Abstract
Background
Looping is a crucial phase during heart development when the initially straight heart tube is transformed into a shape that more closely resembles the mature heart. Although the genetic and biochemical pathways of cardiac looping are well-studied, the biophysical mechanisms that actually effect the looping process remain poorly understood. Using a combined experimental (chick embryo) and computational (finite element modeling) approach, we study the forces driving early s-looping when the primitive ventricle moves to its definitive position inferior to the common atrium.
Results
New results from our study indicate that the primitive heart has no intrinsic ability to form an s-loop and that extrinsic forces are necessary to effect early s-looping. They support previous studies that established an important role for cervical flexure in causing early cardiac s-looping. Our results also show that forces applied by the splanchnopleure cannot be ignored during early s-looping and shed light on the role of cardiac jelly. Using available experimental data and computer modeling, we successfully developed and tested a hypothesis for the force mechanisms driving s-loop formation.
Conclusions
Forces external to the primitive heart tube are necessary in the later stages of cardiac looping. Experimental and model results support our proposed hypothesis for forces driving early s-looping.
Keywords: heart development, s-looping, chick embryo, biomechanics
1 Introduction
The heart is the first major organ to develop in the vertebrate embryo. Its morphogenesis involves a remarkable sequence of complex shape changes that transform it from an initially straight tube to an asymmetric curved organ (Manner, 2000; Martinsen, 2005). While the genetic and biochemical pathways underlying cardiac morphogenesis have received considerable attention (Brand, 2003; Linask & VanAuker, 2007), the physical forces involved in this transformation remain poorly understood. The goal of this study is to investigate the role that forces internal and external to the heart play in a specific part of cardiac morphogenesis, early s-looping.
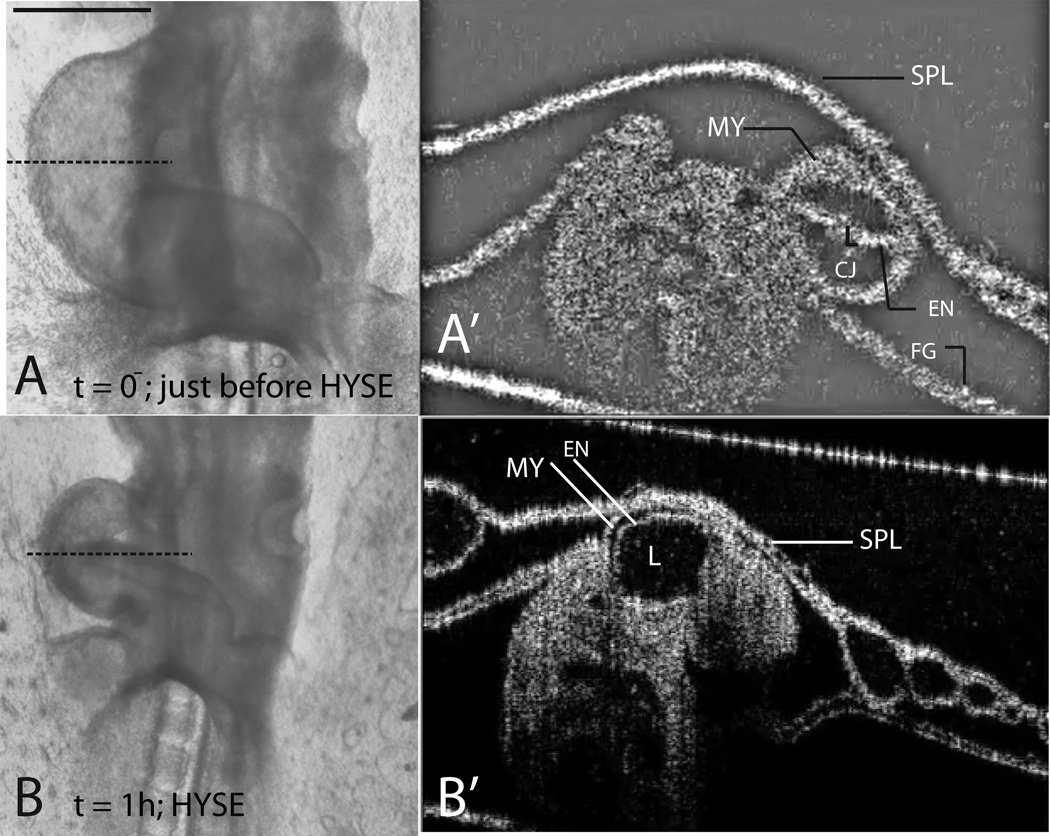
The primitive heart is a tubular structure composed of three distinct layers. An outer layer of myocardium (MY) is separated from an inner layer of endocardium (EN) by the cardiac extracellular matrix (Fig. 6A’). The cardiac ECM is a thick layer of matrix and provides structural support to the developing heart through turgor pressure (Nakamura & Manasek, 1981). It is often referred to as the “cardiac jelly” (CJ)(Davis, 1924). A membrane called the splanchnopleure (SPL) lies on the side of the heart and applies pressure on it (Fig. 6A’)1.
Figure 6.
Effect of ECM ablation on heart morphology. Left lateral views are shown in (A) and (B). (A) heart immediately before injection with hyaluronidase (t=0− is just before injection; time of injection is taken as t=0.). Note the similarity to a fully-filled balloon due to turgor pressure in the ECM. (A’) OCT section of the heart in (A) at the level indicated by the dotted line. The lumen (L) is mostly closed. Cardiac jelly (CJ, the ECM) is plentiful and occupies the space between the myocardium (MY) and the endocardium (EN). (B) Same heart after injection with hyaluronidase and 1 hr. culture. Note the flaccid appearance similar to a deflated balloon. (B’) OCT section of the heart in (B) at the level indicated by the dotted line. Note the near-total absence of cardiac jelly, which allows the lumen to expand dramatically. SPL=splanchnopleure, FG=foregut, HYSE=hyaluronidase-treatment. Scale bar: 400 μm – for (A) and (B) only
Once the primitive heart tube is established, the process of cardiac looping transforms it into a shape that more closely resembles the mature heart (Manner, 2000). This process is divided into two main sequential stages, c-looping and s-looping. During c-looping, the initially straight heart tube bends ventrally and simultaneously rotates to the right. This results in a curved tube where the original ventral side becomes the outer curvature. C-looping is thus a combination of two distinct events: ventral bending and dextral rotation. Prior studies indicate that the bending component is intrinsic to the heart, i.e., hearts cut out of the embryo and cultured in isolation can bend into c-shaped tubes (Butler, 1952; Manning & McLachlan, 1990). Recent evidence also indicates that the rotational component is extrinsic to the heart and that external forces, notably those due to the splanchnopleure (SPL), are necessary for rotation under normal conditions (Voronov et al., 2004; Ramasubramanian et al., 2008).
Early s-looping follows c-looping and is characterized by two distinct morphological changes (Manner, 2000; Manner 2009): (1) the migration of the primitive ventricle from its post c-loop position cranial to the common atrium2 to its definitive position caudal to it and (2) a shortening of the distance between the conotruncus (outflow tract) and the common atrium (Fig. 1). While c-looping has been studied extensively and its mechanics now becoming better understood, relatively little is known about the forces driving early s-looping. Most of the available results are due to Männer, who established a strong link between heart and head/neck morphogenesis (Manner et al., 1993, 1995a,b). We expand on these results by investigating what role, if any, some of the major players in the mechanics of c-looping have on s-looping.
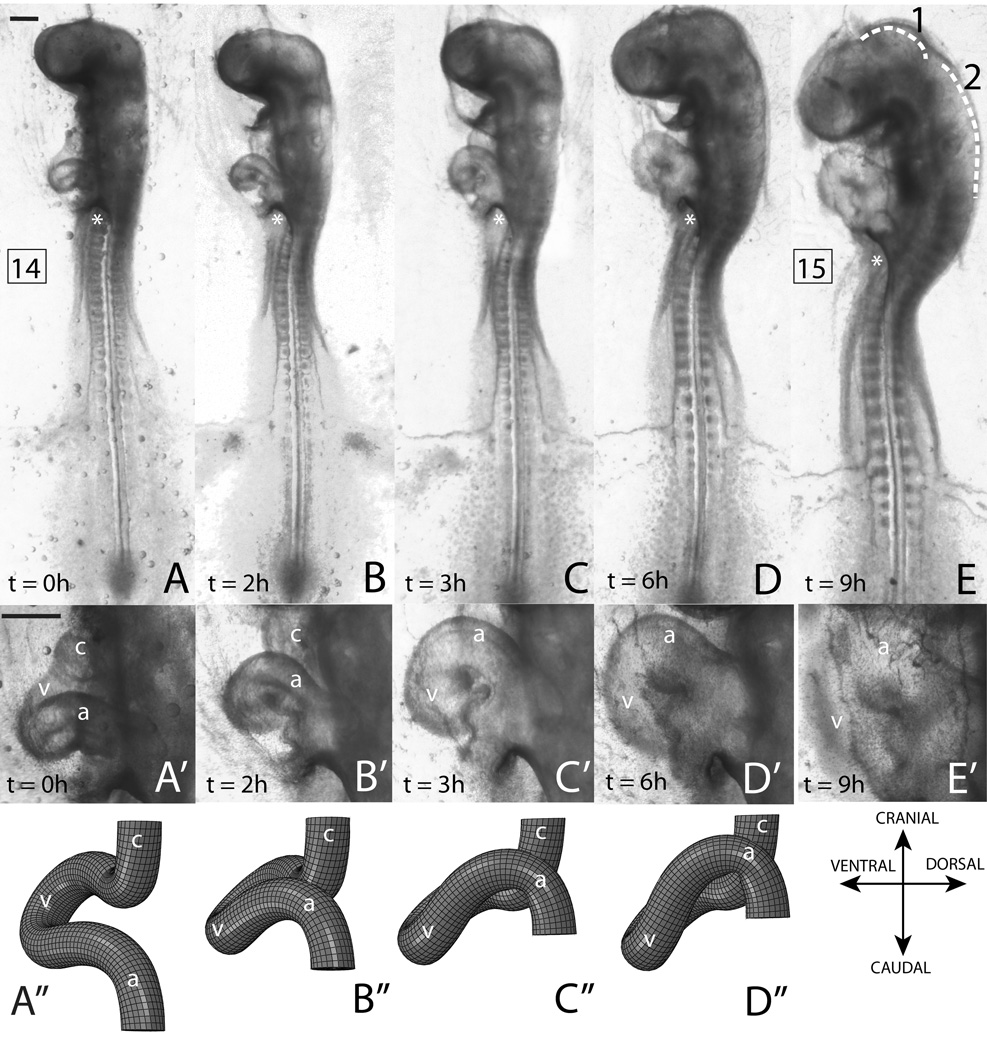
Figure 1.
