Abstract
Rtp801, a stress – related protein triggered by adverse environmental conditions, inhibits mTOR and enhances oxidative stress – dependent cell death. We postulated that Rtp801 acts as potential amplifying switch in the development of cigarette smoke – induced lung injury, leading to emphysema. Rtp801 was overexpressed in human emphysematous lungs and in lungs of mice exposed to cigarette smoke. The upregulation of Rtp801 expression by cigarette smoke in the lung relied on oxidative stress – dependent activation of the CCAAT response element. Rtp801 was necessary and sufficient for NF – κ B activation in cultured cells and, when forcefully expressed in mouse lungs, it promoted NF – kB activation, alveolar inflammation, oxidative stress, and apoptosis of alveolar septal cells. On the other hand, Rtp801 − / − mice were markedly protected against acute cigarette smoke – induced lung injury, partly via increased mTOR signaling, and, when exposed chronically, against emphysema. Our data support the notion that Rtp801 may represent an important molecular sensor and mediator of lung injury to cigarette smoke.
Keywords: Rtp801, cigarette smoke, oxidative stress, apoptosis, inflammation, NF –κB, rapamycin
Introduction
Emphysematous lung destruction is a major component of chronic obstructive pulmonary disease (COPD), a highly prevalent and morbid disease predominantly caused by exposure to cigarette smoke (CSk) and environmental pollutants 1. Lung inflammation and excessive extracellular matrix proteolysis have been implicated as mediators of lung injury by CSk. CSk – triggered oxidative stress initiates lung inflammation and progressively disrupts cellular signaling involved in maintenance of lung integrity, eventually leading to organ aging and cell senescence 2. Amplification of these processes by oxidants generated by inflammatory and parenchymal cells may enhance apoptotic septal destruction 3 and cellular autophagy 4, involving the action of endogenous mediators, such as ceramide 5–7.
Rtp801 8, also known as Redd1 (for regulated in development and DNA damage responses) 9, was identified based on its induction by hypoxia 8 or DNA damage 9. Brain ischemia leads to upregulated neuronal Rtp801 expression 8 and induction of RTP801 in the retinal inner nuclear cell layer is required for neural retina and central vessel endothelial cell death in mice with retinopathy of prematurity 10. Moreover, overexpression of Rtp801 causes apoptosis of cultured cells and lung cells in vivo 8, leading to enhanced oxidative stress 9. Rtp801 inhibits cell growth and protein synthesis directed by the mammalian target of rapamycin (mTOR) 11, 12 via activation of Tsc – 2 (tuberin), a negative regulator of mTOR 11, 13, 14. Decreased mTOR activity downregulates hypoxia inducible factor (HIF) – 1 – dependent vascular endothelial growth factor (VEGF), which has been linked to experimental and human emphysema 1, 15, 16.
In the present study, we addressed whether the axis Rtp801/mTOR might integrate environmental stresses due to CSk with the activation of inflammation and cell death signals, leading to lung tissue damage and emphysematous destruction.
Results
Rtp801 expression in human lung emphysema and in cigarette smoke – exposed mouse lungs
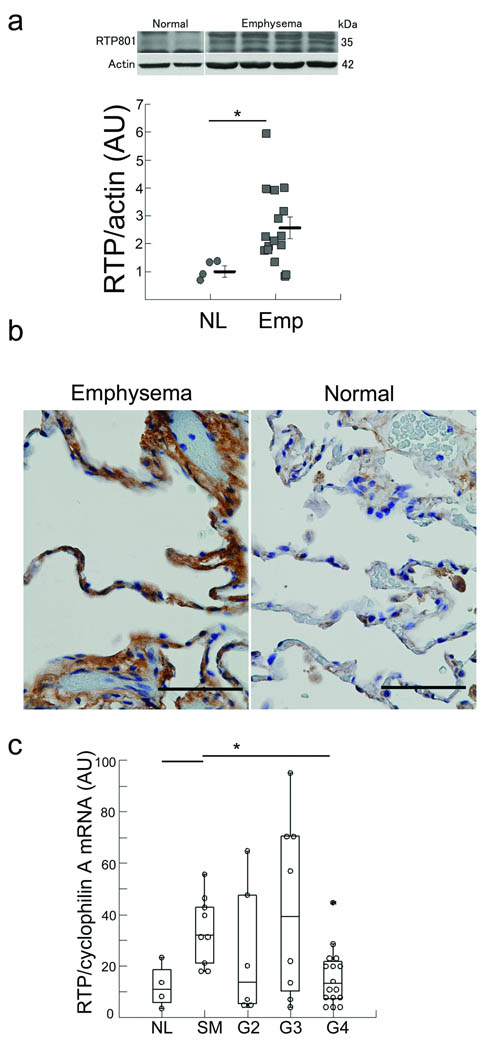
We found a significantly upregulated Rtp801 expression in lungs of patients with advanced emphysema compared with normal lungs (Fig. 1a), notably in alveolar septa of lungs with advanced emphysema when compared with normal lungs (Fig. 1b). Lungs of healthy smokers and patients with mild to moderate COPD had increased expression of Rtp801 mRNA levels, while lungs of patients with severe disease expressed similar levels of Rtp801 transcripts as normal non smoker’s lungs. (Fig. 1c and Supplementary Table 1). These findings suggest that Rtp801 may undergo posttranscriptional stabilization in lungs with advanced COPD, as recently shown with cultured cells exposed to hypoxia 17.
Figure 1. Enhanced expression of Rtp801 in human emphysematous lungs.
