrhea prompted efforts to eliminate the pathogen from captive populations [Kinsey et al., 1976; Mulder, 1971; Pucak et al., 1977; Takeuchi et al., 1968; Wolfensohn, 1998]. More recent studies have implicated Campylobacter coli, Campylobacter jejuni, and Yersinia enteroclitica in enterocolitis [Russell et al., 1988; Sestak et al., 2003; Tribe & Frank, 1980]. Several viral etiologies have been identified, and the association between viral infection and diarrheal disease remains a dynamic research area. Adenovirus was positively associated with colitis in 2003, but recent publications suggest the virus is also prevalent in asymptomatic carriers [Roy et al., 2012; Sestak et al., 2003 Stuker et al., 1979]. A recently characterized rhesus monkey calicivirus may also play a role in macaque colitis [Farkas et al., 2012; Sestak et al., 2012]. Similarly Helicobacter spp., and the protozoal enteric pathogens Balantidium coli, and Giardia lamblia have been isolated from animals with colitis [Fox et al., 2007; Sestak et al., 2003]. Each of these enteric pathogens can be isolated from clinically healthy animals, suggesting that additional factors should be evaluated when developing diarrhea prevention and mitigation strategies.
The influence of environment on diarrhea has not been as well studied. Several reports suggest housing may be a factor in colitis development. Nursery reared animals have the highest rate; diarrhea incidence in indoor, nursery-reared infants is 7.5 times higher than in animals reared with dams [Elmore et al., 1992] and diarrhea percentage among nursery-reared animals is three to four times higher than any other housing type [Hird et al., 1984]. Indoor, group-housed animals and animals in single or pair housing have higher diarrhea rates than outdoor, group-housed animals [Hird et al., 1984; Sestak et al., 2003; Wolfensohn, 1998]. The role of housing type in outdoor-housed animals is less clear, as the only study to evaluate diarrhea risk factors in outdoor-housed animals did not include housing type in the analysis [Hird et al., 1984].
An updated assessment of diarrhea incidence and risk factors in outdoor-housed macaques is clearly needed. The information will help institutions develop focused prevention and mitigation strategies and provide baseline information for evaluating outcomes. Using electronic medical records from our database, we conducted a retrospective cohort study, measuring the incidence of diarrhea-associated morbidity and mortality for rhesus macaques (N = 3,181) housed in outdoor breeding groups at the Oregon National Primate Research Center (ONPRC). We hypothesized that housing type, sex, and age would be positively correlated with diarrhea incidence and diarrhea-associated mortality, and that diarrhea-associated mortality had a seasonal variation. We concluded that housing type and age are risk factors for diarrhea-associated morbidity and mortality. Sex was not a risk factor, but adult females with diarrhea were more likely to experience diarrhea-associated mortality than adult males. There is a seasonal component to diarrhea incidence in our population. Using Classification and Regression Tree Analysis (CART) with proportional random sampling, we also built and tested a predictive model for diarrhea-associated mortality. This information will be used to develop and assess diarrhea mitigation strategies in our outdoor-housed groups. It also provides updated information on the potential role of housing and environment in the development of diarrheal disease and provides baseline data for genetic susceptibility analysis and immune function testing.
METHODS
No living animals were used directly during the course of this study. Rather, the database consisted solely of the electronic health records of animals that were housed at the ONPRC. Thus, the Institutional Animal Care and Use Committee (IACUC) Chair, the Compliance Officer, and the Attending Veterinarian determined that IACUC approval was not required. Nevertheless, those animals on which the electronic health records are based were housed in accordance with standards established by the U.S. Federal Animal Welfare Act and the Guide for Care and Use of Laboratory Animals. All animals were assigned to the ONPRC breeding colonies and managed under the IACUC-approved protocol of the Oregon Health and Science University West Campus. The research also adhered to the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates.
Study Population
The electronic health records of 3,181 Indian rhesus macaques housed in outdoor social groups at the ONPRC between November 1, 2009 and October 31, 2010 were reviewed for this study. All animals were tested annually for simian viruses (Simian Immunodeficiency Virus, Simian Retrovirus 2, Macacine Herpesvirus 1, and Simian T lymphotrophic virus) and received a mammalian old tuberculin test semi-annually. Animals were fed LabDiet 5047 (Ralston Purina, St Louis, MO) twice daily and supplemental produce or other enrichment once daily. Municipal water was available ad libitum. For purposes of the study, animals were grouped by age: infants were <0.7 years; juveniles were greater than 0.7 to <4 years; and adults were ≥4 years. During the period of this study, the animals were group housed in either 1 acre corrals (Fig. 1), sheltered housing (Fig. 2), or temporary housing areas (Fig. 3).
Fig. 1.
One acre corral housing for corral-breeding groups (CBGs) of 125–250 animals.
Fig. 2.
Three-bay sheltered housing for shelter-breeding groups (SBGs) of 22–54 animals.
Fig. 3.
Temporary housing run used for temporary groups (STG) of 18–24 animals.
Six breeding groups, representing 1,459 animals, were housed in 1 acre corrals (Fig. 1). Each corral-breeding group (CBG) contained 125–250 animals from 1 day to 18 years and had been housed together from 2 months to 14 years. Housing density was 174–348 square feet per animal. In addition to the 1 acre open-earth space, each corral had sheltered feed areas with cement floors, radiant heat, and fans. There were play structures and dome-shaped polyethylene shelters for enrichment. Permanently installed sprinklers provided water for vegetation, and a source of cooling water during hot weather. Surface drain areas within each corral, and floor drains within each feed area prevented standing water. The feed areas were cleaned daily, using water wash-down. Animals were observed twice daily by husbandry staff from observation towers, and an area walk-through was conducted daily.