Morphological changes during early s-looping in control conditions. Left lateral views are shown throughout. The same embryo is used in all images. (A–E) Whole embryo at time points indicated. Numbers in boxes are HH stages. Dotted lines in (E) denote cranial (1) and cervical (2) flexures. Rotation of embryo can be observed in the changing orientation of the anterior intestinal portal (marked by a ‘*’). Note the decrease in the head-to-heart distance and progressive crowding in the heart region. (A’–E’) Close-up of hearts at corresponding time points. (A’’–D’’) Finite element (FE) model results at time points corresponding to (A’–D’). Note normal early s-looping progression in both the model and the experiment: the ventricle moves caudal to the common atrium (D’,D’’) and the distance between the conotruncus and common atrium decreases (B’,B’’). Local embryo directions are indicated with the FE results. Please see Fig. 9 for model’s initial configuration, boundary conditions, and loading. v = primitive ventricle, a = common atrium, c = conotruncus. Scale bars: 400 μm
First, as mentioned earlier, previous studies demonstrate the importance of the SPL to the rotational component of c-looping under normal conditions. In this study we investigate the role of the SPL in early s-looping (Sec. 2.2). Second, the role of cardiac jelly (CJ) in cardiac c-looping has long been controversial (Baldwin & Solursh, 1989), although it is now accepted by most researchers that turgor pressure provided by the CJ is not a major player in c-looping (Taber, 2006; Linask et al., 2003). In this study we investigate the role of CJ in early s-looping (Sec. 2.3). Finally, it is known that isolated straight hearts can form a c-loop (Butler, 1952) and here we investigate if the isolated c-looped heart has any intrinsic potential to form an s-loop (Sec. 2.4).
Modeling has been used by several researchers to study cardiac looping. Manner (2004) used an idealized physical model of the tubular heart to study s-looping.Manasek et al. (1984) employed a model of an inflated balloon to study turgor pressure in the CJ. Here, like Ramasubramanian et al. (2006) and Nerurkar et al. (2006), we use finite element modeling with accurate material properties and experimentally-derived boundary conditions.
Like many previous studies, we use chick embryos in our experiments. The classic paper, Hamburger & Hamilton (1951), divides the 21-day incubation period into 46 stages. S-looping is a lengthy process starting at stage 12 (49 hrs) and ending at stage 18 (69 hrs). For simplicity, we focus on the early part of this process, from stage 12 to stage 15 (55 hrs). This is referred to as “early s-looping” in this paper.3
2 Results
The basic premise of loop formation is quite simple: when the two ends of a curved tube are brought together and crossed, a loop is formed (Bremer (1928), also in Romanoff (1960)). The early cardiac s-loop, however, is specific in its directionality and handedness (Manner, 2004). To get the correct morphology, the conotruncus and about two-thirds of the ventricular segment (collectively referred to as “the cranial portion of the heart tube” in this paper) have to be displaced backwards (i.e., away from the observer in a left lateral view) before the ends are brought together. Using computer modeling we show that a combination of head rotation and SPL pressure achieves this (Fig. 1, Fig. 9).
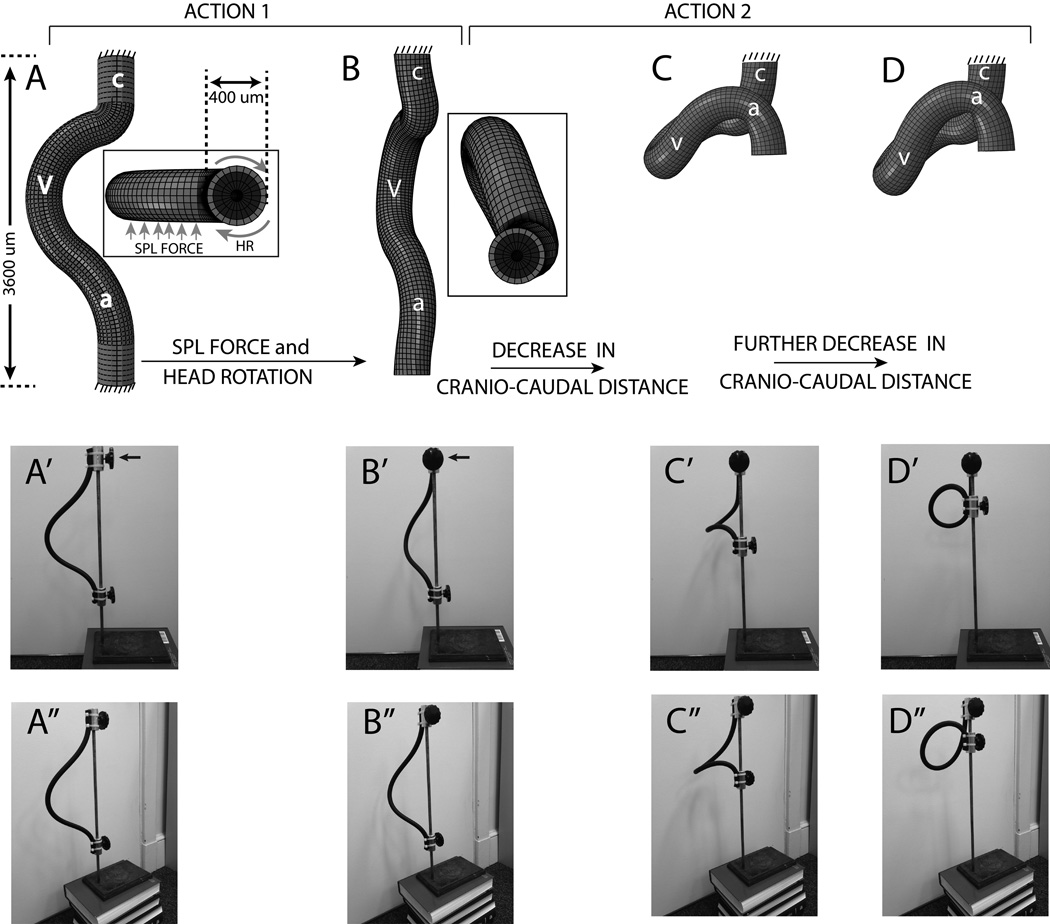
Figure 9.
Illustration of our hypothesis for early s-looping. (A–D) and (A’–D’) show left lateral views in the FE and a rubber tube model respectively. Insert in (A) and (B) show FE model top views (note non-homogenous cross section: light color = MY, dark color = CJ, a circular lumen is present). (A’’–D’’) show ventrolateral views of the rubber tube model. According to Action 1 of our hypothesis, a combination of SPL pressure and/or head rotation ensures that the cranial portion of the heart tube is displaced backwards (i.e., away from the observer in left lateral views) compared to the caudal portion (transitions from A to B, A’ to B’, and A’’ to B’’, forces are shown using arrows in the insert in A. Note that SPL pressure is not included in the rubber tube model. Rotation in the rubber tube model can be visualized by observing the changing position of the top knob, indicated by arrows in A’ and B’). At this point Action 2 takes over and a decrease in cranio-caudal distance caused by cervical flexure brings the ends of the heart tube together to form a loop (transition from B to C to D, B’ to C’ to D’, and B’’ to C’’ to D’’). Though the final shapes are somewhat different looking between the FE and rubber tube models (D, D’), the overall topology is exactly the same and is discussed in detail in Sec. 2.1. v = primitive ventricle, a = common atrium, c = conotruncus, HR = head rotation (used in insert in A). Please see text (Sec. 4.4) for a discussion of model boundary conditions and dimensions. Please also see model and orientations in Fig. 1.
Our finite element model uses a simplified geometry where the arterial and venous poles of the heart are idealized as straight tubes (Fig. 9A). A combination of SPL pressure, head rotation, and shortening of the cranio-caudal distance due to cervical flexure then produces the correct early s-loop morphology (Fig. 9A–D) as described below:
2.1 Heart Morphology during Early S-looping
This paper is primarily concerned with heart development between stages HH 12 and HH 15. The changes in cardiac morphology during this phase are termed “early s-looping” and consist primarily of the following (Fig. 1A’–E’)(Manner, 2000, 2009): (1) the decrease in distance between the conotruncus (outflow tract, sometimes referred to as the truncus arteriosus in literature) and the common atrium and (2) the gradual shift of the primitive ventricle caudal to the common atrium. The s-loop (HH stage 15 or 16, Fig. 1D’,E’) may be described as follows: starting from the inflow tracts which connect to the sinus venosus, the heart tube progresses in a cranial and ventral direction and at its peak is almost in direct contact with the distal end of the primitive head (Fig. 1E). Here it turns around and follows a caudad dorsal course before turning cranialward again with the outflow tract again ending up near the head region where it is connected to the great arteries (Fig. 1D’; see also rubber tube model in Fig. 9A’–D’). Two distinct features distinguish the early s-loop from the c-loop (Manner, 2000, 2009): (1) The common atrium now lies superior to the ventricle, (2) the distance between the outflow (conotruncus) and inflow tracts of the heart is decreased.
When interpreting Fig. 1 and other figures in this paper, it is important to keep rotation of the embryo in mind. Starting at about stage 12, chick embryos start rightward rotation of their bodies around the cranio-caudal body axis (Patten, 1951; Romanoff, 1960; Hamburger & Hamilton, 1951). As a consequence, the right side of the embryo faces the egg shell, while the left side faces the yolk sac. In the whole embryo culture used in this study, embryos are viewed from the side of the yolk sac endoderm. In this view, the s-shaped heart loop is usually seen in left lateral views. Rotation starts in the cephalic region of the embryo and gradually progresses caudad and is completed only at HH stage 20 (Hamburger & Hamilton, 1951; Patten, 1951). Hence, in the stages under consideration in this study, a frontal view through the microscope shows a left lateral view of the cranial portion of the embryo (head and heart) and a ventral view of the caudal portion (lower somites). However, rotation begins early and by stage 13 the portions of the embryo containing the heart have undergone significant torsion (Patten, 1951; Romanoff, 1960). Hence, all of the light microscope pictures in this study are labeled “left lateral views”, with the understanding that the lower somites are still in ventral view.
The heart at this stage is covered on its side by the splanchnopleure (SPL) and this membrane applies a pressure on it (Fig. 6A’). The SPL is not visible in the left lateral views shown in Fig. 1 although it is responsible for the fuzziness of the picture in Fig. 1E’.
All embryos cultured from stage 12 or 13 developed normally for the period considered in this study, i.e., up to stage 15 (n > 50). Finite element modeling using the hypothesis developed in Sec. 3 provides topologies that are consistent with experimental results (Fig. 1A’’—D’’). In both the control experiment and the model, the distance between the conotruncus and the common atrium is shortened while the primitive ventricle moves inferior to the common atrium. Note also the normal head flexure and rotation in Fig. 1A–E, which are prerequisites for normal s-looping (Manner et al., 1993). Moreover, the embryo continues to rotate as s-looping progresses; this can be observed by noting the changing orientation of the anterior intestinal portal (please refer to Fig. 1A–E).