(a) Rtp801 expression in normal human lungs (lanes 1 and 2) or emphysematous lungs (GOLD 4) (lanes 3 – 6) (normalized by actin protein expression). (b) Histological sections showing increased expression of Rtp801 (brown) in a lung with emphysema (left) when compared with a normal lung (right) (arbitrary units (AU); n = 4 normal and 16 advanced emphysema lungs). (c) Determination of RTP801 mRNA expression in lungs of normal non smokers (n=8), normal smokers (n=13), and smoker patients with Gold stages 2 (n=12), 3 (n=12), and 4 (n=20) (normalized by cyclophilin A; signal intensity in arbitrary units (AU)).*: P < 0.05
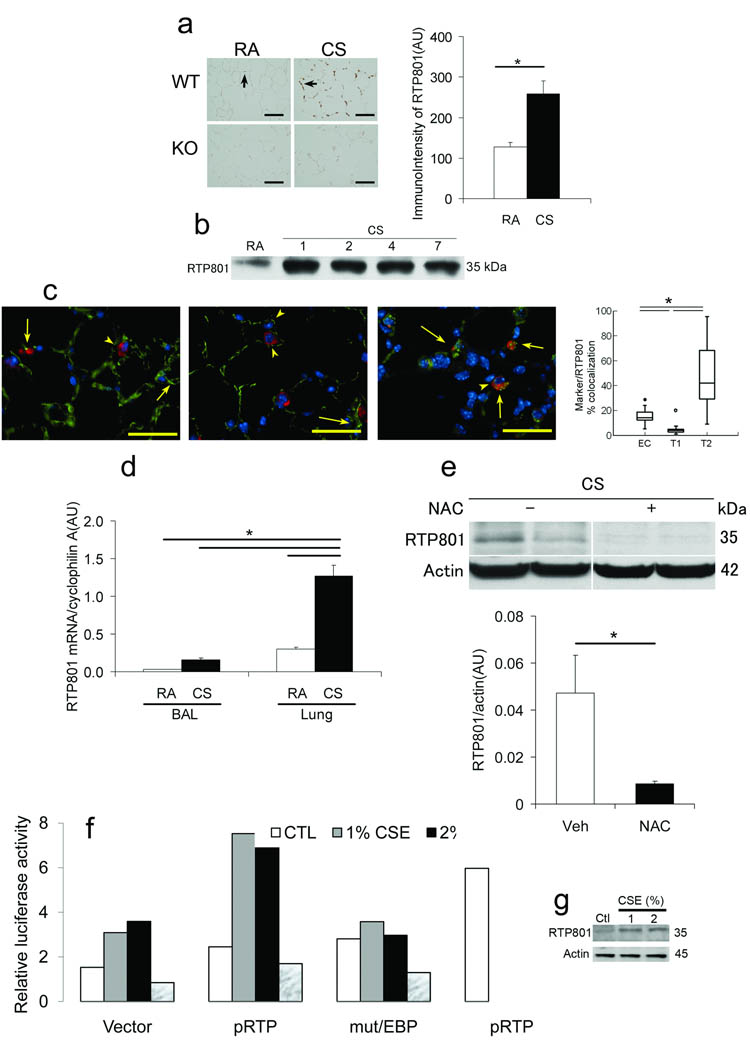
We tested whether Rtp801 expression may be upregulated by CSk – induced lung oxidative stress 18. Mice exposed to CSk for up to 7 days showed increased lung expression of Rtp801 in alveolar septa by immunohistochemistry (IHC) (Fig. 2a) and Western blot analyses (Fig. 2b). Alveolar type II pneumocytes showed the highest levels of Rtp801, followed by that of endothelial cells and minimal expression in type I pneumocytes (Fig. 2c). Of note, expression of Rtp801 appeared to predominate in alveolar septal cells rather than inflammatory cells based on the more modest expression of Rtp801 mRNA (Fig. 2d) and protein levels (data not shown) in cells obtained by bronchoalveolar lavage (Bal) (composed predominantly by inflammatory cells) and lack of the more sensitive IHC signal in macrophages. Mice exposed to CSk for 4 – 6 months also exhibited increased Rtp801 expression levels (Supplementary Fig. 1a) 19, 20.
Figure 2. Cigarette smoke – induced upregulation of Rtp801 expression occurs in lung septal but not resident or infiltrating inflammatory cells and relies on oxidative stress – dependent activation of the CCAAT promoter region.
(a) Lung Rtp801 expression (brown, arrows) in wildtype (upper) or Rtp801 − / − (lower) mice exposed to RA (left) or CSk (right) for 7 days (× 50 µm) and expression levels (AU; n = 3 and 7, respectively). (b) Lung Rtp801 protein expression levels in mice exposed from 0 to 7 days to CSk (pooled n = 3 lungs in each time point). (c) Lungs costained with Rtp801 (red, arrowheads), nuclei (DAPI, bue), endothelial cells (thombomodulin, left), type I epithelial cells (T1α, middle), type II cells (ProSpC, right) (all in green) in mice exposed to CSk for 1 day (superimposed red plus green shown in yellow, arrows). Percent colocalization of alveolar cell specific markers (Marker) (thrombomodulin, T1α, or ProSPC over Rtp801 expression (10 fields, n = 3 lungs/marker; × 50 µm). (d) Rtp801 mRNA expression levels in Bal and lung tissue in wildtype mice exposed to CSk for 1 day or ambient air controls (RA) (AU, n = 3 – 4 mice in each group). (e) Rtp801 expression levels in lungs of Rtp801 wildtype mice treated with NAC (500 mg/kg, i.p.) or vehicle (veh) and exposed to CSk for 1 day (normalized by actin, AU; n = 4 – 5 mice in each group). (f, g) Activity of intact 2.5 kb Rtp801 promoter or with a point mutation within CCAAT binding site (mut/CEBP) or pGL3 plasmid (Vector) – Firefly luciferase in mouse lung fibroblasts (MLF) exposed to media alone (CTL), CSE (1 or 2%), or NAC (10 mM) (positive control: H2O2, 250 µM; pRTP+H2O2) for 12 h (normalized by Renilla luciferase; data representative of 2 independent experiments). (g) Expression of Rtp801 in cells from (f). *: P < 0.05
Role of oxidative stress in cigarette smoke – induced Rtp801 expression
Consistent with the prior observation that oxidative stress induces Rtp801 expression in vitro 8, 21, the antioxidant N – Acetyl – l – Cysteine (NAC, 10mM) completely prevented lung Rtp801 up regulation caused by CSk exposure for 1 day (Fig. 2e). Consistent with these results, lungs of mice lacking the nuclear erythroid-related factor (Nrf) – 2, with heightened sensitivity to CSk – induced lung injury 19, showed increased Rtp801 mRNA signal when compared with wildtype littermates chronically exposed to CSk (Supplementary Fig. 1a). However, the resistant strain CD – 1 and the more sensitive strain C57Bl6/J had similar levels of Rtp801 mRNA when exposed to CSk for 1 day (Fig. 2 and data not shown).
The Rtp801 promoter contains regulatory elements that bind Elk, CCAAT/enhancer binding protein (C/EBP), HNF – 4, NF – κB, p53, and HIF – 1α transcription factors 21. The CSk induction of reporter plasmids 21 driven by the Rtp801 2.5 kB promoter 21 involved the activation of the CCAAT motif as a critical point mutation in this response element reduced CSk – dependent luciferase activity to baseline levels in mouse lung fibroblasts (MLF) (Fig. 2f), human bronchial epithelial cell line Beas2B (Supplementary Fig. 1b), and human fetal kidney epithelial line HEK293 cells (data not shown). Enhanced expression of endogenous Rtp801 in duplicate cell samples paralleled the induction of the Rtp801 – promoter (Fig. 2g and Supplementary Fig. 1c). NAC added with CSE prevented the activation of the Rtp801 promoter reporter construct in MLF (Fig. 2f).
