Abstract
Hyperglycemia is common in critically ill patients and can be caused by various mechanisms, including nutrition, medications, and insufficient insulin. In the past, hyperglycemia was thought to be an adaptive response to stress, but hyperglycemia is no longer considered a benign condition in patients with critical illnesses. Indeed, hyperglycemia can increase morbidity and mortality in critically ill patients. Correction of hyperglycemia may improve clinical outcomes. To date, a definite answer with regard to glucose management in general intensive care unit patients, including treatment thresholds and glucose target is undetermined. Meta-analyses of randomized controlled trials suggested no survival benefit of tight glycemic control and a significantly increased incidence of hypoglycemia. Studies have shown a J- or U-shaped relationship between average glucose values and mortality; maintaining glucose levels between 100 and 150 mg/dL was likely to be associated with the lowest mortality rates. Recent studies have shown glycemic control < 180 mg/dL is not inferior to near-normal glycemia in critically ill patients and is clearly safer. Glycemic variability is also an important aspect of glucose management in the critically ill patients. Higher glycemic variability may increase the mortality rate, even in patients with the same mean glucose level. Decreasing glucose variability is an important issue for glycemic control in critically ill patients. Continuous measurements with automatic closed-loop systems could be considered to ensure that blood glucose levels are controlled within a specific range and with minimal variability.
Keywords: Critical care, Glycemic control, Hyperglycemia, Hypoglycemia, Insulin
INTRODUCTION
Hyperglycemia is common in critically ill patients, even those patients who have not been previously diagnosed with diabetes[1,2]. Increasing evidence indicates that the development of hyperglycemia during acute medical or surgical illness is not a physiological or benign condition[3-7]. Alterations in glucose metabolism occur during critical illness and are mediated by various factors, including increased insulin resistance, change in hormone production, and activation of cytokines[8]. In critically ill patients a hypermetabolic state exists[9], with the predominant cause being the intense activation of counter-regulatory hormones and cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6, which may be important mediators of insulin resistance and result in hyperglycemia[10]. Clinicians have increasingly appreciated the impact of hyperglycemia in patients with diabetes, as well as stress-induced hyperglycemia (SIH) or hospital-related hyperglycemia[11-13]. The vast majority of patients in the intensive care unit (ICU) have SIH, which refers to transient hyperglycemia during illness and is usually restricted to patients without previous evidence of diabetes[14]. Patients without diabetes have a higher mortality risk when admitted to the hospital than do patients with diabetes[15-18].
Hyperglycemia is independently associated with increased ICU mortality[19-23]. Strict control of the blood glucose concentration is considered important because strict control of the blood glucose concentration may reduce mortality and morbidity; however, hypoglycemia is significantly higher in patients with tight glucose control using intensive insulin therapy[24,25]. Glycemic control to a moderately tight range is not inferior to euglycemia and clearly safer in critically ill patients[26,27]. A less than strict approach to managing critical illness-related hyperglycemia while avoiding hypoglycemia is becoming the standard approach in most ICUs.
EPIDEMIOLOGY
The prevalence of hyperglycemia in critically ill patients is difficult to estimate because the diagnosis is variably defined. Approximately 75% of all patients, including diabetics, have blood glucose concentrations > 110 mg/dL at the time of admission, and 12% of all patients have blood glucose concentrations > 200 mg/dL[24]. Another study showed that > 60%, 38% and 23% of patients had blood glucose concentrations > 110 mg/dL, > 150 mg/dL and > 200 mg/dL after admission in the medical ICU of a tertiary care medical center, respectively[28]. Glucose values > 140 mg/dL occur in 51%-58% of patients presenting with acute myocardial infarctions (MIs)[29,30]. Latham et al[31] found that 21% of cardiothoracic surgery patients developed post-operative blood glucose levels of > 200 mg/dL. The prevalence rates of hyperglycemia were 86.7%, 61% and 35.2% for pediatric patients with maximal glucose levels of > 110 mg/dL, > 150 mg/dL and > 200 mg/dL, respectively[32]. Faustino et al[33] reported prevalence data of 75%, 50.1%, and 26.3% in pediatric patients with cut-off values of 120, 150 and 200 mg/dL, respectively.
CAUSES OF HYPERGLYCEMIA AND PATHOPHYSIOLOGY
The factors contributing to hyperglycemia in patients with critical illnesses include the release of stress hormones and the use of medications (exogenous glucocorticoids, vasopressors, lithium, and β-blockers). Overfeeding, intravenous dextrose, commonly used parenteral nutrition, dialysis solutions, and antibiotic solutions, also contribute to hyperglycemia. Insufficient insulin or volume depletion can cause hyperglycemia[34]. Bed rest, even in the absence of obvious disease, leads to impaired skeletal muscle glucose uptake and promotes peripheral insulin resistance; simple bed rest can further aggravate SIH[35].
