Abstract
Background
Prior studies have shown relationships between serum immunoglobulin E (IgE) and asthma.
Objective
To investigate relationships between total and allergen-specific IgE concentrations and lung function in young adults.
Methods
Measurements of total IgE, allergen-specific IgE to 6 common allergens, and spirometry (forced expiratory volume in one second [FEV1], forced vital capacity [FVC], FEV1/FVC, and percent change in FEV1 after bronchodilation) were used to calculate correlations between the logarithmically transformed IgE values and measures of lung function among participants in a birth cohort not selected for risk of allergic disease stratified by current asthma, prior asthma, or no asthma.
Results
The 428 participants were 51.6% female, 93% white, and 18.4 (standard deviation = 0.6) years old. Forty-eight (11.2%) had current asthma, 55 (12.9%) had a history of asthma, and 325 (75.9%) never had asthma. For males with current asthma, correlations between total IgE and FEV1% and FVC% were −0.51 (P =.06) and −0.70 (P = .005), respectively. For females with current asthma, the only significant correlation was between total IgE and the FEV1/FVC ratio (−0.55, P = .001). After excluding smokers and individuals without detectable allergen-specific IgE, the negative correlations for both males and females remained statistically significant. The correlations among males or females with prior asthma or no history of asthma were minimal and not statistically significant. The sum of the allergen-specific IgEs showed the same pattern of relationships to lung function as did total IgE.
Conclusions
Our results show significant negative correlations that vary by gender between both total and allergen-specific IgE and measurements of lung function in young adults with current asthma.
Introduction
Previous studies have shown that increasing serum concentrations of total IgE are strongly related to an increasing risk of a person having asthma.1–3 Sears et al. also showed that serum IgE concentrations were significantly correlated with responsiveness to inhaled methacholine.2 In the study of Burrows et al. the positive correlation between total serum IgE and asthma was stronger than correlations between indicators of allergen-specific IgE and asthma suggesting that a mechanism other than sensitivity to inhalant allergens links IgE to asthma.1
Studies by Grunstein et al. have shown that, in mice, airway smooth muscle cells have both high- (FcεR1) and low- (FcεR2) affinity IgE receptors on their surfaces.4 These are functional IgE receptors as shown by upregulation of cytokine production, especially IL-5 and IL-13.5 Furthermore, IgE activation of airway smooth muscle cells increases smooth muscle reactivity.4 Human studies have also shown associations between IgE and airway responsiveness6 illustrated by studies in which reducing serum IgE concentration with omalizumab improved asthma symptoms.7;8
There are important gender differences in asthma. During childhood, males are more likely to have asthma than females, while in adolescence, prevalence equalizes followed by a greater prevalence in females during adulthood.9 It seems likely that hormonal changes occurring during puberty contribute to this pattern.10;11 These known gender differences prompted us to look for gender differences in correlations between IgE and lung function. Based on the described relationships of IgE to asthma, we evaluated whether total or allergen-specific serum IgE concentrations were associated with decreased lung function in a population-based birth cohort of otherwise healthy young adults after considering their individual histories of asthma. We hypothesized that increasing levels of serum IgE would be associated with reduced lung function primarily seen as reduced airflow measured by percent predicted forced expiratory volume in 1 second (FEV1) and ratio of FEV1 to forced vital capacity (FVC) or (FEV1/FVC). We posited that inverse relationships between IgE and lung function would be found in individuals with current asthma, those with a previous history of asthma and perhaps also those that had never had a diagnosis of asthma.
Methods
The initial selection of the cohort has been previously described.12–14 Briefly, the Childhood Allergy Study (CAS) is a population-based birth cohort conducted among members of a health maintenance organization in northern suburbs of Detroit, Michigan to study environmental influences on the development of allergic diseases and asthma. All pregnant women enrolled in the HMO who were at least 18 years of age, living in a predefined geographic area, and due to deliver in 1987 through 1989, were invited to participate in the study. Eligibility was not related to a history of allergic disease. Only full term infants (at least 36 weeks gestation) with valid cord blood IgE measurements were included in the study.15
Eight hundred thirty-five children were initially enrolled in CAS. Annual interviews were conducted until 6–7 years at which time children were invited to complete a clinic visit.12 Beginning in May 2005, all of the former child participants were contacted by telephone as they attained 18 years of age to obtain information on allergy, asthma, and other health and exposure histories including pets and tobacco smoke exposure, from the ages of 6 through 18 years. All interviewed teens were invited to a clinic visit for blood sample collection and spirometry measurements but some could not come to the clinic because they had moved from the area or for other reasons. Spirometry and blood collection were not performed if the patient reported a viral illness within the previous week. There were no exclusions for the clinic visit and samples were collected throughout the year at the convenience of participants. If a visit was cancelled due to illness it was rescheduled whenever possible.