Rtp801 overexpression and activation of NF – kB due to cigarette smoke
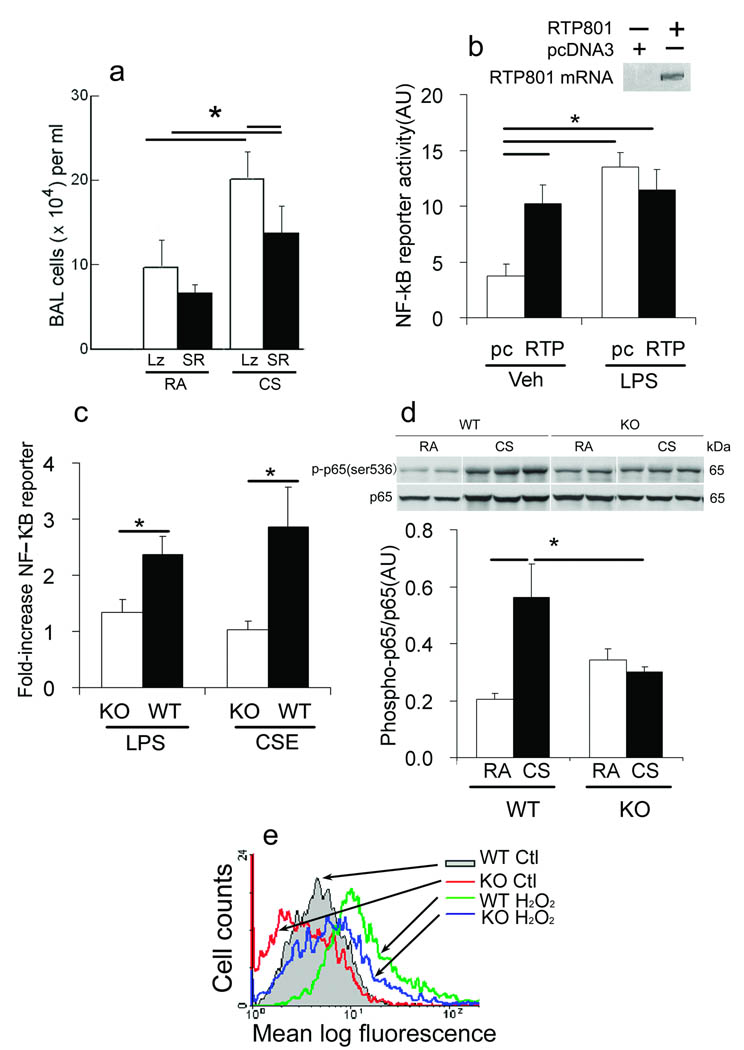
CSk – associated oxidative stress 22 may promote lung inflammation through NF – κB signaling 23. Accordingly, inhibition of NF – κB activation in lungs infected with adenovirus – IκBα super repressor 24 significantly (P<0.05) decreased lipopolysacharide (LPS – positive control) – and, notably, CSk – induced accumulation of inflammatory cells in the Bal (Fig. 3a and Supplementary Figs. 2a – c). However, expression of the IκBα super repressor had no effect on CSk – dependent induction of the oxidative stress markers 3 – nitrotyrosine (Supplementary Fig. 2d) and 8 – oxo – 2 deoxy – guanosine (data not shown) as compared with CSk – exposed control mice. The up regulation of Rtp801 mRNA levels driven by acute CSk exposure was not affected by expression of the IκB super repressor (Supplementary Fig. 2e). LPS, an activator of NF – κB, did not alter Rtp801 gene expression in challenged lungs when compared with PBS – instilled lungs, and, conversely, the IκB super repressor did not affect lung Rtp801 mRNA levels after treatment by LPS (Supplementary Fig. 2f) or in cultured A549 cells treated with CSE or TNF-α (Supplementary Figs. 2g,h). These data positioned the stress – dependent up regulation of Rtp801 by CSk upstream of NF – κB activation both in vivo and in vitro.
Figure 3. Rtp801 – dependent NF – κB activation by cigarette smoke.
(a) Total Bal cells in Rtp801 wildtype mice intratracheally transduced with AAV5 – expressing IκB super repressor (SR) or β – gal (Lz) for 7 – 10 days in ambient air (RA) or exposed to CSk for 1 day (n = 5 – 6 mice in each group). (b) Effect of transfection with RTP801 – (RTP) or empty vector pcDNA3.1+ (pc) on NF – kB – dependent reporter Firefly luciferase activity (lower) in LPS, 1 µg ml−1 or vehicle – (veh) treated rat lung endothelial cells (normalized by Renilla luciferase activity, AU; n = 3 independent experiments), and RTP801 mRNA expression in transfected cells (upper). (c) NF – κB reporter activity in wildtype (WT) or Rtp801 − / − (KO) MLF treated with LPS – (1 ug ml−1) or 1% CSE (normalized AU; n = 3 independent experiments). (d) Expression levels of phosphorylated Ser536 p65 NF – κB (p – p65 NF – κB) in whole lung lysates of Rtp801 wildtype and Rtp801 − / − mice, exposed to CSk for 1 day (normalized by total p65, n = 3 – 5 mice in each group). (e) Vehicle (Ctl) and H2O2 − induced (125 µM, 6 h) ROS levels in Rtp801 wildtype and Rtp801 − / − MEF. *: P < 0.05
Human RTP801 overexpression sufficed to activate NF – κB in rat lung microvascular endothelial cells to levels similar to those elicited by LPS in the same cells (Fig. 3b). Furthermore, Rtp801 was not only sufficient but also required for NF – κB activation since Rtp801 − / − MLF did not activate the NF – κB dependent promoter when stimulated with CSE or LPS (Fig. 3c).
In ambient room air (RA) conditions, Rtp801 − / − lungs showed a trend towards enhanced baseline Serine (Ser) 536 phosphorylation in p65 NF – κB (which closely correlates with NF – κB transcriptional activation 25). However, following exposure to CSk, only wildtype mice demonstrated a sizable NF – κB response with phosphorylation of p65 on Ser 536, whereas this response was abolished in Rtp801 − / − mice (Fig. 3d); MLF displayed similar CSE – induced NF – κB responses (Supplementary Fig. 3a). We also detected increased mRNA expression of the NF – κB – dependent chemokine macrophage inflammatory protein (MIP) – 2α (CXCL – 2) 26 in lungs from wildtype mice exposed to CSk, compared with similarly treated Rtp801 − / − mice (Supplementary Fig. 3b). The stress – and CSk – inducible p38MAPK was probably not involved induced NF – κB activation by CSk as the p38MAPK inhibitor SB239063 did not reduce levels of phosphorylation of p65 NF – κB in Ser536 or in Ser276 in mice exposed to CSk (Supplementary Figs. 3c–e).
Further supporting the observed link between oxidative stress and NF – κB activation26, Rtp801 − / − MEF contained lower levels of reactive oxygen species (ROS) than wildtype cells, either at baseline or following treatment with H2O2 from 6 to 24 h (Fig. 3e and Supplementary Fig. 3f). Likewise, the mouse lung epithelial cell line (MLE) – 15 27 showed decreased oxidative stress caused by H2O2 after transduction of a specific siRNA lowered Rtp801 mRNA levels by approximately 50% as compared with Rtp801 – replete cells treated with a scrambled siRNA (Supplementary Fig. 3g). These findings are in line with higher expression of the inducible anti – oxidant gene heme oxygenase (HO) – 1 mRNA in CSk – exposed Rtp801 − / − lungs compared with wildtype controls (see below and Supplementary Fig. 3).