In patients with diabetes, the cause of hyperglycemia is a combination of insulin resistance and pancreatic β-cell secretory defects. In patients with SIH, the cause of hyperglycemia is a complex interaction of counter-regulatory hormones, cytokines and insulin resistance[36]. Glucagon, epinephrine, and cortisol increase gluconeogenesis and glycogenolysis. Gluconeogenesis is triggered to a greater extent by glucagon than by epinephrine and cortisol[37-39]. TNF-α may promote gluconeogenesis by stimulating glucagon production[40]. Glycogenolysis is triggered primarily by catecholamines and perpetuated under the influence of epinephrine and cortisol[36].
Insulin resistance may be associated with impaired insulin receptor binding or impairment in the activation of early or intermediate components of the insulin signaling pathway[41] and/or with defects in glucose transporter 4[42]. Epinephrine can inhibit insulin-stimulated glucose transport in skeletal muscle. The action of counter-regulatory hormones on insulin resistance in skeletal muscles may be mediated through an elevation in the circulating free fatty acid level in patients with critical illness, despite hyperinsulinemia[43]. Cytokines such as TNF-α and IL-1, inhibit post-receptor insulin signaling[44,45]. The severity of illness is associated with a proportional rise in serum cytokines and insulin resistance[46,47].
ADVERSE EFFECTS OF HYPERGLYCEMIA
It has been reported that pronounced hyperglycemia might lead to complications or a poor clinical outcome[12]. Elevated blood glucose concentrations are associated with increased morbidity and mortality after burns, surgery, strokes, MIs and head trauma[4,48-54]. In the pediatric ICU, peak blood glucose levels and the duration of hyperglycemia are independently associated with mortality[55]. Hyperglycemia can cause polymorphonuclear neutrophil dysfunction[56], and decreased intracellular bactericidal[57,58] and opsonic activity[56,59] which plays a role in the increased incidence of infections in patients with hyperglycemia. High glucose concentrations in cells can damage mitochondrial protein[60], exacerbate inflammatory pathways, modify the innate immune system, and impair endothelial function[61]. High glucose concentrations also reduce vascular reactivity and endothelial nitric oxide production, which may compromise blood flow to the periphery[62]. In addition, acute hyperglycemia enhances proteolysis[63] and is associated with an increased risk of cardiac complications, hemodynamic and electromyocardial disturbances, acute renal failure, and death[64,65].
INSULIN THERAPY PROTOCOLS
Use of a validated protocol to help maintain the glucose level is effective in critically ill patients. The protocol should be developed by a core group of clinicians, including physicians, nurses, pharmacists, and dietitians with guidelines that provide targeting a specific glucose level, insulin dose adjustment, the interval of glucose monitoring, and time for stopping infusion or decreasing the infusion rate to accommodate changes in patient feeding regimes for tests or medications. The risks of complications, such as hypoglycemia, must be addressed. Intravenous insulin therapy is suggested. The initial blood glucose level is monitored every 1-2 h until a stable infusion rate is reached, then decreased to every 4 h while the blood glucose concentration is stable[66]. A protocol is shown in Figure 1 as an example which can be modified for local needs.
Figure 1.
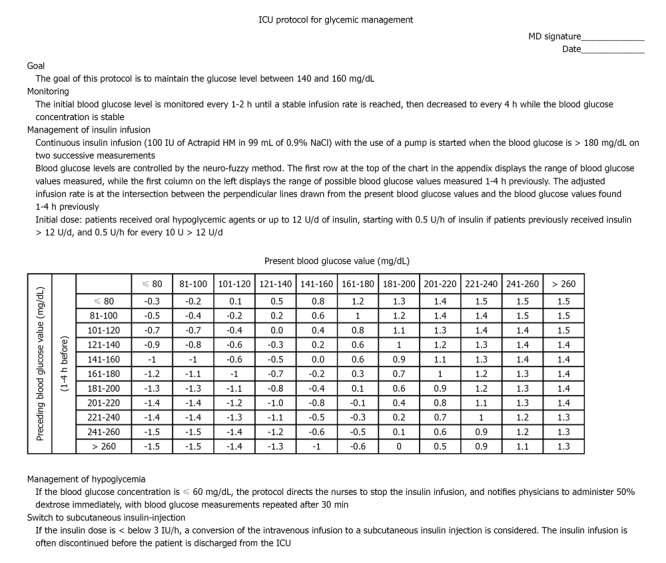
Example of glycemic control protocol in an adult intensive care unit. ICU: Intensive care unit.
Published glycemic management protocols have been documented to significantly improve glucose levels without a significant increase in the risk of hypoglycemia[67]. The advantages of an algorithm or protocol include more consistent glucose control, less of a trial-and-error pattern of treatment, the ability to maintain glycemic control closer to the target range of near-normal, and earlier intervention for hypoglycemia[68,69]. A lack of protocol-based care might be expected to increase glycemic variability[70].
Regular measurement of blood glucose is a burden for nurses; glycemic control by continuous glucose monitoring with automatic closed-loop systems can reduce the clinical burden[71]. Glycemic management protocols in the ICU should focus more on the variability of glycemic control as the treatment target, because glycemic management is related to patient outcome. Continuous monitoring of glucose would allow for the early identification of rapid fluctuations in status associated with changes in insulin requirements. Continuous monitoring of glucose may help prevent the extremes of glucose variability and can maintain optimal blood levels without causing hypoglycemia, thus decreasing the variability in blood glucose concentrations in patients admitted to the ICU[72]. Conversion of an intravenous infusion to subcutaneous insulin injection therapy is often necessary before or at the time of discharge from the ICU[73].
