Abstract
Background
Alcohol consumption and behavioral inhibition share some common underlying genetic mechanisms. The current study examined whether lines of mice selected for high blood ethanol concentrations, attained by heavy drinking in the dark period (DID) of the light-dark cycle that models binge drinking, also exhibit higher levels of drug-naïve inhibition. It also examined whether the administration of ethanol would result in higher levels of disinhibition in these selected lines compared to the founder stock (HS).
Methods
A Go/No-Go task was used to assess baseline inhibition and the effects of acute ethanol on disinhibition (response to a No-Go cue) in the HS line and in mice selected for high levels of DID (HDID-1 and HDID-2).
Results
Lines did not differ in inhibition at baseline and all lines showed increased disinhibition following moderate doses of ethanol. Ethanol decreased responding to Go cues for HDID-2 and HS lines at high doses but not HDID-1 mice.
Conclusions
These data corroborate previous work showing ethanol-induced increases in behavioral disinhibition. The selection paradigm did not result in differential sensitivity to the disinhibiting effects of ethanol, but did result in differential sensitivity to the suppressant effects of ethanol on operant behavior between the two HDID lines.
Keywords: inhibition, Go/No-Go, drinking in the dark, selected lines, alcohol, impulsivity
1. INTRODUCTION
Binge drinking is a serious public health problem that costs billions of dollars each year in medical treatment and property damage (Bouchery et al., 2011). Research suggests that individuals prone to binge drinking (i.e., drinking until they attain high blood ethanol concentrations [BECs]) experience more disinhibition following acute alcohol than individuals who binge drink less frequently or not at all (Marczinski et al., 2007). Data indicate that a number of behavioral phenotypes associated with alcohol drinking are genetically-moderated, including binge drinking and behavioral inhibition (Rhodes et al., 2007; Gubner et al., 2010). The degree to which these genes are common to both phenotypes is unclear, although behavioral correlation and strain studies suggest that some genes that contribute to alcohol consumption/misuse do play a role in impulsivity (reviews: Crabbe et al., 2010; Dick et al., 2010; Mitchell, 2011). Our goal was to compare baseline and post-ethanol levels of inhibitory behavior using a Go/No-Go task in two replicate lines of mice selected from a segregating stock HS/Npt (HS) to achieve high BECs during a drinking in the dark (DID) paradigm (High Drinking in the Dark [HDID]-1 and 2; Crabbe et al., 2009; 2011a). The DID paradigm, in which high BECs are obtained in a short time period, is commonly used to model binge drinking (Crabbe et al., 2011b).
2. METHODS
2.1 Subjects
Male and female mice (32 HS, 27 HDID-1, 31 HDID-2; aged 5–7 weeks) were obtained from Dr. Crabbe at the Portland VA Veterinary Medical Unit (Crabbe et al., 2009, 2011a provides information on line development). Our mice were offspring of breeders from the 19th selection generation of HDID-1 mice and 12th generation of HDID-2 mice (Blood Ethanol Concentration attained: 1.25 ± 0.07 and 1.13 ± 0.10 mg/ml; Crabbe et al., 2013). Mice were housed 2–5 per cage in a temperature-controlled vivarium under a 12:12-h light: dark cycle (lights on at 6 am). Mice were maintained according to the guidelines provided by the Oregon Health & Science University Department of Comparative Medicine, and all procedures were approved by the Institutional Animal Care and Use Committee. After 1 week of habituation and handling, mice were food-restricted and maintained at approximately 90% of their free-feeding bodyweight for the remainder of the study. Subjects participated as a single cohort.
2.2 Apparatus
Sixteen Med-Associates (Med-Associates Inc., St. Albans, VT) operant chambers housed in sound-attenuating boxes were used in the study (see Gubner et al., 2010 for full description). In the chamber, one wall panel contained three nosepoke holes mounted 1.27 cm above the grid floor. Each contained a liquid cup and sensors to identify when nosepokes occurred. Immediately above each was a yellow LED light. Computer-controlled pumps were used to deliver 10% w/v sucrose to the liquid cups. All input and output was controlled and recorded using a program written in MED-PC (Med-Associates Inc.).
