Abstract
We describe the development of efficient benzannulations of siloxy alkynes with pyridinium and isoquinolinium salts. Such reactions are successfully promoted by a stoichiometric amount of silver(I) benzolate under mild reaction conditions. This process proceeds via a formal inverse-electron demand Diels-Alder reaction, followed by fragmentation of the initially produced bicyclic adducts to deliver a range of synthetically useful phenols and naphthols.
Keywords: alkynes, silver, benzannulation, cycloaddition
Benzannulation is a highly modular way of assembling aromatic compounds starting from two or more components.[1] Structural variation of reaction partners enables rapid diversification of the resulting aromatic products. Alkynes have been employed successfully to develop a number of synthetically useful benzannulations.[2] Indeed, our recent study demonstrated that siloxy alkynes underwent benzannulations with pyrones and isoquinoline N-oxides in the presence of a catalytic amount of aq Au(I) phosphine complex.[3] However, pyridine N-oxides were found to be unreactive under such conditions. We now describe a solution to this limitation and demonstrate that reactions between N-alkyl-pyridinium iodides and siloxy alkynes efficiently proceed in the presence of stoichiometric amounts of Ag(I) salts to afford a range of substituted phenols. The process occurs presumably via a formal inverse-electron demand Diels-Alder reaction, followed by fragmentation of the initially produced cycloadducts. The newly developed benzannulation has a broad substrate scope and also works with a variety of N-alkyl-isoquinolinium iodides.
We initially examined a reaction between 1-siloxy-1-hexyne (1) and 1-ethyl-4-(methoxycarbonyl) pyridinium iodide (2). No significant reaction between 1 and 2 occurred either in the absence of additives or in the presence of catalytic amounts of several Ag(I) and Au(I) complexes (Table 1, entries 1-3). However, treatment of solution of 1 and 2 with stoichiometric amounts of several silver salts, which gave suspensions in the employed solvent, was found to promote formation of imine 3. While AgCO2CH3 and AgCO2CF3 were only moderately effective (Table 1, entries 4 and 5), the use of AgCO2Ph resulted essentially in quantitative conversion to 3 within 2 h at 20 °C (Table 1, entry 6). Changing the nature of the Ag(I) salt counterion seemed to have substantial effect on the efficiency of this reaction. For example, the use of Ag2CO3 and Ag2SO4 resulted only in moderate conversion levels (Table 1, entries 7 and 8). In addition, AgF and AgNO2 were unsuccessful in promoting this reaction (Table 1, entries 9 and 10). Substoichiometric amounts of AgCO2Ph did not catalyse this reaction effectively, showing that a full equivalent of such promoter is required (Table 1, entry 11). The use of other Brønsted bases including K2CO3, Cs2CO3, NaCO2CH3 and t-BuOK proved to inefficient in catalyzing this transformation.
Table 1. Initial Reaction Optimization.
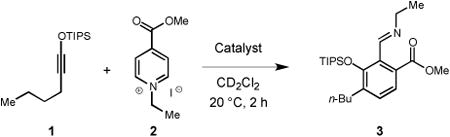
| |||
|---|---|---|---|
|
| |||
| Entry | Catalyst | Catalyst loading | Conversion (%)[b] |
| 1 | LAu(MeCN)SbF6[a] | 5 mol % | 9.1 |
| 2 | AuCl3 | 5 mol % | 11.5 |
| 3 | AgNTf2 | 5 mol % | 8.2 |
| 4 | AgCO2CH3 | 100 mol % | 55 |
| 5 | AgCO2CF3 | 100 mol % | 50 |
| 6 | AgCO2Ph | 100 mol % | 99 |
| 7 | Ag2CO3 | 100 mol % | 48 |
| 8 | Ag2SO4 | 100 mol % | 60 |
| 9 | AgF | 100 mol % | <5 |
| 10 | AgNO2 | 100 mol % | <5 |
| 11 | AgCO2ph | 10 mol % | 10 |
L = Johnphos.