OUTCOMES
Hyperglycemia has been linked to worse outcomes in critically ill patients[74-76]. The SPRINT study showed that tight glycemic control to a mean of 6.0 mmol/L mitigated organ failure faster than conventional control at a higher mean level of 7.2 mmol/L[77]. In 2001, van den Berghe et al[24] published the Leuven study, which demonstrated that tight glycemic control with a target of blood glucose level between 80 and 110 mg/dL had better outcome than conventional control in critically ill surgical patients. ICU mortality, the risk of multi-organ failure, systemic infection and sepsis, the incidence of acute renal failure, critical illness-related polyneuropathy, the need for blood transfusion, and the need for prolonged mechanical ventilator support were reduced from 8% to 4.6%, 34%, 40%, 41%, 44%, 50%, and 50%, respectively. Based on the Leuven study, tight glycemic control was adopted as the standard for critical care patients worldwide. In 2004, Krinsley et al[67] demonstrated that patients in whom the blood glucose concentrations were controlled to <
140 mg/dL had superior survival rates than did patients in whom the blood glucose concentrations were controlled to < 200 mg/dL in medical-surgical ICUs. In 2006, Van den Berghe et al[25] repeated the Leuven study in a medical ICU, but did not demonstrate a survival benefit with tight glycemic control in all critically ill medical patients; however, better outcomes, including ICU, ventilator, and hospital days were noted. For patients in the ICU > 3 d, a survival benefit was reported in the tight glycemic control group. Other studies in which tight glycemic control in the ICU was achieved did not demonstrate a lower mortality rate, less frequent acute renal failure, decreased need for renal replacement therapy, decreased vasopressors, and a lower number of ventilator-free days in the intensive insulin treatment group[26,78-81]. A significantly higher mortality rate was reported in the NICE-SUGAR study[26]. Furthermore, the NICE-SUGAR study did not demonstrate shorter ventilator, ICU, and hospital days, and a lower rate of renal replacement therapy, positive blood cultures, and red cell transfusions, but significantly higher rates of severe hypoglycemia were noted. Wiener et al[82] reviewed 29 randomized controlled trials with a total of 8432 patients in a meta-analysis. Wiener et al[82] showed that hospital mortality did not differ between patients with tight glucose control and patients with usual care, and there was also no significant difference in mortality when stratified by glucose goals. Another meta-analysis study showed that intensive insulin therapy may be beneficial in patients admitted to the surgical ICU, but not the medical ICU or a mixed ICU[83].
Patients with SIH had worse outcomes than patients with a known diabetic history. Umpierrez et al[2] reported that newly diagnosed hyperglycemia (admission or fasting glucose level > 125 mg/dL or random glucose level > 200 mg/dL) was associated with a 16% mortality rate compared to a mortality rate of 3% among patients with known diabetes and a rate of 1.7% among patients without hyperglycemia. Three cohorts of ICU patients concluded that hyperglycemia during an ICU admission had a more significant impact on the risk of mortality among patients without diabetes than among patients with diabetes[72,84,85].
GLYCEMIC VARIABILITY
Blood glucose levels in critically ill patients fluctuate widely, even when continuous feeding and an insulin infusion are used[69]. Glycemic variability is usually expressed as the standard deviation around the mean glucose value or as the mean amplitude of glycemic excursions[86]. Glycemic variability is also associated with outcome in critically ill patients; specifically, greater glycemic variability is associated with a significantly higher mortality rate[87-89]. Non-survivors of critical illnesses were shown to have a higher standard deviation and coefficient of variation (CV) of glucose (standard deviation/mean glucose level) during the ICU stay. A blood glucose level standard deviation > 20 mg/dL was associated with a 9.6-fold increase in mortality compared with a blood glucose level standard deviation < 20 mg/dL[89]. A deleterious effect resulting from increased glycemic variability was noted among non-diabetic patients, but not among patients with diabetes. The mortality rate among non-diabetic patients with a mean glucose level of 70-99 mg/dL during the ICU stay was 10.2% for patients with a glucose CV of < 15% vs 58.3% for patients with a glucose CV above 50%[88]. Increased glycemic variability not only increased the mortality rate, but also morbidities, such as nosocomial infections and hospital length of stay[90]. In a recent retrospective study involving surgical ICU patients, Hermanides and co-workers reported serum glucose variance and combined with high serum glucose levels was associated with the highest mortality, and glucose variability was more important than glucose levels in predicting outcome[91]. Dossett et al[92] reported that glucose variability was associated with increased mortality, but the mean blood glucose level was not associated with increased mortality in patients with sepsis.