Of the 835 initial child participants, interviews were completed on 670 participants and of these 566 had serum samples collected between the ages of 18–20 years for measurement of total and allergen specific IgE concentrations. A total of 432 participants completed pre- and post-bronchodilator spirometry providing 428 with complete data from questionnaires, IgE measurements and spirometry results for analysis. Current asthma was defined as a participant report of a previous doctor diagnosis of asthma and a report of asthma symptoms or asthma medication use in the last 12 months. Prior asthma was defined as a participant report of having received a doctor diagnosis of asthma in those who had no asthma symptoms or medications in the previous year. Those denying ever having received a doctor’s diagnosis of asthma were defined as those without asthma. Smoking was defined as a report of smoking one or more cigarettes per week. All aspects of this research were approved by the Henry Ford Hospital Institutional Review Board, and the Georgia Health Sciences University Human Assurance Committee and written informed consent was obtained from the subjects as required.
Measurements of total and allergen specific IgE were performed following the standard manufacturer’s protocols using the Pharmacia UniCAP system (Phadia USA, Portage, MI). Allergen-specific IgE was measured to dog, cat, dust mite (Dermatophagoides farina), timothy grass, Alternaria alternata and short ragweed and values of ≥0.35 kIU/l were considered positive as in previous publications. Atopy was defined as one or more positive values for allergen-specific IgE. For analyses, the allergen-specific IgE values for each allergen were summed to produce a single value which is referred to as the sum of allergen-specific IgE. We also evaluated using the number of positive allergen-specific IgE values. Since there were no differences in the overall results when using the sum or number of positive allergen-specific results, only the analyses with the sum of the allergen-specific IgE are presented. One percent of all IgE assays were repeated in a different assay run on a different day to provide estimates of interassay reliability. The geometric mean coefficient of interassay variation was 5.9% for total IgE and 6.7% for allergen-specific IgE.
Lung function was recorded with a handheld spirometer (SpiroPro spirometer Jaeger, Hoechberg, Germany). If a subject was taking a short-acting bronchodilator, the medication was withheld for 6 hours prior to testing but all controller medications were continued (including leukotriene modifying agents, inhaled corticosteroids, and combinations of inhaled corticosteroids and long-acting beta-agonists). The subjects were coached to engage in maximal forced expiratory maneuvers while standing with the use of nose clips. Spirometry was performed in accordance with American Thoracic Society – European Respiratory Society standards16 and findings were considered to be acceptable if the subject made a good effort and if two forced exhalation maneuvers showed reproducibility (±5%) for FVC and FEV1. Spirometry was repeated for all subjects a second time 15 minutes after having the subject inhale 2 puffs of pirbuterol (Maxair Authohaler, Graceway Pharmaceuticals, Bristol, TN) from a metered dose inhaler and the percent change in FEV1 was calculated from the best of three attempts. Spirometry results included; the percent predicted forced expiratory volume in one second (FEV1%), forced vital capacity (FVC%), the ration of FEV1/FVC, and the percent change in FEV1 post pirbuterol. All spirometry was examined for proper effort and consistency and results from inadequate evaluations were removed before data analysis which reduces the number of subjects in some analyses.
Statistical Analysis
The distribution of the total serum IgE and the sums of the allergen-specific IgE values was skewed but after natural logarithmic transformation the distribution adequately approximated a normal distribution. Total IgE data are presented as geometric means (GM) and 95% confidence intervals (95% CI). Chi-square analysis was used to compare charcteristics of participants and nonparticipants. All analyses were performed with log-transformed IgE values. Bivariate associations between two continuous variables, such as log-transformed IgE and FEV1%, were assessed with Pearson’s correlations. Kruskal-Wallis tests were used to look for differences across the groups stratified by asthma status and sex (current, prior, never). Teen participant smoking status was adjusted for using partial correlation analysis. All statistical analyses were performed using SAS software (version 9.2: SAS Institute, Cary, N.C.). P-values less than 0.05 were considered statistically significant in all analyses.