2.3 Procedure
Subjects finished two training phases to acquire the Go/No-Go task (Gubner et al., 2010 for description), then performed the task for 15 sessions. The last five sessions provided baseline data. Mice then entered the injection phase of the study. All animals received four doses of ethanol (0.0 [vehicle], 1.0, 1.5, and 2.0 g/kg) administered intraperiotoneally (i.p.) immediately before beginning the Go/No-Go task on Tuesdays and Fridays. On other weekdays, animals completed the task without an injection occurring. Once animals had received each of the four doses, they received each dose a second time (order followed an incomplete Latin Square).
The Go/No-Go task was identical to that used by Gubner et al (2010). Briefly, each session lasted until animals either completed 60 trials (30 Go and 30 No-Go) or 40 min elapsed. Each trial began with a precue period lasting 9 – 24 s, signaled by illuminating the houselight. Responses made during the last 3 s of the precue period reset the trial to prevent premature responding. Following the precue period was a 5-s cue period, during which either the Go cue or the No-Go cue occurred. One cue was the illumination of the light over the left or right nosepoke (counterbalanced between subjects), and the other was a continuous 65-dB 2.5 kHz tone (tone and light counterbalanced as Go or No-Go cue between subjects; there were no systematic differences in performance for any configuration). If the animal responded during the Go cue, the cue terminated and the 3-s reward period began, during which a “click” sounded and 20 μl of 10% sucrose was delivered to the liquid cup. A 10-s inter-trial interval (ITI), during which the house light was off, followed. No response during the Go cue terminated the cue after 5 s and initiated the ITI. Conversely, not responding during the No-Go period was reinforced at the end of the cue period, while responding lead directly to the ITI. All lines were able to discriminate between the Go and No-Go cues as indicated by d’ values being significantly greater than 0 (HS: 0.53 ± 0.12, HDID-1: 0.33 ± 0.11, HDID-2: 0.62 ± 0.12; ts > 3.08, ps < 0.005).
2.4 Drugs
Ethanol (200 proof) was mixed with 0.9% saline to yield a 20% v/v ethanol solution. Doses were varied by altering injection volume. Vehicle was 0.9% saline. Injection volume was equivalent to that used for the 1.0g/kg ethanol dose.
2.5 Data Analysis
The primary measures of inhibition were false alarms (responses to the No-Go cue) and the precue rate (responses during the precue period as a fraction of the total precue time in responses/s). Hits (responses during the Go period) were also of interest as a measure of motivation and operant activity. Because the two HDID lines were selected separately (Crabbe et al., 2009; 2011a), the two lines should not be directly compared, so separate analyses compared the HS mice to HDID-1 mice and to the HDID-2 mice. We examined the baseline data (sessions 11 – 15 before the injection phase) using 2 x 2 ANOVAs (sex x line). Ethanol effects were examined using 4 x 2 x 2 ANOVAs (dose x sex x line). Because there were no systematic effects between the two occasions on which a specific ethanol dose was given in preliminary analyses, data were collapsed across session for each dose. Huynh-Feldt corrections were used when sphericity was violated, and Bonferroni post-hoc tests were used to follow-up significant effects.
3. RESULTS
3.1 Baseline
As shown in Table 1, HDID-1 mice did not differ on measures of inhibition (false alarms and precue response rates: Fs < 2.71, ps > 0.10) compared with HS mice, but responded less in the presence of the Go cue (hits) than HS mice (F(1,55) = 4.80, p < 0.05). HDID-2 and HS mice did not differ in either inhibition levels or hits. While there were line x sex interactions for both hits and the precue response rate for HDID-2 and HS mice (hits: F(1,59) = 6.63, p < 0.05; precue: F(1,59) = 6.49, p < 0.05), there were no significant differences found between the subgroups after Bonferroni corrections.
Table 1.
Mean ± SEM for false alarms, precue response rate, and hits at baseline.
| Line | n | False Alarms | Precue Response Rate (responses/s) | Hits |
|---|---|---|---|---|
| HS | 32 | 15.43 ± 0.68 | 0.11 ± 0.01 | 20.20 ± 1.12 |
| HDID-1 | 27 | 13.75 ± 0.62 | 0.13 ± 0.01 | 16.86 ± 0.93* |
| HDID-2 | 31 | 15.84 ± 0.76 | 0.12 ± 0.02 | 21.28 ± 1.06 |
Note. The maximum number of false alarms and hits is 30, determined by the total number of No-Go and Go trials/session.