Why is glycemic variability associated with poorer outcomes? Glycemic variability may reflect more attention to detail in medical and nursing care, which may be the real determinants of better outcomes. Less glycemic variability may be associated with severe illness[93]. Induced fluctuation in glycemic levels is more likely to produce apoptosis than sustained hyperglycemia[94,95]. These effects may be mediated via wide changes in osmolarity that in turn could affect cellular and organ function[96]. Oxidative stress was produced in much higher concentrations by alterations in glycemic levels than by sustained hyperglycemia[97]. Indeed, increased oxidative stress can result in endothelial dysfunction and contributed to vascular damage. Oxidative stress may be one of the unifying mechanisms underpinning the vasoconstriction, microvascular thrombosis, and inflammation associated with hyperglycemia and glycemic variability[98,99]. Rapid changes in glucose levels can also induce monocyte adhesion to endothelial cells[100]. Another reason why increased glycemic variability may be associated with poorer ICU outcomes is the fact that significant hypoglycemia could occur undetected[101].
In past trials involving intensive insulin therapy, there were discrepancies in mortality outcomes. All of the data regarding glycemic variability were unavailable in these trials; however, glycemic variability may account for the different mortality rates.
HYPOGLYCEMIA
A plasma glucose concentration < 70 mg/dL is the most common threshold used to define hypoglycemia[102]; however, most of the studies involving glucose control in the ICU have defined severe hypoglycemia arbitrarily as values < 40 mg/dL whether or not the patients had associated symptoms[24,25,67,79,81]. Emerging data suggest that hypoglycemia may have a negative impact on the clinical status and outcome of ICU patients[103,104]. ICU patients may tolerate hypoglycemia poorly and also exhibit impaired counter-regulatory responses or have delayed detection of hypoglycemia. The most severe complications of severe hypoglycemia, such as seizures and death, are easy to measure; more subtle manifestations of neuroglycopenia, such as headaches, fatigue, confusion, dysarthria, or impaired judgment, may be difficult or impossible to diagnose in critically ill patients[105,106]. Hypoglycemia is more common in medical and septic sub-groups of patients[107]. Female gender, a history of diabetes, the APACHE II score, mechanical ventilation, continuous veno-venous hemodialysis, and ICU length of stay are independent predictors of hypoglycemia[108]. Spontaneous episodes of severe hypoglycemia are rare and observed mainly in patients with fulminant hepatic failure and adrenal failure secondary to septic shock, and especially in patients with severe co-morbidities, such as liver cirrhosis, chronic renal failure, and malnutrition[26,109].
Based on the Leuven study in 2001, intensive insulin therapy was widely used in many ICUs. Many studies have shown that intensive insulin therapy is associated with significantly more episodes of severe hypoglycemia than conventional insulin therapy[78-81,110]. In the VISEP[80] and Glucocontrol trials[81], the studies were terminated early because of significantly more hypoglycemic episodes in the intensive insulin treatment group. In two meta-analyses studies, intensive insulin therapy also showed a significantly increased risk of hypoglycemia[82,83]. Because intensive insulin therapy has been associated with a significantly higher risk of hypoglycemia, there is increased concern about the safety of intensive insulin therapy, which has become an obstacle to strict glycemic control.
Is the hypoglycemic episode directly responsible for an increased risk of death in patients with critical illnesses? One study revealed the degree of hypoglycemia parallels the increase in the risk of death[111]. Even a single episode of severe hypoglycemia is independently associated with an increased risk of mortality[104]; however, some studies have shown that the occurrence of hypoglycemic is not associated with an increased risk of mortality[108,112].
GLYCEMIC GOAL
Considerable uncertainty remains regarding the optimal target levels of glucose for patients in the ICU. A safe upper limit for blood glucose level during insulin therapy has not been precisely determined in critically ill patients. The Surviving Sepsis Campaign Guidelines advocate a goal of glucose control < 150 mg/dL, in part to limit hypoglycemia[64]. A large body of observational cohort study data from heterogeneous populations strongly suggests that a J- or U-shaped mortality curve exists among acutely and critically ill patients[28,113,114]. Both high and low blood glucose values are independently associated with hospital mortality, with the lowest mortality occurring among those patients with mean glucose levels during their stay in the range of 5.60-8.69 mmol/L and higher rates of mortality for those patients with levels below or above this range[107]. In a recent study, moderate glycemic control was superior to tight glycemic control with decreased mortality and major complications for patients undergoing isolated coronary artery bypass grafting[27]. Patients with a glucose level of 127-179 mg/dL had the lowest mortality and major complications; specifically, sepsis, prolonged ventilation, post-operative renal failure, and the need for new dialysis were highest in the tight glucose control group. Another study also showed that a glucose level of 140-180 mg/dL was associated with the best risk-benefit ratio[103]. The American Association of Clinical Endocrinologists and the American Diabetes Association have increased the treatment threshold to values > 180 mg/dL and a target glucose level between 140 and 180 mg/dL for ICU patients[115].
CONCLUSION
Acute hyperglycemia associated with insulin resistance is common in critically ill patients. Both hyperglycemia and hypoglycemia harm our patients. The appropriate glycemic target has not been established and may indeed be different for different patient populations. At the same mean blood glucose value, the nature of glycemic control can be quite different with respect to glycemic variability. Not only is the blood glucose level important, but glycemic variability is also important. An attempt to minimize glycemic variability might have a significant beneficial impact on the outcomes of patients without diabetes. New strategies should be developed to achieve glycemic control with a minimal risk of hypoglycemia and large glucose variations. More effort should be focused on the quality of blood glucose measurement devices and blood glucose monitoring modalities.