Results
Table 1 shows the characteristics of the study participants and compares the gender and racial distributions of those included and excluded from analysis. The gender and racial proportions did not differ significantly for those included and excluded. Males were more likely to be atopic than females but this difference did not reach statistical significance (92.9% versus 67.6%, respectively, p=0.08). Total serum IgE concentrations were strongly correlated with the sum of the allergen-specific IgE measurements. For males with current asthma the correlation was 0.88 and for females with current asthma it was 0.74. Of the 432 subjects 49 (11.3%) had asthma, 57 (13.2%) prior asthma and 326 (75.5%) never had asthma, Of the Of 49 subjects classified as having current asthma: 21 stated they only took albuterol and an additional 16 said they had a medicine to relieve asthma but could not provide the name, 4 took a leukotriene modifying drug, 4 took an inhaled corticosteroid, 3 took a combination inhaled corticosteroid plus long acting beta-agonist and 1 took an intranasal steroid medication. Based on the medications reportedly taken, only 11 (22%) of the 49 subjects had persistent asthma.
Table 1.
Description of participants and comparison of those included and excluded from analysis
| Included in Analysis |
Excluded from Analysis* |
Chi- square P-value |
|
|---|---|---|---|
| N = 432 | N = 403 | ||
| Gender, n (%) | 0.61 | ||
| Male | 209 (48.4%) | 202 (50.1%) | |
| Female | 223 (51.6%) | 201 (49.9%) | |
| Race, n (%) | 0.70 | ||
| White | 413 (95.6%) | 383 (95.0%) | |
| Other | 19 (4.4%) | 20 (5.0%) | |
| Mean age at spirometry (s.d.) | 18.4 (0.6) | ||
| Asthma status at follow up visit | |||
| Current | 49 (11.3%) | ||
| Prior | 57 (13.2%) | ||
| Never | 326 (75.5%) | ||
| Days teen smoked in 30 days prior to follow up | |||
| 0 (None) | 325 (75.2%) | ||
| 1–29 days (some) | 52 (12.1%) | ||
| All 30 days | 55 (12.7%) | ||
Four hundred seven individuals were excluded from analysis either because they did not participate or because they did not complete all elements of the clinical evaluation.
Table 2 shows the spirometry data obtained from study participants stratified by asthma status. The only significant differences were between those with prior asthma who had lower FEV1% and FVC% compared to those with no history of asthma. The percent change in FEV1 post bronchodilator was not greater in those with current asthma compared to those without asthma. After stratification by asthma history (data not shown) the only significant difference in lung function between males and females was with FEV1% in those with no history of asthma. In this group males had greater average FEV1% than females (102.1% versus 97.6%, respectively, p=0.004).
Table 2.
Summary of Spirometry Measures in Study Participants Stratified by Asthma History
| FEV1%* mean (s.d.) |
FVC%* mean (s.d.) |
FEV1/FVC% mean (s.d.) |
% change FEV1** mean (s.d.) |
|
|---|---|---|---|---|
| All subjects | 99.3 (12.9) n=432 | 103.3 (11.5) n=401 | 83.2% (7.7) n=401 | 3.1 (7.8) n=415 |
| Current asthma | 99.8 (12.6) n=49 | 103.8 (14.2) n=46 | 84.3% (7.0) n=46 | 2.6 (7.0) n=45 |
| Prior asthma | 94.3 (14.5) n=57 | 99.1 (12.3) n=54 | 81.7% (9.6) n=53 | 3.5 (7.7) n=53 |
| Never asthma | 100.1 (12.5) n=326 | 104.0 (10.7) n=301 | 83.3% (7.4) n=301 | 3.1 (7.9) n=317 |
| P value for differences between asthma groups | 0.012† | 0.030† | 0.45 | 0.67 |
Percent predicted FEV1 and FVC.
Percent change in FEV1 15 minutes after receiving two sprays of pirbuterol from a metered dose inhaler.
For both FEV1% and FVC% the differences between prior and never asthma are significant P<0.01.
Table 3 shows the geometric mean values of total serum IgE concentrations for participants stratified by gender and asthma history. The proportion of males with asthma (6.7%) is significantly less than the proportion of females with asthma (15.7%), p=0.003.The geometric mean IgE is statistically significantly different in both males and females by asthma history, (p=0.004 and 0.007, respectively) largely because of the significantly higher geometric mean in those with a history of current asthma compared to those who never had asthma (p = 0.003, p=0.002, respectively). While the geometric mean of those with current asthma was higher than those with prior asthma and the geometric mean of those with prior asthma was greater than for those without asthma, these differences did not reach statistical significance for either males or females.
Table 3.
