Abstract
Intraperitoneal (IP) chemotherapy is more effective than systemic chemotherapy for treating advanced ovarian cancer, but is typically associated with severe complications due to high dose, frequent administration schedule, and use of non-biocompatible excipients/delivery vehicles. Here, we developed paclitaxel (PTX)-loaded microspheres composed of di-block copolymers of poly(ethylene glycol) and poly(sebacic acid) (PEG-PSA) for safe and sustained IP chemotherapy. PEG-PSA microspheres provided efficient loading (~ 13% w/w) and prolonged release (~ 13 days) of PTX. In a murine ovarian cancer model, a single dose of IP PTX/PEG-PSA particles effectively suppressed tumor growth for more than 40 days and extended the median survival time to 75 days compared to treatments with Taxol® (47 days) or IP placebo particles (34 days). IP PTX/PEG-PSA was well tolerated, with only minimal to mild inflammation. Our findings support PTX/PEG–PSA microspheres as a promising drug delivery platform for IP therapy of ovarian cancer, and potentially other metastatic peritoneal cancers.
Keywords: drug delivery, controlled release, chemotherapy, biodegradable polymers
Ovarian cancer is the fifth leading cause of death from malignancies among women worldwide, with an estimated 275,100 deaths globally in 2011 [1]. It often remains clinically silent until tumors have disseminated beyond the ovaries into the peritoneal cavity, leaving patients with poor prognosis [2]. Intraperitoneal (IP) chemotherapy, which elevates peritoneal drug concentrations, suppresses ovarian tumors more effectively than conventional systemic treatment [3, 4]. Nevertheless, IP chemotherapy can lead to significant side effects, due to transient exposure to high levels of chemo drugs and frequent injections [3–6]. In contrast, IP controlled release systems can maintain therapeutically effective yet moderate levels of chemo in the peritoneal cavity to suppress tumors for a prolonged period of time with reduced side effects [7–9]. While significant advances have been made in the development of IP delivery systems [10–15], new platforms with enhanced efficacy and biocompatibility are still needed. In particular, systems with optimized particle size, surface properties and degradation kinetics may provide greater particle stability, reduced immunogenicity, and optimal clearance time to improve particle-based IP chemotherapy [7–9].
Biodegradable polymers, including polyesters and polyanhydrides, are widely used to develop drug delivery systems that release therapeutic molecules in a sustained fashion [16–18]. One of the advantages of polyanhydrides is that they can be tailored to degrade at predictable rates and release drug in a surface erosion-driven and tunable manner [16, 19, 20]. A variety of polyanhydride-based copolymers, such as poly (ether-anhydrides) [21–25] and poly (ester-anhydrides) [26–31], have been developed and used for drug delivery applications.
Here, we report the development of a microsphere-based delivery system, composed of poly(ethylene glycol)-co-poly(sebacic acid) (PEG-PSA), for IP delivery of paclitaxel (PTX) against ovarian cancer. PSA is a polyanhydride polymer that has been widely studied and is used in Gliadel® wafer, an FDA-approved product [16]. Since sustained release of therapeutic molecules from polyanhydride particles occurs concurrently with the erosion of particles, minimal residual polymer is expected upon depletion of the drug. PEG, a hydrophilic polyether polymer, has a demonstrated history of safe use in FDA-approved pharmaceutical products [32]. During the synthesis of particles composed of PEG-containing amphiphilic co-polymers, PEG partitions to the particle surface, forming a dense coating that improves particle stability and reduces immunogenicity, and thus improves the biocompatibility of the particles [32–35].