Footnotes
Peer reviewer: Philip C Spinella, MD, FCCM, Associate Professor, Director, Translational Research Program, Washington University School of Medicine, Pediatric Critical Care Medicine, Campus Box 8116 - NW Tower 10th floor, 1 Children's Place, St. Louis, MO 63110, United States
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM
References
- 1.Levetan CS, Passaro M, Jablonski K, Kass M, Ratner RE. Unrecognized diabetes among hospitalized patients. Diabetes Care. 1998;21:246–249. doi: 10.2337/diacare.21.2.246. [DOI] [PubMed] [Google Scholar]
- 2.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 3.Norhammar AM, Rydén L, Malmberg K. Admission plasma glucose. Independent risk factor for long-term prognosis after myocardial infarction even in nondiabetic patients. Diabetes Care. 1999;22:1827–1831. doi: 10.2337/diacare.22.11.1827. [DOI] [PubMed] [Google Scholar]
- 4.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 5.Levetan CS, Magee MF. Hospital management of diabetes. Endocrinol Metab Clin North Am. 2000;29:745–770. doi: 10.1016/s0889-8529(05)70162-6. [DOI] [PubMed] [Google Scholar]
- 6.Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40:1748–1754. doi: 10.1016/s0735-1097(02)02483-x. [DOI] [PubMed] [Google Scholar]
- 7.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 8.Robinson LE, van Soeren MH. Insulin resistance and hyperglycemia in critical illness: role of insulin in glycemic control. AACN Clin Issues. 2004;15:45–62. doi: 10.1097/00044067-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 9.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 10.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15:533–551. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo PJ. Blood glucose control during surgery. Anesthesiology. 1981;55:94–95. doi: 10.1097/00000542-198108000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Pomposelli JJ, Baxter JK, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22:77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 13.Walts LF, Miller J, Davidson MB, Brown J. Perioperative management of diabetes mellitus. Anesthesiology. 1981;55:104–109. doi: 10.1097/00000542-198108000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373:1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapik P, Nadziakiewicz P, Urbanska E, Saucha W, Herdynska M, Zembala M. Cardiopulmonary bypass increases postoperative glycemia and insulin consumption after coronary surgery. Ann Thorac Surg. 2009;87:1859–1865. doi: 10.1016/j.athoracsur.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 16.Estrada CA, Young JA, Nifong LW, Chitwood WR. Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg. 2003;75:1392–1399. doi: 10.1016/s0003-4975(02)04997-4. [DOI] [PubMed] [Google Scholar]
- 17.McAlister FA, Man J, Bistritz L, Amad H, Tandon P. Diabetes and coronary artery bypass surgery: an examination of perioperative glycemic control and outcomes. Diabetes Care. 2003;26:1518–1524. doi: 10.2337/diacare.26.5.1518. [DOI] [PubMed] [Google Scholar]
- 18.Jones KW, Cain AS, Mitchell JH, Millar RC, Rimmasch HL, French TK, Abbate SL, Roberts CA, Stevenson SR, Marshall D, et al. Hyperglycemia predicts mortality after CABG: postoperative hyperglycemia predicts dramatic increases in mortality after coronary artery bypass graft surgery. J Diabetes Complications. 2008;22:365–370. doi: 10.1016/j.jdiacomp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell I, Knight E, Gissane J, Tamhane R, Kolli R, Leditschke IA, Bellomo R, Finfer S. A phase II randomised controlled trial of intensive insulin therapy in general intensive care patients. Crit Care Resusc. 2006;8:289–293. [PubMed] [Google Scholar]
- 20.Coursin DB, Connery LE, Ketzler JT. Perioperative diabetic and hyperglycemic management issues. Crit Care Med. 2004;32:S116–S125. doi: 10.1097/01.ccm.0000115623.52021.c0. [DOI] [PubMed] [Google Scholar]
- 21.Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314:1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orford N, Stow P, Green D, Corke C. Safety and feasibility of an insulin adjustment protocol to maintain blood glucose concentrations within a narrow range in critically ill patients in an Australian level III adult intensive care unit. Crit Care Resusc. 2004;6:92–98. [PubMed] [Google Scholar]
- 23.Orford NR. Intensive insulin therapy in septic shock. Crit Care Resusc. 2006;8:230–234. [PubMed] [Google Scholar]
- 24.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 25.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 26.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 27.Bhamidipati CM, LaPar DJ, Stukenborg GJ, Morrison CC, Kern JA, Kron IL, Ailawadi G. Superiority of moderate control of hyperglycemia to tight control in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2011;141:543–551. doi: 10.1016/j.jtcvs.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cely CM, Arora P, Quartin AA, Kett DH, Schein RM. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest. 2004;126:879–887. doi: 10.1378/chest.126.3.879. [DOI] [PubMed] [Google Scholar]
