Abstract
This study sought to characterize neuropsychological functioning in MJ-using adolescents and emerging adults (ages 18–26) and to investigate whether gender moderated these effects. Data were collected from 59 teens and emerging adults including MJ users (n = 23, 56% female) and controls (n = 35, 50% female) aged 18–26 (M = 21 years). Exclusionary criteria included independent Axis I disorders (besides SUD), and medical and neurologic disorders. After controlling for reading ability, gender, subclinical depressive symptoms, body mass index, and alcohol and other drug use, increased MJ use was associated with slower psychomotor speed/sequencing ability (p< .01), less efficient sustained attention (p< .05), and increased cognitive inhibition errors (p< .03). Gender significantly moderated the effects of MJ on psychomotor speed/sequencing ability (p< .003) in that males had a more robust negative relationship. The current study demonstrated that MJ exposure was associated with poorer psychomotor speed, sustained attention and cognitive inhibition in a dose-dependent manner in young adults, findings that are consistent with other samples of adolescent MJ users. Male MJ users demonstrated greater cognitive slowing than females. Future studies need to examine the neural substrates underlying with these cognitive deficits and whether cognitive rehabilitation or exercise interventions may serve as a viable treatments of cognitive deficits in emerging adult MJ users.
Keywords: Adolescents, Young adults, Neuropsychology, Cognition, Marijuana, Drug effects, Gender
INTRODUCTION
Marijuana (MJ) is the most commonly used illicit drug, with 42% 12th graders and over 50% of young adults using MJ in their lifetime (Johnston, O’Malley, Bachman, & Schulenberg, 2010; Johnston, O’Malley, Bachman, & Schulenberg, 2009). For most of the world, individuals begin using MJ when they are adolescents and use tends to peak in the emerging adult years of 18–25 (Degenhardt et al., 2008). Due to ongoing neurodevelopment occurring throughout adolescence and emerging adulthood, chronic MJ exposure may result in greater neurocognitive deficits as what is reported in adults.
Several brain regions, including the prefrontal cortex (PFC), parietal cortex, and cerebellum, continue to undergo gray matter synaptic pruning into the mid-20s (Giedd, Snell, et al., 1996; Gogtay et al., 2004; Lenroot & Giedd, 2006; Sowell et al., 2004; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Sowell, Trauner, Gamst, & Jernigan, 2002). Maturation of white matter, yielding improvements in efficient neural conductivity, appears to continue into the early thirties (Ashtari et al., 2007; Barnea-Goraly et al., 2005; Giedd, Blumenthal, et al., 1999; Jernigan & Gamst, 2005; Nagel et al., 2006; Paus et al., 1999). It should also be noted that boys and girls differ in the timing and rate of neurodevelopment (see Lenroot & Giedd, 2010 for review). In girls, substantial gray matter pruning in PFC, parietal and temporal cortices begins earlier than boys, while there are greater age-related white matter increases in males; overall, this results in relatively larger brain volumes in boys compared to girls (Giedd, Vaituzis, et al., 1996; Lenroot et al., 2007; Lenroot & Giedd, 2010; Nagel et al., 2006). The endogenous cannabinoid system is also undergoing developmental changes during the adolescent and emerging adult years, with the CB1 receptor density peaking during the adolescent years (Belue et al., 1995; Howlett et al., 2002). Some have hypothesized that the endocannabinoid system plays a direct role in brain development, moderating neurotransmitter release and neurogenesis (Viveros et al., 2005). Given this, it is suggested that exposure to exogenous cannabinoids may disrupt healthy neuronal development and alter the developmental trajectory for boys and girls.
Indeed, the literature has suggested younger animals may be more susceptible to damage caused by exposure to high doses of THC (delta-9-tetrahydrocannabinol), one of the major psychoactive compounds in MJ. Animal studies have found increased cellular changes associated with THC exposure during adolescence compared to adulthood (Cha, White, Kuhn, Wilson, & Swartzwelder, 2006; Kang-Park, Wilson, Kuhn, Moore, Swartzwelder, 2007; Quinn et al., 2008; Rubino et al., 2008; Schneider & Koch, 2003), and THC exposure during adolescence has been associated with long-term cognitive impairment and poorer synaptic connections in the hippocampus, lasting into adulthood (Rubino et al., 2009). Consistent with gender differences in adolescent brain development, female adolescent rats displayed greater CB1 desensitization in the prefrontal cortex, hippocampus, periaqueductal gray matter, and ventral midbrain following THC exposure compared to males, while no gender effect was noted among adult rats in the same conditions (Burston, Wiley, Craig, Selley, & Sim-Selly, 2010). Results suggest a vulnerability of the female adolescent rat brain to THC-associated CB1 receptor signaling changes; however, authors also posited that such increased desensitization could reflect protective adaptation. Taken together, these findings suggest that chronic MJ use during adolescence and emerging adulthood may differentially impact the male and female adolescent or young adult brain compared to exposure during adult years.