Thirty breeding groups within sheltered housing contained 1,581 animals ranging in age from 1 day to 16 years and who were typically housed together for 1–5 years (Fig. 2). Housing density was 18–46 square feet per animal. Shelter-breeding groups (SBG) contained 22–54 animals in one of two housing designs. Twenty-four housing areas were three-bay, 1,300 square feet units and 8 housing areas were two-bay, 1,000 square feet units. The three-bay units have a central bay with a retractable garage door and the two-bay units have external retractable garage doors. Play structures were located in each unit. The floors were sealed concrete. The ceilings and outer bay walls were stainless steel wire mesh. The roof over each duplex was clear polycarbonate to provide greenhouse lighting and heat. There was radiant heat in the floor of the central unit. Fans with misters provided cooling. Structures were cleaned daily, with water wash-down. Observations were made each morning prior to washing. The doors between bays were closed during observation, to ensure that animals could be adequately assessed.
One hundred forty-one animals were housed in seven temporary groups during the study. Typically, these temporary groups (STG) were together for 3–6 months and contained 20 juveniles and 2–4 adults awaiting permanent assignment to a breeding group. Housing density was 50–54 square feet per animal. Housing was either in the shelter housing as described above (Fig. 2), or runs attached to a building (Fig. 3) and described below. The circular exterior portions measured approximately 1,200 square feet, and the rectangular interior portions measured approximately 200 square feet each. The floors and walls were poured concrete, covered with a sealant. The interior area was environmentally controlled. The exterior area contained play structures, perching areas along all walls, and was covered by a galvanized wire mesh. All floors, walls, and play structures were cleaned using water wash-down daily.
Medical Records
Clinical cases were opened by the veterinary staff for each animal receiving medical treatment. Cases were entered through the Integrated Research Information System (IRIS), an electronic medical records system which uses case classifiers, Systematic Nomenclature of Medicine (SNOMED) codes, and free text to capture routine husbandry practices, veterinary medical care, and clinical pathology data. At case opening, a Master Problem was assigned. The Master Problem was selected by the user from a pre-defined menu of 14 case types and captures the primary reason for patient treatment. Typically, Master Problem identifies the major body system affected by disease but diarrhea is such a frequent reason for presentation that Gastrointestinal (GI) - Diarrhea is a distinct Master Problem. Only cases assigned the Master Problem “GI-Diarrhea” were included in the study. Cases that were opened for another reason, such as wound or dystocia, and subsequently developed diarrhea as a secondary problem or complication were not included in the study. IRIS records for all rhesus macaques maintained in outdoor social housing between November 1, 2009 and October 31, 2010 were included in the study. Records for abortion and still birth were not included in the study. Records were evaluated by housing type, sex, age, and death date (if applicable), Master Problem, previous diarrhea diagnosis, and previous hospitalization of any type. Every tenth record was manually reviewed. All records with the Master Problem “GI-Diarrhea,” and all death records were also manually reviewed to confirm data validity.
Inclusion Criteria
All 3,181 Specific Pathogen Free (SPF) Indian rhesus macaques housed in outdoor social groups between November 1, 2009 and October 31, 2010 were included in the study. During daily husbandry checks, animals noted with signs consistent with dehydration, including lethargy, sunken eyes, dull coat, inappetance, hunched posture, loose stool, and/or dysentery, were removed from social groups and transported to the hospital for evaluation by the veterinary staff. The veterinary staff diagnosis of diarrhea was initially made following a history review and physical examination. The diagnosis, and subsequent assignment of the Master Problem “GI-Diarrhea,” was confirmed with diagnostic tests. Diagnostic techniques included, but were not limited to, complete blood cell count, rectal fecal culture, rectal fecal parasitology, and either a serum chemistry or iSTAT +8 analysis. Hydrated animals not requiring treatment were returned to the social group. Animals determined to require medical care were hospitalized and treated. Treatment included, but was not limited to IV fluids, IV and oral antibiotics, anti-parasitics, and caloric support.
Data Collection and Analysis
IRIS records from November 1, 2009 to October 31, 2010 were included in the study. Data mining of the IRIS database was accomplished using SQL Microsoft SQL Server 2012 (Microsoft Corporation, Seattle, WA). Records were sorted by housing type, sex, age, group assignment and release date, and (where applicable) death date, and Master Problem assigned during clinical treatment. The resulting dataset was used for statistical analysis.
Descriptive statistics were calculated for the population as a whole, and by housing type, sex, and age. One assumption for the two-sample t-test is an equal variance. For populations that did not have equal variance, Satterswaite’s method was used to adjust the degrees of freedom for the t-test [Satterthwaite, 1946]. Diarrhea incidence and diarrhea-associated mortality were calculated for the entire population and by the variables housing type, sex, age, and month of year. For animals experiencing more than one episode of diarrhea, only the initial episode was included in the incidence calculation. Multiple logistic regression analysis was performed to examine the risk factors affecting diarrhea-associated mortality. Risk factors included housing type, sex, and age. To test the joint significance of logistic regression coefficients, a Wald chi-squared test was used. The Wald chi-squared test uses the square of the difference between the estimate and the true parameter, divided by variance of estimate, to compare a chi-squared distribution [Harrell, 2001].
CART methods were used to allow further consideration of combinations of the variables age, sex, and housing type that may discriminate diarrhea-associated mortality. Using proportional random sampling, the data were divided into a model building set (70% of the data) and validation set (30% of the data). The CART method was applied to the model building set (N = 2,228). The predictive model from the CART procedure was then applied to a randomly selected validation set (N = 953), and the sensitivity and specificity of diarrhea-associated mortality were calculated as cross-validation measures. All analysis was done with Statistical Analysis System (SAS, Inc., Cary, NC) and Salford Predictive Model (Salford Systems, San Diego, CA). Statistical significance was determined at the significant level of 0.05 using a 2-tailed test. Diarrhea incidence or diarrhea-associated morbidity was calculated by dividing the numerator—the number of unique diarrhea cases during the study period—by the denominator—total population—and multiplying by 1,000. Animals who died acutely from diarrhea, and those who developed chronic colitis and were subsequently euthanized were included in the mortality analysis. Diarrhea-associated mortality, or the proportion of deaths attributed to diarrhea, was calculated by dividing all diarrhea-associated deaths by the total population. Diarrhea case fatality rate was calculated by dividing all diarrhea-associated deaths by all deaths and multiplying by 1,000.