- 29.Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, Krumholz HM. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111:3078–3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 30.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus JA. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117:1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 31.Latham R, Lancaster AD, Covington JF, Pirolo JS, Thomas CS. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607–612. doi: 10.1086/501830. [DOI] [PubMed] [Google Scholar]
- 32.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 33.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 34.Kovalaske MA, Gandhi GY. Glycemic control in the medical intensive care unit. J Diabetes Sci Technol. 2009;3:1330–1341. doi: 10.1177/193229680900300613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37:802–806. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 36.Lena D, Kalfon P, Preiser JC, Ichai C. Glycemic control in the intensive care unit and during the postoperative period. Anesthesiology. 2011;114:438–444. doi: 10.1097/ALN.0b013e3182078843. [DOI] [PubMed] [Google Scholar]
- 37.Lang CH, Bagby GJ, Blakesley HL, Spitzer JJ. Importance of hyperglucagonemia in eliciting the sepsis-induced increase in glucose production. Circ Shock. 1989;29:181–191. [PubMed] [Google Scholar]
- 38.McGuinness OP, Shau V, Benson EM, Lewis M, Snowden RT, Greene JE, Neal DW, Cherrington AD. Role of epinephrine and norepinephrine in the metabolic response to stress hormone infusion in the conscious dog. Am J Physiol. 1997;273:E674–E681. doi: 10.1152/ajpendo.1997.273.4.E674. [DOI] [PubMed] [Google Scholar]
- 39.Fujiwara T, Cherrington AD, Neal DN, McGuinness OP. Role of cortisol in the metabolic response to stress hormone infusion in the conscious dog. Metabolism. 1996;45:571–578. doi: 10.1016/s0026-0495(96)90026-8. [DOI] [PubMed] [Google Scholar]
- 40.Blumberg D, Hochwald S, Burt M, Donner D, Brennan MF. Tumor necrosis factor alpha stimulates gluconeogenesis from alanine in vivo. J Surg Oncol. 1995;59:220–24; discussion 220-24;. doi: 10.1002/jso.2930590404. [DOI] [PubMed] [Google Scholar]
- 41.Fan J, Li YH, Wojnar MM, Lang CH. Endotoxin-induced alterations in insulin-stimulated phosphorylation of insulin receptor, IRS-1, and MAP kinase in skeletal muscle. Shock. 1996;6:164–170. [PubMed] [Google Scholar]
- 42.Fink RI, Wallace P, Brechtel G, Olefsky JM. Evidence that glucose transport is rate-limiting for in vivo glucose uptake. Metabolism. 1992;41:897–902. doi: 10.1016/0026-0495(92)90174-9. [DOI] [PubMed] [Google Scholar]
- 43.McGuinness OP, Snowden RT, Moran C, Neal DW, Fujiwara T, Cherrington AD. Impact of acute epinephrine removal on hepatic glucose metabolism during stress hormone infusion. Metabolism. 1999;48:910–914. doi: 10.1016/s0026-0495(99)90228-7. [DOI] [PubMed] [Google Scholar]
- 44.Ishizuka K, Usui I, Kanatani Y, Bukhari A, He J, Fujisaka S, Yamazaki Y, Suzuki H, Hiratani K, Ishiki M, et al. Chronic tumor necrosis factor-alpha treatment causes insulin resistance via insulin receptor substrate-1 serine phosphorylation and suppressor of cytokine signaling-3 induction in 3T3-L1 adipocytes. Endocrinology. 2007;148:2994–3003. doi: 10.1210/en.2006-1702. [DOI] [PubMed] [Google Scholar]
- 45.He J, Usui I, Ishizuka K, Kanatani Y, Hiratani K, Iwata M, Bukhari A, Haruta T, Sasaoka T, Kobayashi M. Interleukin-1alpha inhibits insulin signaling with phosphorylating insulin receptor substrate-1 on serine residues in 3T3-L1 adipocytes. Mol Endocrinol. 2006;20:114–124. doi: 10.1210/me.2005-0107. [DOI] [PubMed] [Google Scholar]
- 46.Whitcomb BW, Pradhan EK, Pittas AG, Roghmann MC, Perencevich EN. Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations. Crit Care Med. 2005;33:2772–2777. doi: 10.1097/01.ccm.0000189741.44071.25. [DOI] [PubMed] [Google Scholar]
- 47.Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, Schneeweiss B, Zauner C. Severity of insulin resistance in critically ill medical patients. Metabolism. 2007;56:1–5. doi: 10.1016/j.metabol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Ljungqvist O, Nygren J, Thorell A. Insulin resistance and elective surgery. Surgery. 2000;128:757–760. doi: 10.1067/msy.2000.107166. [DOI] [PubMed] [Google Scholar]
- 50.Kagansky N, Levy S, Knobler H. The role of hyperglycemia in acute stroke. Arch Neurol. 2001;58:1209–1212. doi: 10.1001/archneur.58.8.1209. [DOI] [PubMed] [Google Scholar]
- 51.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 52.Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626–2632. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 54.Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67–71. doi: 10.1212/wnl.59.1.67. [DOI] [PubMed] [Google Scholar]