Subtle brain structure abnormalities have been found in human adolescent MJ users, including relationships between increased MJ use and larger left hippocampal (Medina, Schweinsburg, et al., 2007) and cerebellar vermis volumes (Jarvis et al., 2008; Medina, Nagel, & Tapert, 2010) and larger PFC volumes that correlated with poorer executive functioning (Medina et al., 2008). With one exception (Delisi et al., 2006), reduced white matter integrity has also been found in adolescent and young adult MJ users (Arnone et al., 2008; Ashtari et al., 2009; Bava et al., 2009). Altered white matter metabolite levels in several brain regions have also been observed in MJ-dependent young adult males, suggesting glial cell disruption (Silveri, Jensen, Rosso, Sneider, & Yurgelun-Todd, 2011). Young adults that initiate MJ use early (younger than 16 years old) demonstrate greater brain functioning abnormalities compared to late onset users (Becker, Wagner, Gouzoulis-Mayfrank, Spuentrup, & Daumann, 2010) and adolescent MJ users have shown abnormal PFC, limbic, cerebellar, and parietal activation patterns compared to controls in response to cognitive inhibition (Tapert et al., 2007), verbal working memory (Jacobsen, Pugh, Constable, Westerveld, Mencl, 2007; Jager, Block, Luijten, & Ramsey, 2010), and spatial working memory (Schweinsburg et al., 2008, 2010) tasks. Furthermore, our laboratory has reported gender differences in the effects of MJ on brain structure (Medina et al., 2008; McQueeny et al., 2011). Taken together, these studies suggest that heavy MJ exposure during this neurodevelopmental stage may result in interrupted pruning, especially in girls, reduced white matter myelination and cognitive inefficiency.
Despite the high rate of MJ use during this age period and the aforementioned evidence of subtle neurologic abnormalities, relatively few studies have focused on the long-term neuropsychological consequences of repeated MJ use in otherwise healthy adolescents and young adults without comorbid psychiatric disorders. Two studies thus far have reported more severe cognitive consequences (including attention, verbal memory, overall intelligence and verbal fluency) of MJ exposure if individuals start using MJ regularly before the age of 17 (Ehrenreich et al., 1999; Wilson et al., 2000). In a longitudinal study following adolescents with substance use disorders into young adulthood, Tapert and colleagues (2002) found that greater cumulative MJ use over an 8-year follow-up period was associated with poorer attention. Thus far, studies have reported subtle cognitive deficits associated with MJ use in teens and young adults, including processing speed (Fried, Watkinson, & Gray, 2005; Medina, Hanson, et al., 2007), attention (Hanson et al., 2010; Harvey, Sellman, Porter, & Frampton, 2007; Medina, Hanson, et al., 2007), memory (Fried et al., 2005; Hanson et al., 2010; Harvey et al., 2007; Medina, Hanson, et al., 2007; Schwartz, Gruenewald, Klitzner, & Fedio, 1989), Visuospatial (Hanson et al., 2011), and executive functioning (Hanson et al., 2010; Harvey et al., 2007; Medina, Hanson, et al., 2007). For example, our group (Medina, Hanson, et al., 2007) previously compared neuropsychological functioning in a sample of demographically matched healthy controls and MJ using adolescents (16–19 years old) without comorbid psychiatric disorders who underwent a month of monitored abstinence. During the month of abstinence a subgroup was also administered a small neuropsychological battery at day 1, week 2, and week 3. After only a few days of abstinence, MJ users had poorer initial verbal memory list learning, sustained attention, and working memory (Hanson et al., 2010). After 3 weeks of abstinence, only initial verbal learning significantly improved (Hanson et al., 2010) and the larger sample of adolescent MJ also showed deficits in complex attention, verbal story learning, sequencing ability, and slower psychomotor speed compared to controls after a month of abstinence (Medina, Hanson, et al., 2007). Still, relatively few studies have attempted to replicate these findings in a different sample that includes a sufficient number of females to address potential gender differences.
Therefore, the purpose of the current study is to further characterize the effects of chronic MJ use on cognition in a sample of adolescents and young adults (ages 18–26; 53% female) who do not meet criteria for independent Axis I psychiatric disorders (besides substance use disorders). Consistent with adolescent findings (e.g., Medina, Hanson, et al., 2007), it is hypothesized that increased MJ use will significantly predict slower psychomotor speed and poorer complex attention and executive functioning in a sample of young adults. We will also explore whether these MJ effects are moderated by gender.
METHODS
Participants
Participants were chosen from ongoing studies conducted at the University of Cincinnati; one examines the cognitive effects of marijuana and ecstasy use (Medina, Shear, & Corcoran, 2005) and the other is an ongoing imaging genetics study (NIH 1R03 DA027457-01; PI: Lisdahl). All participants were originally recruited through advertisements in a local free newspaper and fliers located around the University of Cincinnati. Inclusion criteria for the current analysis included fluent English, 18–26 years of age and participants had to fit into one of two groups: (1) current marijuana (MJ) users (≥10 joints in past year; ≥50 joints lifetime; ≤10 episodes of any other illicit drug in past year and ≤100 illicit drugs in lifetime) or (2) demographically matched normal controls (NC) (≤10 joints past year, ≤50 joints in lifetime; ≤10 illicit drugs used in past year; ≤20 episodes of any other illicit drugs in lifetime). Exclusion criteria for both groups included body mass index (BMI)<18 (Gunstad et al., 2008); history of chronic medical or neurologic illness or injury [meningitis, HIV, epilepsy, brain tumor, traumatic head injury with >2 minutes LOC and post-concussive symptoms, stroke, cerebral palsy, Parkinson’s disease, high blood pressure or diabetes, migraines, recent (past 2 months) and/or multiple concussions]; history of a learning disability or mental retardation; use of psychoactive medication; <80 on WRAT-4 Reading subtest; and refusal to remain abstinent for 7 days before testing. Individuals who met past year or lifetime diagnostic criteria (independent of their substance use) for any psychotic disorder, bipolar disorder, major depressive disorder, or anxiety disorder (generalized anxiety disorder, panic disorder, and post traumatic stress disorder) were excluded from the current sample. Participants include 23 young adult MJ users (56% female) and 35 healthy controls (50% female).
Procedures
The Institutional Review Board at the University of Cincinnati approved all aspects of this study. To determine eligibility, all participants were screened over the phone by trained research assistants. Past year and lifetime Axis I psychotic, anxiety, and mood disorders were screened by phone using a semi-structured interview based on DSM-IV-TR criteria. Interested participants who had positive responses to the screening questions were discussed in committee; if clear decisions could not be reached then they were re-contacted and administered the additional diagnostic questions based on the SCID I/P (determined by KLM) (First et al., 2002).