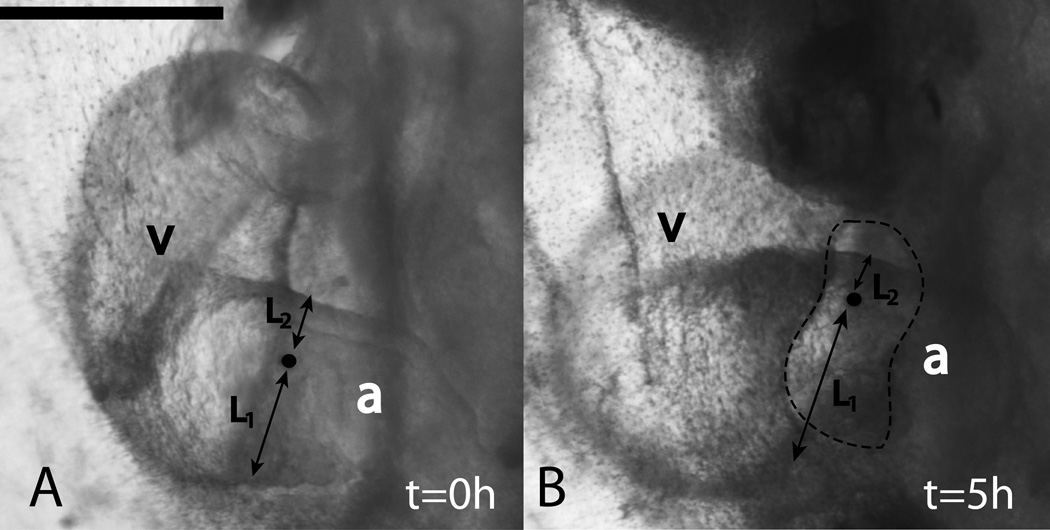
Torsion in the ventricular loop is a major component of c-looping (Stalsberg & DeHaan (1969);Manasek et al. (1972); de la Cruz (1998); also see Taber (2006), Manner (2000), and Manner (2009) for reviews). Torsion is also present in the later stages of looping; in particular, the AV canal, which connects the common atrium to the ventricular segment, undergoes considerable rotation during the s-looping stage (reviewed in Manner (2009)). We used India ink markers to confirm the presence of rotation in the inflow region (n=8). A label placed roughly in the middle of the atrial tube starts moving toward the periphery, indicating the presence of torsion about the tubular axis (Fig. 2). If L1 and L2 denote the perpendicular distance from the label to the caudal and cranial ends of the aortic tube respectively, then the ratio L2/L1 decreases as looping progresses (Fig. 2). This decrease was found to be statistically significant (p < 0.001). Please note: This atrial torsion should not be confused with the ninety degree rotation of the head which leads to the boundary condition for the top of the heart tube in the FE model (Sec. 4.4).
Figure 2.
Torsion in the common atrium during early cardiac s-looping. At t=0, a small hole was made in the SPL and an India ink label (black dot, highlighted) was placed in the common atrium (A). At t=5h, the label’s position relative to the borders of the atrial tube changes, i.e., the ratio of lengths L2/L1 becomes smaller (B). This indicates that there is torsion in this portion of the developing heart about the axis of the cylindrical common atrium. Dotted outline in (B) indicates the SPL hole that was made. v = primitive ventricle, a = common atrium. Scale bar: 400 μm
The subsequent sections describe the effects of various forces on the early s-looping heart. Sec. 2.2 discusses the effect of the force from the SPL, Sec. 2.3 discusses the effect of cardiac jelly pressure, and Sec. 2.4 discusses the effect of head forces on heart looping. Using these data, we develop a comprehensive hypothesis for the morphological changes during early s-looping (Sec. 3.1).
2.2 Effect of Removing the Splanchnopleure (SPL)
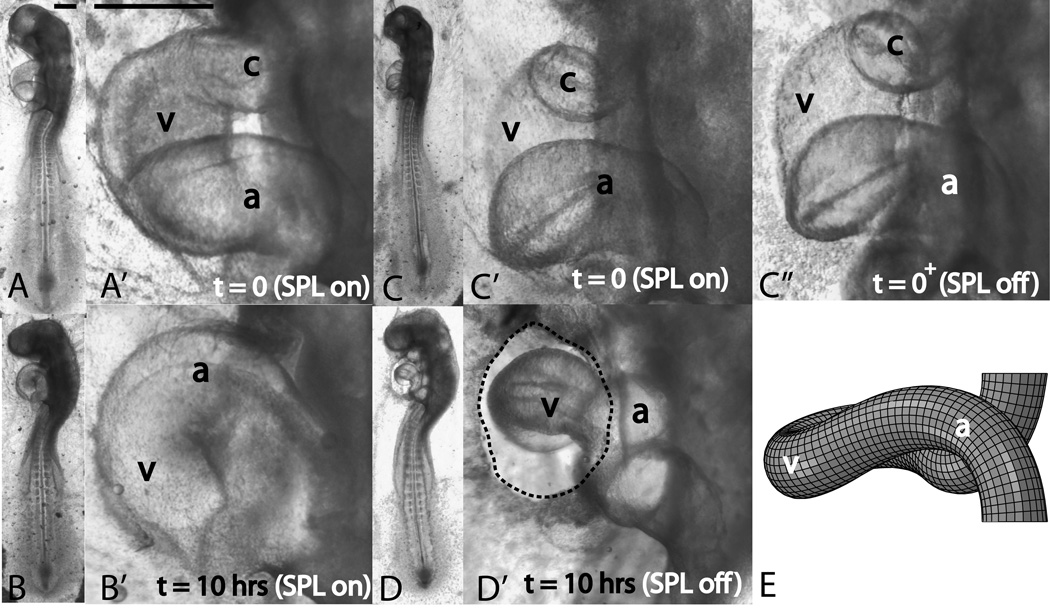
The conotruncus (outflow tract) and the sinus venosus (inflow tracts) act as anchor points and attach the s-looping heart tube to the body of the embryo. The conotruncus forms the attachment at the cranial end of the heart tube while the sinus venosus forms the attachment at its caudal end. During normal looping, the SPL applies a compressive force on the developing heart (Fig. 6A’). Immediately upon SPL removal (n=56), this compressive force is lost, and the heart, using the two hinge points, swings forward (i.e., toward the observer in transition from Fig. 3C’ to C’’). Short term culture (t=5hrs) with SPL removal revealed several interesting effects.
Figure 3.
Effect of SPL removal on early cardiac s-looping. Whole embryo (unprimed labels) and heart close-up (primed labels) pictures are shown. Left lateral views are shown throughout. Panels A and B (heart close-ups in A’ and B’) depict the control embryo. Panels C and D (heart close-ups in C’, C’’, and D’) depict the perturbed embryo. Control embryo and its heart develop normally when cultured for 10 hours (A,A’ denote t=0h and B,B’ denote t=10hrs). The ventricle moves inferior to the common atrium and cranial and cervical flexures increase. When the SPL is removed (C,C’=embryo and its heart prior to SPL removal, C’’=heart just after SPL removal), the heart immediately moves forward (i.e., toward the observer in the left lateral view shown). When SPL-lacking embryos are cultured for 10 hours (D,D’), the heart diameter decreases markedly and the caudal movement of the ventricle is severely disrupted. Note that this embryo with its improperly-looped heart still has normal cranial and cervical flexures. c=conotruncus, v=primitive ventricle, a=common atrium. Dotted line in D’ traces the cut SPL boundary. Partial SPL regrowth did occur compared to C’’, but the heart remained uncovered. (E) Model-predicted shape with no SPL pressure. Except for the absence of SPL pressure, all model parameters are the same as those in Fig. 1D’’. Initial configuration is shown in Fig. 9A. Note that the model matches the experiment and here also the caudal movement of the ventricle is severely disrupted (compare with Fig. 1D’’). Scale bars: 400 μm
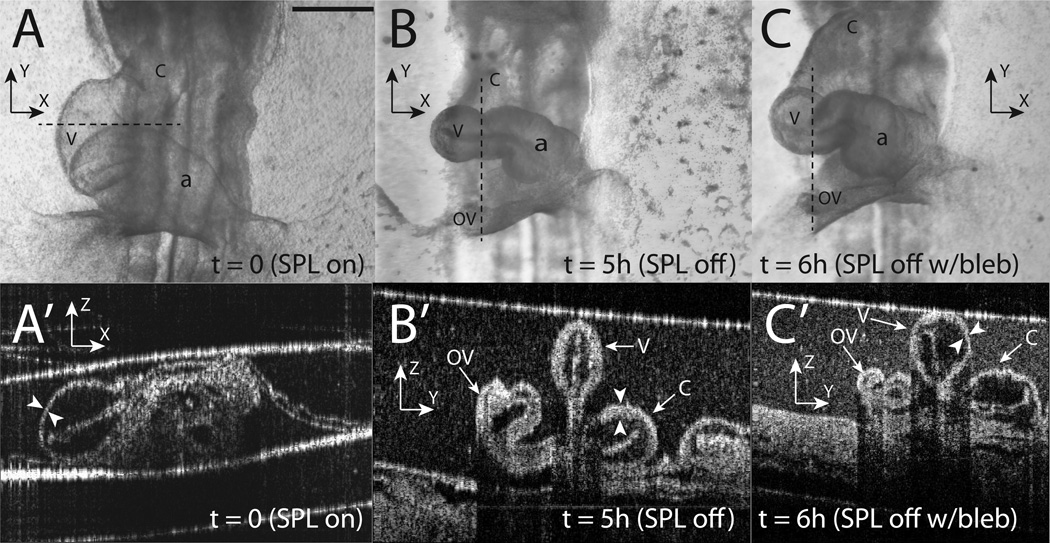
First, the diameter of the heart decreased significantly compared to controls (compare ventricular diameters in Fig. 4A and Fig. 4B). The heart diameter before (395 ± 49 μm)4 and after (273 ± 37 μm) SPL removal are statistically different (p << 0.001). To investigate if this decrease in diameter is due to cytoskeletal contraction, we incubated hearts that already experienced five hours of SPL-free culture in blebbistatin (n=14), a specific and potent inhibitor of non-muscle myosin (Straight et al., 2003). The heart diameters recovered in blebbistatin culture (303 ± 49 μm), but they did not reach the diameters observed in SPL-intact hearts (Fig. 4A–C). Heart function (i.e., pumping) in embryos treated with blebbistatin was less vigorous.
Figure 4.
Effect of blebbistatin on SPL-removed hearts. Same heart is shown in all pictures. (A–C) Light microscope pictures at time points indicated. Dotted line in each picture shows the approximate locations for corresponding OCT sections (A’–C’). We strove to obtain circular sections through the heart at all time points. This is the reason for horizontal sections in (A) and vertical sections in (B) and (C). At t = 0 with SPL intact, heart diameter is normal (A) and the MY wall is relatively thin (A’, marked by arrowheads). Following five hours of SPL-free culture, the heart diameter is smaller (B) and its wall is thicker (B’). At this point, the SPL-free embryo was incubated in a 30 μM solution of blebbistatin for 1 hour and then imaged (C,C’). Note that heart diameter recovers somewhat (C) and the heart wall becomes thinner again although not uniformly so around the circumference (C’). The bright white line along the top of (A’–C’) denotes the surface of the liquid growth media in the culture dish and should not be confused with the SPL. The liquid surface is coincident with the SPL in (A’). Coordinate axes are drawn in all pictures to help orient the reader (z axis points out of the page in A–C). When interpreting B’ and C’, it is helpful to use the liquid surface (bright white line at top) as a guide to orientation. v = primitive ventricle, a = common atrium, c = conotruncus, OV = omphalomesenteric vein. Scale bar: 400 μm (A–C)
Second, OCT imaging (n=10) revealed that following five hours of SPL-free culture, the myocardial wall became considerably thicker (Fig. 4A’,B’). To investigate if this thickening is due to cytoskeletal contraction, we again used blebbistatin. We found that, upon exposure to blebbistatin, the MY wall became thinner again although not uniformly so around the circumference (Fig. 4C’).