RESULTS
Records for 3,181 SPF rhesus macaques assigned to outdoor housing groups between November 1, 2009 and October 31, 2010 were included in the study. The 1,459 animals in CBGs were divided into six corrals. SBGs included 1,581 animals divided between 30 shelters, and 141 animals were placed in seven temporary groups. There was a statistically significant difference in age between animals in CBGs and those in temporary groups (Table I) (one-way ANOVA: F = 3.31, df = 2, P = 0.04). No statistically significance difference in age was observed between SBGs and temporary groups, or between animals in CBGs and SBGs. Among all animals, there was a statistically significant difference in age between males and females (Table I) (two-sample t-test: t = 14.51, Satterthwaite-adjusted df = 3,097.3, P < 0.01).
TABLE I.
Descriptive Statistics of Age in Years for Housing Type and Sex
| Variable | N | Mean | Standard Deviation | Median | Lower 95% | Upper 95% | Min. | Max. |
|---|---|---|---|---|---|---|---|---|
| Corral-breeding group | 1,459 | 3.98 | 3.67 | 2.67 | 3.79 | 4.16 | 0.04 | 18.26 |
| Shelter-breeding group | 1,581 | 4.14 | 3.59 | 2.71 | 3.97 | 4.32 | 0.02 | 22.52 |
| Shelter temporary group | 141 | 4.73 | 3.44 | 3.71 | 4.16 | 5.31 | 0.26 | 20.52 |
| Male | 1,213 | 3.02 | 2.83 | 2.46 | 2.86 | 3.18 | 0.04 | 20.72 |
| Female | 1,968 | 4.75 | 3.90 | 3.75 | 4.58 | 4.93 | 0.02 | 22.52 |
| Total | 3,181 |
Notes: No statistically significant difference in age was observed between shelter-breeding group and temporary groups or between corral-breeding groups and shelter-breeding groups. There was a statistically significant difference in age between animals in corral-breeding groups and those in temporary groups (One-way ANOVA: F = 3.31, df = 2, P = 0.04). Among all animals, there was a statistically significant difference in age between males and females (two sample t-test: t = 14.51, Satterthwaite adjusted df = 3,179, P < 0.01).
A total of 1,818 clinical cases were opened during the study period. Diarrhea was the second most common reason for treatment, accounting for 446 cases and 24.53% of all treatments. Two hundred fifty monkeys experienced at least one episode of diarrhea. The diarrhea incidence rate in outdoor-housed animals was 78.59 per 1,000 animals (Table II). Animals in SBGs and temporary groups had diarrhea incidence rates double that of animals in CBGs (Table II). Diarrhea rates declined with age; infant diarrhea rates were almost double those of adult animals (Table II). Males were slightly more likely to develop diarrhea than females and females with diarrhea were more likely to experience diarrhea-associated mortality (Table II).
TABLE II.
Diarrhea Incidence and Mortality by Housing Type, Sex, and Age
| Variable | N (1) | Diarrhea Incidence (2) | Diarrhea Incidence Rate (per 1,000) (2)/(1)* 1,000 | Diarrhea-Associated Mortality (3) | Diarrhea-Associated Mortality Rate (per 1,000) (3)/(1)* 1,000 |
|---|---|---|---|---|---|
| Corral-breeding group | 1,459 | 71 | 48.66 | 24 | 16.45 |
| Shelter-breeding group | 1,582 | 165 | 104.30 | 73 | 46.14 |
| Temporary group | 140 | 14 | 100.00 | 6 | 42.86 |
| Male | 1,213 | 118 | 97.28 | 35 | 28.85 |
| Female | 1,968 | 132 | 67.07 | 68 | 34.55 |
| Infant (<0.7 years) | 551 | 55 | 99.81 | 22 | 39.93 |
| Juvenile (0.7 to <4 years) | 1,381 | 122 | 88.34 | 50 | 36.21 |
| Adult (4 years and older) | 1,249 | 73 | 58.45 | 31 | 24.82 |
| Total | 3,181 | 250 | 78.59 | 103 | 32.38 |
Notes: The total population (N = 3,181) is subdivided by housing type, sex, and age. The diarrhea incidence was 78.59 per 1,000 (N1 = 250) and the diarrhea-associated mortality was 32.38 per 1,000 (N2 = 103).
During the study period, 3.2% of all animals and 41% of the animals with diarrhea developed chronic colitis that precluded assignment to a social group, breeding group or research project. These animals were medically culled from the breeding population, and euthanized. Of the 103 euthanasia deaths attributed to diarrhea, 22 were infants, 50 juveniles, and 31 adults (Table II). Overall diarrhea-associated mortality, the proportion of deaths from diarrhea divided into the population at risk, was 32.38 per 1,000 animals. There was a strong association between clinical diarrhea and death (chi-squared test: χ2 = 162.31, df = 1, P = <0.01) and monkeys who experienced diarrhea had 6.56 times as large odds of death as monkeys who did not experience diarrhea.