Rtp801 overexpression in mouse lungs and alveolar injury
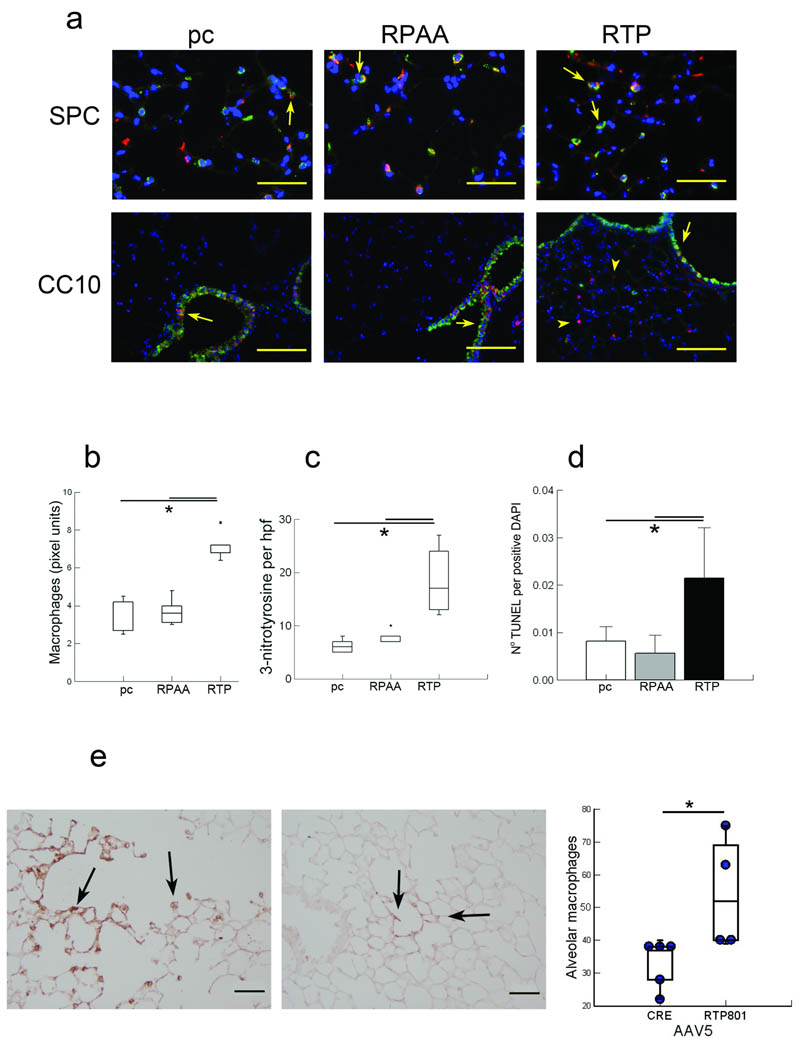
We addressed next whether forced overexpression of Rtp801 in mouse lungs elicits features similar to those caused by CSk exposure. Intratracheally – instilled human RTP801 cDNA led to increased Rtp801 expression, localized predominantly in alveolar type II cells rather than small airway cells (Fig. 4a), when compared with lungs instilled with the loss – of – function mutant Rtp801 – RPAA (which is unable to remove the inhibitory protein 14 – 3 – 3 from Tsc – 2 and consequently block TORC1 activity 28) or the empty vector pcDNA3. Up regulation of RTP801 triggered lung inflammation, oxidative stress, and alveolar cell death, (Figs. 4b – d and Supplementary Fig. 4a).
Figure 4. Forced in vivo overexpression of humanRTP801 in mouse lungs enhances oxidative stress, inflammation, and alveolar cell apoptosis.
Effect of human RTP801 – expressing (RTP, right), mutant Rtp801 – RPAA (RPAA, middle), or control pcDNA3.1 (pc, left) injected i.v. (24 h) in Rtp801 wildtype lungs costained (a) with RTP801 (red), type II cells (ProSPC, red, upper), or airway epithelial cells (CC10, green, lower) with coexpression highlighted by arrows (in yellow) (upper row: × 50 µm; lower row: × 200 µm). Numbers of infiltrating lung macrophages (b), 3 – nitrotyrosine positive cell profiles per high power field (hpf) (c), and alveolar cell apoptosis (TUNEL, normalized by DAPI – positive nuclei) (d) (10 fields, n = 5 – 10 mice in each group). (e) Infiltrating lung macrophages (brown, upper) in alveolar septa along an alveolar duct (arrows) in mice infected i.t. with AAV5 – RTP801 (left) or AAV5 – Cre (right) (negative control) for 4 weeks with quantification of numbers of alveolar macrophages per high power field (lower; 10 fields, n = 4 – 5 mice in each group). *: P < 0.05.
Intra – tracheal infection of wildtype mice with adenoassociated – virus serotype 5 (AAV5) – RTP801 (AAV5 infects lung epithelial cells for prolonged periods of time without causing inflammation 29 ) for 4 weeks augmented the numbers of macrophages in airspaces compared with negative control AAV – Cre transduced lungs (Fig. 4e). However, we did not observe significant (P>0.05) airspace enlargement, which is often seen with longer (i.e., 6 months) exposures to Csk (data not shown).
Rtp801 and cigarette smoke – induced acute and chronic pulmonary alveolar injury
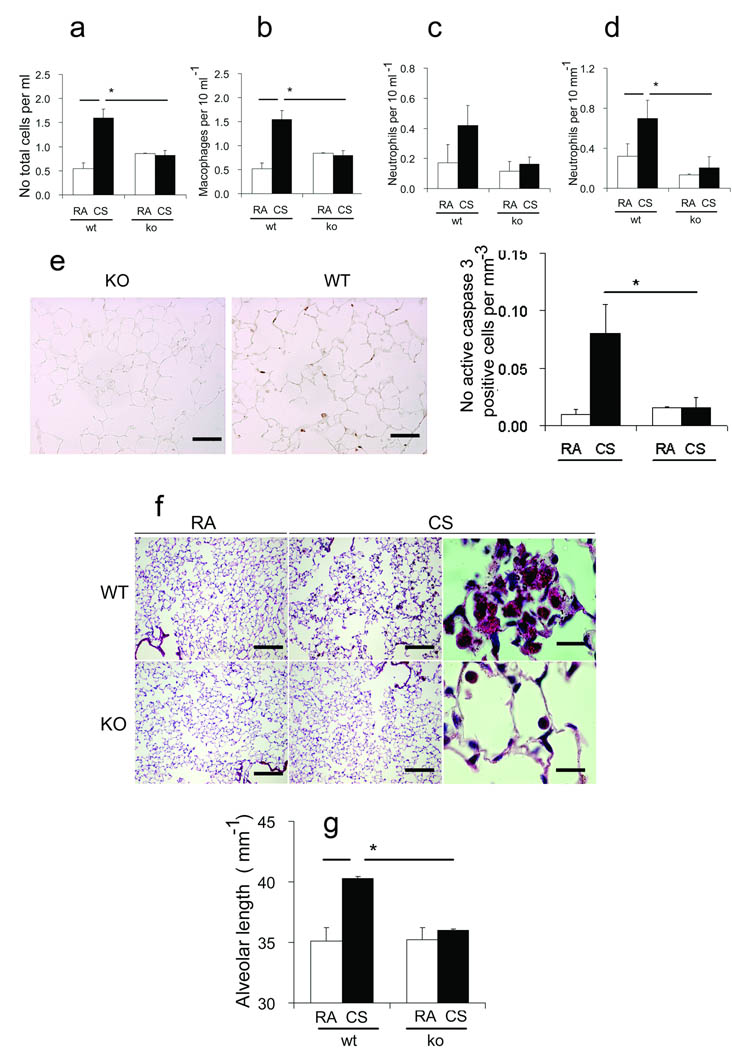
Rtp801 − / − mice have normal lung structure at 1, 2, 4, and 12 weeks when compared with wildtype littermates, based on analyses of lung sections stained with hematoxylin eosin, elastic, type II cell pro surfactant protein C expression, and terminal airway epithelial Clara cell antigen IHC (data not shown). Following the demonstration that forced RTP801 expression in mouse lungs resulted in oxidative stress, apoptosis, and inflammation, we next assessed whether Rtp801 expression is necessary for CSk-induced lung pathology. Rtp801 − / − mice, when exposed to CSk for up to 7 days, showed complete protection against acute inflammation, including reduced numbers of total cells (Fig. 5a), macrophages (Fig. 5b), and neutrophils (Fig. 5c) in Bal fluid and reduced neutrophil influx in lung parenchyma (Fig. 5d) when compared with similarly treated wildtype mice. Rtp801 − / − mice also displayed significantly reduced (P<0.05) numbers of apoptotic cells as assessed by IHC staining of activated caspase 3 (Fig. 5e) and poly – ADP ribose polymerase (PARP) cleavage in immunoblots (Supplementary Fig. 4b). Furthermore, when exposed to CSk, Rtp801 − / − mice had increased expression of Hif – 1α – dependent lung protective genes, such as VEGF120, and the glucose transporter (GLUT) – 1 mRNA (Supplementary Figs. 3i – j).