Before beginning the study, informed consent was obtained. All participants completed a brief background questionnaire, psychological questionnaires, and were administered a drug use interview. (Those completing the imaging genetics protocol also underwent MRI scanning, although these data are not being used for the current study.) They also completed a brief neuropsychological evaluation focused on memory, attention and executive functioning. At the conclusion of the study, participants were paid $35 if they only completed the behavioral session or $110 for completing the full imaging genetics study and all were reimbursed for parking. Participants were also given drug and alcohol treatment referrals.
Measures
Demographic information
Participants completed a Background Questionnaire (Medina et al., 2005) outlining demographic variables including age, gender, ethnicity, self and biological parents’ educations, incomes, and employments, nicotine smoker status, marital status, confirmed absence of significant medical or neurologic illness, psychological disorders or use of psychiatric medication, and learning disability. Participants weight and heights were collected and standard BMI calculations were made [weight in kilograms/(height in meters1height in meters)].
Mood
Participants completed the Beck Depression Inventory-II (BDI-II; Beck, 1996), which contains 21 items measuring levels of depressive symptoms on a 0–3 point scale.
Drug use frequency/quantity
A modified version of the Time-Line Follow-Back (Sobell, Maisto, Sobell, & Cooper, 1979) technique was used, which uses memory cues of common holidays and personal events to measure frequency of drug use over the past year (assessed month-by-month) (Medina et al., 2005). Additionally, a semi-structured interview was administered to measure frequency/quantity of lifetime drug use. For each drug, participants were asked their average weekly use each year that they used. Memory cues appropriate to adolescents and young adults (developmental milestones, school grades, and relationship changes) were used. The following drug categories were assessed: ecstasy, marijuana, alcohol, sedatives (e.g., downers, ketamine, GHB), stimulants (amphetamine, methamphetamine, cocaine, crack cocaine), hallucinogens (mushrooms, PCP, LSD, peyote), opioids (heroin, opium), and inhalants (nitrous oxide, paint, glue, household cleaners, gas). The participant’s drug use was measured in standard units (tablets for ecstasy; standard drinks for alcohol; joints for marijuana; grams for stimulants; number of hits for inhalants, hallucinogens, and opioids; and pills or hits for sedatives).
Premorbid intelligence
The Wide Range Achievement Test-4th edition (WRAT-4) Reading subtest (Wilkinson, 2006) was used as an estimate of intelligence and quality of education for group comparison purposes (see Manly, Jacobs, & Touradji, 2002).
Neuropsychological Battery & Composite Scores
To reduce the number of dependent variables, a hybrid method using composite scores was used (Medina et al., 2007); this approach considered both the established categorization of cognitive domains (Lezak, Howieson, & Loring, 2004) as well as the results of reliability analyses (for discussion see Delis, Jacobson, Bondi, Hamilton, & Salmon, 2003). Each individual raw score was first converted to Z-scores based on the whole sample (N = 59). Average Z-scores were calculated for each composite score (if more than one variable was used). Standardized Chronbach’s alpha coefficients were examined to determine internal consistency of the composite scores; if <.50 then the composite scores were reevaluated. This resulted in 11 composite scores/individual variables covering six total areas of cognitive functioning:
Verbal memory
Immediate Memory was assessed with Trial 1 and total recall from the California Verbal Learning Test-II (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2001). Delayed Memory was measured by Short delay free recall and long delay free recall from the CVLT-II. Recognition Memory was assessed by the recognition discriminability variable from the CVLT-II.
Sequencing ability/psychomotor speed
Delis-Kaplan Executive Functioning Scale (D-KEFS; Delis & Kaplan, 2000) Trail Making Test (TMT-B) switching score.
Sustained attention
Sustained attention speed and accuracy measures from the Ruff 2 & 7 were used to assess sustained attention (Ruff & Allen, 1996).
Verbal fluency
D-KEFS Verbal Fluency FAS subtest total correct.
Design Fluency
D-KEFS Design Fluency total correct and total accuracy score.
Cognitive inhibition
D-KEFS Color Word Interference Test Inhibition and Inhibition Switching combined total correct and total accuracy (errors).
Data Analysis
Analyses of variance (ANOVAs) and χ2 tests compared groups on important demographic and drug use variables. A series of standard multiple regressions were then run to examine whether past year MJ use independently, or inter-actively with gender, predicted cognitive functioning after controlling for possible confounds (gender, BMI, WRAT-4 Reading subtest- a verbal IQ estimate, past year alcohol use, past year other drug use, and BDI-2 depressive symptoms). More specifically, the first block included all main effects (past year MJ use, gender, covariates) and the second block included MJ use*gender interactions. Interpretations of statistical significance were made if p< .05.
RESULTS
Demographic, BMI, & Mood Information
ANOVA and χ2 tests were conducted to determine whether the MJ users and controls differed (see Table 1). The two groups did not differ in age [F(1,58) = .58; p = .49], gender composition [χ2(1) = .24; p = .62], ethnicity [χ2(2) = 5.2; p = .07], WRAT-3 Reading standard score [F(1,58) = 1.67; p = .20] (Wilkinson, 1993; Manly et al., 2002), education [F(1,58) = 3.04; p = .09], BDI-II depressive symptoms [F(1,58) = .29; p = .59], percent of subjects characterized as overweight according to the 25 BMI cut-off [χ2(1) = 1.5; p = .22], although there was a trend toward MJ users having slightly higher total BMI [F(1,58) = 2.51; p = .11] and their weight was significantly greater compared to controls [F(1,58) = 4.01; p = .05]. Because BMI has been associated with neurocognitive functioning in youth (Bauer, Kaplan, & Hesselbrock, 2010; Lisdahl & Price, under review) and adults (Ho et al., 2011; Volkow et al., 2009), BMI was controlled for in all regressions.