Finally, we observed that the heart rate increased relative to controls when the SPL is removed. Upon five hours of SPL-free culture, the average heart rate with SPL off was 25% higher compared to control embryos which were incubated for five hours with SPL intact. We observed that all of the short term effects – decreased diameter, increased wall thickness, and increased heart rate – are first observed at about 2 hours of SPL-free culture, although it takes about five hours for the full effects to manifest themselves.
Longer term (10 hrs or greater) culture revealed that SPL removal had a detrimental effect on normal s-looping morphogenesis. Out of the total 56 embryos, 46 (82%) had abnormal looping, i.e., the ventricle did not move inferior to the common atrium, resulting in an incomplete loop (Fig. 3D’). Only 10 out of 56 (18%) had normal looping, but even here, the heart tube was markedly thinner and the heartrate was increased. We note here that head morphogenesis, i.e., the development of cranial and cervical flexures and progression of head rotation, was normal in most cases of SPL-free culture (Fig. 3). Please compare flexures in the control embryo (Fig. 3B, Fig. 1E) with those in the SPL-removed embryo (Fig. 3D).
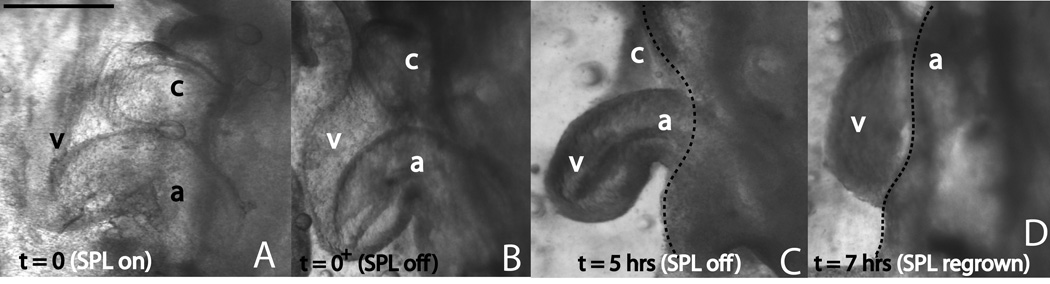
The model with SPL pressure removed (all other parameters are the same as the control model in Fig. 1) also shows a similar morphology to the experiment. Here also the caudal movement of the ventricle is disrupted (Fig. 3E; compare with control model in Fig. 1D’’). Partial re-growth of the SPL was sometimes observed. In these hearts, the development of the portion of the heart directly under the re-grown SPL appeared normal (Fig. 5).
Figure 5.
Effect of SPL regrowth on early cardiac s-looping. Same heart is shown in all four panels. (A) Stage 14 heart with SPL intact. (B) heart following SPL removal. (C) heart following five hours of SPL-free culture. (D) heart following seven hours of culture. Note that the portion of the heart under the re-grown SPL resembles the control heart in Fig. 3B’. Forward swing (i.e., more toward the observer in the left lateral view shown) of the ventricle did occur in the transition from (A) to (B), although this is not readily apparent in the images shown. Dotted lines in (C) and (D) denote the boundary of the re-grown SPL. c=conotruncus, v=primitive ventricle, a=common atrium. Scale bar: 400 μm
2.3 Effect of Dissolving the Cardiac Jelly
In all the embryos treated with the enzyme (hyaluronidase), the hearts were shrunken, indicating a loss of turgor pressure normally attributed to hydration of the cardiac jelly (CJ). None of the control embryos or those with sham injections showed any shrinkage. Fig. 6A,B shows the gross morphological effect of treatment with hyaluronidase. Note the shrunken and flaccid appearance of the heart, but note also that the overall c-shape is retained. Fig. 6A’,B’ shows the cross-sections at locations indicated by dotted lines in Fig. 6A,B. Prior to injection of the enzyme, the lumen is essentially closed and the transparent CJ fills the space between the myocardium (MY) and the endocardium (EN). The enzyme ablates most of the CJ resulting in an almost direct apposition of the MY and EN. As others have remarked (Manasek et al., 1984), the CJ-intact heart can be compared to an inflated balloon; CJ ablation makes the heart look like a deflated balloon (compare Fig. 6A and Fig. 6B).
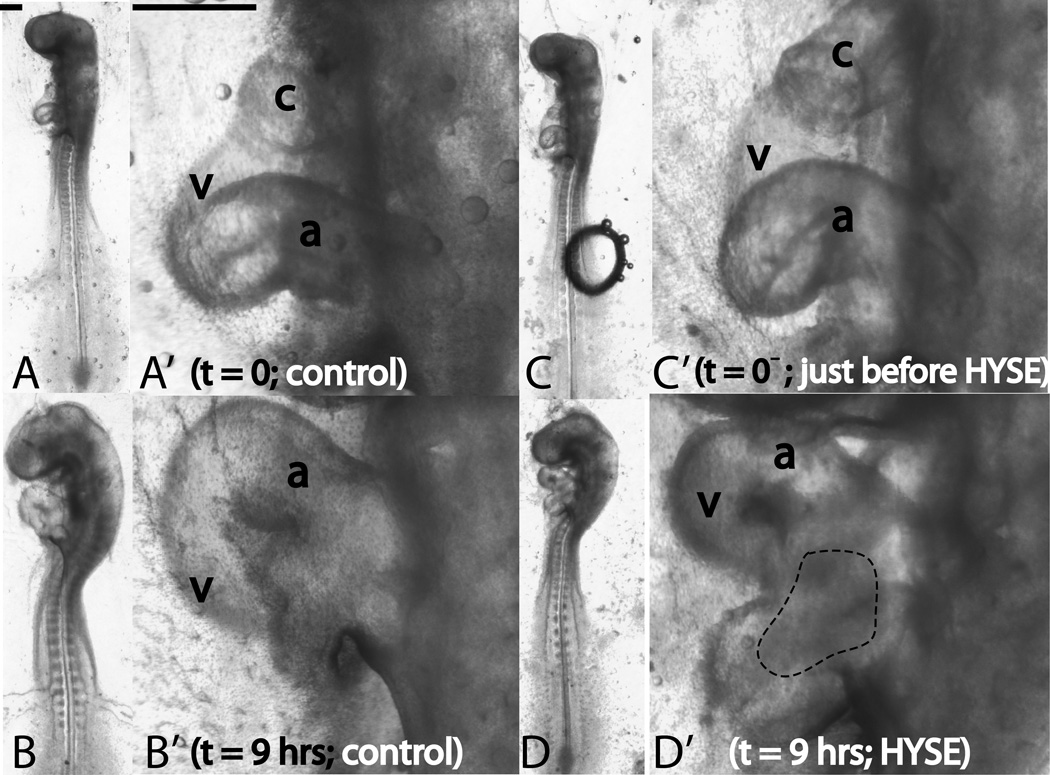
In all, we treated 52 embryos with the enzyme. Of these, looping was arrested in 13 embryos (25%, looping did not complete and the ventricle remained superior to the common atrium), delayed in 28 embryos (54%, at any given time point, the looping morphology lagged behind that in control hearts), and normal in 11 embryos (21%, looping morphology same as that in controls). A sample embryo from the delayed category is shown in Fig. 7. The enzyme-treated heart loop (Fig. 7D’) is not yet fully formed compared to control (Fig. 7B’). The enzyme has no effect on head flexures and both cranial and cervical flexures in the hyaluronidase-treated embryos are no different from those in the control embryo (Fig. 7B,D). Note, however, that the entire head region in the enzyme-treated embryo appears shrunken compared to control (Fig. 7B,D).
Figure 7.
Effect of ECM ablation on early cardiac s-looping. Whole embryo (unprimed labels) and heart close-up (primed labels) pictures are shown. Left lateral views are shown throughout. Panels A and B (heart close-ups in A’ and B’) depict the control embryo. Panels C and D (heart close-ups in C’ and D’) depict the perturbed embryo. Control embryo and its heart develop normally when cultured for 9 hours (A,A’ denote t=0h and B,B’ denote t=9hrs). The ventricle moves inferior to the common atrium and cranial and cervical flexures increase. When hearts are injected with hyaluronidase and cultured for the same time period, looping is delayed, but not disrupted (C,C’ denote t=0− and D,D’ denote t=9hrs; t=0− is just before injection; time of injection is taken as t=0.). Note that the s-loop in D’ is immature compared to the control loop in B’. The hyaluronidase-treated embryos have normal cranial and cervical flexures (compare head flexures in B and D. Note, however, the shrunken appearance of the head in D. This could be possibly due to matrix ablation in the head region caused by transport of hyaluronidase through the blood stream). c=conotruncus, v=primitive ventricle, a=common atrium, HYSE=hyaluronidase treatment. The dotted region in (D’) denotes the area where blood has pooled. Please ignore the air bubble in (C). It was removed soon after this picture was taken. Scale bars: 400 μm
The enzyme was injected in the ventricular region and it is no surprise that these regions appear more shrunken (Fig. 7D’). In about 50% of the treated embryos, we also observed a pooling of blood in the caudal region of the heart.
2.4 Effect of Culturing Hearts in Isolation
It is known that isolated hearts in culture can transform from a straight tube into a curved tube; hence the bending component of c-looping is intrinsic to the heart (Butler, 1952). To see if isolated hearts have any potency to form an s-loop, hearts were dissected from c-looped embryos and cultured in isolation, completely free of any extra-cardiac forces. The results are shown in Fig. 8. Since there was considerable variability in the observed shapes, two representative samples are shown.
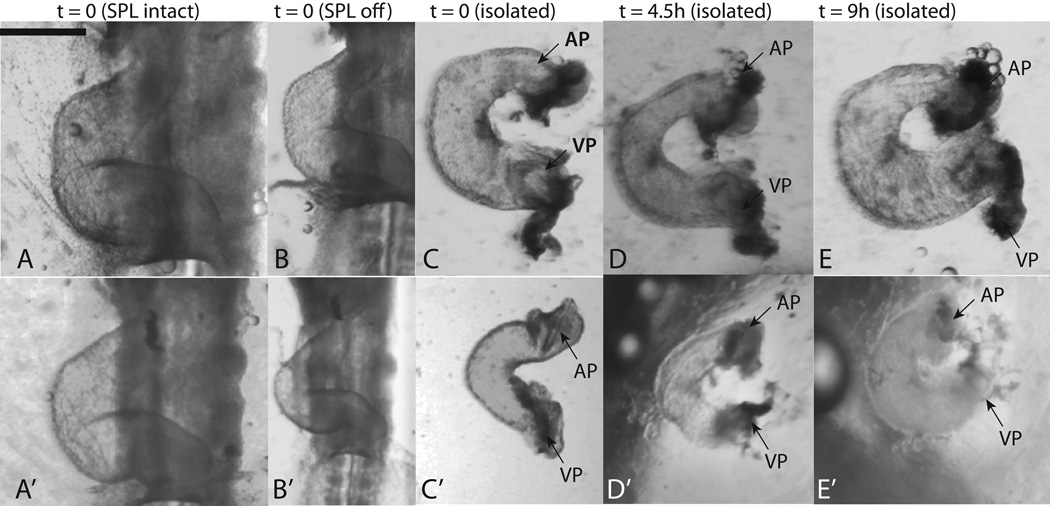
Figure 8.