The odds of diarrhea-associated death for animals in SBGs were 3.05 times the odds of diarrhea-associated mortality of animals in CBGs (multiple logistic regression with contrast: 95% CI: 1.91–4.86, Wald chi-square = 21.97, df = 1, P < 0.01). This was true even after adjusting for age and sex. Similarly, animals in temporary groups had odds of diarrhea-associated mortality 2.88 times the odds of diarrhea-associated mortality of animals in CBGs (multiple logistic regression: 95% CI: 1.14–7.23, Wald chi-square = 5.05, df = 1, P = 0.02) even after adjusting for age and sex. There was no statistically significant difference between the odds of diarrhea-associated mortality between SBGs and temporary groups.
Within our study population, infants had the highest diarrhea-associated mortality of 39.93 per 1,000 monkeys (Table II). An infant’s odds of diarrhea-associated mortality were 1.95 times the odds of diarrhea-associated mortality of adult animals (multiple logistic regression: 95% CI: 1.11–3.41, Wald chi-square = 5.45, df = 1, P = 0.02) even after adjusting for housing type and sex. Juvenile animals had a slightly lower rate than infants. A juvenile’s odds of diarrhea-associated mortality were 1.71 times the odds of diarrhea-associated mortality of adults (multiple logistic regression: 95% CI: 1.07–2.72, Wald chi-square = 5.04, df = 1, P = 0.02). As with infants, this was true even after adjusting for housing type and sex. There was no statistically significant difference between juvenile and infant groups.
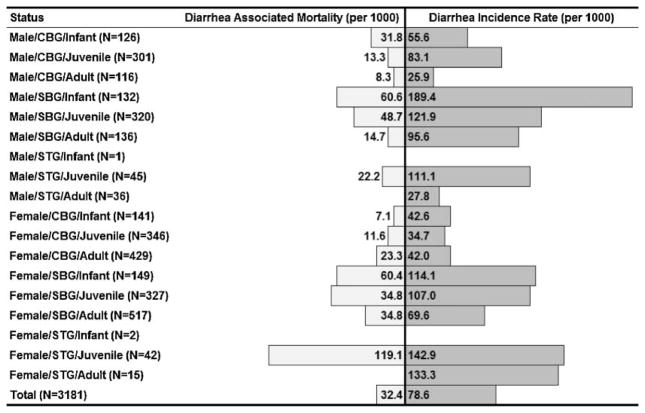
Sex was not statistically associated with odds of diarrhea-associated mortality. However, when diarrhea-associated mortality and diarrhea incidence are plotted, adult females experiencing diarrhea are more likely to develop diarrhea-associated mortality. Adult males, by contrast, are less likely to develop diarrhea-associated mortality (Fig. 4).
Fig. 4.
Diarrhea-associated mortality and diarrhea incidence shown for each combination of housing, sex, and age. Groups are sorted by sex (male and female), housing type (CBG, Corral-Breeding Group, SBG, Shelter-Breeding Group, and STG, Shelter Temporary Group), and age (infants were <0.7 years, juveniles were >0.7 to <4 years, and adults were ≥4 years). There is a linear relationship between diarrhea incidence and diarrhea-associated mortality for most combinations of age, sex, and housing type (Pearson correlation: r = 0.67, N = 25, P < 0.05). Juvenile females in STG, and both infants and female juveniles in SBG have higher mortality rates. Adult males and females in STG, juvenile males in STG and CBG, and adult males in SBG have lower mortality rates.
The incidence of diarrhea, and diarrhea-associated mortality was calculated and graphed for all possible combinations of age, sex, and housing type (Fig. 4). There is linear relationship between diarrhea incidence and diarrhea-associated mortality for most combinations of age, sex, and housing type (Pearson correlation: r = 0.67, N = 25, P < 0.05). Higher mortality rates were observed among juvenile females in temporary groups and female juveniles and infants in SBGs. Lower than expected mortality rates were seen in adult females in temporary groups, juvenile males in both temporary and corral groups, and adult males in SBGs (Fig. 4).
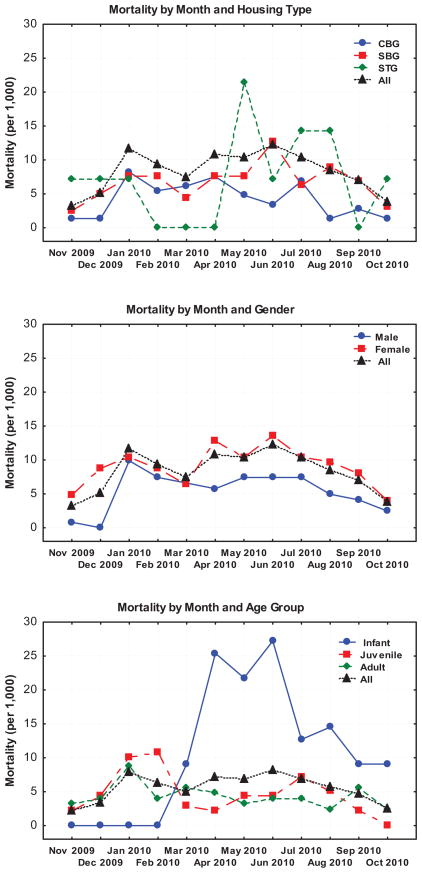
Diarrhea-associated mortality did have a seasonal variance (Fig. 5). January had the highest diarrhea-associated mortality rate and November had the lowest. The number of cases declined in early spring, and increased during the summer. Most infant-associated mortality occurred during late spring and through the summer, while juvenile rates were highest in winter (Fig. 5). Patterns were relatively consistent between housing type, with the exception of temporary groups which experienced an increase in diarrhea-associated mortality from May through August. There was a significant difference between males and females, with males experiencing lower diarrhea-associated mortality throughout the year (Fig. 5).
Fig. 5.