Table 1.
Demographic, mood, BMI, and drug use variables by group
| MJ users (n = 23) |
Controls (n = 36) |
|
|---|---|---|
| % or M ± SD (range) | % or M ± SD (range) | |
| Gender (male) | 44% | 50% |
| Ethnicity (Caucasian) | 70% | 92% |
| Age (years) | 21.2±2.8 (18–28) | 20.7±2.8 (18–25) |
| Education (years) | 12.7±1.9 (9–17) | 13.6±1.7 (11–18) |
| Reading standard score (WRAT-3) | 108.3±12.3 (85–133) | 104.8±8.6 (83–120) |
| Body mass index (BMI) | 27.2±7.3 (20.7–47.0) | 24.7±4.7 (18.9–39.75) |
| Beck Depressive Inventory-2 Total Score | 7.0±7.2 (0–27) | 6.1±5.6 (0–26) |
| Length of abstinence from MJ (days) | 50±109 (7–407) | N/A |
| Age first used marijuana* | 15±2 (11–19) | 17±2 (14–20) |
| Age first used alcohol* | 16±2 (13–20) | 17±2 (13–22) |
| Past year alcohol use (episodes) * | 304±372 (0–1254) | 132±193 (0–878) |
| Past year marijuana use (episodes) * | 208±198 (10–728) | 1±3 (0–10) |
| Past year other drug use (episodes)* | 4±7 (0–31) | 0±0.9 (0–5) |
| Lifetime alcohol use (drinks) * | 1423±1677 (51–6191) | 434±606 (0–2359) |
| Lifetime marijuana use (joints) * | 1014±1126 (50–3976) | 7±11 (0–41) |
| Lifetime other drug use (episodes)* | 13±18 (0–62) | 1±3 (0–16) |
Note. Group differences:
p<.01.
Drug Use Information
Based on self-report, participants were abstinent from MJ for at least 7 days (approximately half of the MJ users did have urine toxicology screens, although they were allowed to be positive for THC as THC-COOH can stay in the system for up to 3 weeks in heavy users; positive results for all other illicit drugs excluded the participant). Participants were not allowed to smoke nicotine within one hour of the neuropsychological battery. The average length of abstinence from any MJ use for MJ-users was 50 days (±109, range: 7–407 days). As expected, MJ-users reported earlier age of first MJ use [F(1,58) = 8.9; p< .005], greater past year [F(1,58) = 39.4; p< .001] and lifetime [F(1,58) = 29.0; p< .001] MJ joints used compared to controls. MJ-users also had more lifetime [F(1,58) = 10.5; p< .002] and past year [F(1,58) = 5.4; p< .03] alcohol drinks than NC. Although MJ users divulged more intake of other drugs (measured by number of hits of stimulants, sedatives, inhalants, opiates, and hallucinogens) than controls [F(1,58) = 7.6; p< .008], such use was limited to 31 past year hits in the MJ users, most commonly recreational use of stimulants (especially prescription) or hallucinogens (see Table 1).
Gender Status & Covariates
In the MJ users, there were no significant differences in demographic or drug use variables between the genders, with the exception of first age of regular MJ use [F(1,58) = 5.23; p< .04]; the female MJ users had an average age of first use of 14.2 years, compared to 16.0 years old in the males.
Neuropsychological Functioning
To assist with interpretation of the findings, Table 2 displays the mean composite Z-scores on the individual and composite neuropsychological scores used in the primary analyses. As seen in the table, the MJ users on average performed −.15 standard deviations below the controls across all the cognitive tests.
Table 2.
Neuropsychological composite variables by group
| MJ users (n = 23) |
Controls (n = 36) |
||
|---|---|---|---|
| % or M ± SD (range) | % or M ± SD (range) | (MJ-Control) | |
| CVLT-II Immediate Memory | −.10 ± .91 (−1.60–1.68) | .60 ± .94 (−1.53–2.25) | −.70 |
| CVLT-II Delayed Memory | −.04 ± .51 (−1.00–.67) | .03 ± .69 (−1.91–1.07) | −.07 |
| CVLT-II Recognition Memory | .22 ± .69 (−1.76–.92) | −.14 ± 1.14 (−3.37–.92) | .36 |
| TMT-B Sequencing Ability/Psychomotor Speed | −.09 ± 1.29 (−3.44–1.34) | .06 ± .77 (−2.14–1.20) | −.15 |
| Ruff 2 & 7 Sustained Attention Speed | −.18 ± .87 (−1.95–1.26) | .11 ± 1.07 ( −2.04–2.82) | −.29 |
| Ruff 2 & 7 Sustained Attention Accuracy | .21 ± 1.12 (−1.95–1.26) | −.13 ± 1.13 (−2.35–1.56) | .34 |
| Verbal Fluency Total Correct | .01 ± .86 (−1.27–2.51) | −.01 ± .84 (−1.66–1.96) | .02 |
| Design Fluency Total Correct | −.17 ± .95 (−2.25–1.95) | .11 ± 1.02 (−1.77–2.19) | −.28 |
| Design Fluency Accuracy | −.33 ± 1.01 (−3.77–1.27) | .22 ± .94 ( −2.26–1.27) | −.55 |
| Cognitive Inhibition Total Correct | −.05 ± .89 (−3.28–1.52) | .03 ± .93 (−2.32–1.57) | −.08 |
| Cognitive Inhibition Accuracy | −.14 ± .92 ( −2.20–1.09) | .09 ± 1.05 (−4.39–1.09) | −.23 |
Note. CVLT-2 = California Verbal Learning Test-2nd Edition; WRAT-3 = Wide Range Achievement Test-3rd Edition. (MJ-Control) indicates difference in mean Z-scores between MJ and control groups; average Z-score difference across all composites revealed that MJ users performed −0.15 SD below the controls on the neuropsychological battery.