Isolated culture of c-looped hearts. Since there was considerable variability in the observed shapes, two representative specimens are shown. All images in sequence A–E are from the same heart as are those in sequence A’–E’. (A,A’ ) hearts in embryo with SPL still intact; (B,B’) hearts immediately after removal of SPL; (C,C’) hearts immediately after isolation; (D,D’) isolated hearts after 4.5 hours of culture; (E,E’) isolated hearts after 9 hours of culture. In (C–E) and (C’–E’), we see a shortening of the distance between the arterial (labeled ‘AP’, original cranial end) and venous poles (labeled ‘VP’, original caudal end) of the heart. During imaging of isolated hearts, the presence of out-of-plane deformations was noted, indicating that the topologies shown are not entirely planar. Scale bar: 400 μm
The distance between the arterial and venous poles of the heart nearly always decreased during 9 hours of culture (Fig. 8C–E and C’–E’). In hearts that were harvested from stage 12 and 13 embryos, this distance decreased in 59 out of 74 cases. In 13 out of the remaining 15 cases, the distance was already quite small to begin with and there was not much scope for further decrease. The poles separated and the distance increased in two out of 74 cases.
In many of the images of the isolated heart, we could not take precise quantitative distance measurements because tissues warped a great deal locally and we could not determine the exact location of the arterial and venous ends of the heart tube. We therefore selected a subset of 20 embryos where the ends could be located (relatively) accurately. In these embryos the distance between the arterial and venous poles of the isolated heart was 217±78 μm at t=0 and 80 ± 67 μm at t=9h. The decrease in distance was statistically significant (p < 0.001).
We also noted that the initial shape of isolated hearts was not planar and out-of-plane deformations were present in the conotruncus region. However, the percentage of hearts without-of-plane deformations decreased with time spent in culture (data not shown). Although hearts cultured in isolation displayed one characteristic of a mature heart loop, the decrease in distance between arterial and venous poles of the heart, the final shape was quite different from a normal s-loop. The three dimensional topology described in Sec. 2.1 was not realized and although out-of-plane deformations were initially noted in the conotruncus region, the final loop in isolated culture lay nearly in a single plane (Fig. 8). In the whole embryo culture, the primitive ventricle shifts caudad because of an out-of-plane deformation in the ventricular loop (Fig. 1, Fig. 9). Heart morphogenesis in isolated culture, having no such out-of-plane deformation in the ventricular loop, does not lead to a similar ventricular repositioning (compare hearts in Fig. 8E,E’ with the s-looped controls in Fig. 1C’, Fig. 3B’ , and Fig. 7B’).
3 Discussion
C-looping represents the first easily visible manifestation of morphological L-R asymmetries in the originally bilaterally symmetric embryo. The biophysical mechanisms driving it are now becoming better understood (please see Taber (2006) for a review) as are the molecular mechanisms (please see Linask & VanAuker (2007), Brand (2003), and Mercola & Levin (2001) for reviews). A three dimensional map of cell-proliferation and cell-growth is also available for the c-looping stages (Soufan et al., 2006).
By contrast, little is known about the forces involved in s-looping and the process has attracted scant attention (Manner, 2000). Because s-looping in the chick embryo covers a roughly 20-hour time span from stage 12 to stage 18, we restricted our attention to a 6-hour span from stage 12 to stage 15. We refer to this period as “early s-looping”. During early s-looping, the ventricle moves inferior to the common atrium and distance between the conotruncus and the common atrium decreases (Sec. 2.1, Fig. 1).
Most of our knowledge on the biophysical mechanisms leading to s-looping come from a series of papers by Jörg Männer (Manner et al., 1993, 1995a,b; Manner, 2000, 2004). These prior studies posit a strong causal role for external forces, particularly those due to cervical flexure, in s-looping.
Our results support and significantly extend Männer’s results (please see Sec. 3.2–Sec. 3.5). In this study, we considered some of the major players in the mechanics of c-looping and studied their effect on early s-looping. The following is a summary of our major new findings:
First, it is known that the SPL plays an important role in c-looping (Voronov et al., 2004; Nerurkar et al., 2006; Ramasubramanian et al., 2008); our experiments show that its role continues into early s-looping (Sec. 2.2). Second, while it is accepted that cardiac jelly inflation is not necessary for c-looping, our experiments show that the matter is not so black and white for early s-looping (Sec. 2.3). Finally, while isolated straight hearts have the capability to bend on their own during c-looping, we found that isolated c-looped hearts do not have the capability to form an s-loop (Sec. 2.4). In summary, we tested the role of intrinsic and extrinsic forces in early cardiac s-looping and our results show that the latter are much more important than the former.
As far as we know, we are the first group to develop a three-dimensional computer model for early cardiac s-looping that includes both the myocardium and the cardiac jelly. The model uses stage 12 topologies and measured nonlinear material properties for the myocardium and the cardiac jelly. We used the model to propose and test the following hypothesis for the mechanics of early cardiac s-looping.
3.1 A Hypothesis for Early S-looping
Action 1: Following c-looping, continued pressure from the SPL, possibly in combination with head rotation, ensures that the cranial portion of the c-looped heart tube is laterally displaced (i.e., away from the observer in a left lateral view) compared to the caudal portion (Fig. 9: Transition from A to B, A’ to B’, A’’ to B’’).
Action 2: The formation and rapid deepening of cervical flexure compresses the inner surface of the neck region where the heart is located. This results in a shortening of the distance between the conotruncus and the common atrium (Fig. 9: Transition from B to C, B’ to C’, B’’ to C’’).
Outcome: Continued application of these two actions results in a 3D loop where the ventricle moves inferior to the common atrium (Fig. 9D, D’, D’’).
Support for Action 1 comes from our results: when the SPL is removed, the cranial portion of the heart swings forward in an arc (i.e., comes more toward the observer in a left lateral view, transition from Fig. 3C’ to Fig. 3C’’); this indicates that the SPL is pushing on the heart. Also, rotation of the embryo starts at the head and gradually progresses caudad (Patten, 1951). This can also ensure that the cranial portion of the heart tube is pushed back (i.e., away from the observer in a left lateral view) compared to its caudal portion. Support for Action 2 comes from several previous studies which have shown that the heart tube becomes compressed during s-looping (Patten, 1951; Romanoff, 1960; Manner, 2000). Our results (Sec. 3.2) and those of Manner et al. (1993) indicate strongly that this is the result of cervical flexure. It is noted that Actions 1 and 2 are not sequential events and occur simultaneously.
Manner (2004) contains a similar idea (the SPL is not included). In our study, we tested our hypothesis by computer modeling. The model with forces specified as per our hypothesis produces topologies that are similar to those in experiment (Fig. 1). Our model also has good predictive capabilities: when the simulation is run without the SPL pressure, a topology that is remarkably similar to experiment (Fig. 3D’, E) is obtained. Our hypothesis relies heavily on external forces applied by the SPL and head morphogenesis. As expected, the removal of external forces does not produce the complex 3D structure observed in whole embryos (Sec. 2.4).
Natural biological variability is an important issue in development which has not received the attention it deserves (please see von Dassow & Davidson (2011) and references contained therein). The stark natural morphological differences even in embryos at the same stage is an important consideration when studying cardiac looping. For instance, embryos in Fig. 3A and Fig. 3C both correspond to HH stage 13 and both have SPL intact (at t=0); however, their hearts look quite different (compare Fig. 3A’ and Fig. 3C’; see also heart in Fig. 5A for yet another variation in shape). Accordingly, we have refrained from fine-tuning the model parameters to match any particular image; rather, the primary aim of the model presented in this paper is to capture the general topology of the loop as described in Sec. 2.1.
A note on the rubber tube model
The rubber tube model (Fig. 9A’–D’ and A’’–D’’) is intended as a convenient visual aid to understand the complex morphogenetic process of early s-looping; here, the ability of the tube to form an s-loop without the equivalent of an SPL pressure is not significant. A slender rubber tube whose ends are brought together will always form a loop. The rubber tube model does not have realistic cross-sectional geometry (the tube is uniformly solid) or material properties. The FE model includes a cross section that includes the myocardium, cardiac jelly, and a lumen (insert in Fig. 9A). It also uses realistic stage 12 material properties. The FE model is therefore a more realistic simulation and it correctly predicts the altered morphology when the SPL is removed (Fig. 3D’,E; see also Sec. 3.3).
Similarities to Gut Looping
The formation of a three-dimensional loop is not unique to cardiac development. During days 3–6, the avian gut also undergoes s-looping with the final topology having a number of similarities to the s-looped heart (Romanoff, 1960; Davis et al., 2008).
3.2 Remarks on the Link between Heart and Head Morphogenesis
Two of the most visible developmental changes in vertebrate embryos are the flexure and rotation of the embryonic axis. These morphological changes transform the initially straight embryonic axis into a curved axis leading to the familiar “fetal position”. The curvature of the embryonic axis is seen in embryos of reptiles, birds, and mammals possibly due to the space limitations inside an egg or uterine cavity; it is not seen in fish and amphibian embryos which develop in open water without any space constraints (Patten, 1951). Since reptilian, avian, and mammalian embryos normally develop with the ventral side facing the yolk, flexing is prevented by the presence of the yolk. Hence, the embryo must first rotate so that its lateral side now faces the yolk so that flexing, which brings the anterior and posterior ends together, can now commence (Patten, 1951).
In the chick embryo, flexion and rotation occur simultaneously, starting at about stage 11. By stage 20, the embryo is fully rotated on its side. By about stage 13 rotation has progressed to the regions containing the heart and two prominent bends, the cranial flexure and the cervical flexure can now be seen (Fig. 1). The location of cranial flexure is remote from the looping heart and its influence in early s-looping has been ruled out (Manner et al., 1995b).
Cervical flexure, on the other hand, occurs in close proximity to the heart and leads to a prominent bend in the cervical region (Fig. 1A–E). Recall that during early s-looping, the two ends of the heart, the conotruncus and the common atrium, come into close proximity to each other (Fig. 1A’–E’). It is clear from looking at the morphology that either of these processes can lead to the other – shortening of the distance between the ends of the heart can pull the cervical portion into a curved configuration; or, cervical flexure can lead to early s-looping in the heart.Flynn et al. (1991) and Waddington (1937) support the former idea; these researchers severed the conotruncus and noted that these embryos, where the heart can no longer exert any forces in the neck region, lacked cervical flexure.
The converse idea, i.e., cervical flexure leads to early s-looping, is not new and was proposed by early researchers such as Patten (1951) and Romanoff (1960). A classic experiment performed by Männer and his colleagues lends strong support to this theory: they inserted a human hair into the neural tube of a stage-12 (completed c-looping) embryo; the hair, being stiffer than the developing cephalic region, prevents cervical flexure (Manner et al., 1993). It was noted that preventing cervical flexure in this manner led to an arrest of cardiac morphogenesis in the c-looped state. Separately Männer also showed that the lack of cervical flexure in Flynn et al. (1991) is due to oxygen deprivation and that heart-deprived embryos can still form normal flexures when cultured in an oxygen-rich environment (Manner et al., 1995a).