Figure 5. Rtp801 − / − mice are protected against cigarette smoke – induced pulmonary inflammation, apoptosis, and emphysema.
Bal total cell counts (a), including numbers of macrophages (b), and neutrophils (c), infiltrating neutrophils in alveolar lung tissue (d) (µm−1 alveolar length), active caspase 3 – positive cells (brown, arrows) (upper) (× 50 µm) and numbers of active caspase 3 – positive cells (µm−1 alveolar septa) (lower) in Rtp801 wildtype and Rtp801 − / − mice kept in RA or exposed to CSk for 7 days (n = 3 and 7 mice, respectively). Alveolar morphology in Rtp801 wildtype CSk for 6 months (f) showing airspace enlargement and large clusters of alveolar macrophages containing smoking pigment in the cytoplasm when compared with Rtp801 − / − mouse lungs (× 250 µm, right and middle; × 25 µm, left). Alveolar length represent mean linear intercepts (g) (n = 5 to 7 mice in each group). *: P < 0.05
Consistent with protection against pathology caused by short – term CSk exposure, Rtp801 − / − lungs had preserved alveolar structure after 6 months of smoking 30, with no increases in mean linear intercepts (vs. RA – exposed mice) and sparse accumulation of intraalveolar macrophages. On the other hand, wildtype mice exhibited the classic morphologic features of airspace enlargement and accumulation of alveolar macrophages filled with smoking pigment 30 (Figs. 5f,g). These findings were accompanied by decreased expression and nuclear accumulation of NF – κB in Rtp801 − / − when compared with wildtype lungs (Supplementary Fig. 4c). These results indicate that Rtp801 might have a critical role in the pathogenesis of experimental CSk – induced pulmonary injury and emphysema.
mTOR and protection of Rtp801 − / − mice against cigarette smoke – induced injury
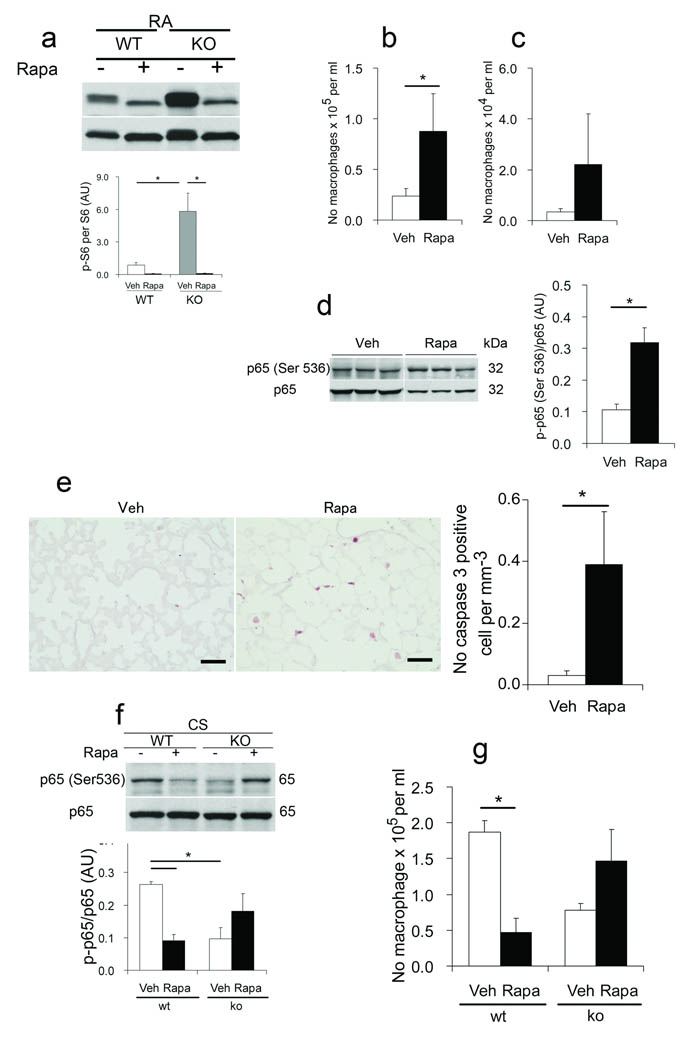
Rtp801 negatively regulates mTOR activity, via dissociation of the inhibitory protein 14 – 3 – 3 from Tsc – 2, leading to its blockade of mTOR11, 28, evidenced by decreased phosphorylation of S6 Kinase, its phosphorylation target S6, and 4EBP1 31. Accordingly, we found enhanced S6 phosphorylation on Ser235/236 (p-S6) in Rtp801 − / − RA – exposed lungs and MLF compared with wildtype lungs and MLF, respectively (Fig. 6a and Supplementary Figs. 5a,b). The selective mTORC1 inhibitor rapamycin pronouncedly blocked p-S6 in mouse lungs and MLF under control conditions (Fig. 6a and Supplementary Fig. 5b).
Figure 6. mTOR regulates lung homeostasis and inflammatory responses due to cigarette smoke in Rtp801 wildtype and Rtp801 − / − mice.
Effect of vehicle ( – ) or rapamycin (rapa) treatment on phosphorylated serine 235/236 S6 (p – S6) protein expression (normalized by S6, AU) (a), on inflammatory cell counts of Bal macrophages (b) and neutrophils (c), lung p – p65 NF – κB protein expression (d) (normalized by p65, AU), on lung expression of active caspase 3 (e) (brown, left, × 50 µm), and total counts (right) of active caspase 3 – positive cellular profiles (normalized by µm alveolar length) in RA – exposed C57Bl6 mice. Effect of vehicle or rapa treatment on p – p65 NF – kB expression (f) (normalized by total p65, AU) and numbers of macrophages (g) in Bal fluid from lungs of wildtype and Rtp801 − / − mice exposed to CSk for 1 day (10 fields, n = 4/5 mice in each group). *: P < 0.05).