Multivariate Relationships
Primary regression analysis: Frequency/quantity of past year marijuana use
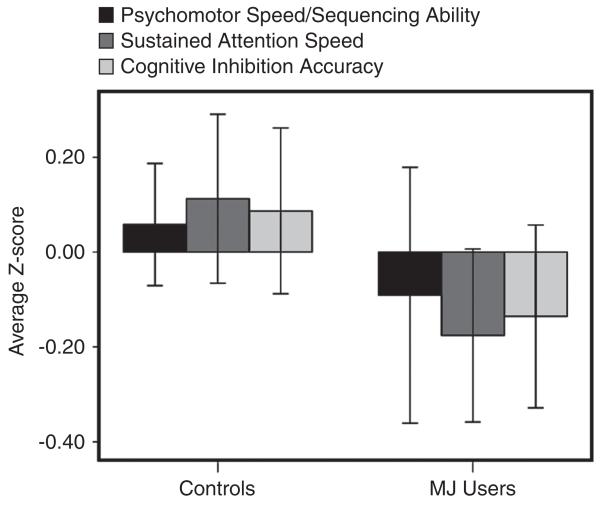
After controlling for gender, WRAT reading ability (verbal IQ estimate), depressive symptoms, BMI, and past year alcohol and other drug use, and increased MJ use significantly predicted poorer sequencing ability/psychomotor speed (beta = −.38; f2 = .12, p< .01), sustained attention speed (beta = −.28; f2 = .09; p< .05), and cognitive inhibition accuracy (beta = −.32; f2 = .12; p< .03). Although the analysis examined the dose-dependent impact of past year MJ use, Figure 1 is included to demonstrate the average Z-scores on these cognitive variables for the MJ users and controls to help with interpretation of the findings. MJ-use did not significantly predict performance on verbal memory, selective attention accuracy, or verbal and design fluency tasks.
Fig. 1.
Average Z-scores (± 1 standard error) on Trail Making Test-B (psychomotor speed/sequencing ability), Ruff 2 & 7 (sustained attention speed) and D-KEFS Color-Word Interference Test (cognitive inhibition accuracy) by group.
Gender*MJ use interaction
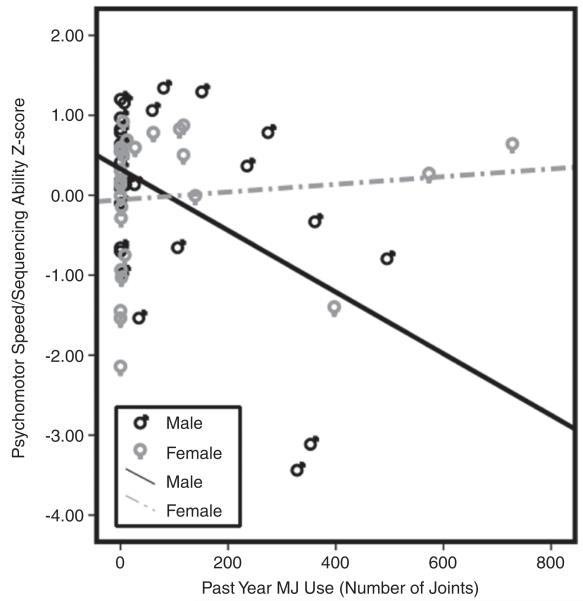
Gender interacted with past year MJ use in predicting sequencing ability/psychomotor speed (beta = .40; f2 = .20; p< .003). Despite similar levels of past year MJ use, males demonstrated a more robust relationship between increased MJ use and poorer sequencing ability/psychomotor speed compared to females (see Figure 2).
Fig. 2.
Bivariate scatterplot demonstrating the relationship between past year MJ use and psychomotor speed/sequencing ability separately by gender.
Past year alcohol & other drug use
Independent of MJ use, increased past year alcohol use independently predicted poorer delayed memory (beta = 2.30; p< .05) on the CVLT-2. Other drug use did not predict cognitive functioning, although as previously mentioned, other drug use was low in this sample.
Length of abstinence from marijuana
Pearson correlations were run in the MJ users to examine the relationship between length of abstinence from MJ and cognitive functioning. As shown in Table 3, with the exception of design fluency accuracy, no significant relationships were observed. Still, MJ users demonstrated medium sized correlations between increased MJ abstinence and improved immediate verbal recall (r = .33; p = .12) and sustained attention accuracy (r = .31; p = .16) suggesting preliminary evidence for recovery of cognitive function with abstinence.
Table 3.
Simple relationships between neuropsychological components and length of abstinence from marijuana (MJ users only)
| Length of abstinence from MJ (n = 23) |
P | |
|---|---|---|
| CVLT-II Immediate Memory | .33 | .12 |
| CVLT-II Delayed Memory | .25 | .25 |
| CVLT-II Recognition Memory | .25 | .25 |
| TMT-B Sequencing Ability/Psychomotor Speed |
−.03 | .87 |
| Ruff 2 & 7 Sustained Attention Speed | .02 | .95 |
| Ruff 2 & 7 Sustained Attention Accuracy | .31 | .16 |
| Verbal Fluency Total Correct | −.28 | .19 |
| Design Fluency Total Correct | −.26 | .22 |
| Design Fluency Accuracy | −.51 | .02 |
| Cognitive Inhibition Total Correct | −.10 | .62 |
| Cognitive Inhibition Accuracy | −.19 | .36 |
Note. CVLT-2 = California Verbal Learning Test-2nd Edition; TMT-B = Trail Making Test.