New experiments performed in this study support the results of Manner et al. (1993) . Two of our perturbations, SPL removal and ablation of the cardiac jelly, led to irregularities in the looping process, but did not have any effect on cervical flexure or head rotation (Fig. 3 and Fig. 7). This implies that cardiac morphogenesis is not a necessary cause for cervical flexure. Also, our experiments with isolated heart cultures indicate that external forces are necessary for s-looping (Fig. 8).
Material property measurements also support the hypothesis that cervical flexure causes s-looping and not vice-versa. Results from the Taber group indicate that the developing neural tissue is about 10 times stiffer compared to developing cardiac tissue at stage 12 (Zamir & Taber, 2004; Xu et al., 2010). It is of course easier for a stiffer tissue (i.e., developing brain) to deform a softer tissue (i.e., developing heart) than vice-versa.
There is thus strong evidence that the formation of the cervical flexure is crucial for early cardiac development. As pointed to in Manner et al. (1993), this could be the reason why some congenital malformations of the neck are accompanied by congenital cardiac malformations. The actual mechanism of cervical flexure formation is not currently known although differential cell proliferation in the axial structures (neural tube, notochord, etc.) and cell-shape changes caused by actin filaments have been proposed as possible mechanisms (Goodrum & Jacobson, 1981; Schoenwolf & Smith, 1990).
Although simulations strongly suggest a role for head rotation (our results – please see Sec. 3.1 and Fig. 9; the idea is also mentioned in Manner (2004)), so far no study has done experiments on the effect of head rotation on cardiac s-looping. Our hypothesis does not say anything about the relative importance of head rotation and SPL pressure during Action 1. This warrants further study.
3.3 Remarks on SPL removal
The splanchnopleure (SPL) is a membrane that lies on the side of the pre-looped heart (Fig. 6A’) and applies pressure on it. Experiments performed by other researchers indicate a strong role for the SPL in c-looping (Voronov et al., 2004). New results from this study indicate a similarly important role for it in early s-looping. We first consider the short-term effects of SPL removal:
Immediately following SPL removal, the heart swings forward (i.e., toward the observer in a left lateral view). This effect has been observed in a previous study (Filas et al., 2007) and suggests that the SPL applies a compressive force, removing which, the heart is free to swing in an arc (Fig. 3C’,C’’). The heart tube also becomes markedly thinner (Fig. 3B’,D’ and Fig. 4A,B) and the heart wall becomes considerably thicker (Fig. 4A’,B’). These effects are first noticed at t=2h following SPL removal and become established at t=5h. There is some recovery in both effects when SPL-lacking hearts are incubated in the presence of blebbistatin, i.e., the heart diameter increases (Fig. 4B,C) and the heart wall becomes thinner again (Fig. 4B’,C’). These results indicate potential activation of cytoskeletal contraction when the SPL is removed. Since circumferential contraction results in a smaller-diameter tube, there must be some thickening in the radial direction due to material incompressibility.
The short-term effects of SPL removal on s-looping have similarities to those observed during SPL removal on c-looping.Nerurkar et al. (2006) and Ramasubramanian et al. (2008) also report a decrease in heart diameter following SPL removal during c-looping. And as mentioned earlier, Filas et al. (2007) also report a forward swing (i.e, toward the observer in a left lateral view) of the heart tube immediately following SPL removal in the c-looping stages.
The long term (10 hours of SPL-free culture) effects of SPL removal are also significant: the ventricle still has the potency to move caudad, but not to the extent seen in control hearts (Fig. 3B’,D’). Our hypothesis for early s-looping posits that SPL pressure together with head rotation is necessary to displace the cranial portion of the heart tube (Sec. 3.1, Fig. 9). This sets the stage for cervical flexure to complete the process. Removal of the SPL pressure possibly results in an inadequately displaced cranial portion, which when acted on by cervical flexure, results in an immature heart loop. The model with SPL pressure removed, but head rotation retained, results in topologies that are remarkably similar to experiment (Fig. 3D’,E). The importance of the SPL to early s-looping is also demonstrated by experiments in which the SPL manages to regrow during culture (Fig. 5). These embryos tended to loop normally.
Looping could also be inhibited because of the observed reduction in heart size: it is possible that the crowding effect of cervical flexure formation on the embryonic heart tube may be less strong in an abnormally small heart loop within a normal-sized pericardial cavity as compared to a normal-sized heart loop within a normal-sized pericardial cavity.
Long-term effects of SPL removal are quite different between c and s-looping. Normal c-looping is only delayed following SPL removal. As reported in Nerurkar et al. (2006), a secondary mechanism involving asymmetric cytoskeletal contraction manifests itself about five hours following the perturbation and normal c-looping is restored 10 hours following the perturbation. In contrast we found that, following SPL removal, normal s-looping was not restored in longer-term (> 10hrs) cultures.
While prior studies do not mention anything about the effects of SPL removal on heart rate during c-looping, our results indicate a jump in heart rate following the same perturbation. It is possible that the more rapid pumping is a compensatory mechanism since the heart tube is now thinner and more frequent beats are needed to achieve the same volume of blood flow. Another possible reason for the increase in heartrate is the change in fluid pressure in the coelomic cavity brought about by SPL removal. Experiments with elasmobranch fish (sharks and rays) and sturgeon show that altering the fluid pressure in the various body cavities produces significant alterations in cardiac function (Shabetai et al., 1985; Gregory et al., 2004).
We note here that head flexure and rotation are not impaired by SPL removal even though heart looping is. This lends support to the study of Manner et al. (1993) which claims that cardiac s-looping is caused by head flexure and not vice-versa as claimed by other studies (Flynn et al., 1991; Waddington, 1937).
3.4 Remarks on Cardiac Jelly Inflation
The cardiac extracellular matrix, the cardiac jelly (CJ), is a significant component of the looping heart, occupying a large volume between the myocardium (MY) and the endocardium (EN) (Fig. 6). The CJ is not a passive substrate and plays an active role in many aspects of cardiac morphogenesis (please see Bowers & Baudino (2010) for a review). Recent research indicates that the CJ is also implicated in cell convection leading to endocardial morphogenesis (Aleksandrova et al., 2012). Despite its name, the CJ is structurally sound with a modulus that is roughly 25% of that of the MY (Zamir & Taber, 2004). Furthermore, when the MY is removed, the isolated CJ can hold its shape (Nakamura & Manasek, 1978).
There was considerable debate regarding the role of CJ in c-looping. Taking inspiration from tubular worms whose shape is determined by internal turgor pressure (Wainwright et al., 1982), Manasek proposed that the c-loop in the tubular heart is also formed by turgor pressure due to hydration-driven expansion of the CJ (Manasek et al., 1984). This hypothesis was contradicted by experiments of Baldwin & Solursh (1989), who found that hearts whose CJ was ablated by the enzyme hyaluronidase looped normally. Later experiments by Linask et al. (2003) also indicated that CJ inflation was not necessary for c-looping. This view is now widely accepted (Taber, 2006).
Our results from s-looping show many similarities to these previous studies on c-looping. Like the results of Manasek et al. (1984) and Baldwin & Solursh (1989), our hearts also became flaccid when treated with hyaluronidase. These previous studies report that enzyme treatment resulted in a greatly expanded lumen with the MY and EN almost directly apposed. OCT imaging confirmed the same finding in our results (Fig. 6). Our OCT data also show an unevenly thick CJ layer with the original (i.e., pre c-looped) left and right sides having the greatest thickness (Fig. 6A’). These results are in agreement with previous studies (Manner et al., 2008, 2010).
New results from our study shed light on the role of CJ inflation in s-looping morphogenesis; we found that looping was negatively affected (delayed or inhibited) in 79% of the cases (Sec. 2.3). S-looping was normal in the remaining 21% of the cases. It is curious that CJ removal has no influence on c-looping (Baldwin & Solursh, 1989; Linask et al., 2003), but delays development in the morphogenetic phase immediately following it (our results, Section 2.3). We offer three explanations:
First, similar to the effect of SPL removal, the decrease in heart size could make looping more difficult to accomplish by the application of head forces, i.e., the crowding effect of cervical flexure formation is less strong in an abnormally small heart loop within a normal-sized pericardial cavity compared to a normal-sized heart loop within a normal-sized pericardial cavity.
Second, looping could be affected because blood flow is interrupted. Assuming circular cross sections for the MY and EN and a 20% shortening of the MY during systole, Barry showed that the tubular heart at the stages considered in this study cannot effectively pump blood unless a layer of CJ is present between the MY and the EN (Barry, 1948). By culturing rat embryos in the presence of hyaluronidase,Baldwin et al. (1994) showed that ablation of cardiac jelly during looping leads to “substantial hemodynamic alterations” compared to control embryos. A more recent OCT study found that the CJ layer is not perfectly circular in cross section. The latter study also suggests that the uneven thickness of the CJ leads to an elliptical endocardial tube during diastole with a higher pumping efficiency (Manner et al., 2008).
There is thus considerable evidence that the CJ layer is needed for heart function. Treatment with hyaluronidase results in a lumen that is open all the time (Fig. 6), disrupting normal blood flow and possibly leading to the pooling of blood visible in 50% of our enzyme-treated hearts (Fig. 7D’). Our results also show that the hyaluronidase-treated hearts have MY and EN layers that are almost directly apposed. The lumen is no longer closed shut during systole and the heart ceases to be an effective pump. Although blood flow and heart beat are not necessary for c-looping (Manasek & Monroe, 1972), their effects on s-looping are not known. It is therefore possible that morphogenesis is interrupted because normal blood flow is interrupted.
Third, enzyme-treated hearts are flaccid and we found it somewhat challenging to determine if they had looped or not. By our criteria, a heart is not considered normal unless the loop had the same morphology as control at the same time point. It is possible that Baldwin & Solursh (1989) and Linask et al. (2003) found similar distortions due to flaccidity, but nonetheless classified looping as normal based purely on whether the heart acquired a c-loop at the end of culturing.
Effect of Hyaluronidase on Head Morphogenesis
Previous studies have found that the developing head region has a high concentration of glycosaminoglycans (Toole, 1976). Hence, it is possible that shrinkage in the head region, which we observed in many of our enzyme-treated embryos (Fig. 7), is due to transport of the enzyme to the head region. A previous study also found that rat embryos cultured in hyaluronidase showed a slight reduction in neuroepithelial cell number (Morriss-Kay et al., 1986).
3.5 Remarks on Isolated Heart Cultures
Isolated straight stage 10 hearts have the potency to bend and form a c-shape (Latacha et al., 2005; Butler, 1952; Manning & McLachlan, 1990; Flynn et al., 1991). New results from the current study show that isolated c-looped hearts do not have the potency to form an s-loop (Sec. 2.4).