Significantly different from HS mice, p < 0.05, see text.
3.2 Ethanol
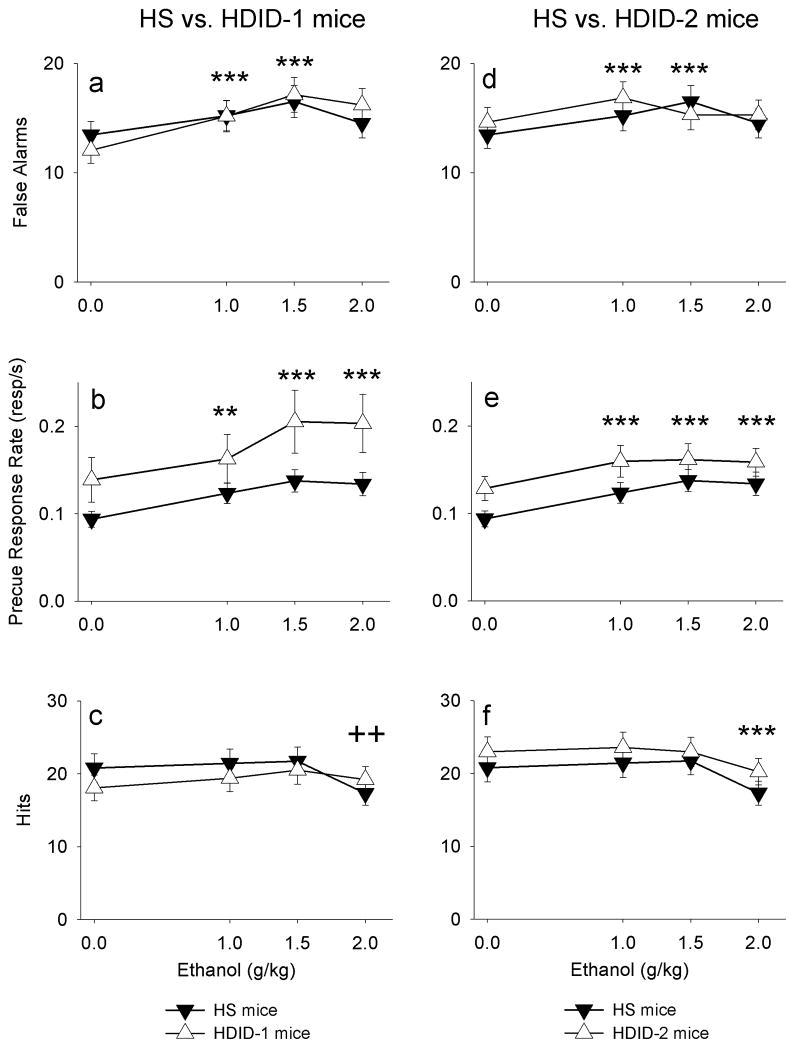
Relative to vehicle, ethanol increased disinhibition (increased false alarms and precue response rate) similarly in both HDID-1 and HS lines of mice (Figure 1a, 1b: (F(2.9, 159.0) = 17.46, p < 0.001; F(2.1, 114.7) = 22.68, p < 0.001; no dose x line interactions). Conversely, ethanol decreased hits at the highest dose (F(2.9,158.3) = 7.83, p < 0.001; see Figure 1c). This was complicated slightly by a significant dose x line interaction, coupled with post-hoc tests, which indicated a steeper decline in responding at the 2 g/kg dose in HS mice (F(2.9, 188.3) = 5.21, p < 0.005). For both false alarms and hits, males responded more than females (main effect of sex: F(1, 55) = 11.91, p < 0.005 and F(1, 55) = 10.70, p < 0.005; data not shown).
Figure 1.
Ethanol increased mean false alarms and precue response rate (behavioral disinhibition) in all lines of mice (a,b,d,e). For comparison, ethanol’s effects on hits are also shown (c,f). Notice: the maximum number of false alaram and hits is 30 based on the number of No-Go and Go trials/session.