DISCUSSION
Approximately one-third of young adults have used MJ within the past year (Johnston et al., 2009) and for the majority of the world, the age of onset of MJ use is during the teenage years (Degenhardt et al., 2008). Animal and human research suggest that adolescence may be a vulnerable period for negative environmental impact on brain health due to critical neurodevelomental processes (see Spear, 2010). The primary goal of the current study was to examine whether past year MJ exposure was associated with neuropsychological functioning in a sample of older adolescents and emerging adults following a minimum of 1 week of abstinence. The primary finding was that after controlling for reading ability, gender, depressive symptoms, body mass index, and alcohol and other drug use, increased past year MJ exposure was independently associated with poorer psychomotor speed/sequencing ability, sustained attention efficiency, and cognitive inhibition in a dose-dependent manner in otherwise healthy young adults. The effect sizes were in the medium range (f2 = .09–.12). Male MJ users demonstrated more robust relationship between increased MJ use and poorer psychomotor speed/sequencing ability (large effect size, f2 = .20) compared to females.
These results buildings upon previous studies that have reported subtle cognitive deficits associated with MJ use in late adolescence, primarily including processing speed (Fried et al., 2005; Medina, Hanson, et al., 2007), attention (Hanson et al., 2010; Harvey et al., 2007; Medina, Hanson, et al., 2007), and executive functioning (Hanson et al., 2010; Harvey et al., 2007; Medina, Hanson, et al., 2007). In contrast with previous findings (Fried et al., 2005; Hanson et al., 2010; Harvey et al., 2007; Medina, Hanson, et al., 2007; Schwartz et al., 1989), the current study did not find a dose-dependent relationship between MJ exposure and memory functioning, which was instead predicted by past year alcohol use. These discrepant results may be due to differences in length of abstinence across the samples or differential levels of alcohol exposure. Hanson and colleagues (2010) found significant improvements in verbal list learning following 2 weeks of abstinence in adolescent MJ users and our sample had an average of 50 days of abstinence (range, 7–407 days based on self-report). Indeed, our study found medium sized correlation between superior immediate verbal memory and length of abstinence, suggesting some evidence for recovery of function. Furthermore, the current study did not include a story memory paradigm, which was shown to be impaired following 1 month of abstinence in adolescent MJ users (Medina, Hanson, et al., 2007).
The psychomotor speed/sequencing ability deficits were particularly present in the male MJ users, even though they had similar levels of MJ use and had a later age of onset of regular MJ use. Thus, it may be possible that males are particularly vulnerable to white matter changes leading to slower psychomotor speed. This result is somewhat in contrast with our prior studies finding increased PFC and amygdala volumes only in females MJ users, suggesting a disrupting of the pruning process (Medina et al., 2008; McQueeny et al., 2011). However, Bava and colleagues (2009) reported reduced white matter integrity in a primarily male sample of adolescent MJ users (Bava et al., 2009); therefore, future research is necessary to examine whether similar findings are seen in female MJ users. Given developmental differences in CB1 sensitivity (Burston et al., 2010) and the timing and rate of neurodevelopment between genders (Lenroot & Giedd, 2010), it is possible that chronic MJ exposure differentially impacts gray and white matter development in males and females.
In general, this neuropsychological profile is consistent with the brain structural findings reported in adolescent and emerging adult MJ users, including abnormal prefrontal cortex (PFC) and cerebellar structure (Jarvis et al., 2008; Medina et al., 2009, 2010), aberrant hippocampal-memory relationships (Medina, Schweinsburg, et al., 2007), reduced frontal-parietal white matter integrity (Bava et al., 2009), and altered white matter metabolite levels suggesting glial cell disruption (Silveri et al., 2011). Functional MRI studies on teenage MJ users have primarily reported abnormal PFC, limbic, cerebellar and parietal activation patterns in MJ users compared to controls in response to cognitive inhibition (Tapert et al., 2007), verbal working memory (Jacobsen et al., 2007; Jager et al., 2010), and spatial working memory (Schweinsburg et al., 2008) tasks. It is of note that the majority of these studies included primarily males, so further research is needed to examine potential gender differences in these MJ effects. Interestingly, the current study did not report verbal memory deficits, suggesting that the medial temporal lobe and hippocampal regions may be relatively spared compared to the more distributed fronto-cerebellar, fronto-parietal, and fronto-subcortical networks, which is consistent with a recent finding by Schweinsburg and colleagues (2011). Given these neuropsychological findings, longitudinal studies using multimodal imaging techniques with MRI and diffusion tensor imaging are needed to understand the impact of heavy MJ exposure on underlying gray and white matter development in males and females throughout adolescence and young adulthood.
It is important to note some limitations of this research. Risk factors associated with early drug experimentation (such as poor cognitive inhibition, attention problems, conduct disorder, and family history of substance use disorders) are themselves related to subtle cognitive and brain abnormalities (Aronowitz et al., 1994; Hanson, Medina, et al., 2010; Hill, Muddasani, et al., 2007; Hill, Kostelnik, et al., 2007; Nigg et al., 2004; Ridenour et al., 2009; Schweinsburg et al., 2004; Spadoni, Norman, Schweinsburg, & Tapert, 2008; Tapert & Brown, 2000; Tapert, Baretta et al., 2002). Therefore, longitudinal research in youth before MJ exposure is needed to explore the influence of early drug use on adolescent neurodevelopment. Second, results may not generalize to other samples with different lengths of abstinence, patterns of substance use, gender or ethnic distribution, or SES. On balance, the current results may not generalize to heavier users who are unwilling or unable to abstain from MJ use for 7 days. Third, nicotine use was not thoroughly addressed in this sample and nicotine users were required to remain abstinent for at least an hour before the cognitive testing. Because nicotine withdrawal may affect cognitive variables, especially immediate memory and attention (Jacobsen et al., 2007; Fried, Watkinson, & Gray, 2006), future studies will need to rule out the influence of nicotine use on the current results. Finally, we did not use drug toxicology testing when assessing length of abstinence. Still, every attempt was made to maximize the reliability of the self-reported use and abstinence—including guaranteed confidentiality, privacy, and the fact that the last date of use was assessed on two separate occasions. Furthermore, the Time Line Follow-Back technique which has shown high re-test reliability, high convergent and discriminant validity compared, high agreement with informants and with patient’s urine assays (Fals-Stewart, O’Farrell, Freitas, McFarlin, & Rutligliano, 2000). Still, future studies will need to incorporate monitored abstinence to rule out potential influence of acute usage and withdrawal on cognitive performance.