Geometric Mean Total Serum IgE in Males and Female Stratified by Asthma Status
| Strata | Number of subjects |
Total IgE, IU/ml Geometric Mean (95% CI) |
P-value* |
|---|---|---|---|
| Males | |||
| Current asthma | 14** | 119.4 (63.8, 223.4) | 0.004 |
| Prior asthma | 32 | 60.5 (35.6, 102.9) | |
| No asthma | 163 | 34.0 (26.3, 43.9) | |
| Females | |||
| Current asthma | 35** | 63.1 (40.2, 99.1) | 0.007 |
| Prior asthma | 25 | 35.3 (19.5, 63.8) | |
| No asthma | 163 | 27.8 (22.5, 34.4) | |
Comparison of IgE by asthma status for males: current, prior and never - P=0.004, current versus never asthma – P=0.003, current versus prior – P=0.16, and prior versus never P=0.07. For females: current, prior and never - P=0.007, current versus never asthma – P=0.002, current versus prior – P=0.07, and prior versus never P=0.61.
The proportion of males with asthma (6.7%) is significantly less than the proportion of females with asthma (15.7%), p=0.003.
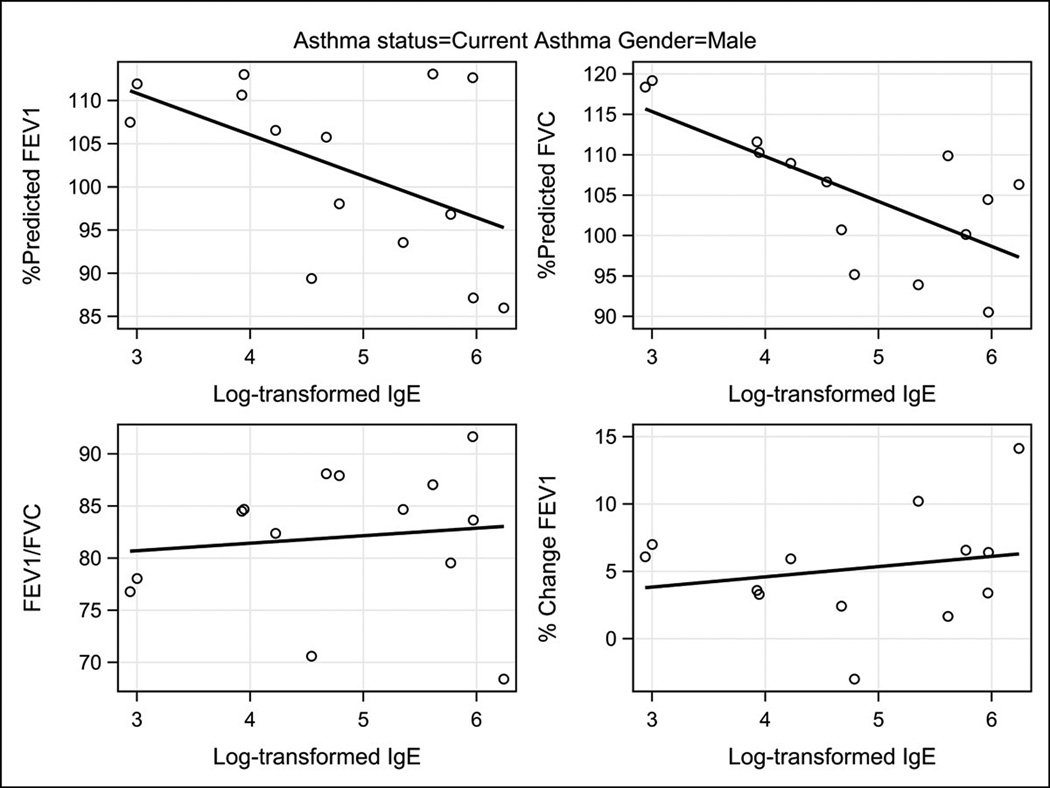
Table 4 describes the correlations between total and allergen-specific IgE concentrations and lung function variables in the participants stratified by gender and asthma status. Figures 1 and 2 illustrate the correlations between total serum IgE concentrations and lung function variables in the participants stratified by gender among those with current asthma. In males with current asthma the inverse correlations between total serum IgE and both FEV1% and FVC% were relatively large, −0.51 and −0.70, respectively. The correlation between IgE and FVC% reached statistical significance (p=0.005) while that with FEV1% was of borderline significance (p=0.06). The allergen-specific IgE inverse correlation with FVC% was relatively strong but did not reach statistical significance. Among males with a prior asthma history and those never having asthma, the total serum IgE and allergen-specific IgE correlations were minimal and none approached statistical significance.
Table 4.