Diarrhea-associated mortality calculated by month and either housing type (CBG, Corral-Breeding Group, SBG, Shelter-Breeding Group, and STG, Shelter Temporary Group), sex (male, female, and all), or age (infants were <0.7 years, juveniles were >0.7 to <4 years, and adults were ≥4 years) demonstrated a seasonal variance. Overall, January had the highest diarrhea-associated mortality rate and November had the lowest. Most infant-associated mortality occurred during late spring and summer. Juvenile rates were highest in winter.
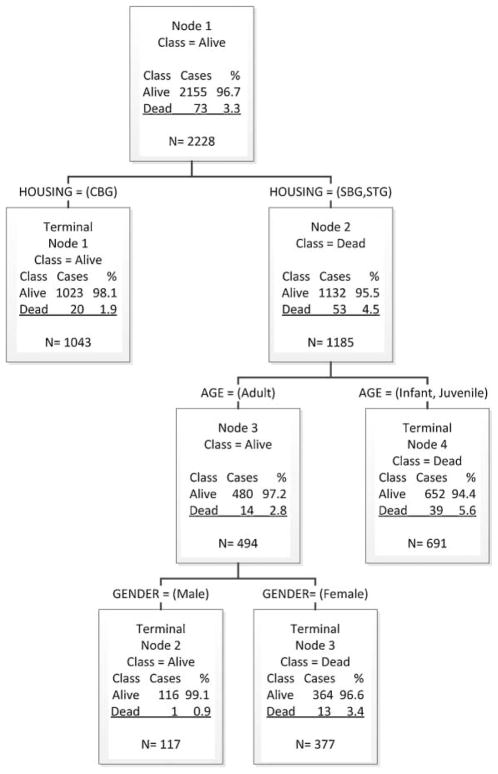
Following our data analysis, CART was used to construct a diarrhea mortality risk model (Fig. 6). Using proportional random sampling of diarrhea-associated deaths, the data were divided into a model building set (70% of the data, N = 2,227) and a validation set (30% of the data, N = 954). To build a predictive model, the CART procedure was carried out on the model building set (N = 2,227). Housing type, sex, and age were used as predictors of diarrhea-associated mortality. The resulting tree identified subgroups of monkeys at higher likelihood of diarrhea-associated mortality [Breiman et al., 1984]. Infants and juveniles in either SBGs or temporary groups were identified as animals at risk for diarrhea-associated mortality (Fig. 2). Adult female monkeys residing in SBGs were also identified as animals at risk for diarrhea-associated mortality. The complete model with model building set achieved overall sensitivity of 71.23% (52 out of 73) and a specificity of 52.85% (1,139 out of 2,155). The predictive model from the CART procedure was then applied to a randomly selected validation set (N = 953), and the sensitivity and specificity of diarrhea-associated mortality were 83.33% (25 out of 30) and 50.49% (466 out of 890), respectively.
Fig. 6.
Classification and Regression Tree (CART) developed from the model building set (70% of the data, N = 2,228), using the predictor variable diarrhea-associated mortality. The algorithm searched the response variables housing type, age, and sex to find a split that was most predictive, and generated a binary partition at each such point. Node 1 included all 2,228 cases, classified (Class) by alive (N = 2,155) and dead (N = 73). The first partition resulted in a terminal node for animals in corral housing (CBG, corral-breeding group) and shows 1,023 (98.1%) of these animals are alive and 20 (1.9%) are dead. Further partition of this node did not yield statistically relevant data, so the algorithm stopped. Node 2 included all animals in sheltered housing (SBG, shelter-breeding group) and temporary housing (STG, shelter temporary group). This group was large enough for additional partition. The algorithm identified age as the next predictive response variable, and partitioned this dataset by age (adult at Node 3 and infants and juveniles at Node 4). The algorithm generated a similar partition for the response variable sex, partitioning Node 3 into terminal Node 2 for male and terminal Node 3 for female. The complete model building set achieved overall sensitivity of 71.23% (52 out of 73) and a specificity of 52.85% (1,139 out of 2,155).
DISCUSSION
Summary and Interpretation
This study provides diarrhea morbidity and mortality data for outdoor-housed rhesus macaques, demonstrates that housing type and age are risk factors, and presents a predictive model for diarrhea risk factors. The diarrhea incidence in outdoor-housed rhesus macaques at the ONPRC from November 1, 2009 to October 31, 2010 was 78.59 per 1,000 animals, and the diarrhea-associated mortality rate was 32.38 per 1,000 animals. Diarrhea incidence was highest in the smaller housing types (shelter and temporary housing areas) and among younger animals, with infants being particularly vulnerable. The relationship between diarrhea incidence and diarrhea-associated mortality is linear for most combinations of housing, sex, and age. However, juvenile females in temporary groups have higher than predicted diarrhea-associated mortality rates while males and adult females in temporary groups have lower predicted diarrhea-associated mortality rates. Parametric and non-parametric statistical models of diarrhea-associated mortality were successfully constructed, and both indicate young animals in smaller housing areas (shelter and temporary housing areas) are at greatest risk. There is a strong association between animals presenting with diarrhea and death; monkeys experiencing diarrhea have a 5.44 higher chance of death than monkeys who do not experience diarrhea. Time of year may be an important factor in diarrhea development, with the highest incidence in January and a second peak in late spring and early summer.
Diarrhea incidence among our outdoor-housed rhesus macaques is similar to that reported in 1984 [Hird et al., 1984]. Hird’s study provided a broad assessment of diarrhea among non-human primates, reporting data from 16 facilities and 9 species but the generalizability of these results to our population, and to other facilities with large populations of outdoor, group-housed rhesus macaques is problematic. The data were heavily skewed by input from two facilities which “accounted for 32% of the animals, 54% of diarrhea cases, and 58% of diarrhea-related deaths” [Hird et al., 1984]. Also, differences in diarrhea incidence were found among all types of indoor housing, yet outdoor housing was assessed collectively, rather than by type. Assessing outdoor housing collectively, rather than by type, obscures diarrhea risk factors in outdoor-housed populations. Our descriptive statistics, as well as both parametric and non-parametric methods, demonstrate differences in diarrhea-associated mortality by housing type. We also demonstrate that age is a significant risk factor and that sex, while not a statistically significant risk factor, should be taken into account when formulating prevention and mitigation strategies.