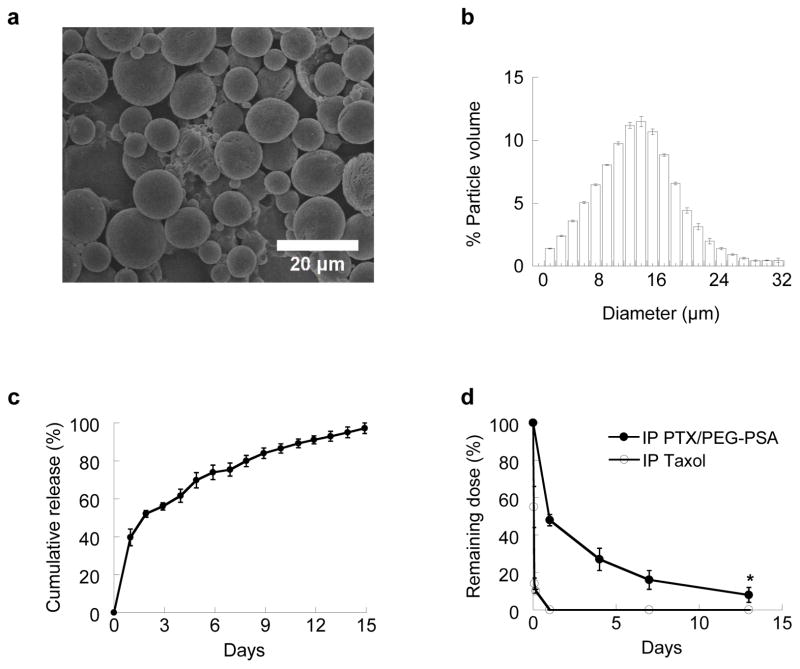
We first formulated PTX-encapsulated PEG-PSA microspheres (PTX/PEG-PSA) using an oil-in-water emulsion method, and characterized their physicochemical properties in vitro. Detailed methods are provided in the Electronic Supplementary Material online. All data represent mean ± standard error of the mean (S.E.M.) unless otherwise specified. Scanning electron micrographs show that PTX/PEG-PSA particles possessed smooth surfaces without drug precipitates (Fig. 1a). The mean diameter of PTX/PEG-PSA particles measured by a Coulter Multisizer was 14.2 μm, with standard deviation of 5.8 μm (Fig. 1b). Submicron particles (e.g., < 1 μm) smaller than the openings of peritoneal lymphatic ducts may be cleared rapidly by lymphatic drainage [7, 8]; thus, the relatively large size of PTX/PEG-PSA particles may facilitate particle retention in the peritoneal cavity.
Fig. 1.
In vitro and in vivo characterizations of PTX/PEG-PSA particles. (a) Scanning electron micrograph, (b) volume-weighted size distribution, and (c) in vitro release kinetics for PTX/PEG-PSA particles. (d) Intraperitoneal retention of PTX delivered by Taxol® or PTX/PEG-PSA particles. Data represent mean ± S.E.M. (n = 3). * indicates statistical differences at all time points (p < 0.05)
We next tested three different target loading levels of PTX to optimize the drug loading in PEG-PSA particles (Table 1). At a target loading of 20%, we achieved an optimal PTX loading of 13 ± 1% with 67 ± 6% encapsulation efficiency. We further characterized the release of encapsulated PTX from PEG-PSA particles in vitro. As shown on Fig. 1c, PTX was released from PEG-PSA particles for over 2 weeks with limited burst effects. Further tuning of drug loading and drug release kinetics may be achieved by adjusting the molecular weight (MW) and/or hydrophobicity of the polymer [23]. While the system described here is engineered for IP delivery of PTX, we have previously shown that PEG-PSA particles can efficiently encapsulate and provide sustained release of other molecules, such as etoposide [25]. PEG-PSA particles may also be suitable for various types of peritoneal indications other than ovarian cancer, including metastatic cancers in the peritoneal cavity such as pancreatic cancer, and peritoneal inflammation such as gastroenteritis.
Table 1.