Pearson Correlation Coefficients for Total and Sums of Allergen-Specific Serum IgE and Lung Function Variables Stratified by Gender and Asthma Group: Current Asthma, Prior Asthma, and Never Asthma*
| All Individuals Participating in Study | ||||
|---|---|---|---|---|
| Asthma status | FEV1% ** | FVC%** | FEV1/FVC | % change FEV1# |
| Males | ||||
| Current Asthma | ||||
| Total Serum IgE | −0.51 | −0.7 | 0.12 | 0.2 |
| p=0.06 | p=0.005 | p=0.69 | p=0.50 | |
| n=14 | n=14 | n=14 | n=13 | |
| Allergen Specific IgE | −0.48 | −0.7 | 0.17 | −0.04 |
| p=0.09 | p=0.006 | p=0.57 | p=0.97 | |
| n=14 | n=14 | n=14 | n=13 | |
| Prior Asthma | ||||
| Total Serum IgE | 0.14 | −0.03 | 0.25 | 0.05 |
| p=0.46 | p=0.88 | p=0.18 | p=0.78 | |
| n=32 | n=31 | n=31 | n=30 | |
| Never Asthma | ||||
| Total Serum IgE | −0.08 | −0.07 | −0.01 | 0.03 |
| p=0.32 | p=0.37 | p=0.96 | p=0.70 | |
| n=163 | n=156 | n=156 | n=161 | |
| Females | ||||
| Current Asthma | ||||
| Total Serum IgE | −0.19 | 0.07 | −0.53 | 0.1 |
| p=0.28 | p=0.71 | p=0.002 | p=0.57 | |
| n=35 | n=32 | n=32 | n=32 | |
| Allergen Specific IgE | −0.07 | 0.12 | −0.42 | 0.13 |
| p=0.71 | p=0.51 | p=0.017 | p=0.48 | |
| n=35 | n=32 | n=32 | n=32 | |
| Prior Asthma | ||||
| Total Serum IgE | −0.32 | −0.23 | −0.21 | 0.09 |
| p=0.12 | p=0.30 | p=0.34 | p=0.69 | |
| n=25 | n=23 | n=23 | n=23 | |
| Never Asthma | ||||
| Total Serum IgE | −0.09 | −0.09 | 0.01 | 0.03 |
| p=0.25 | p=0.31 | p=0.89 | p=07 | |
| n=163 | n=145 | n=145 | n=156 | |
Correlations for the sums of the allergen-specific IgE are not included for those with prior or no asthma because the correlations were all very small and similar to those of the total IgE. The numbers of subjects varies slightly depending on participation and adequate performance of all spirometry
Percent predicted FEV1 and FVC.
Percent change in FEV1 15 minutes after receiving two sprays of pibuterol from a metered dose inhaler.
Figure 1.
Scatter plot of total serum IgE concentrations as natural logarithms in relationship to the lung function measurements FEV1%, FVC%, FEV1/FVC, and percent change in FEV1 post-bronchodilator among males with current asthma.
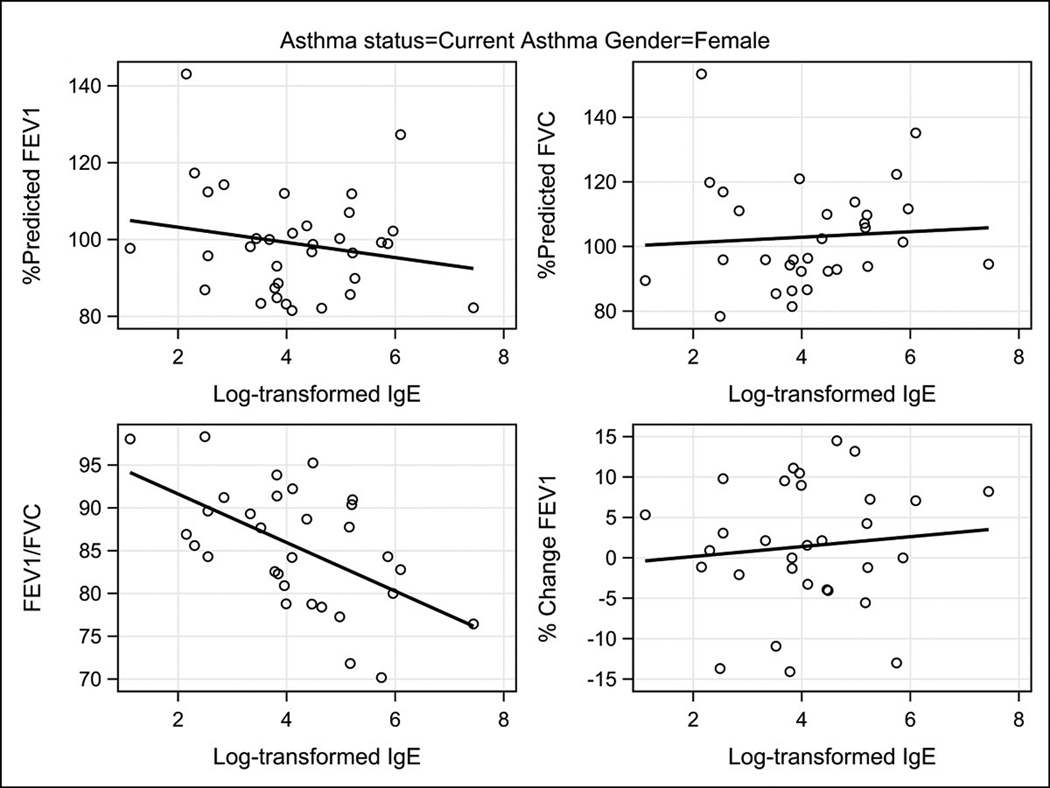
Figure 2.