Conversion is measured by 500 MHz 1H NMR analysis of the crude reaction mixtures
Having identified AgCO2Ph as the best promoter for this reaction, we then studied the effect of the counterion and the N-alkyl group of the pyridinium salt on the reaction efficiency. Various counterions were investigated including Cl-, Br-, I-, BF4- and PF6-. Halogen counterions proved to be the most effective, with I- leading to the highest conversion levels. On the contrary, having BF4- and PF6- counterions was much less effective leading to complex mixtures of products or no reactivity. Also, examination of N-(n-Bu), N-ethyl, and N-isopropyl pyridinium salts revealed that N-ethyl, and N-isopropyl pyridinium salts were equally reactive towards siloxy alkynes.
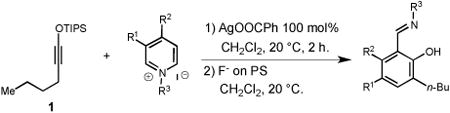
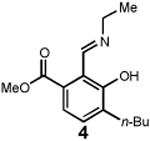
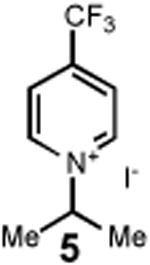
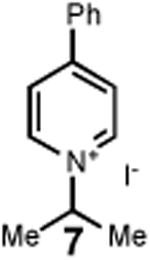
Having identified an optimum set of conditions for benzannulation of siloxy alkyne 1 with N-isopropyl pyridinium iodide 2, we next investigated the scope of this transformation in respect to the pyridinium salt. This study is summarized in Table 2. Pyridinium salts bearing electron deficient substituents such as 2 (R2= CO2CH3) and 5 (R2= CF3) successfully reacted with siloxy hexyne 1. Subsequent desilylation using polymer supported fluoride source gave the corresponding phenols 4 and 6 in 70 and 85% yields, respectively (Table 2, entries 1 and 2). Since the presence of electron-withdrawing groups may activate the pyridium moiety towards the initial cycloaddition with siloxyalkyne, we examined a range of other pyridinium salts that would not have such activating substituent. Subjection of arylcontaining pyridinium salt 7 to the standard reaction conditions delivered the corresponding phenol 8 in 85% yield (Table 2, entry 3). Furthermore, fully unsubstituted pyridinium iodide 9 successfully reacted with alkyne 1 to give phenol 10 (Table 2, entry 4). Substitution of the pyridinium ring with either one or two methyl groups was also well tolerated (Table 2, entries 5 and 6). As expected, further increase in the electron-donating ability of pyridinium substituents rendered such substrates substantially less reactive. Indeed, introduction of methoxy group at the 4-position of the pyridinium ring resulted in no reaction. However, 3-substituted pyridinium salt 15 delivered the corresponding benzannulation product 16 (Table 2, entry 7).
Table 2.
Ag-promoted benzannulations of siloxy alkynes with N-alkylpyridinium iodides.
| |||
|---|---|---|---|
|
| |||
| Entry | Pyridinium salt | Product | Yield, %[a] |
| 1 |
|
|
70 |
| 2 |
|
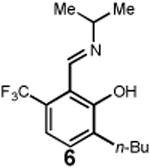
|
85 |
| 3 |
|
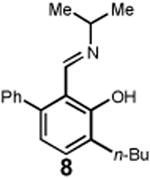
|
85 |
| 4 |
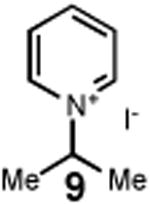
|
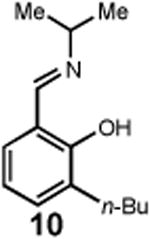
|
70 |
| 5 |

|

|
66 |
| 6 |
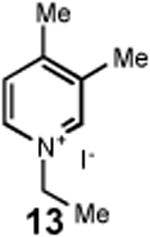
|
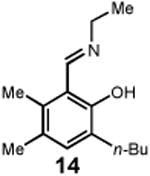
|
55 |
| 7 |
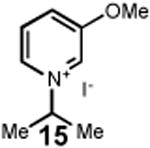
|

|
61 |
Isolated yields after standard chromatographic purification.