** p < 0.01, *** p < 0.001 compared to vehicle for both lines.
++ p < 0.01 compared to vehicle for HS mice only.
Similarly, in both HDID-2 and HS mice, ethanol increased disinhibition (false alarms: F(3,177) = 6.30, p < 0.001; precue response rate: F(3, 177)= 12.67, p < 0.001; see Figure 1d,e). A significant dose x line effect for false alarms (F(3, 177) = 2.84, p < 0.05) suggested that HDID-2 mice exhibited a leftward-shift in sensitivity relative to the HS line, although no significant differences were found between groups at individual doses. Also, for both measures of disinhibition males responded more than females (false alarms: F(1, 59) = 5.79, p < 0.05; precue response rate: F(1, 59) = 5.63, p < 0.05; data not shown). Accompanying the changes in disinhibition measures was a three-way interaction for hits: dose x line x sex (F(2.8, 163.6) = 4.42, p < 0.01), which reflected a complex interaction of line and sex on the effects of dose that could not be easily disambiguated using post-hoc tests.
4. DISCUSSION
Binge drinking is associated with impaired self-control (Lyvers, 2000), and deficient behavioral inhibition (impulsivity) is thought to be one of the primary mechanisms by which alcohol impairs self-control (Fillmore, 2003; Abroms, 2003). Consistent with this, we found ethanol-induced increases in both precue responses and false alarms for all three strains, indicating an increase in behavioral disinhibition. This is similar to other reports indicating that low doses of ethanol increase behavioral disinhibition (Moschak et al., 2013; Oliver et al., 2009; Weafer and Fillmore, 2012). Additionally, HDID-2 mice appeared to be slightly more sensitive to ethanol’s effects on disinhibition than HS mice, although the lack of differences at individual doses and the increased disinhibition after vehicle makes this effect difficult to interpret. Thus, although other research has suggested common influences for both behavior inhibition and heavy drinking (Filbey et al., 2012), the effect of ethanol on inhibition was generally not influenced by the genetic selection for high DID consumption.
All lines showed increased impulsivity following ethanol treatment at low doses. However, the highest ethanol dose decreased hits, suggesting that the processes underlying these measures are differentially affected by ethanol, rather than a general failure in stimulus control. Furthermore, ethanol’s effect on hits was significantly weaker in the HDID-1 mice than in the HS mice. This difference could be due to a reduced sensitivity to ethanol in the HDID-1 line, which may be related to the higher BECs achieved in these animals (Crabbe et al., 2011a). While these mice may be less sensitive to the aversive effects of ethanol (Barkley-Levenson et al., 2013), they have shown increased sensitivity to other effects of ethanol. For example, HDID-1 mice have previously exhibited higher locomotor activity in a parallel rod task in response to ethanol than HDID-2 or HS mice (Crabbe et al., 2012b), which could also contribute to the difference in hits observed in this study.
In addition to the ethanol-induced changes in disinhibition, the HDID-1 mice had fewer hits than HS mice at baseline, which may be related to their resistance to ethanol’s suppressant effects on hits. Several factors could be responsible, including differences in learning, motivation, attention, and activity. More targeted measures of these individual behaviors are needed to determine the source of this difference. However, similar to the HDID-1 line, mice selected for high methamphetamine drinking also had fewer hits in this task (Moschak et al., 2012), which may suggest a relationship between high drug consumption and low response to the Go cue.
While we did not directly compare the HDID-1 and 2 selected lines, our data show that these lines differ in certain aspects of their Go/No-Go responses both at baseline and in the presence of ethanol. Although the selection processes for these two lines were identical (high BECs following a DID paradigm; see Crabbe et al., 2009 and 2011a), the target behavior is highly complex and almost certainly driven by a large number of genes. Thus, it is unsurprising that these lines, while both showing high BECs in DID, differ on other behaviors, including the present impulsivity task. For example, these lines have been shown to differ in their loss of righting reflex and footslips following acute ethanol, but have similar responses on a balance beam test, in acute withdrawal severity, and in hypothermic responses to ethanol (Crabbe et al., 2012a, 2012b). Thus, while this selection process successfully captured the target behavior, a subset of other alcohol-related behaviors, including Go/No-Go performance, was not captured identically across these two lines.