Drug loading and encapsulation efficiency of PTX/PEG-PSA particles
| Target loading (% w/w) | Actual loading (% w/w) | Encapsulation efficiency (%) |
|---|---|---|
| 10% | 7 ± 1% | 74 ± 3% |
| 20% | 13 ± 1% | 67 ± 6% |
| 30% | 13 ± 1% | 43 ± 5% |
We next investigated the in vivo release of PTX/PEG-PSA particles injected into the mouse peritoneal cavity. Residual PTX was recovered at different time points by performing a peritoneal lavage using PBS, and then quantified by high performance liquid chromatography (HPLC). Fig. 1d showed that Taxol® (the clinical formulation of PTX) was quickly cleared from the peritoneal cavity, with only 14% of the initial dose recovered by 2 hr and no detectable drug level by 24 hr. In contrast, ~ 50% of the initial dose delivered by PTX/PEG-PSA particles remained in the peritoneal cavity at 24 hr, and ~ 8% was recovered on day 13. The drug retention profile of PTX/PEG-PSA particles in vivo was consistent with the in vitro release kinetics, implying that particles were largely stable and cleared minimally from the peritoneal cavity. The improved pharmacokinetics of PTX delivered by PEG-PSA microspheres demonstrate the advantage of this particle system for sustained IP drug delivery.
We also measured plasma drug concentrations at pre-defined time points following treatment (Table 2). In mice receiving 20 mg/kg IP Taxol®, plasma levels of PTX were high (5.2 ± 0.4 μg/mL) at 2 hr after treatment, and then declined to 1.9 ± 0.3 μg/mL at 4 hr, consistent with the documented rapid clearance of Taxol® from the peritoneal cavity into systemic circulation [36]. The half-life of IP Taxol® is ~ 3 hr in mice, leading to a rapid decline in plasma PTX concentration to ~ 0.1 μg/mL at 30 hr following IP Taxol® at 18 mg/kg [36]. In comparison, the plasma level of PTX in mice receiving IP PTX/PEG-PSA remained relatively constant at ~ 1 μg/mL from day 1 up to day 14, suggesting the microspheres provided sustained release of PTX within the peritoneal cavity leading to prolonged systemic exposure to PTX but at relatively low levels.
Table 2.
Plasma PTX concentrations following IP Taxol® or PTX/PEG-PSA
| Treatment | Time | Plasma PTX concentration (μg/mL) |
|---|---|---|
| Taxol® | 5 min | 0 |
| 1 hr | 3.2 ± 0.6 | |
| 2 hr | 5.2 ± 0.4 | |
| 4 hr | 1.9 ± 0.3 | |
| PTX/PEG-PSA | 1 day | 0.9 ± 0.1 |
| 7 days | 1.2 ± 0.1 | |
| 14 days | 1.1 ± 0.1 |
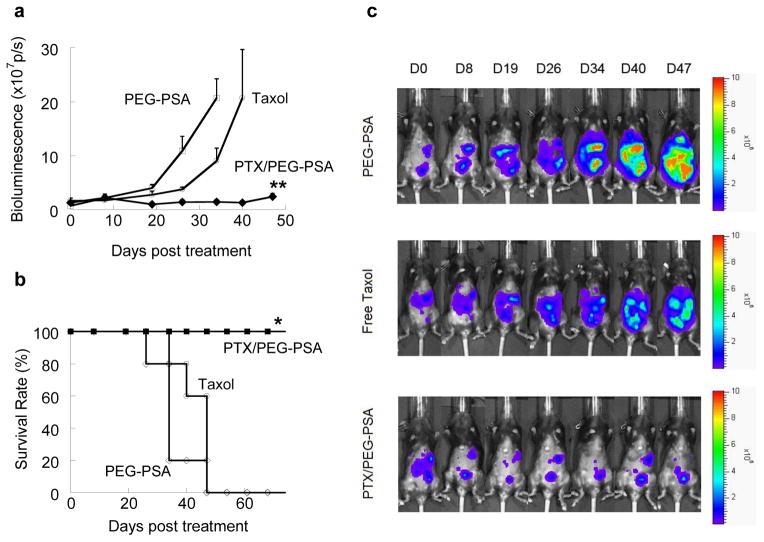
We then evaluated the efficacy of IP PTX/PEG-PSA particles in a previously established murine ovarian tumor model using luciferase-expressing mouse ovarian surface epithelial cells (MOSEC-luc), which allows us to evaluate tumor burden and, thus, the efficacy of new therapies in a non-invasive yet quantitative fashion via bioluminescence measurements [37, 38]. Female C57BL/6 mice were inoculated intraperitoneally with the MOSEC-luc cells. About 4 weeks later, mice bearing tumors were treated by IP administration of a single dose of PTX/PEG-PSA particles (20 mg/kg), Taxol® (20 mg/kg) or placebo PEG-PSA particles. Only mice receiving Taxol® showed signs of distress immediately upon administration, implying the anaphylactic effects of