We also examined the propensity of quinolinium and isoquinolinium salts to participate in the same transformation. While the use of several quinolinium salts resulted in formation of complex mixtures of products, isoquinolinium iodides proved to be highly effective substrates (Table 3). Electron-deficient isoquinolinium salt 17 (R5 = NO2) afforded the expected naphthol 18 in 70% yield (Table 3, entry 1). Subjection of the unsubstituted isoquinolinium salt 19 (R5 = H) to the general benzannulation protocol furnished the desired product 20 in 88% yield. Furthermore, the use of halogenated isoquinolinium salt 21 (R5 = Br) successfully gave the corresponding naphthol 22 (Table 3, entry 3). Electron-donating groups were also well tolerated. Reaction of siloxy alkyne 1 with isoquinolinium salt 23 (R5 = OBn) successfully furnished the expected product in 80% yield (Table 3, entry 4). This study also demonstrated that broad substitution pattern of siloxy alkynes can be well tolerated (Table 3, entries 5-10). It is also interesting to note that ynamines are known to react with quinolinium and isoquinolinium salts to produce structurally different products under thermal conditions,[4] which points to a unique role of silver(I) salt in promoting the formation of the observed aromatic products.
Table 3.
Ag-promoted benzannulations of siloxy alkynes with N-alkylisoquinolinium iodides.
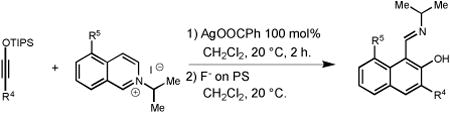
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | R4 | R5 | Product | Yield, %[a] |
| 1 | n-Bu | NO2 (17) |
18 | 70 |
| 2 | n-Bu | H (19) |
20 | 88 |
| 3 | n-Bu | Br (21) |
22 | 90 |
| 4 | n-Bu | OBn (23) |
24 | 80 |
| 5 |

|
Br | 25 | 96 |
| 6 |
|
Br | 26 | 96 |
| 7 |
|
Br | 27 | 90 |
| 8 |
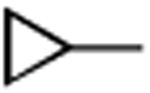
|
Br | 28 | 93 |
| 9 |
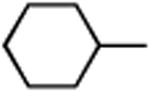
|
Br | 29 | 98 |
| 10 |
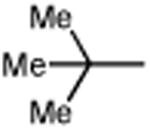
|
Br | 30 | 93 |
Isolated yields after standard chromatographic purification.
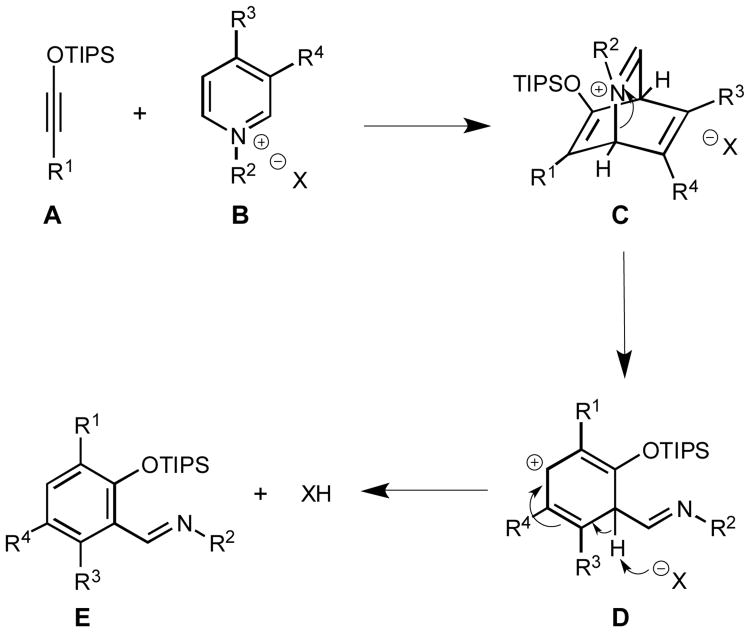
Formation of benzannulation products can be rationalized using a mechanistic hypothesis depicted in Scheme 1. [4+2] Cycloaddition between siloxy alkynes A and pyridinium salts B leads to the bicyclic intermediate C that subsequently undergoes C-N bond cleavage to afford intermediate cation D. Subsequent loss of a proton produces final aromatic product E. Currently, the role of Ag(I) salt in promoting this reaction is not entirely clear since this additive can facilitate the reaction by either activating the siloxy alkyne or promoting pyridinium anion exchange. Therefore, the observed regioselectivity of this transformation is difficult to rationalize at the current stage. However, further mechanistic studies are currently in progress.