To further define the potential function of mTOR in lung homeostasis in RA and in response to CSk, we investigated the influence of rapamycin on pulmonary inflammation and apoptosis in wildtype mice in RA and in wildtype and mice after exposure to CSk. CSk led to a marked increase in p-S6 in wildtype lungs (close to levels in Rtp801 − / − lungs) on day 1 when compared with RA (Supplementary Fig. 5a). In RA – kept wildtype mice, rapamycin led to increased inflammatory cells in Bal (Figs. 6 b,c) with lung enhanced expression of phosphorylated Ser536 p65 NFκB (Fig. 6d) and heightened numbers of apoptotic alveolar cells in lung parenchyma (Fig. 6e). Furthermore, rapamycin pretreatment partially abrogated the protection against CSk – induced inflammation observed in the Rtp801 − / − mice. Paradoxically, rapamycin decreased alveolar inflammation and phosphorylated Ser536 p65 NF– κB levels in wildtype mice exposed to CSk (Figs. 6 f,g). The aggregate of these findings indicates that mTOR activity contributes to maintenance of lung homeostasis under normal conditions and partly accounts for the resistance to CSk – induced alveolar inflammation in Rtp801 − / − mice.
Discussion
Our findings indicate that Rtp801 plays a critical role in the pathogenesis of alveolar injury caused by CSk, amplifying inflammatory and cell death responses by regulating negatively mTOR signaling and activating NF – κB. Enhanced NF – κB activation in vitro and particularly in vivo leads to expression of cytokines (e.g., MIP-2α 32) responsible for the recruitment of neutrophils and macrophages 33. Moreover, RTP801 also directs alveolar cell apoptosis due to acute CSk, which might further promote lung inflammation, oxidative stress, and extracellular matrix degradation 3. In rodents, acute CSk causes fragmentation of lung elastin 34, generating chemotactic elastin peptides 5, and a collagen – degradation product, the tripeptide, proline – glycin – proline (PGP), which binds to CXCR – 2 and stimulates neutrophil lung infiltration and alveolar destruction 7. Alveolar injury caused by acute CSk exposures might therefore have relevance to chronic emphysematous lung destruction. Our finding that type II and endothelial cells (rather than inflammatory cells) up regulate Rtp801 in response to CSk underscore their critical role in the pathogenesis of alveolar destruction in emphysema and cell specific responses that drive lung pathology in COPD. This central role of alveolar septal cells has been supported by models of emphysema caused by lung epithelial cell overexpression of cytokines 35 and by targeted apoptosis of lung capillary endothelial cells 36.
Oxidative stress is a potential key mechanism that links CSk with lung inflammation and tissue injury, including alveolar septal cell apoptosis 37. Activation of Rtp801 expression relies on oxidative stress and Rtp801 − / − enhances oxidative stress when overexpressed 8, 38. These unique dual properties position Rtp801 as a potential key amplifier of ROS in acute and chronic pathological conditions and determinant of tissue injury caused by CSk. Indeed, in the present study, Rtp801 overexpression in lung epithelial cells in vivo not only promoted septal cell apoptosis 8, but also enhanced lung macrophage infiltration. These properties may promote aseptic innate inflammation 39 and therefore contribute to persistence of inflammation in COPD despite smoking cessation, via stimulation of auto – immunity 40, 41. Moreover, Rtp801 appears not to directly affect Nrf – 2 signaling based on gene expression profiling of wildtype vs. Rtp801 − / − lungs exposed to CSk (Yoshida et al, unpublished observations). Of note, our results indicate that Rtp801 is both required and sufficient for the activation of NF – κB in vitro and in vivo by both CSk and LPS (no general abnormalities were seen in peripheral blood cells in Rtp801 − / − mice (EF, unpublished observation)), allowing for further amplification of oxidant generation by inflammatory and parenchymal cells 42. The finding that the mutant Rtp801 – RPPA, which does not activate Tsc – 2, did not increase lung injury when acutely overexpressed implies that inhibition of mTOR signaling may be mechanistically involved in CSk – triggered proinflammatory and apoptotic effects of Rtp801 43. Rtp801 may therefore link environmental stresses, the ensuing oxidative stress, with the activation of innate immunity via downregulation of mTOR signaling.
Consistent with the inhibitory role of RTP801 in mTOR signaling, the CSk – resistant Rtp801 − / − mice had increased lung mTOR signaling under normal conditions when compared with susceptible wildtype lungs. Of note, we also found that, when compared with wildtype mice, Rtp801 − / −lungs express higher levels of lung protective genes including VEGF121 and the inducible HO – 1, which produces the antioxidant and anti – inflammatory metabolites, i.e., carbon monoxide, biliverdin – IXα/bilirubin, and ferrous iron 44, 45. The protective role of enhanced mTOR in Rtp801 − / − lungs against acute CSk was disrupted by rapamycin, leading to alveolar inflammation and NF – κB phosphorylation in CSk – exposed mice. These results and the observed alveolar injury in wildtype mice by rapamycin in RA are in line with the described actions of rapamycin in promoting inflammation or apoptosis 46–48. However, mTOR is a critical signaling pathway not only in lung parenchymal cells but also in infiltrating inflammatory cells, possibly playing cell – and injury – context specific roles, which might lead to opposing effects in models of disease. The cell – specific (i.e., alveolar vs. inflammatory cells) regulation of RTP801 may explain the finding of increased p – S6 expression in wildtype lungs exposed to CSk. In contrast to the effects in Rtp801 − / − mice, rapamycin protected against acute CSk inflammation in Rtp801 wildtype mice. This beneficial effect of rapamycin may be due to blockade of mTOR activation in inflammatory cells by CSk – induced AKT phosphorylation (49 and Yoshida et al, unpublished observations) or IKKβ activation 50. These opposing roles of mTOR are supported by the observations that rapamycin causes lymphocytic pneumonitis in transplant recipients 51, while it protects against experimental asthma 52. The timing and lung cell targets of mTOR inhibition might therefore be critical to define its beneficial vs. pathological roles in disease (Supplementary Fig. 6).
In conclusion, our data provide a novel insight into the role of Rtp801 in stress – response sensing as an integrator of CSK – induced oxidative stress, NF – κB activation, and alveolar cell apoptosis, ultimately involved in the pathogenesis of emphysema. Genes, such as Rtp801, which are involved in environmental stress responses, particularly those converging in mTOR, may provide first – tier tissue responses prior to the action of inflammation, extracellular matrix proteolysis, or triggers of apoptosis.
Methods
Antibodies and reagents are outlined in the Supplementary Methods.
Human lungs
Human normal and diseased lung tissues were obtained, processed, and assessed histologically as previously described 6. Lung samples for RT – PCR were obtained from National Jewish Health (normals and smokers) and from the Lung Tissue Repository Consortium (Supplementary Table 1). The experimental protocol was approved by the Western Institutional Review Board in lieu of the Johns Hopkins University and the Colorado Human Subject Review Boards.