- 55.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 56.Rassias AJ, Marrin CA, Arruda J, Whalen PK, Beach M, Yeager MP. Insulin infusion improves neutrophil function in diabetic cardiac surgery patients. Anesth Analg. 1999;88:1011–1016. doi: 10.1097/00000539-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Nielson CP, Hindson DA. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes. 1989;38:1031–1035. doi: 10.2337/diab.38.8.1031. [DOI] [PubMed] [Google Scholar]
- 58.Perner A, Nielsen SE, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. 2003;29:642–645. doi: 10.1007/s00134-002-1628-4. [DOI] [PubMed] [Google Scholar]
- 59.Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982;72:439–450. doi: 10.1016/0002-9343(82)90511-3. [DOI] [PubMed] [Google Scholar]
- 60.Van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114:1187–1195. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amour J, Brzezinska AK, Jager Z, Sullivan C, Weihrauch D, Du J, Vladic N, Shi Y, Warltier DC, Pratt PF, et al. Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms. Anesthesiology. 2010;112:576–585. doi: 10.1097/ALN.0b013e3181cded1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nazir FS, Alem M, Small M, Connell JM, Lees KR, Walters MR, Cleland SJ. Blunted response to systemic nitric oxide synthase inhibition in the cerebral circulation of patients with Type 2 diabetes. Diabet Med. 2006;23:398–402. doi: 10.1111/j.1464-5491.2006.01815.x. [DOI] [PubMed] [Google Scholar]
- 63.Flakoll PJ, Hill JO, Abumrad NN. Acute hyperglycemia enhances proteolysis in normal man. Am J Physiol. 1993;265:E715–E721. doi: 10.1152/ajpendo.1993.265.5.E715. [DOI] [PubMed] [Google Scholar]
- 64.Cheung NW, Napier B, Zaccaria C, Fletcher JP. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care. 2005;28:2367–2371. doi: 10.2337/diacare.28.10.2367. [DOI] [PubMed] [Google Scholar]
- 65.Burkett E, Keijzers G, Lind J. The relationship between blood glucose level and QTc duration in the critically ill. Crit Care Resusc. 2009;11:8–13. [PubMed] [Google Scholar]
- 66.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 67.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 68.Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28:2418–2423. doi: 10.2337/diacare.28.10.2418. [DOI] [PubMed] [Google Scholar]
- 69.Brown G, Dodek P. Intravenous insulin nomogram improves blood glucose control in the critically ill. Crit Care Med. 2001;29:1714–1719. doi: 10.1097/00003246-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Egi M, Bellomo R, Stachowski E, French CJ, Hart G, Stow P. Circadian rhythm of blood glucose values in critically ill patients. Crit Care Med. 2007;35:416–421. doi: 10.1097/01.CCM.0000253814.78836.43. [DOI] [PubMed] [Google Scholar]
- 71.Signal M, Pretty CG, Chase JG, Le Compte A, Shaw GM. Continuous glucose monitors and the burden of tight glycemic control in critical care: can they cure the time cost? J Diabetes Sci Technol. 2010;4:625–635. doi: 10.1177/193229681000400317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yatabe T, Yamazaki R, Kitagawa H, Okabayashi T, Yamashita K, Hanazaki K, Yokoyama M. The evaluation of the ability of closed-loop glycemic control device to maintain the blood glucose concentration in intensive care unit patients. Crit Care Med. 2011;39:575–578. doi: 10.1097/CCM.0b013e318206b9ad. [DOI] [PubMed] [Google Scholar]
- 73.Moghissi ES. Addressing hyperglycemia from hospital admission to discharge. Curr Med Res Opin. 2010;26:589–598. doi: 10.1185/03007990903566822. [DOI] [PubMed] [Google Scholar]
- 74.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 75.Worthley MI, Shrive FM, Anderson TJ, Traboulsi M. Prognostic implication of hyperglycemia in myocardial infarction and primary angioplasty. Am J Med. 2007;120:643.e1–643.e7. doi: 10.1016/j.amjmed.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 76.Fogelholm R, Murros K, Rissanen A, Avikainen S. Admission blood glucose and short term survival in primary intracerebral haemorrhage: a population based study. J Neurol Neurosurg Psychiatry. 2005;76:349–353. doi: 10.1136/jnnp.2003.034819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chase JG, Pretty CG, Pfeifer L, Shaw GM, Preiser JC, Le Compte AJ, Lin J, Hewett D, Moorhead KT, Desaive T. Organ failure and tight glycemic control in the SPRINT study. Crit Care. 2010;14:R154. doi: 10.1186/cc9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 79.Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 80.National Institute of Health. Glucontrol Study: Comparing the Effects of Two Glucose Control Regimens by Insulin in Intensive Care Unit Patients. Available from: http://www.clinicaltrial.gov/show/NCT00107601. Access: Jun 12, 2011.