Scatter plot of total serum IgE concentrations as natural logarithms in relationship to the lung function measurements FEV1%, FVC%, FEV1/FVC, and percent change in FEV1 post-bronchodilator among females with current asthma.
The pattern of the correlations between IgE and lung function in females was different from that seen in males. Among females with current asthma strong inverse correlations were observed between total and allergen-specific IgE and the FEV1/FVC ratio,( −0.55, p = 0.001 and , −0.36, p=0.043, respectively). The strong negative correlations between serum IgE concentration and both FEV1% and FVC% seen in the males with current asthma were not seen in the females with current asthma.
Smoking has been reported to increase serum IgE and to reduce lung function and the relationship between atopy and total serum IgE is well known.17 We therefore considered the possibility that the correlations between serum IgE and lung function variables were influenced by subject smoking or by atopic status. When we statistically adjusted the correlations shown in Table 4 for smoking status there were no important changes in the results. The results of restricting analysis to only atopic, non-smoking males and females were then examined For males with current asthma, the correlation between total IgE and the percent predicted FVC increased to −0.81 (p=0.002) and that for FEV1% remained similar in direction and magnitude. For atopic, nonsmoking females with current asthma, the correlation between total and allergen-specific IgE with the FEV1/FVC ratio increased in magnitude (−0.62, p=0.003, −0.65, p=0.001). In this group of females the correlations of total and allergen-specific IgE with FVC% were much stronger (r=.41, p=0.065 for total IgE; r=0.47, p=0.033 for allergen-specific IgE). All other correlations in this group remained inconsequential (p>0.50).
Discussion
While our results are consistent with our initial hypothesis that increasing serum IgE concentrations would be negatively correlated with lung function we believe our observation of gender differences between IgE and lung function are unique. Increasing total IgE was associated with lower FVC% in males but the association was in the opposite direction for females. The reverse was true for FEV1/FVC. The same pattern of relationships was observed when the sum of allergen-specific IgE values were examined in relationship to the lung function measurements. The negative correlations we observed were only in participants with current asthma and not among participants with either a prior or a negative history for asthma. As has previously been reported for this age range, females were more likely to have current asthma than males.
As expected we found that total IgE concentrations were significantly higher in those with asthma than in those without asthma and intermediate in those with prior asthma histories.1–3 Vollmer et al. showed that as IgE increased the percentage of persons with a FEV1% < 80% increased in one of their two cohorts. However, they did not explore differences by asthma history or gender.3 Sears et al. reported that regardless of their asthma status, children with increased levels of serum IgE had increased airway responsiveness to methacholine challenges.2 However, we did not perform methacholine challenges in our cohort as done by Sears et al., and the change in FEV1 after administration of a bronchodilator in our data was not related to IgE regardless of asthma status. The minimal changes we observed post-bronchodilator may have been related to only withholding short-acting beta-agonists and not withholding controller medications, especially those containing long-acting beta-agonists, prior to testing or to the relatively small dose of beta-agonist administered.