excipients such as Cremophor EL® in Taxol®. As shown in Fig. 2a, tumors in mice receiving IP placebo particles grew steadily, with total bioluminescence signal levels reaching 2×108 p/s by day 34 post treatment, at which point the extremely high tumor load necessitated humane sacrifice. IP Taxol® showed suppression, albeit modest, of tumor growth compared to placebo particles after the first week post treatment, with average tumor load reaching 2 ×108 p/s by day 40. In contrast, IP delivery of PTX/PEG-PSA particles effectively inhibited tumor growth over an extended period of time compared to Taxol® and placebo particles. By day 40, the total bioluminescence signals for mice treated with IP PTX/PEG-PSA were still comparable to initial signal levels at day 0 (1.3×107 p/s). The median survival times of mice receiving placebo and Taxol® were only 34 days and 47 days, respectively, with 0% survival on day 60 for both groups (Fig. 2b). In contrast, mice receiving PTX/PEG-PSA particles demonstrated a median survival time of > 75 days, with all mice surviving at day 60 (Fig. 2b).
Fig. 2.
In vivo efficacy of IP delivered PTX/PEG-PSA microspheres, Taxol® or blank PEG-PSA microspheres in mice bearing IP MOSEC-luc tumors. (a) Bioluminescence signals from MOSEC-luc tumors. PTX/PEG-PSA particles better suppressed tumor growth than other treatments. ** indicates statistical difference between PTX/PEG-PSA and other groups starting from Day 19 (p < 0.01). (b) Kaplan-Meier survival curves. PTX/PEG-PSA particles significantly extended animal survival to > 75 days compared to blank PEG-PSA particles (34 days) and Taxol® (47 days). * indicates statistical difference between PTX/PEG-PSA and other groups (p < 0.05). (c) Representative bioluminescence images of IP tumor burden. Data represent mean ± S.E.M. (n = 5 per treatment set)
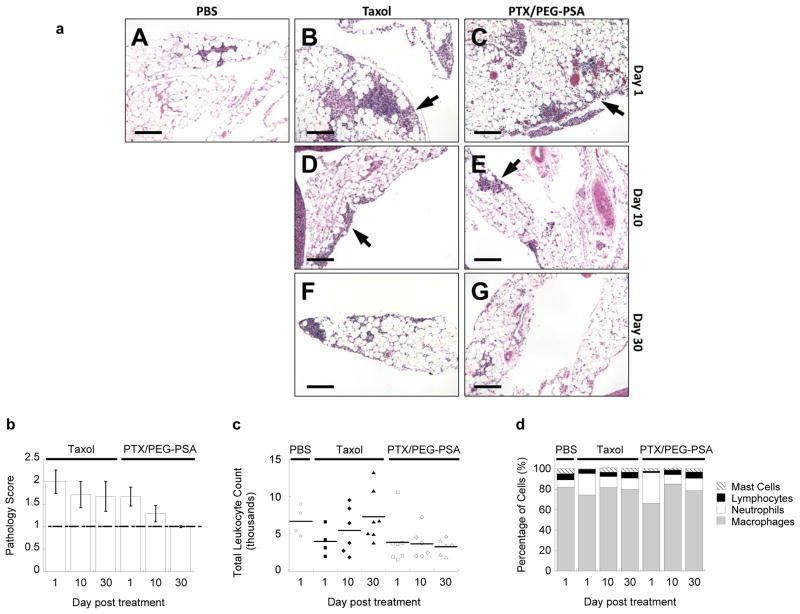
Finally, we evaluated the biocompatibility of IP PTX/PEG-PSA particles. No morphologic anomalies were noted in the major peritoneal organs of mice receiving IP Taxol®, PTX/PEG-PSA particles, or PBS on days 1, 10 and 30 following a single dose administered on day 0. For all groups, minimal to mild inflammatory infiltrates were found in the mesentery but not in other major organs (Fig. 3a). Average pathology scores associated with PTX/PEG-PSA treatment (1.67, 1.29 and 1.00 on days 1, 10 and 30, respectively) were lower than those for Taxol® (2.00, 1.71, and 1.69 on days 1, 10 and 30, respectively), with complete return to baseline by day 30 (Fig. 3b). Total peritoneal leukocyte counts for both treatment groups did not increase significantly at any time point compared to the PBS control (Fig. 3c). However, the fraction of neutrophils increased slightly after 1 day in both treatment groups, likely due to immediate exposure to PTX, but recovered after 10 and 30 days (Fig. 3d). Throughout the entire course of the study, no signs of GI toxicity, such as emesis, diarrhea, or significant weight loss, were observed in any of the treatment groups. These results demonstrate that IP therapy using PTX/PEG-PSA particles was well tolerated, with superior local biocompatibility to that of Taxol®.