In conclusion, ethanol dose-dependently increased behavioral disinhibition in these lines, although this effect was not moderated by the selection of genes for high DID consumption. In addition, ethanol decreased hits in HDID-2 and HS mice, but not HDID-1 mice, suggesting a differential sensitivity to the effects of ethanol on this measure in the HDID-1 line. While the data suggest that the DID selection method for high BECs does not result in parallel changes in impulsivity, these experiments do offer insight into the effect of acute ethanol doses on behavioral inhibition.
Acknowledgments
The authors would like to thank William Guethlein & Vanessa Wilson for creating some of the macros & programs used to analyze the data and Katherine Stang and Ryan McLaughlin for running the experimental sessions.
Footnotes
Contributors
SHM designed the study. Data analysis and interpretation was performed by TMM, with assistance from MT and SHM. MT was responsible for writing the early drafts of the manuscript with TMM and SHM involved in editing and refining the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Role of funding source
This research was supported by the Portland Alcohol Research Center (P60 AA10760). MET was supported by NIAAA F32AA022011. TMM was supported by NIAAA T32 training grant AA007468 and F31 AA020741. All research was conducted in compliance with laws in the United States of America.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abroms BD, Fillmore MT, Marczinski CA. Alcohol-induced impairment of behavioral control: effects on the alteration and suppression of prepotent responses. J Stud Alcohol. 2003;64:687–695. doi: 10.15288/jsa.2003.64.687. [DOI] [PubMed] [Google Scholar]
- Barkley-Levenson AM, Cunningham CL, Smitasin PJ, Crabbe JC. Rewarding and aversive effects of ethanol in High Drinking in the Dark selectively bred mice. Addict Biol. 2013 doi: 10.1111/adb.12079. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Colville AM, Kruse LC, Cameron AJ, Spence SE, Schlumbohm JP, Huang LC, Metten P. Ethanol tolerance and withdrawal severity in high drinking in the dark selectively bred mice. Alcohol Clin Exp Res. 2012a;36:1152–1161. doi: 10.1111/j.1530-0277.2011.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011b;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Kruse LC, Colville AM, Cameron AJ, Spence SE, Schlumbohm JP, Huang LC, Metten P. Ethanol sensitivity in high drinking in the dark selectively bred mice. Alcohol Clin Exp Res. 2012b;36:1162–1170. doi: 10.1111/j.1530-0277.2012.01735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Belknap JK, Spence SE, Cameron AJ, Schlumbohm JP, Huang LC, Barkley-Levenson AM, Ford MM, Phillips TJ. Progress in a replicated selection for elevated blood ethanol concentrations in HDID mice. Genes Brain Behav. 2013 doi: 10.1111/gbb.12105. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet. 2010;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011a;45:427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus ED, Morgan M, Forester GR, Hutchison K. Dopaminergic genes modulate response inhibition in alcohol abusing adults. Addict Biol. 2012;17:1046–1056. doi: 10.1111/j.1369-1600.2011.00328.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Gubner NR, Wilhelm CJ, Phillips TJ, Mitchell SH. Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol Clin Exp Res. 2010;34:1353–1362. doi: 10.1111/j.1530-0277.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Exp Clin Psychopharmacol. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Abroms BD, Van Selst M, Fillmore MT. Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacology (Berl) 2005;182:452–459. doi: 10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Moschak TM, Stang KA, Mitchell SH. Mice bred for severity of acute alcohol withdrawal respond differently in a go/no-go task. Alcohol Clin Exp Res. 2013;37:1483–1490. doi: 10.1111/acer.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschak TM, Stang KA, Phillips TJ, Mitchell SH. Behavioral inhibition in mice bred for high vs. low levels of methamphetamine consumption or sensitization. Psychopharmacology. 2012;222:353–365. doi: 10.1007/s00213-012-2650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver YP, Ripley TL, Stephens DN. Ethanol effects on impulsivity in two mouse strains: similarities to diazepam and ketamine. Psychopharmacology (Berl) 2009;204:679–692. doi: 10.1007/s00213-009-1500-0. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Comparison of alcohol impairment of behavioral and attentional inhibition. Drug Alcohol Depend. 2012;126:176–182. doi: 10.1016/j.drugalcdep.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31:1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]