Animals
Male Rtp801 wildtype (C57BL/6 × 129SvEv F1) (Taconic) and Rtp801 − / − mice (C57BL/6 × 129SvEv, 2 to 6 months – old) (generated by Lexicon Genetics Inc. for Quark Pharmaceuticals Inc.) were used 10. All experiments conducted in mice were approved by the Animal Care Use Committees of The Johns Hopkins University, Harvard University, and University of Colorado.
Cigarette smoke exposure
Mice were divided into RA – exposed control or exposed to CSk (n = 3 – 7 mice in each group) as described 19. The antioxidant NAC (500 mg/Kg body weight) was intraperitoneally injected to wildtype mice twice (day 0 and 1) before initiation of CSk exposure for 1 day and mice were sacrificed just after smoking. Six months exposure to CSk was performed as described 30. Rtp801 expression was also investigated in lung samples from C57Bl/6 wildtype and Nrf – 2 − / − mice exposed to 4 – 6 months CSk 20.
RTP801 − / − cDNA and AAV – RTP801 constructs and administration to mice
Human RTP801 and Cre cDNAs were cloned into an intermediate vector flanked by AAV internal inverted repeats. Expression was confirmed by Western blot analysis of transfected HEK293 cells. AAV5 – hRTP801 and AAV5 – Cre viruses were prepared as previously described 53. Rtp801 wildtype mice received 2 × 1010 of AAV5 – hRTP801 or AAV5 – Cre viral particles via intratracheal instillation.
IκBα super repressor experiments
In vivo: RTP801 wildtype (C57BL/6 × 129SvEv or C57Bl6/J mice as LPS controls; 2 month – old) were instilled intratracheally with 1 × 109 plaque forming units of Adenovirus – 5 (Ad 5) IκB super repressor or Ad5 LacZ (in 50 µL of sterile PBS). Seven to ten days later, the mice were exposed to CSk. Controls consisted of RA – exposed mice. LPS was instilled in 50 µl sterile PBS (30 ug ml−1) and euthanized 16 h later. In vitro: A549 cells were infected with an adenovirus construct containing IκB super repressor (S32/36A) or lacZ at approximately 10 pfu per cell. Twenty four – 36 h later, 2 and 5% CSE for 6 h (defined in preliminary studies with concentrations varying from 1 to 10% for 6, 12, 18 and 24 h) and TNF-α (10 ng ml−1) for 1 hr as positive control were added.
Rapamycin experiments
In vivo: We used a protocol developed for rapamycin – induced blockade of experimental transplant rejection based on intraperitoneal administration 54, 55 or intratracheal instillation of rapamycin (1 mg/kg) or vehicle (1.8% DMSO) to wildtype and Rtp801 − / − mice for 7 days prior to exposure to Csk for 4 days or RA. In vitro: MLF were prepared as described and were pretreated with 10 nM of rapamycin for 3 days prior to CSE exposure, and then treated with 1% CSE for the indicated period.
Cell culture and cigarette smoke extract preparation
MLF and MEF were isolated from Rtp801 wildtype and Rtp801 − / − mice by collagenase digestion and were grown as outlined in the Supplementary Methods. Beas2B cells were cultured with bronchial epithelial basal supplement. MLFs and MEFs were used between the 3rd and 7th passages seven, while RLMEC and Beas2B were used up to passage 20. CSE was prepared as described previously 6.
Rtp801 and NF – κB promoter assays
For Rtp801 promoter assay, MLF, HEK293, and Beas2B were transfected with firefly luciferase expression vectors of the full – length Rtp801 2.5 kb promoter or with a point mutation within C/EBP binding site or with an empty vector (pGL3 basic) 21. Two independent experiments, were performed each in triplicate (the standard deviation for each individual measurement within the triplicates was up to 10% of the mean). Parallel cell lysates samples were prepared for Rtp801 Western blot analysis.
For NF – kB promoter assay, approximately 8~11 × 104 MLFs and rat lung microvascular endothelial cells were transfected with pHTS NF – kB reporter vector encoding the Firefly luciferase gene and/or hRTP801 (779 bp) plasmid DNA or pcDNA3.1 (+). Transfection conditions are detailed in the Supplementary Methods.
Bronchoalveolar lavage and alveolar morphometry 19, 30, immunohistochemistry and immunofluorescence 19, 56, Western blotting, Determination of intracellular ROS production and cell death assay, isolation of RNA and quantitative RT – PCR are detailed in the Supplementary Methods.
Statistical analysis
Data are represented mean ± SEM. Multiple comparison analyses were done by ANOVA with Tukey’s posthoc test or Kruskal – Wallis non – parametric ANOVA with Dunnet’s T3 posthoc test, or Student’s t – test or Wilcoxon rank – sum test, when involving two groups. Horizontal lines represent significant statistical (P < 0.05) comparisons among the listed (x – axis) experimental groups.
Supplementary Material
Acknowledgements
We thank Dr T. Stevens (Univ. of South Alabama) for providing the primary rat lung microvascular endothelial cells, Dr. J. Whitsett (Univ. of Cincinnati) for allowing us to use the MLE – 15 cells, Dr. T. Flotte and Mr. P. Cruz (Univ. of Florida and Massachusetts) for preparing the AAV5 vectors, Dr. Y. Chen (Univ. of Indiana) for the Rtp801 promoter constructs, Dr. I. Rahman (Univ. of Rochester) for sharing cigarette smoke – sensitive and resistant mouse lung specimens, Dr. D. Brenner (Univ. of California, San Diego) for the adenovirus IκB super repressor, Dr. L. Ellison (Dana Farber Cancer Center, Boston) for providing the Rtp801 – RPAA construct, and Dr. S. Reynolds (National Jewish Health) for the anti – mouse CC10 antibody. This work was supported by the grants R01 NHLBI 66554, Alpha 1 Foundation, Flight Attendant Medical Research Institute (FAMRI), and Quark Pharmaceuticals (to RMT), R01 NHLBI 077328 (IP), COPD SCCOR P01 – NHLBI 085609, Project 1 (to RMT and IP) and Project 2 (to SB), RO1 NHLBI RO1 081205 (SB), and NHLBI R01 HL082541 – 01 (SDS). Anonymous, non – transplanted, non – diseased lung samples were obtained from Tissue Transformation Technologies, Inc., Edison, NJ.
Footnotes
Authors’ contributions:
TY, EF, and RMT designed the experiments, analyzed the data, and composed the manuscript; IM, AKB, JB, MP, LZ, AG, UC, TM, AR, EB HA, NN, AG, and IP performed several of the in vitro and in vivo experiments; RKT, TR, TS and SB provided mouse lung samples for Rtp801 expression studies; CG provided normal human lung samples for Rtp801 expression studies; MM and SD performed the chronic exposure to cigarette smoke.