However, when c-looped hearts are cultured in isolation, they bend into a shape where the two ends of the heart come together (Sec. 2.4, Fig. 8). These findings are consistent with the results of Flynn et al. (1991) who did a similar experiment. However, our conclusions are quite different. The earlier study posits that bending of a c-shaped heart tube into an o-shaped one is the cause for cervical flexure. Our conclusions are more in agreement with those of Manner et al. (1993) and are the exact opposite, i.e., cervical flexure is responsible for heart looping (Sec. 3.2).
Since forces due to cervical flexure are not found in isolated heart cultures, the question arises as to what causes these hearts to continue bending.Latacha et al. (2005) showed that cell shape changes driven by actin polymerization cause the straight heart tube to bend into a c-shaped one. In the absence of external constraints, it is possible that these forces cause continued bending into an o-shaped tube. Indeed some of the straight hearts in Latacha et al. (2005) were cultured for 24 hours (c-looping only takes 12 hours to complete) and these were found to bend into an o-shaped tube with the arterial and venous poles almost in contact.
We found that many of our isolated hearts had out-of-plane deformations, an effect that diminished over time. This suggests a slow relieving of stresses that were present when the heart was still attached to the embryo. Ramasubramanian & Taber (2008) suggests some feedback mechanisms by which this can be achieved.
3.6 Limitations
While the model-predicted topologies match experiment, there is some difference between their respective shapes (Fig. 1). There are several possible reasons: First, our hypothesis focuses on the role of external forces and internal loads are not included. Previous studies have shown that cell-shape changes brought out by actin polymerization drive the bending component of c-looping (Latacha et. al., 2005) while cytoskeletal contraction plays an important role in the restoration of the rotational component of c-looping when the SPL is removed (Nerurkar et. al., 2006). The role of these forces in s-looping is currently under investigation (Chu-LaGraff et. al., unpublished) and they are not included in our hypothesis. CJ inflation is also not included in the model. Second, the model dimensions are chosen to demonstrate s-loop formation in a tubular heart of fixed length and diameter; no attempt has been made to accurately capture the changing length and diameter (Fig. 1A’–E’) of the heart tube as it undergoes s-looping. Finally, there are stark differences in the shape of the normal s-loop (Sec. 3.1) due to natural biological variability, and the model does not attempt to capture the heart shape of any particular embryo. Rather, it only captures the general topology that is present in all control hearts (as described in Sec. 2.1).
The earliest hypothesis for c-looping was put forth in 1922 and even now there is debate regarding the forces driving it. While we have demonstrated a strong role for external forces in early s-looping, we realize that our hypothesis is but a starting point. It will need to be modified when further data become available.
4 Experimental Procedures
4.1 Embryo Culture, SPL Removal, Labeling, Heart Rate Measurement
Fertile white leghorn chicken eggs were incubated in a forced-draft incubator up to stage 12. Embryos were then harvested from the eggs and staged according to the system of Hamburger & Hamilton (1951). Embryos were cultured under a thin layer of growth media in an oxygen-rich atmosphere using the procedure in Voronov & Taber (2002). One liter of growth media contains 890 ml of Dulbecco’s Modified Eagle’s Medium (DMEM), 100 ml of chick serum, and 10 ml of antibiotics (all from Sigma-Aldrich).
Submerging the embryo in this fashion eliminates the deleterious effects of surface tension. Although Voronov & Taber (2002) mentions that this culture technique can be used up to stage 18, the majority of prior studies done using this technique culture embryos only up to stage 12. For this reason, a number of control embryos were tested and we made sure that embryos developed normally when cultured from stage 12 to stage 15. An example of proper control embryo development can be seen in Fig. 1.
For those experiments involving SPL removal, the membrane was removed using microdissection. We then incubated SPL-lacking hearts for several hours to observe effects on heart development. To investigate if cytoskeletal contraction plays a role following SPL removal, we incubated some SPL-lacking hearts (already incubated for five hours with SPL removed) in a 30 μM solution of blebbistatin (Sigma Aldrich) for one hour. Imaging using Optical Coherence Tomography (OCT) was used to observe the changes in the cross-section at various time points.
To measure the heart rate in beats per minute (BPM), we counted the total beats for three minutes and then divided the result by three. Unless measurements are made in physiological conditions in ovo, there is tremendous variability in heart rate measurements (Barry, 1940). This study focused on heart morphogenesis rather than heart function and BPM was measured in vitro and outside the incubator. Hence, there was considerable variability in our heart rate data. Given this, absolute values of heart rate may be misleading; hence, we only report how much the heart rate increased relative to that in control hearts. A large number of data points was collected (n=69 for control and n=78 for SPL off) to reduce measurement error to the extent possible. To observe rotation in the atrial region, a tiny hole was made in the SPL and India ink was used to label small sections of the myocardial tissue. The dye was sufficiently diluted and injected using a pneumatic pico pump (WPI).
4.2 Isolated Heart Culture
In order to test if isolated hearts are capable of s-looping, c-looped hearts were cut out from the embryo and cultured in 96-well plates on a surface of semi-solid agar infused with culture media. The hearts were again submerged in a small amount of growth media to eliminate surface tension artifacts and cultured in an oxygen-rich environment. This procedure ensures that there is good mechanical support for the heart without any adhesion of the heart to the agar (Manning & McLachlan, 1990); the same procedure has been used in previous studies on isolated hearts at earlier stages (Latacha et al., 2005; Manning & McLachlan, 1990).
When cultured in ovo, early s-looping takes about six hours to complete. Since hearts cut out from the embryo and cultured in vitro are not in their natural environment, we cultured isolated hearts for up to 12 hours to see if they are capable of s-looping on their own. We also repeated the experiments of Butler (1952) and confirmed that isolated stage 10 hearts can indeed c-loop (results not shown). This served as a validation of our culture technique.
All imaging was done under a Leica M125 stereo microscope. In many of the isolated hearts, we observed that the loop did not lie entirely in one plane; rather, the loop had three-dimensional structure. Taking inspiration from Manner (2004), we reconstructed the three-dimensional structure of the heart loop by shaping a rubber tube to match the image seen under the microscope. No quantitative characterization was attempted. Instead, we simply determined if the out-of-plane character changed over time.
While analyzing the results, we looked for characteristics of the normal s-loop in the final shape formed by the isolated hearts. First, we investigated if the arterial and venous poles approached each other; next, we tested if the observed out-of-plane deformations matched those found in normal s-looping (Sec. 2.1). Finally, we compared the overall shape of the hearts in isolated cultures to those cultured in control conditions.
4.3 Ablation of Cardiac ECM
Following the procedure in Nakamura & Manasek (1981), a 1 mg/ml solution of bovine testicular hyaluronidase (Sigma) was used to disrupt the various components of the cardiac extracellular matrix, the cardiac jelly. The enzyme was dissolved in PBS and directly injected into the CJ through a micro-needle positioned using a micromanipulator. A pneumatic pico pump (WPI) was used to deliver small volumes of the enzyme. The injections were performed under a stereo dissecting microscope (Leica M125) and direct visualization of the needle into the CJ space was possible. Transverse sections of about one in five enzyme-treated embryos were imaged using OCT to confirm CJ ablation. All enzyme-treated embryos were then cultured to observe the effect of CJ ablation on early s-looping.
Both the SPL and the MY had to be punctured to reach the CJ and any effect of these punctures on morphogenesis has to be ruled out. In addition, it is possible that the injection itself could influence morphogenesis, e.g., by altering the pressure inside the heart. Sham experiments were therefore carried out: in one set of experiments, we injected an equivalent volume of PBS without the enzyme; in another, the SPL and MY were punctured, but nothing was injected.
4.4 Finite Element Modeling
Nonlinear finite element (FE) modeling was used to test our hypothesis for the mechanics of early s-looping (Sec. 3). We started with a fully c-looped heart tube as the initial configuration. The cranial and caudal ends are idealized as straight tubes (Fig. 9A). The model is fully three dimensional and has one symmetry plane in the initial configuration. Similar to experiment (Fig. 6A’), our model has a cross-section with a thin layer of myocardium (MY) enclosing a thick layer of cardiac jelly (CJ). The model cross-section is visible in the top views in Fig. 9A. Model dimensions (Fig. 9A) are chosen to simulate an ideal tubular heart of uniform cross-section; variations in heart diameter along the length of the heart tube are currently not included.
Material properties of the stage 12 MY and CJ were taken from Zamir & Taber (2004). As per our hypothesis, the following loads were applied during Action 1 (Fig. 9A): (1) a positive pressure was applied to the surface of the heart to simulate the load applied by the SPL and (2) a ninety degree rotation was applied to the top of the heart tube to simulate head torsion. During Action 2, the distance between the conotruncus and the common atrium was decreased as is done by cervical flexure (Manner et al., 1993). Cardiac jelly inflation is not included in the model. For boundary conditions, the two ends of the heart tube are initially held fixed as SPL pressure and rotation are applied (Action 1, Fig. 9A,B). Next, the caudal end is displaced cranialward (while the cranial end is still fixed) to simulate the compressive forces applied by the deepening cervical flexure (Action 2, Fig. 9B,C,D). The geometry was created in SolidWorks (Dassault Systemes) and a dense hexahedral mesh was created in Patran (MSC Software). ABAQUS/Standard (Dassault Systemes) was used as the analysis solver.
4.5 Statistics
To compare measured quantities before and after treatment, we used the two-sample t-test. Here “measured quantities” refers to the ratio of the distances from the label to the ends of the atrial tube (Sec. 2.1), heart diameters before and after SPL removal (Sec. 2.2), and the distance between the arterial and venous poles of the heart during isolated heart culture (Sec. 2.4). All statistical computations were done using the MATLAB Statistics Toolbox (The MathWorks, Inc).
Bullet Points.
The embryonic heart has no intrinsic capability to form an s-loop.
External forces supplied by the splanchnopleure and cervical flexure are necessary for cardiac s-loop formation.
Computer modeling can be used to study the forces involved in s-loop formation.
Acknowledgements
The project described was supported by Award Number R15HL110009 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. Support also came from Union College’s Undergraduate Summer Fellowship program, Union College Faculty Research Fund, and the National Science Foundation (NSF DUE 0850242). We would like to thank Dr. Kim Latacha, Dr. Larry Taber, and Dr. Dmitry Voronov for their help. Many thanks to Dr. Andrew Rapoff for help with statistical analysis. We also thank the reviewers of this paper for their many helpful suggestions.
Footnotes
Here, “pressure” is used as in engineering, i.e., a positive pressure always acts perpendicular to the surface and applies a pushing (compressive) force on it.
Please note the region denoted “common atrium” in the text and figures gives rise to both the left atrium and the right atrium in future stages (Manner, 2009).
In Manner (2009), stages 13—18 comprise “early s-looping”.
Values are shown as mean � standard deviation.