Fig. 3.
Toxicity associated with IP Taxol® and PTX/PEG-PSA particles. (a) Representative histological images of mesenteric inflammatory cells for each treatment and time point. PBS (A), Taxol (B, D, F) and PTX/PEG-PSA (C, E, G) treatment groups are shown at day 1 (A, B, C), day 10 (D, E) and day 30 (F, G) post treatment. Arrows indicate groups of inflammatory cells. Scale bars are 100 μm. (b) Pathology scores of representative hematoxylin and eosin-stained tissue sections of abdominal organs from mice receiving Taxol® or PTX/PEG-PSA particles. Dashed line represents the baseline score of PBS-treated mice. (c) Total leukocyte count from peritoneal lavage samples. No significant differences were noted between groups (p > 0.05). (d) Relative proportion of different leukocytes in the peritoneal lavage samples from mice receiving Taxol® or PTX/PEG-PSA particles. Data represent mean ± S.E.M. (n ≥ 4 per treatment set)
The overall PTX dose administered in this study (20 mg/kg) is equivalent to ~ 60 mg/m2 in humans [39], which is consistent with the single IP Taxol® dose used previously in a pivotal clinical trial [4]. However, IP Taxol® is commonly combined with intravenous or IP platinum-based chemotherapy (cisplatin or carboplatin) in the clinic, and treatments are often given once every three weeks for six cycles [4], which significantly increases overall systemic exposure to chemotherapeutic drugs and likely leads to severe systemic side effects including myelotoxicity. Since PTX/PEG-PSA particles showed markedly higher efficacy than free PTX treatment in our studies, we expect that PTX/PEG-PSA particles may be used at a lower dose and dosing frequency to achieve similar or greater efficacy than the current standard IP Taxol® treatment. In addition, our results suggest that PTX is released from PTX/PEG-PSA particles in a sustained fashion in the peritoneal cavity and gradually absorbed into systemic circulation, leading to a sustained but relatively low level of plasma PTX. Thus, we anticipate a lower incidence and lower severity of systemic toxicity due to PTX/PEG-PSA treatment compared to that caused by current standard IP chemotherapy.
Our results show that IP PTX/PEG-PSA significantly suppressed the growth of ovarian tumor compared to standard Taxol® treatment, with better biocompatability. The substantial improvement in efficacy is likely due to the improved pharmacokinetics of PTX in the peritoneal cavity when delivered in PEG-PSA particles. Upon IP administration, Taxol® was cleared by systemic absorption within hours (Fig. 1d). In contrast, the PTX/PEG-PSA microspheres may effectively avoid systemic drainage and last for weeks. Additionally, PEG molecules present on particle surfaces can shield the particles from biological constituents and immune cells in the peritoneal cavity, minimizing particle aggregation and immune elimination. Compared to irritating exipients such as Cremophor EL and ethanol in Taxol®, the components of PEG-PSA microspheres are more biocompatible and less agitating. Overall, PTX/PEG-PSA particles may persist stably and continuously release PTX in the peritoneal cavity, exposing tumors to elevated levels of PTX over longer periods of time with minimal side effects.