Although the cognitive effects of MJ exposure may have been subtle, considering that almost half of high school seniors have tried MJ (Johnston et al., 2010), even small reductions in cognitive functioning are of concern. Furthermore, combined negative impacts of acute drug and alcohol use (such as hangovers), sleep deprivation caused by drug use (Cohen-Zion et al., 2009), and chronic effects on brain structure may lead to even more pronounced cognitive problems in current MJ using youth. Subtle brain abnormalities and cognitive deficits in adolescents and young adults may lead to important psychosocial consequences. Students may miss information presented in class due to poorer processing speed, sustained attention and executive functioning. This cognitive disadvantage may lead to lower than expected school performance, risky decision-making, and poorer emotional regulation (Kloos, Weller, Chan, & Weller, 2009). Given these concerns, it is critical to disseminate these research findings to clinicians, teachers, school administrators, pediatricians, and parents in an effort to prevent heavy drug use.
In conclusion, the results suggested that, after a minimum of 1 week of abstinence, increased past year MJ use was significantly associated in a dose-dependent manner with poorer sequencing ability/psychomotor speed, sustained attention, and cognitive inhibition in teens and young adults. Males appeared to be more susceptible to MJ-associated cognitive slowing. Further research is necessary to examine the impact of age of onset of regular MJ use and to examine whether recovery of cognitive function occurs with sustained abstinence. Given the current findings, research examining the efficacy of programs aimed at treating marijuana use disorders and improving neurocognition, including approaches such as exercise (Crews, Nixon, & Wilkie, 2004; Leasure & Nixon, 2010; Pereira et al., 2007), in MJ using emerging adults needs further development.
ACKNOWLEDGMENTS
The information in this manuscript and the manuscript itself has never been published either electronically or in print. This research was supported by NIDA (R03DA027457-01 and R01DA030354; PI: K.M. Lisdahl, aka K.L. Medina) and the University of Cincinnati Center for Environmental Genetics pilot grant (P30 ES006096; PI: K.M. Lisdahl, aka K.L. Medina). The authors thank Tim McQueeny and Claudia Padula for their assistance in running the research protocols. Finally, the authors would like to acknowledge the vast scientific contributions of our collaborators and undergraduate research assistants in the UWM Brain Imaging and Neuropsychology (BraIN) Laboratory.
Footnotes
All authors reported no financial interests or potential conflicts of interest.
REFERENCES
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT. Corpus callosum damage in heavy marijuana use: Preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage. 2008;41(3):1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Aronowitz B, Liebowitz MR, Hollander E, Fazzini E, Durlach-Misteli C, Frenkel M, DelBene D. Neuropsychiatric and neuropsychological findings in conduct disorder and attention-deficit hyperactivity disorder. Journal of Neuropsychiatry & Clinical Neurosciences. 1994;6:245–249. doi: 10.1176/jnp.6.3.245. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43(3):189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Kumra S. White matter development during late adolescence in healthy males: A cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35(2):501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Reiss AL. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cerebral Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Kaplan RF, Hesselbrock VM. P300 and the Stroop effect in overweight minority adolescents. Neuropsychobiology. 2010;61(4):180–187. doi: 10.1159/000297735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research. 2009;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory-2nd edition. Psychological Corporation; New York: 1996. [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset marijuana use on functional brain correlates of working memory. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34(6):837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol. 1995;17(1):25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selly LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated delta9-tetrahydrocannabinol exposure. British Journal of Pharmacology. 2010;161:103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta(9)-THC on learning in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2006;83(3):448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Cohen-Zion M, Drummong SPA, Padula CB, Winward J, Kanady J, Medina KL, Tapert SF. Sleep Architecture in Adolescent marijuana and Alcohol Users during Acute and Extended Abstinence. Addictive Behaviors. 2009;34(11):967–969. doi: 10.1016/j.addbeh.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33(1):63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, Wells JE. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO World Mental Health Surveys. PLoS Medicine. 2008;5(7):e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Jacobson M, Bondi MW, Hamilton JM, Salmon DP. The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society. 2003;9:936–946. doi: 10.1017/S1355617703960139. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E. Delis-Kaplan Executive Functioning Scale Manual. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test- Second edition. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Hoehe MR. Specific attentional dysfunction in adults following early start of marijuana use. Psychopharmacology. 1999;142(3):295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutligliano P. The Timeline Followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- First Michael B, Spitzer Robert L, Gibbon Miriam, Williams Janet BW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute; (SCID-I/P) New York: Nov, 2002. [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana-a comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27(2):231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of cigarette smoking in young adults-a comparison with pre-drug performance. Neurotoxicology and Teratology. 2006;28(4):517–525. doi: 10.1016/j.ntt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: Ages 4-18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4-18 years. The Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. The Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. International Journal of Neuroscience. 2008;118(11):1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Nagel BJ, Spadoni AD, Gorlick A, Tapert SF. Hippocampal volumes in adolescents with and without a family history of alcoholism. The American Journal of Drug and Alcohol Abuse. 2010;36:16–167. doi: 10.3109/00952991003736397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. How does adolescent alcohol and drug use affect neuropsychological functioning in young adulthood?: 10-year outcomes. Journal of Child & Adolescent Substance Abuse. 2011;20:135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35(11):970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent marijuana use and cognition. Drug and Alcohol Review. 2007;26(3):309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, Keshavan M. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcoholism, Clinical and Experimental Research. 