References
- Aleksandrova A, Czirok A, Szabo A, Filla MB, Hossain MJ, Whelan PF, Lansford R, Rongish BJ. Convective tissue movements play a major role in avian endocardial morphogenesis. Dev Biol. 2012;363:348–361. doi: 10.1016/j.ydbio.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HS, Lloyd TR, Solursh M. Hyaluronate degradation affects ventricular function of the early postlooped embryonic rat heart in situ. Circ Res. 1994;74:244–252. doi: 10.1161/01.res.74.2.244. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Solursh M. Degradation of hyaluronic acid does not prevent looping of the mammalian heart in situ. Dev Biol. 1989;136:555–559. doi: 10.1016/0012-1606(89)90281-9. [DOI] [PubMed] [Google Scholar]
- Barry A. Age changes in the pulsation frequency of the embryonic chick heart. J Exp Zool. 1940;85:157–170. [Google Scholar]
- Barry A. The functional significance of the cardiac jelly in the tubular heart of the chick embryo. Anat Rec. 1948;102:289–298. doi: 10.1002/ar.1091020304. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Baudino TA. Laying the groundwork for growth: Cell-cell and cell-ECM interactions in cardiovascular development. Birth Defects Res C Embryo Today. 2010;90:1–7. doi: 10.1002/bdrc.20168. [DOI] [PubMed] [Google Scholar]
- Brand T. Heart development: Molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Bremer JL. Experiments on the aortic arches in the chick. Anat Rec. 1928;37:225–254. [Google Scholar]
- Butler JK. M. Phil thesis. University of Texas; 1952. An experimental analysis of cardiac loop formation in the chick. [Google Scholar]
- Davis C. The cardiac jelly of the chick embryo. Anat Rec. 1924;27:201–202. [Google Scholar]
- Davis NM, Kurpios NA, Sun X, Gros J, Martin JF, Tabin CJ. The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev Cell. 2008;15:134–145. doi: 10.1016/j.devcel.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz MV. Living Morphogenesis of the Heart. Birkhauser. 1998:99–119. Chapter: Torsion and looping of the cardiac tube and primitive cardiac segments: anatomical manifestations. [Google Scholar]
- Filas BA, Efimov IR, Taber LA. Optical coherence tomography as a tool for measuring morphogenetic deformation of the looping heart. Anat Rec (Hoboken) 2007;290:1057–1068. doi: 10.1002/ar.20575. [DOI] [PubMed] [Google Scholar]
- Flynn ME, Pikalow AS, Kimmelman RS, Searls RL. The mechanism of cervical flexure formation in the chick. Anat Embryol. 1991;184:411–420. doi: 10.1007/BF00957902. [DOI] [PubMed] [Google Scholar]
- Goodrum GR, Jacobson AG. Cephalic flexure formation in the chick embryo. J Exp Zool. 1981;216:399–408. doi: 10.1002/jez.1402160308. [DOI] [PubMed] [Google Scholar]
- Gregory JA, Graham JB, Cech JJ, Dalton N, Michaels J, Chin LN. Pericardial and pericardioperitoneal canal relationships to cardiac function in the white sturgeon (acipenser transmontanus) Comp Biochem Physiol , Part A Mol Integr Physiol. 2004;138:203–213. doi: 10.1016/j.cbpb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Latacha KS, Remond MC, Ramasubramanian A, Chen AY, Elson EL, Taber LA. Role of actin polymerization in bending of the early heart tube. Dev Dyn. 2005;233:1272–1286. doi: 10.1002/dvdy.20488. [DOI] [PubMed] [Google Scholar]
- Linask KK, Han MD, Linask KL, Schlange T, Brand T. Effects of antisense misexpression of CFC on downstream flectin protein expression during heart looping. Dev Dyn. 2003;228:217–230. doi: 10.1002/dvdy.10383. [DOI] [PubMed] [Google Scholar]
- Linask KK, Vanauker M. A role for the cytoskeleton in heart looping. ScientificWorldJournal. 2007;7:280–298. doi: 10.1100/tsw.2007.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasek FJ, Kulikowski RR, Nakamura A, Nguyenphuc Q, Lacktis JW. Growth of the Heart in Health and Disease. New York: Raven Press; 1984. pp. 105–130. Chapter: Early heart development: a new model of cardiac morphogenesis. [Google Scholar]
- Manasek FJ, Burnside MB, Waterman RE. Myocardial cell shape change as a mechanism of embryonic heart looping. Dev Biol. 1972;29:349–371. doi: 10.1016/0012-1606(72)90077-2. [DOI] [PubMed] [Google Scholar]
- Manasek FJ, Monroe RG. Early cardiac morphogenesis is independent of function. Dev Biol. 1972;27:584–588. doi: 10.1016/0012-1606(72)90196-0. [DOI] [PubMed] [Google Scholar]
- Manner J. Cardiac looping in the chick embryo: A morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec. 2000;259:248–262. doi: 10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Manner J. On rotation, torsion, lateralization, and handedness of the embryonic heart loop: New insights from a simulation model for the heart loop of chick embryos. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:481–492. doi: 10.1002/ar.a.20036. [DOI] [PubMed] [Google Scholar]
- Manner J. The anatomy of cardiac looping: A step towards the understanding of the morphogenesis of several forms of congenital cardiac malformations. Clin Anat. 2009;22:21–35. doi: 10.1002/ca.20652. [DOI] [PubMed] [Google Scholar]
- Manner J, Seidl W, Steding G. Correlation between the embryonic head flexures and cardiac development. an experimental study in chick embryos. Anat Embryol (Berl) 1993;188:269–285. doi: 10.1007/BF00188218. [DOI] [PubMed] [Google Scholar]
- Manner J, Seidl W, Steding G. Formation of the cervical flexure: An experimental study on chick embryos. Acta Anat (Basel) 1995a;152:1–10. doi: 10.1159/000147677. [DOI] [PubMed] [Google Scholar]
- Manner J, Seidl W, Steding G. The role of extracardiac factors in normal and abnormal development of the chick embryo heart: Cranial flexure and ventral thoracic wall. Anat Embryol (Berl) 1995b;191:61–72. doi: 10.1007/BF00215298. [DOI] [PubMed] [Google Scholar]
- Manner J, Thrane L, Norozi K, Yelbuz TM. High-resolution in vivo imaging of the cross-sectional deformations of contracting embryonic heart loops using optical coherence tomography. Dev Dyn. 2008;237:953–961. doi: 10.1002/dvdy.21483. [DOI] [PubMed] [Google Scholar]
- Manner J, Wessel A, Yelbuz TM. How does the tubular embryonic heart work? looking for the physical mechanism generating unidirectional blood flow in the valveless embryonic heart tube. Dev Dyn. 2010;239:1035–1046. doi: 10.1002/dvdy.22265. [DOI] [PubMed] [Google Scholar]
- Manning A, McLachlan JC. Looping of chick embryo hearts in vitro. J Anat. 1990;168:257–263. [PMC free article] [PubMed] [Google Scholar]
- Martinsen BJ. Reference guide to the stages of chick heart embryology. Dev Dyn. 2005;233:1217–1237. doi: 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- Mercola M, Levin M. Left-right asymmetry determination in vertebrates. Annu Rev Cell Dev Biol. 2001;17:779–805. doi: 10.1146/annurev.cellbio.17.1.779. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM, Tuckett F, Solursh M. The effects of streptomyces hyaluronidase on tissue organization and cell cycle time in rat embryos. J Embryol Exp Morphol. 1986;98:59–70. [PubMed] [Google Scholar]
- Nakamura A, Manasek FJ. Experimental studies of the shape and structure of isolated cardiac jelly. J Embryol Exp Morphol. 1978;43:167–183. [PubMed] [Google Scholar]
- Nakamura A, Manasek FJ. An experimental study of the relation of cardiac jelly to the shape of the early chick embryonic heart. J Embryol Exp Morphol. 1981;65:235–256. [PubMed] [Google Scholar]
- Nerurkar NL, Ramasubramanian A, Taber LA. Morphogenetic adaptation of the looping embryonic heart to altered mechanical loads. Dev Dyn. 2006;235:1822–1829. doi: 10.1002/dvdy.20813. [DOI] [PubMed] [Google Scholar]
- Patten BM. Early embryology of the chick. 4th Edition. New York: McGraw-Hill; 1951. [Google Scholar]
- Ramasubramanian A, Latacha KS, Benjamin JM, Voronov DA, Ravi A, Taber LA. Computational model for early cardiac looping. Ann Biomed Eng. 2006;34:1655–1669. doi: 10.1007/s10439-005-9021-4. [DOI] [PubMed] [Google Scholar]
- Ramasubramanian A, Nerurkar NL, Achtien KH, Filas BA, Voronov DA, Taber LA. On modeling morphogenesis of the looping heart following mechanical perturbations. J Biomech Eng. 2008;130:061018. doi: 10.1115/1.2978990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubramanian A, Taber LA. Computational modeling of morphogenesis regulated by mechanical feedback. Biomech Model Mechanobiol. 2008;7:77–91. doi: 10.1007/s10237-007-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanoff AL. The avian embryo: Structural and functional development. New York: Macmillan; 1960. [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: Traditional viewpoint and recent advances. Development. 1990;109:243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Shabetai R, Abel DC, Graham JB, Bhargava V, Keyes RS, Witztum K. Function of the pericardium and pericardioperitoneal canal in elasmobranch fishes. Am J Physiol. 1985;248:H198–H207. doi: 10.1152/ajpheart.1985.248.2.H198. [DOI] [PubMed] [Google Scholar]
- Soufan AT, van den Berg G, Ruijter JM, de Boer PA, van den Hoff MJ, Moorman AF. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ Res. 2006;99:545–552. doi: 10.1161/01.RES.0000239407.45137.97. [DOI] [PubMed] [Google Scholar]
- Stalsberg H, DeHaan RL. The precardiac areas and formation of the tubular heart in the chick embryo. Dev Biol. 1969;19:128–159. doi: 10.1016/0012-1606(69)90052-9. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Taber LA. Biophysical mechanisms of cardiac looping. Int J Dev Biol. 2006;50:323–332. doi: 10.1387/ijdb.052045lt. [DOI] [PubMed] [Google Scholar]
- Toole BP. Neuronal Recognition. New York: Plenum Press; 1976. pp. 275–329. Chapter: Morphogenetic role of glycosaminoglycans (acid mucopolysaccharides) in brain and other tissues. [Google Scholar]
- von Dassow M, Davidson LA. Physics and the canalization of morphogenesis: A grand challenge in organismal biology. Phys Biol. 2011;8 doi: 10.1088/1478-3975/8/4/045002. 045002-3975/8/4/045002. Epub 2011 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronov DA, Alford PW, Xu G, Taber LA. The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev Biol. 2004;272:339–350. doi: 10.1016/j.ydbio.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Voronov DA, Taber LA. Cardiac looping in experimental conditions: Effects of extraembryonic forces. Dev Dyn. 2002;224:413–421. doi: 10.1002/dvdy.10121. [DOI] [PubMed] [Google Scholar]
- Waddington C. The dependence of head curvature on the development of the heart in the chick embryo. J Exp Biol. 1937;14:229–231. [Google Scholar]
- Wainwright SA, Biggs W, Currey J, Gosline J. Mechanical design in organisms. Princeton University Press; 1982. [Google Scholar]
- Xu G, Kemp PS, Hwu JA, Beagley AM, Bayly PV, Taber LA. Opening angles and material properties of the early embryonic chick brain. J Biomech Eng. 2010;132:011005. doi: 10.1115/1.4000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir EA, Taber LA. Material properties and residual stress in the stage 12 chick heart during cardiac looping. J Biomech Eng. 2004;126:823–830. doi: 10.1115/1.1824129. [DOI] [PubMed] [Google Scholar]