In summary, we developed a PEG-PSA based microsphere delivery system for sustained IP chemotherapy with PTX. We demonstrated that PTX/PEG-PSA particles provided sustained released of PTX in vitro and retention of PTX in the peritoneal cavity over 2 weeks. In a murine model of metastatic ovarian cancer, we demonstrated superior tumor suppression by IP PTX/PEG-PSA particles compared to Taxol®. We also showed that IP PTX/PEG-PSA particles were well tolerated in vivo. The sustained release properties and improved biosafety of PTX/PEG-PSA microspheres may further advance IP chemotherapy for ovarian cancer, and potentially other metastatic peritoneal cancers.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants U54CA151838 (J.H.), P50CA098252 (T.W.), R01CA114425 (T.W.), and a National Science Foundation Graduate Research Fellowship (Y.-Y. W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. We thank M. Koontz, N. Forbes-McBean and M. Chen for technical and experimental assistance.
Footnotes
Disclosure of conflicts of interest
The authors declare no conflicts of interest.
Declaration of Ethical Standards
The experiments in this work comply with the current laws of the United States of America.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3(7):502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 3.Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277–83. doi: 10.1016/s1470-2045(03)01074-x. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 2006;100(1):27–32. doi: 10.1016/j.ygyno.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Tummala MK, Alagarsamy S, McGuire WP. Intraperitoneal chemotherapy: standard of care for patients with minimal residual stage III ovarian cancer? Expert Rev Anticancer Ther. 2008;8(7):1135–47. doi: 10.1586/14737140.8.7.1135. [DOI] [PubMed] [Google Scholar]
- 7.Lu Z, Wang J, Wientjes MG, Au JLS. Intraperitoneal therapy for peritoneal cancer. Future Oncology. 2010;6(10):1625–41. doi: 10.2217/fon.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj G, Yeo Y. Drug delivery systems for intraperitoneal therapy. Pharmaceutical research. 2010;27(5):735–8. doi: 10.1007/s11095-009-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Souza R, Zahedi P, Allen CJ, Piquette-Miller M. Polymeric drug delivery systems for localized cancer chemotherapy. Drug Deliv. 2010;17(6):365–75. doi: 10.3109/10717541003762854. [DOI] [PubMed] [Google Scholar]
- 10.Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30(30):6006–16. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, et al. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32(33):8548–54. doi: 10.1016/j.biomaterials.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JL-S. Tumor-Penetrating Microparticles for Intraperitoneal Therapy of Ovarian Cancer. Journal of Pharmacology and Experimental Therapeutics. 2008;327(3):673–82. doi: 10.1124/jpet.108.140095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahedi P, Stewart J, De Souza R, Piquette-Miller M, Allen C. An injectable depot system for sustained intraperitoneal chemotherapy of ovarian cancer results in favorable drug distribution at the whole body, peritoneal and intratumoral levels. Journal of Controlled Release. 2012;158(3):379–85. doi: 10.1016/j.jconrel.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj G, Kim MR, Mohammed SI, Yeo Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. Journal of Controlled Release. 2012;158(3):386–92. doi: 10.1016/j.jconrel.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong DK, Fleming GF, Markman M, Bailey HH. A phase I trial of intraperitoneal sustained-release paclitaxel microspheres (Paclimer) in recurrent ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103(2):391–6. doi: 10.1016/j.ygyno.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Advanced drug delivery reviews. 2002;54(7):889–910. doi: 10.1016/s0169-409x(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 17.Pillai O, Panchagnula R. Polymers in drug delivery. Current opinion in chemical biology. 2001;5(4):447–51. doi: 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 18.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chemical reviews. 1999;99(11):3181–98. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 19.Gopferich A, Tessmar J. Polyanhydride degradation and erosion. Advanced drug delivery reviews. 2002;54(7):911–31. doi: 10.1016/s0169-409x(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 20.Leong KW, Brott BC, Langer R. Bioerodible polyanhydrides as drug-carrier matrices. I: Characterization, degradation, and release characteristics. Journal of biomedical materials research. 1985;19(8):941–55. doi: 10.1002/jbm.820190806. [DOI] [PubMed] [Google Scholar]