- 81.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 82.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 83.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180:821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rady MY, Johnson DJ, Patel BM, Larson JS, Helmers RA. Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus. Mayo Clin Proc. 2005;80:1558–1567. doi: 10.4065/80.12.1558. [DOI] [PubMed] [Google Scholar]
- 85.Krinsley JS. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2006;18:317–325. doi: 10.1053/j.semtcvs.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 87.Ali NA, O'Brien JM, Dungan K, Phillips G, Marsh CB, Lemeshow S, Connors AF, Preiser JC. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36:2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krinsley JS. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol. 2009;3:1292–1301. doi: 10.1177/193229680900300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waeschle RM, Moerer O, Hilgers R, Herrmann P, Neumann P, Quintel M. The impact of the severity of sepsis on the risk of hypoglycaemia and glycaemic variability. Crit Care. 2008;12:R129. doi: 10.1186/cc7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: Hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9:361–366. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 91.Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. 2010;38:838–842. doi: 10.1097/CCM.0b013e3181cc4be9. [DOI] [PubMed] [Google Scholar]
- 92.Dossett LA, Cao H, Mowery NT, Dortch MJ, Morris JM, May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008;74:679–85; discussion 685. doi: 10.1177/000313480807400802. [DOI] [PubMed] [Google Scholar]
- 93.Egi M, Bellomo R, Reade MC. Is reducing variability of blood glucose the real but hidden target of intensive insulin therapy? Crit Care. 2009;13:302. doi: 10.1186/cc7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924–E930. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 95.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 96.Otto NM, Schindler R, Lun A, Boenisch O, Frei U, Oppert M. Hyperosmotic stress enhances cytokine production and decreases phagocytosis in vitro. Crit Care. 2008;12:R107. doi: 10.1186/cc6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schiekofer S, Andrassy M, Chen J, Rudofsky G, Schneider J, Wendt T, Stefan N, Humpert P, Fritsche A, Stumvoll M, et al. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor kappaB in PBMCs. Diabetes. 2003;52:621–633. doi: 10.2337/diabetes.52.3.621. [DOI] [PubMed] [Google Scholar]
- 98.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 99.Hirsch IB. Glycemic variability: it's not just about A1C anymore! Diabetes Technol Ther. 2005;7:780–783. doi: 10.1089/dia.2005.7.780. [DOI] [PubMed] [Google Scholar]
- 100.Watada H, Azuma K, Kawamori R. Glucose fluctuation on the progression of diabetic macroangiopathy--new findings from monocyte adhesion to endothelial cells. Diabetes Res Clin Pract. 2007;77 Suppl 1:S58–S61. doi: 10.1016/j.diabres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 101.Krinsley J, Preiser JC. Intensive insulin therapy to control hyperglycemia in the critically ill: a look back at the evidence shapes the challenges ahead. Crit Care. 2010;14:330. doi: 10.1186/cc9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 103.Preiser JC, Devos P. Clinical experience with tight glucose control by intensive insulin therapy. Crit Care Med. 2007;35:S503–S507. doi: 10.1097/01.CCM.0000278046.24345.C7. [DOI] [PubMed] [Google Scholar]
- 104.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med. 2007;35:2262–2267. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 105.Gerich JE, Mokan M, Veneman T, Korytkowski M, Mitrakou A. Hypoglycemia unawareness. Endocr Rev. 1991;12:356–371. doi: 10.1210/edrv-12-4-356. [DOI] [PubMed] [Google Scholar]
- 106.Boord JB, Graber AL, Christman JW, Powers AC. Practical management of diabetes in critically ill patients. Am J Respir Crit Care Med. 2001;164:1763–1767. doi: 10.1164/ajrccm.164.10.2103068. [DOI] [PubMed] [Google Scholar]
- 107.Bagshaw SM, Egi M, George C, Bellomo R. Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009;37:463–470. doi: 10.1097/CCM.0b013e318194b097. [DOI] [PubMed] [Google Scholar]
- 108.Arabi YM, Tamim HM, Rishu AH. Hypoglycemia with intensive insulin therapy in critically ill patients: predisposing factors and association with mortality. Crit Care Med. 2009;37:2536–2544. doi: 10.1097/CCM.0b013e3181a381ad. [DOI] [PubMed] [Google Scholar]
- 109.Bagshaw SM, Bellomo R, Jacka MJ, Egi M, Hart GK, George C. The impact of early hypoglycemia and blood glucose variability on outcome in critical illness. Crit Care. 2009;13:R91. doi: 10.1186/cc7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 111.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, Hegarty C, Bailey M. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc. 2010;85:217–224. doi: 10.4065/mcp.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, Roos YB, Schultz MJ, Rosendaal FR, Hoekstra JB. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med. 2006;34:2714–2718. doi: 10.1097/01.CCM.0000241155.36689.91. [DOI] [PubMed] [Google Scholar]
- 113.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 114.Falciglia M, Freyberg RW, Almenoff PL, D'Alessio DA, Render ML. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001–3009. doi: 10.1097/CCM.0b013e3181b083f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kavanagh BP, McCowen KC. Clinical practice. Glycemic control in the ICU. N Engl J Med. 2010;363:2540–2546. doi: 10.1056/NEJMcp1001115. [DOI] [PubMed] [Google Scholar]