The use of CART is unique in our study. The “if-then” algorithm generates a decision tree that is particularly useful for data mining, when there is little a priori knowledge. Additionally, the “if-then” design provides a simple, easily visualized model of both classifier and predictor variables [Hill & Lewicki, 2006; Merkle & Shaffer, 2010]. Using diarrhea-associated mortality as the response variable, we demonstrated first, that CART modeling produces the same explanatory variables as logistic regression. The response variable “diarrhea-associated mortality,” predicted that infants and juveniles in either sheltered housing or temporary housing were at greatest risk, and that adult females in sheltered housing were also at risk. We also demonstrated that CART could be used to develop a predictive model with high sensitivity and good specificity for the predictor variable “diarrhea-associated mortality.” This modeling could be a powerful tool for animal management, particularly with new or complex diseases where retrospective data analysis is needed to identify risk factors. Examples at our center include the association between group movement and fighting and production changes associated with new group formation.
Generalizability
Our study is the most comprehensive analysis of diarrhea risk factors in outdoor-housed rhesus macaques published to date. This information is particularly useful for facilities with outdoor-housed populations of rhesus macaques, and can both inform prevention and mitigation strategies, and shape future research. As with previous studies, we demonstrate a strong correlation between diarrhea morbidity and mortality [Elmore et al., 1992; Good et al., 1969; Sauer et al., 1960; Schneider et al., 1960]. We also identify risk factors and highlight specific groups—females in shelters—with lower diarrhea incidence rates and higher diarrhea-associated mortality rates, and groups—generally male—with higher diarrhea incidence rates and lower diarrhea-associated mortality rates.
The high diarrhea incidence among animals in smaller housing (shelters and temporary housing) is a consideration for institutions with high-density housing. Small housing is attractive, easy to build and maintain, and rhesus reportedly develop behaviors that limit aggression in high-density housing situations [Judge & De Waal, 1997]. Additional studies, evaluating stress behaviors and cortisol levels, may enhance our understanding of space and housing density limitations for this species, and identify diarrhea prevention points. For example, corral animals are exposed to bare earth, while animals in shelters and temporary groups are not. This, and differences in cleaning, may affect the enteric microbiome. Similarly, the proximity to human care takers may cause stress differences between the two groups. Quantifying stress levels between the various housing types, and determining how housing design, daily cleaning and husbandry, and density alter stress levels and affect the micro-biome could significantly improve our management of this species.
Veterinary care of ill animals should be tailored to provide additional support for vulnerable animals, particularly for young animals in small housing types. Clinically ill infants, juveniles and adults with diarrhea may benefit from increased nutritional support and specialized housing both during acute disease and convalescence. Targeting preventative strategies and resources at vulnerable young animals may include creep feeders with high calorie chow, and avoiding high stress activities such as group movement or formation in early spring or late fall. Anecdotally, there may be a genetic predisposition to diarrhea. We have identified dams that have produced multiple colitis infants, irrespective of sire. Currently, we remove these animals from the breeding population. Genetic susceptibility may be a factor in diarrhea development and deserves further study [Rogers et al., 2005].
Our use of the non-parametric CART predictive modeling provided an additional statistical technique for identifying diarrhea-associated mortality risk factors. This method is particularly useful in retrospective studies, for informing predictor variable selection. Since CART can be used for all types of data, including continuous, ordinal, and categorical values, it may provide a powerful modeling tool for predicting other associations such as the relationship between new group formation or group movement and diarrhea and fighting.
LIMITATIONS
The retrospective nature of our study has some limitations. We attempted to limit selection bias by including all outdoor-housed animals in the study, but differences in housing design may have artificially lowered the incidence of diarrhea in CBGs. Animals in sheltered housing were, at most 50 feet away from observers. Animals in corrals may be several hundred feet from observers and are thus more difficult to assess. Misclassification or information bias may have artificially increased or decreased diarrhea rates. Cases were counted if they include the Master Problem “GI Diarrhea” and at least one SNOMED code indicating diarrhea, and all mortality cases were manually reviewed to ensure clinical treatment was consistent with diarrhea. However, it is possible cases were erroneously classified.
The 1-year time frame may artificially inflate several risk factors. More groups were established and moved this year than in the previous 3 years. Social group formation is typically stressful, and may have resulted in an over-estimation of diarrhea risk. The 1-year period may have also generated a bias from the weather. Seasonal temperatures may fluctuate from 1 year to the next, and a longer time period could have mitigated this. Both of these factors could have generated an over-estimation of the diarrhea rate. Similarly, our decision to exclude sedation and movement may have generated an over-estimation of the diarrhea rate. For example, all animals in each social group are sedated every 6 months for physical examination, tuberculosis testing, viral screening, and anti-parasitic administration. Thus, each animal in the study was sedated at least once during the study period, but some would have been sedated twice. Some groups would also have been sedated and moved to adjacent housing structures during routine maintenance and repair cycles. The effect of this on diarrhea rates is unclear, and a follow-up analysis is needed.