The gender differences we found in the correlations between IgE and lung function parameters are intriguing because they suggest differences in asthma pathophysiology between males and females. Many studies have shown gender differences in airway reactivity and in asthma prevalence.18–21 We hypothesize that the loss of FVC% in males with increasing IgE is related to increased air trapping resulting in greater residual volume. Consistent with this hypothesis, Cohen et al. demonstrated greater methacholine induced air trapping with end-expiratory CT scans in males than in females.22 In comparison, the negative correlation between IgE and FEV1/FVC in females suggests that increasing airway obstruction in females is more likely associated with airway inflammation than with air trapping. Supporting this interpretation is the demonstration by Cohen et al of greater lung inflammation in females as measured by exhaled nitric oxide.22 Greater inflammation in female humans is consistent with studies using mouse models of allergic asthma showing that female mice develop greater lung inflammation and remodeling than do male mice.23;24 A more recent paper by Collins et al showed that bronchial hyperresponsiveness was more common and severe in females than in males.25 Goksor et al demonstrated that female gender was the strongest predictor of airway obstruction in young adults who had been hospitalized for wheezing at less than 2 years of age.26 Unfortunately, our data do not allow us to more precisely distinguish the origins of these gender differences in airway obstruction related to IgE concentrations. It was notable that our gender differences became more striking when removing non-atopic asthmatics (which reduced the sample more in females), suggesting that these sex differences are most important in asthma associated with allergic sensitivity.
An important strength of our study is that fact that it was done in a large, prospective, population-based, birth cohort. The parents of the study subjects were questioned repeatedly about asthma and respiratory illnesses from the time the subjects were born until they were 7 years old. Asthma status at 18 – 20 years of age was self-reported but the subjects came from families that were frequently enrolled in a health maintenance plan suggesting that access to medical care was likely to be adequate for all participants.12
Weaknesses of our study include the loss of some subjects and the failure of some to participate in all parts of the follow up examination. Study subjects were predominantly Caucasian limiting the ability to generalize to other racial groups. Additionally, our subjects were only from one geographic region. More detailed lung volume studies might have provided additional information to further elucidate gender differences but such studies were not feasible in this study. There has been research showing that females have decreased FEV1 during their menstrual cycle9 but we did not ask participating women about menstruation during their visits so we could not adjust for this in our analyses. To determine allergic sensitization only six allergens were tested but others have shown that a limited number of tests is sufficient for epidemiologic studies.27 Finally, the number of individuals with current asthma limited our statistical power in some comparisons.
In conclusion, we found significant inverse relationships between serum IgE concentrations and lung function among those with current asthma. Additionally we found that the IgE – lung function correlations differed markedly by gender suggesting gender differences in asthma pathophysiology.
Acknowledgements
The Maxair Autohalers used were donated by Graceway Pharmaceutials, Bristol, TN. The study was funded by the National Institute of Allergy and Infectious Diseases, grants R01 AI051598 and R01 AI59415 and by the Fund for Henry Ford Hospital.
Biographies
Chathruckan Rajendra B.S. conceived, initiated and directed the analysis presented and was the principal author of the paper as part of a summer medical student research project.
Edward Zoratti, M.D. is the co-principal investigator of grant funding the 18 year follow up of the cohort studied. He was critically involved in all phases of the study and critically reviewed the manuscript.
Suzanne Havstad, M.A. performed the data analyses, authored the section on data analysis and critically reviewed all aspects of the manuscript.
Charlotte Nicholas, MPH was the primary coordinator of the 18 year follow up study and directly supervised the collection of the data from participants. She also critically reviewed the manuscript and the methods for accuracy.
Ganesa Wegienka, Ph.D. was involved in the design of the 18 year follow up study and helped supervise the conduct of the study with Dr. Zoratti.
M. Todd Cross, M.D. assisted with interpretation of the pulmonary function data, the physiologic meanings of the gender differences found and provided critical background information. He also critically reviewed the manuscript.
Christine C. Johnson, Ph.D., MPH has been a key co-investigator and epidemiologist who assisted with the initial design, recruitment and follow up of the cohort from birth. She is also the co-principal investigator of the 18 year follow up study and critically reviewed the manuscript.
Dennis Ownby, M.D. was the principal investigator of the grant funding the initial recruitment of the cohort with follow up to 7 years of age. He was a co-investigator of the 18 year follow up and the primary mentor of Mr. Rajendra for his research project.
Footnotes
None of the authors have any real or potential conflicts of interest with any of the information presented in the manuscript.