- 21.Jiang HL, Zhu KJ. Preparation, characterization and degradation characteristics of polyanhydrides containing poly(ethylene glycol) Polym Int. 1999;48(1):47–52. [Google Scholar]
- 22.Fu J, Fiegel J, Krauland E, Hanes J. New polymeric carriers for controlled drug delivery following inhalation or injection. Biomaterials. 2002;23(22):4425–33. doi: 10.1016/s0142-9612(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 23.Fu J, Fiegel J, Hanes J. Synthesis and characterization of PEG-based ether-anhydride terpolymers: Novel polymers for controlled drug delivery. Macromolecules. 2004;37(19):7174–80. [Google Scholar]
- 24.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(46):19268–73. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang BC, Fu J, Watkins DN, Hanes J. Enhanced efficacy of local etoposide delivery by poly(ether-anhydride) particles against small cell lung cancer in vivo. Biomaterials. 2010;31(2):339–44. doi: 10.1016/j.biomaterials.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erdmann L, Uhrich KE. Synthesis and degradation characteristics of salicylic acid-derived poly(anhydride-esters) Biomaterials. 2000;21(19):1941–6. doi: 10.1016/s0142-9612(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 27.Slivniak R, Domb AJ. Stereocomplexes of enantiomeric lactic acid and sebacic acid ester-anhydride triblock copolymers. Biomacromolecules. 2002;3(4):754–60. doi: 10.1021/bm0200128. [DOI] [PubMed] [Google Scholar]
- 28.Shikanov A, Vaisman B, Krasko MY, Nyska A, Domb AJ. Poly(sebacic acid-co-ricinoleic acid) biodegradable carrier for paclitaxel: in vitro release and in vivo toxicity. Journal of biomedical materials research Part A. 2004;69(1):47–54. doi: 10.1002/jbm.a.20101. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer BA, Burdick JA, Langer R. Formulation and surface modification of poly(ester-anhydride) micro- and nanospheres. Biomaterials. 2005;26(2):117–24. doi: 10.1016/j.biomaterials.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Shikanov A, Domb AJ. Poly(sebacic acid-co-ricinoleic acid) biodegradable injectable in situ gelling polymer. Biomacromolecules. 2006;7(1):288–96. doi: 10.1021/bm050648+. [DOI] [PubMed] [Google Scholar]
- 31.Shikanov A, Vaisman B, Shikanov S, Domb AJ. Efficacy of poly(sebacic acid-co-ricinoleic acid) biodegradable delivery system for intratumoral delivery of paclitaxel. Journal of biomedical materials research Part A. 2010;92(4):1283–91. doi: 10.1002/jbm.a.32429. [DOI] [PubMed] [Google Scholar]
- 32.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angewandte Chemie International Ed In English. 2010;49(36):6288–308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 33.Gref R, Domb A, Quellec P, Blunk T, Muller RH, Verbavatz JM, et al. The Controlled Intravenous Delivery of Drugs Using Peg-Coated Sterically Stabilized Nanospheres. Advanced drug delivery reviews. 1995;16(2–3):215–33. doi: 10.1016/0169-409X(95)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–3. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 35.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International journal of pharmaceutics. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Innocenti F, Danesi R, Di Paolo A, Agen C, Nardini D, Bocci G, et al. Plasma and tissue disposition of paclitaxel (taxol) after intraperitoneal administration in mice. Drug metabolism and disposition: the biological fate of chemicals. 1995;23(7):713–7. [PubMed] [Google Scholar]
- 37.Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin-expressing ovarian cancer using adoptive transfer of mesothelin peptide-specific CD8+ T cells. Gene Ther. 2007;14(12):921–9. doi: 10.1038/sj.gt.3302913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CL, Tsai YC, He L, Wu TC, Hung CF. Cancer immunotherapy using irradiated tumor cells secreting heat shock protein 70. Cancer research. 2007;67(20):10047–57. doi: 10.1158/0008-5472.CAN-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(3):659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.