Unlike previous studies, we did not evaluate the association between enteric pathogens and diarrhea morbidity and mortality. This decision was an acknowledgment of diarrheal disease complexity. Certainly pathogens play a role in diarrhea development, but less than half of the animals with diarrhea have an identifiable pathogen. Two of most commonly implicated bacterial agents, Shigella and Campylobacter spp., are frequently isolated from clinically healthy animals [Hird et al., 1984; Schneider et al., 1960; Sestak et al., 2003]. Studies have linked adenovirus with diarrhea in captive macaques [Oberste et al., 2008; Sestak et al., 2003] but adenovirus DNA is also readily found in fecal samples of asymptomatic monkeys [Roy et al., 2012]. Since all animals in group housing have equal exposure to these and other pathogens, we felt it important to focus on the role of environment. We also did not evaluate the role of diet in development of colitis. Gluten sensitivity has been identified in some animals, and may be a factor in colitis development [Sestak et al., 2011]. Future studies evaluating the effect of diet, would be beneficial.
CONCLUSIONS
The problem of diarrhea in captive-housed rhesus macaques is well known, and many excellent studies have attempted to identify bacterial, viral, and parasitic risk factors for diarrheal disease. By design, our study did not evaluate these. Instead, we hypothesized that housing type, sex, and age were positively correlated with diarrhea incidence and diarrhea-associated mortality. We established diarrhea incidence rates and diarrhea-associated mortality rates for our outdoor population, and demonstrated that young animals in smaller housing groups have the highest diarrhea-associated mortality rates. These results support our hypothesis and suggest that further evaluation of housing and age-associated risks is essential to develop diarrhea mitigation strategies. Our data did not support a statistical association between sex and diarrhea mortality. However, adult females who develop diarrhea had higher mortality rates than other groups. The reasons for this are unclear, and further study is warranted. We also demonstrated seasonal variation in diarrhea-associated mortality, particularly with infants and juveniles. Targeting support and intervention strategies during these more vulnerable periods may decrease diarrhea-associated mortality. Finally, we used a non-parametric statistical model, CART, to develop a predictive model for diarrhea-associated mortality. The information in this study will be used to develop tailored mitigation strategies. The outcome of changes in housing design, feed, and husbandry practices will be evaluated, using metrics from this study. Future directions include quantifying stress differences and differences in the enteric microbiome by housing type, immune function testing, and genetic susceptibility analysis.
Acknowledgments
We thank Mr. Gary Jones for invaluable assistance for developing the database query. This study was supported by funds from the National Center for Research Resources (P51 RR000163) to the ONPRC, a fully AAALAC-accredited facility.
References
- Blanchard JL, Baskin GB, Watson EA. Generalized amyloidosis in rhesus monkeys. Vet Pathol. 1986;23:425–430. doi: 10.1177/030098588602300412. [DOI] [PubMed] [Google Scholar]
- Breiman L, Friedman JH, Olshen R, Stone C. Classification and Regression Trees. Belmont (CA): Wadsworth; 1984. p. 368. [Google Scholar]
- Desrosiers RC. The value of specific pathogen-free rhesus monkey breeding colonies for AIDS research. AIDS Res Hum Retroviruses. 1997;13:5–6. doi: 10.1089/aid.1997.13.5. [DOI] [PubMed] [Google Scholar]
- Elfenbein HA, McCowan B. The epidemiology of non-pathogenic diarrhea in captive rhesus macaques. J Am Assoc Lab Anim Sci. 2012;52:100. [Google Scholar]
- Elmore DB, Anderson JH, Hird DW, Sanders KD, Lerche NW. Diarrhea rates and risk factors for developing chronic diarrhea in infant and juvenile rhesus monkeys. Lab Anim Sci. 1992;42:356–359. [PubMed] [Google Scholar]
- Farkas T, Falkenstein KP, Bohm RP, Pecotte J, Sestak K. High incidence of rhesus enteric calicivirus infections and diarrhea in captive juvenile macaques: a likely association. J Med Primatol. 2012;41:325–328. doi: 10.1111/j.1600-0684.2012.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JG, Boutin SR, Handt LK, et al. Isolation and characterization of a novel helicobacter species, “Helicobacter macacae,” from rhesus monkeys with and without chronic idiopathic colitis. J Clin Microbiol. 2007;45:4061–4063. doi: 10.1128/JCM.01100-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JW, Lerche NW. Electrolyte abnormalities associated with diarrhea in rhesus monkeys: 100 cases (1986–1987) J Am Vet Med Assoc. 1990;196:1654–1658. [PubMed] [Google Scholar]
- Gomez HF, Ochoa TJ, Carlin LG, Cleary TG. Human lactoferrin impairs virulence of Shigella flexneri. J Infect Dis. 2003;187:87–95. doi: 10.1086/345875. [DOI] [PubMed] [Google Scholar]
- Good RC, May BD, Kawatomari T. Enteric pathogens in monkeys. J Bacteriol. 1969;97:1048–1055. doi: 10.1128/jb.97.3.1048-1055.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann RT, Williams FP., Jr Diseases seen at necropsy of 708 Macaca mulatta (rhesus monkey) and Macaca philippinensis (cynomolgus monkey) Am J Vet Res. 1957;18:419–426. [PubMed] [Google Scholar]
- Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York (NY): Springer-Verlag; 2001. p. 600. [Google Scholar]