References
- 1.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 3.Vollmer WM, Buist AS, Johnson LR, McCamant LE, Halonen M. Relationship between serum IgE and cross-sectional and longitudinal FEV1 in two cohort studies. Chest. 1986;90:416–423. doi: 10.1378/chest.90.3.416. [DOI] [PubMed] [Google Scholar]
- 4.Grunstein MM, Hakonarson H, Leiter J, et al. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;282(3):L520–L528. doi: 10.1152/ajplung.00343.2001. [DOI] [PubMed] [Google Scholar]
- 5.Xia YC, Schuliga M, Shepherd M, et al. Functional Expression of IgG-Fc Receptors in Human Airway Smooth Muscle Cells. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2009-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunyer J, Anto JM, Sabriá J, et al. Relationship between serum IgE and airway responsiveness in adults with asthma. J Allergy Clin Immunol. 1995;95:699–706. doi: 10.1016/s0091-6749(95)70175-3. [DOI] [PubMed] [Google Scholar]
- 7.Milgrom H, Fick RB, Jr, Su JQ, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 8.Johansson SG, Nopp A, Oman H, et al. The size of the disease relevant IgE antibody fraction in relation to 'total-IgE' predicts the efficacy of anti-IgE (Xolair) treatment. Allergy. 2009;64(10):1472–1477. doi: 10.1111/j.1398-9995.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 9.Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4(Suppl B):S133–S146. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- 10.Dimitropoulou C, White RE, Ownby DR, Catravas JD. Estrogen reduces carbachol-induced constriction of asthma airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol. 2004;32:239–247. doi: 10.1165/rcmb.2004-0331OC. [DOI] [PubMed] [Google Scholar]
- 11.Jeon YH, Yang HJ, Pyun BY. Lung function in Korean adolescent girls: in association with obesity and the menstrual cycle. J Korean Med Sci. 2009;24(1):20–25. doi: 10.3346/jkms.2009.24.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 13.Misiak RT, Wegienka G, Havstad S, Ownby DR, Johnson CC, Zoratti EM. Specific allergic sensitization in parents and their 18 year-old offspring in the Detroit Area Childhood Allergy Study. J Allergy Clin Immunol. 2009;123:1401–1406. doi: 10.1016/j.jaci.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas C, Wegienka G, Havstad S, Ownby D, Johnson CC, Zoratti E. How accurately do young adults recall childhood pets? A validation study. Am J Epidemiol. 2009;170(3):388–392. doi: 10.1093/aje/kwp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ownby DR, McCullough J, Johnson CC, Peterson EL. Evaluation of IgA measurements as a method for detecting maternal blood contamination of cord blood samples. Pediatric Allergy and Immunology. 1996;7:125–129. doi: 10.1111/j.1399-3038.1996.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 17.Burrows B, Halonen M, Lebowitz MD, Knudson RJ, Barbee RA. The relationship of serum immunoglobulin E, allergy skin tests and smoking to respiratory disorders. J Allergy Clin Immunol. 1982;70:199–204. doi: 10.1016/0091-6749(82)90042-2. [DOI] [PubMed] [Google Scholar]
- 18.Turner SW, Devereux G. Early life influences on the development of allergy and asthma - how early is early? Clin Exp Allergy. 2007;37:163–165. doi: 10.1111/j.1365-2222.2007.02661.x. [DOI] [PubMed] [Google Scholar]
- 19.Schatz M, Clark S, Camargo CA., Jr Sex differences in the presentation and course of asthma hospitalizations. Chest. 2006;129(1):50–55. doi: 10.1378/chest.129.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson PM, Kjellman B. Asthma from childhood to adulthood: course and outcome of lung function. Respir Med. 2000;94(5):466–474. doi: 10.1053/rmed.1999.0763. [DOI] [PubMed] [Google Scholar]
- 21.deMarco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J, Douma WR, Ten Hacken NH, Oudkerk M, Postma DS. Physiology of the small airways: A gender difference? Respir Med. 2008;102(9):1264–1271. doi: 10.1016/j.rmed.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Antunes MA, Abreu SC, Silva AL, et al. Gender-specific lung remodeling and inflammation changes in experimental chronic allergic asthma. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.00333.2010. [DOI] [PubMed] [Google Scholar]
- 24.Melgert BN, Postma DS, Kuipers I, et al. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35(11):1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 25.Collins RA, Parsons F, Deverell M, Hollams EM, Holt PG, Sly PD. Risk factors for bronchial hyperresponsiveness in teenagers differ with sex and atopic status. J Allergy Clin Immunol. 2011;128(2):301–307. doi: 10.1016/j.jaci.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Goksor E, Gustafsson PM, Alm B, Amark M, Wennergren G. Reduced airway function in early adulthood among subjects with wheezing disorder before two years of age. Pediatr Pulmonol. 2008;43(4):396–403. doi: 10.1002/ppul.20798. [DOI] [PubMed] [Google Scholar]
- 27.Bousquet PJ, Hooper R, Kogevinas M, Jarvis D, Burney P. Number of allergens to be tested to assess allergenic sensitization in epidemiologic studies: results of the European Community Respiratory Health Survey I. Clin Exp Allergy. 2007;37(5):780–787. doi: 10.1111/j.1365-2222.2007.02714.x. [DOI] [PubMed] [Google Scholar]