2007;31(12):2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2007;61(1):41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Thompson PM. The effects of physical activity, education, and body mass index on the aging brain. Human Brain Mapping. 2011;32(9):1371–1382. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent marijuana users. Biological Psychiatry. 2007;61(1):31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Marijuana use and memory brain function in adolescent boys: A cross-sectional multicenter functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(6):561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis K, DelBello MP, Mills N, Elman I, Strakowski SM, Adler CM. Neuroanatomic comparison of bipolar adolescents with and without cannabis use disorders. Journal of Child and Adolescent Psychopharmacology. 2008;18(6):557–563. doi: 10.1089/cap.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T, Gamst A. Changes in volume with age: Consistency and interpretation of observed effects. Neurobiology of Aging. 2005;26(9):1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2008. Volume II: College students and adults ages 19-50. National Institute on Drug Abuse; Bethesda, MD: 2009. p. 306. NIH Publication No. 09-7403. [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2009. National Institute on Drug Abuse; Bethesda, MD: 2010. NIH Publication No. 10-7583. [Google Scholar]
- Kang-Park MH, Wilson WA, Kuhn CM, Moore SD, Swartzwelder HS. Differential sensitivity of GABA A receptor-mediated IPSCs to cannabinoids in hippocampal slices from adolescent and adult rats. Journal of Neurophysiology. 2007;98(3):1223–1230. doi: 10.1152/jn.00091.2007. [DOI] [PubMed] [Google Scholar]
- Kloos A, Weller RA, Chan R, Weller EB. Gender differences in adolescent substance abuse. Current Psychiatry Reports. 2009;11(2):120–126. doi: 10.1007/s11920-009-0019-8. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Price JS. Greater body mass index is associated with poorer cognitive inhibition and sustained attention in healthy young adults. under review. [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcoholism, Clinical and Experimental Research. 2010;34(3):404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4th ed. Oxford University Press; New York: 2004. [Google Scholar]
- Manly JJ, Jacobs DM, Touradji P. Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society. 2002;8(3):341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- McQueeny TM, Padula C, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behavioural Brain Research. 2011;224(1):128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson K, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after 30 days of abstinence. Journal of the International Neuropsychological Society. 2007;13(5):807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson K, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism, Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang T, Tapert SF. Prefrontal morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addiction Biology. 2009;14(4):457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, McQueeny T, Park A, Tapert SF. Depressive symptoms in adolescents: Associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry. 2007;48(6):592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Cerebellar vermis abnormality in adolescent marijuana users. Psychiatry Research: Neuroimaging. 2010;182(2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Shear PK, Corcoran K. Ecstasy (MDMA) exposure and neuropsychological functioning: A polydrug perspective. Journal of the International Neuropsychological Society. 2005;11(6):1–13. doi: 10.1017/S1355617705050915. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Medina KL, Yoshii J, Schweinsburg AD, Moadab I, Tapert SF. Age related changes in prefrontal white matter volume across adolescence. Neuroreport. 2006;17(13):1427–1431. doi: 10.1097/01.wnr.0000233099.97784.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: Findings in early adolescence. Journal of Abnormal Psychology. 2004;113(2):302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Evans AC. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, McGregor IS. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33(5):1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Tarter RE, Reynolds M, Mezzich A, Kirisci L, Vanyukov M. Neurobehavior disinhibition, parental substance use disorder, neighborhood quality and development of cannabis use disorder in boys. Drug and Alcohol Dependence. 2009;102(1-3):71–77. doi: 10.1016/j.drugalcdep.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19(8):763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano’ D, Realini N, Guidali C, Braida D, Capurro V, Parolaro D. Chronic delta(9)-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33(11):2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Allen CC. Ruff 2 & 7 Selective Attention Test. Psychological Assessment Resources, Inc.; Odessa, Florida: 1996. [Google Scholar]
- Schneider M, Koch M. Chronic pubertal but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory and performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1790. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases in Children. 1989;143(10):1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research. 2008;163(1):40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010;42(3):401–412. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106(3):564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Jensen JE, Rosso IM, Sneider JT, Yurgelun-Todd DA. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Research. 2011;191(3):201–211. doi: 10.1016/j.pscychresns.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of corticalthickness and brain growth in normal children. The Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Developmental Medicine & Child Neurology. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcoholism, Clinical and Experimental Research. 2008;32(7):1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. Neuroscience and Biobehavioral Reviews. 2010;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Baratta MV, Abrantes AM, Brown SA. Attention dysfunction predicts substance involvement in community youths. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(6):680–686. doi: 10.1097/00004583-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence, and neuropsychological functioning in adolescence. Addiction. 2000;95:1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychology Society. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berlin) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Moreno E, Marco EM. Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav Pharmacol. 2005;16(5-6):353–362. doi: 10.1097/00008877-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Pradhan K. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity (Silver Spring) 2009;17(1):60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. Wide Range Achievement Test, 3rd Edition (WRAT-3) Manual. Wide Range, Inc.; Wilmington, DE: 1993. [Google Scholar]
- Wilkinson G. Wide Range Achievement Test, 4th edition (WRAT-4) manual. Wide Range, Inc.; Wilmington, DE: 2006. [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: A magnetic resonance and positron emission tomography study. Journal of Addictive Diseases. 2000;19(1):1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]