- Hill T, Lewicki P. Statistics: methods and applications. Tulsa (OK): StatSoft, Inc; 2006. p. 813. [Google Scholar]
- Hird DW, Anderson JH, Bielitzki JT. Diarrhea in nonhuman primates: a survey of primate colonies for incidence rates and clinical opinion. Lab Anim Sci. 1984;34:465–470. [PubMed] [Google Scholar]
- Holmberg CA, Leininger R, Wheeldon E, Slater D, Henrickson R, Anderson J. Clinicopathological studies of gastrointestinal disease in macaques. Vet Pathol Suppl. 1982;7:163–170. [PubMed] [Google Scholar]
- Judge PG, De Waal FBM. Rhesus monkey behaviour under diverse population densities: coping with long-term crowding. Anim Behav. 1997;54:643–662. doi: 10.1006/anbe.1997.0469. [DOI] [PubMed] [Google Scholar]
- Kinsey MD, Formal SB, Dammin GJ, Giannella RA. Fluid and electrolyte transport in rhesus monkeys challenged intracecally with Shigella flexneri 2a. Infect Immunol. 1976;14:368–371. doi: 10.1128/iai.14.2.368-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGuire JG, Christe KL, Yee JL, Kalman-Bowlus AL, Lerche NW. Serologic evaluation of clinical and subclinical secondary hepatic amyloidosis in rhesus macaques (Macaca mulatta) Comp Med. 2009;59:168–173. [PMC free article] [PubMed] [Google Scholar]
- Merkle EC, Shaffer VA. Binary recursive partioning: background, methods, and applied psychology. Br J Math Stat Psychol. 2010;64:161–181. doi: 10.1348/000711010X503129. [DOI] [PubMed] [Google Scholar]
- Mulder JB. Shigellosis in nonhuman primates: a review. Lab Anim Sci. 1971;21:734–738. [PubMed] [Google Scholar]
- Munoz-Zanzi CA, Thurmond MC, Hird DW, Lerche NW. Effect of weaning time and associated management practices on postweaning chronic diarrhea in captive rhesus monkeys (Macaca mulatta) Lab Anim Sci. 1999;49:617–621. [PubMed] [Google Scholar]
- Naumenko ES, Krylova RI. Amyloidosis in macaques in Adler Primatological center. Bull Exp Biol Med. 2003;136:80–83. doi: 10.1023/a:1026005416942. [DOI] [PubMed] [Google Scholar]
- Oberste MS, Jiang X, Maher K, et al. The complete genome sequences for three simian enteroviruses isolated from captive primates. Arch Virol. 2008;153:2117–2122. doi: 10.1007/s00705-008-0225-4. [DOI] [PubMed] [Google Scholar]
- Pucak GJ, Orcutt RP, Judge RJ, Rendon F. Elimination of the Shigella carrier state in rhesus monkeys (Macaca mulatta) by trimethoprim-sulfamethoxazole. J Med Primatol. 1977;6:127–132. doi: 10.1159/000459732. [DOI] [PubMed] [Google Scholar]
- Rogers J, Bergstrom M, Garcia RT, et al. A panel of 20 highly variable microsatellite polymorphisms in rhesus macaques (Macaca mulatta) selected for pedigree or population genetic analysis. Am J Primatol. 2005;67:377–383. doi: 10.1002/ajp.20192. [DOI] [PubMed] [Google Scholar]
- Rothschild BM. Primate spondyloarthropathy. Curr Rheumatol Rep. 2005;7:173–181. doi: 10.1007/s11926-996-0036-0. [DOI] [PubMed] [Google Scholar]
- Roy S, Sandhu A, Medina A, Clawson DS, Wilson JM. Adenoviruses in fecal samples from asymptomatic rhesus macaques, United States. Emerg Infect Dis. 2012;18:1081–1088. doi: 10.3201/eid1807.111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RG, Krugner L, Tsai CC, Ekstrom R. Prevalence of Campylobacter in infant, juvenile and adult laboratory primates. Lab Anim Sci. 1988;38:711–714. [PubMed] [Google Scholar]
- Satterthwaite EW. An approximate distribution of estimates of variance components. Biometrics Bull. 1946;2:110–114. [PubMed] [Google Scholar]
- Sauer RM, Atta AG, Ayres PE, et al. Care and diseases of the research monkey. Ann N Y Acad Sci. 1960;85:735–992. [Google Scholar]
- Schneider NJ, Prather EC, Lewis AL, Scatterday JE, Hardy AV. Enteric bacteriological studies in a large colony of primates. Ann N Y Acad Sci. 1960;85:935–941. doi: 10.1111/j.1749-6632.1960.tb50013.x. [DOI] [PubMed] [Google Scholar]
- Sestak K, Merritt CK, Borda J, et al. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun. 2003;71:4079–4086. doi: 10.1128/IAI.71.7.4079-4086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K, Conry L, Aye P, Smriti M, Doxiadis GG, Kaushal D. Improved xenobiotic metabolism and reduced susceptibility to cancer in gluten-sensitive macaques upon introduction of a gluten-free diet. PLoS ONE. 2011;6:e18648. doi: 10.1371/journal.pone.0018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K, Feely S, Fey B, et al. Experimental inoculation of juvenile rhesus macaques with primate enteric caliciviruses. PLoS ONE. 2012;7:e37973. doi: 10.1371/journal.pone.0037973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuker G, Oshiro LS, Schmidt NJ, et al. Virus detection in monkeys with diarrhea: the association of adenoviruses with diarrhea and the possible role of rotaviruses. Lab Anim Sci. 1979;29:610–616. [PubMed] [Google Scholar]
- Takeuchi A, Formal SB, Sprinz H. Experimental acute colitis in the rhesus monkey following peroral infection with Shigella flexneri. Am J Pathol. 1968;52:503–529. [PMC free article] [PubMed] [Google Scholar]
- Tribe GW, Frank A. Campylobacter in monkeys. Vet Rec. 1980;106:365–366. doi: 10.1136/vr.106.16.365-a. [DOI] [PubMed] [Google Scholar]
- Wilk JL, Maginnis GM, Coleman K, Lewis A, Ogden B. Evaluation of the use of coconut to treat chronic diarrhea in rhesus macaques (Macaca mulatta) J Med Primatol. 2008;37:271–276. doi: 10.1111/j.1600-0684.2008.00313.x. [DOI] [PubMed] [Google Scholar]
- Wolfensohn S. Shigella infection in macaque colonies: case report of an eradication and control program. Lab Anim Sci. 1998;48:330–333. [PubMed] [Google Scholar]