References
- 1.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. Cellular and Molecular Mechanisms of Alveolar Destruction in Emphysema: An Evolutionary Perspective. Proc Am Thorac Soc. 2006;3:503–510. doi: 10.1513/pats.200603-054MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuder RM, et al. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am. J. Respir. Cell Mol. Biol. 2003;29:88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- 4.Ryter SW, Chen ZH, Kim HP, Choi AM. Autophagy in chronic obstructive pulmonary disease: homeostatic or pathogenic mechanism? Autophagy. 2009;5:235–237. doi: 10.4161/auto.5.2.7495. [DOI] [PubMed] [Google Scholar]
- 5.Houghton AM, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–759. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrache I, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weathington NM, et al. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 8.Shoshani T, et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol. Cell. Biol. 2002;22:2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellisen LW, et al. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol. Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 10.Brafman A, et al. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol. Vis. Sci. 2004;45:3796–3805. doi: 10.1167/iovs.04-0052. [DOI] [PubMed] [Google Scholar]
- 11.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18:2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell Biol. 2005;25:5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- 15.Arsham AM, Howell JJ, Simon MC. A Novel Hypoxia-inducible Factor-independent Hypoxic Response Regulating Mammalian Target of Rapamycin and Its Targets. J Biol Chem. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- 16.Hudson CC, et al. Regulation of hypoxia-inducible factor 1 αexpression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katiyar S, et al. REDD1, an inhibitor of mTOR signalling, is regulated by the CUL4A-DDB1 ubiquitin ligase. EMBO Rep. 2009 doi: 10.1038/embor.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman I, et al. 4-Hydroxy-2-Nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 19.Rangasamy T, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sussan TE, et al. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Stringfield TM, Shi X, Chen Y. Arsenite induces a cell stress-response gene, RTP801, through reactive oxygen species and transcription factors Elk-1 and CCAAT/enhancer-binding protein. Biochem. J. 2005;392:93–102. doi: 10.1042/BJ20050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marwick JA, et al. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Resp Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 23.Vlahos R, et al. Differential protease, innate immunity, and NF-kappaB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am. J Physiol Lung Cell Mol. Physiol. 2006;290:L931–L945. doi: 10.1152/ajplung.00201.2005. [DOI] [PubMed] [Google Scholar]
- 24.Iimuro Y, et al. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin. Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell KJ, Perkins ND. Post-translational modification of RelA(p65) NF-kappaB. Biochem. Soc. Trans. 2004;32:1087–1089. doi: 10.1042/BST0321087. [DOI] [PubMed] [Google Scholar]
- 26.Zampetaki A, Mitsialis SA, Pfeilschifter J, Kourembanas S. Hypoxia induces macrophage inflammatory protein-2 (MIP-2) gene expression in murine macrophages via NF-κB: the prominent role of p42/ p44 and PI3 kinase pathways. FASEB J. 2004 doi: 10.1096/fj.03-0991fje. 03-0991fje. [DOI] [PubMed] [Google Scholar]
- 27.Wikenheiser KA, et al. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl. Acad. Sci. U. S. A. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flotte TR, Carter BJ. Adeno-associated virus vectors for gene therapy. Gene Ther. 1995;2:357–362. [PubMed] [Google Scholar]
- 30.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 31.Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol. Mol Biol Rev. 2005;69:79–100. doi: 10.1128/MMBR.69.1.79-100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi MM, Chong I, Godleski JJ, Paulauskis JD. Regulation of macrophage inflammatory protein-2 gene expression by oxidative stress in rat alveolar macrophages. Immunology. 1999;97:309–315. doi: 10.1046/j.1365-2567.1999.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerassimov A, et al. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am. J. Respir. Crit. Care Med. 2004;170:974–980. doi: 10.1164/rccm.200309-1270OC. [DOI] [PubMed] [Google Scholar]
- 34.Churg A, et al. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am. J. Respir. Crit. Care Med. 2003;167:1083–1089. doi: 10.1164/rccm.200212-1396OC. [DOI] [PubMed] [Google Scholar]
- 35.Elias JA, Kang MJ, Crouthers K, Homer R, Lee CG. State of the art. Mechanistic heterogeneity in chronic obstructive pulmonary disease: insights from transgenic mice. Proc Am Thorac Soc. 2006;3:494–498. doi: 10.1513/pats.200603-068MS. [DOI] [PubMed] [Google Scholar]
- 36.Giordano RJ, et al. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem. 2008;283:29447–29460. doi: 10.1074/jbc.M804595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuder RM, Petrache I, Elias JA, Voelkel NF, Henson PM. Apoptosis and Emphysema: The Missing Link. Am J Resp Cell Mol Biol. 2003;28:551–554. doi: 10.1165/rcmb.F269. [DOI] [PubMed] [Google Scholar]
- 38.Gery S, et al. RTP801 is a novel retinoic acid-responsive gene associated with myeloid differentiation. Exp. Hematol. 2007;35:572–578. doi: 10.1016/j.exphem.2007.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 41.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 42.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 43.Kang MJ, et al. Cigarette smoke selectively enhances viral PAMP and viruses-induced pulmonary innate immunity and remodeling responses. J Clin. Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HP, et al. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4:887–895. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 45.Gibbs PE, Maines MD. Biliverdin inhibits activation of NF-kappaB: reversal of inhibition by human biliverdin reductase. Int. J Cancer. 2007;121:2567–2574. doi: 10.1002/ijc.22978. [DOI] [PubMed] [Google Scholar]
- 46.Weichhart T, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 48.Bhaskar PT, et al. mTORC1 hyperactivity inhibits serum deprivation-induced apoptosis via increased hexokinase II and GLUT1 expression, sustained Mcl-1 expression, and glycogen synthase kinase 3beta inhibition. Mol Cell Biol. 2009;29:5136–5147. doi: 10.1128/MCB.01946-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q, Adiseshaiah P, Kalvakolanu DV, Reddy SP. A phosphatidylinositol 3-kinase-regulated Akt-independent signaling promotes cigarette smoke-induced FRA-1 expression. J Biol Chem. 2006;281:10174–10181. doi: 10.1074/jbc.M513008200. [DOI] [PubMed] [Google Scholar]
- 50.Lee DF, et al. IKK[beta] Suppression of TSC1 Links Inflammation and Tumor Angiogenesis via the mTOR Pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 51.Morelon E, Stern M, Kreis H. Interstitial pneumonitis associated with sirolimus therapy in renal-transplant recipients. N Engl J Med. 2000;343:225–226. doi: 10.1056/NEJM200007203430317. [DOI] [PubMed] [Google Scholar]
- 52.Fujitani Y, Trifilieff A. In vivo and in vitro effects of SAR 943, a rapamycin analogue, on airway inflammation and remodeling. Am. J Respir. Crit Care Med. 2003;167:193–198. doi: 10.1164/rccm.200205-455OC. [DOI] [PubMed] [Google Scholar]
- 53.Petrache I, et al. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuhaus P, Klupp J, Langrehr JM. mTOR inhibitors: an overview. Liver Transpl. 2001;7:473–484. doi: 10.1053/jlts.2001.24645. [DOI] [PubMed] [Google Scholar]
- 55.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 56.McGrath-Morrow SA, et al. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir. Cell Mol. Biol. 2005;32:420–427. doi: 10.1165/rcmb.2004-0287OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.