Scheme 1.
Proposed reaction mechanism.
In closing, we have developed a mild, silver-promoted benzannulation of siloxy alkynes with N-alkyl-pyridinium iodides. Investigation of the substrate scope revealed that this transformation tolerated a broad range of pyridinium salts and siloxy alkynes. Furthermore, high levels of efficiency were also observed for a variety of N-alkyl-isoquinolinium. This new benzannulation reaction enables rapid access to a range of synthetically useful, highly functionalized phenols and naphthols.
Experimental Section
N-Alkyl-pyridinium or N-alkyl-isoquinolinium iodide (0.15 mmol) and AgCO2Ph (0.15 mmol) were suspended in CH2Cl2 (1.5 mL). The resulting mixture was treated with siloxy alkyne (0.165 mmol) and stirred at 20 °C for 2 h under a nitrogen atmosphere. The precipitate was removed by filtration and the solvent was evaporated under reduced pressure. The crude reaction mixture was then dissolved in CH2Cl2 (1 mL) and treated with fluoride on polymer support (100 mg). After desilylation was complete, as verified by TLC, the reaction mixture was filtered. Following removal of solvent under reduced pressure, the product was purified by flash chromatography on silica gel using hexane containing 1% Et3N (for N-alkyl-pyridinium substrates) and 3% Et3N (for N-alkyl-isoquinolinium substrates) as eluent.
Supplementary Material
Acknowledgments
Financial support of this project was provided by the National Institutes of Health (P50 GM086145) and the Chicago Biomedical Consortium, with support from the Searle Funds at the Chicago Community Trust.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/adsc.201######.
References
- 1.Saito S, Yamamoto Y. Chem Rev. 2000;100:2901–2916. doi: 10.1021/cr990281x. [DOI] [PubMed] [Google Scholar]
- 2.a) Asao N, Nogami T, Lee S, Yamamoto Y. J Am Chem Soc. 2003;125:10921–10925. doi: 10.1021/ja036927r. [DOI] [PubMed] [Google Scholar]; b) Asao N, Takahashi K, Lee S, Kasahara T, Yamamoto Y. J Am Chem Soc. 2002;124:12650–12651. doi: 10.1021/ja028128z. [DOI] [PubMed] [Google Scholar]; c) Gevorgyan V, Takeda A, Yamamoto Y. J Am Chem Soc. 1997;119:11313–11314. [Google Scholar]; d) Asao N, Aikawa H, Yamamoto Y. J Am Chem Soc. 2004;126:7458–7459. doi: 10.1021/ja0477367. [DOI] [PubMed] [Google Scholar]; e) Danheiser RL, Brisbois RG, Kowalczyk JJ, Miller RF. J Am Chem Soc. 1990;112:3093–3100. [Google Scholar]; f) Austin WF, Zhang Y, Danheiser RL. Org Lett. 2005;7:3905–3908. doi: 10.1021/ol051307b. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Danheiser RL, Gee SK. J Org Chem. 1984;49:1672–1674. [Google Scholar]; h) Türkmen YE, Montavon TJ, Kozmin SA, Rawal VH. J Am Chem Soc. 2012;134:9062–9065. doi: 10.1021/ja302537j. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Shindo M, Sato Y, Shishido K. J Org Chem. 2001;66:7818–7824. doi: 10.1021/jo015929w. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera-Pardo JR, Chai DI, Liu S, Mrksich M, Kozmin SA. Nat Chem. 2013;5:423–427. doi: 10.1038/nchem.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuks R, King GSD, Viehe HG. Angew Chem Int Ed. 1969;8:675. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.