Abstract
We have identified Tspan33 as a gene encoding a transmembrane protein exhibiting a restricted expression pattern including expression in activated B cells. TSPAN33 is a member of the tetraspanin family. TSPAN33 is not expressed in resting B cells, but is strongly induced in primary human B cells following activation. Human 2E2 cells, a Burkitt’s lymphoma-derived B cell model of activation and differentiation, also upregulate TSPAN33 upon activation. TSPAN33 is expressed in several lymphomas including Hodgkin’s and Diffuse large B Cell Lymphoma. TSPAN33 is also expressed in some autoimmune diseases where B cells participate in the pathology, including rheumatoid arthritis patients, systemic lupus erythematosus (SLE), and in spleen B cells from MRL/Faslpr/lpr mice (a mouse model of SLE). We conclude that TSPAN33 may be used as a diagnostic biomarker or as a target for therapeutic antibodies for treatment of certain B cell lymphomas or autoimmune diseases.
Keywords: Tetraspanin 33, B cells, lymphoma, Lupus erythematosus, Rheumatoid arthritis, biomarker
1. Introduction
The discovery and characterization of lineage specific markers has been instrumental for the identification of cell subsets that underlie the complexity of the immune system. Cell surface markers, such as CD3ε (pan T cell marker), CD4 (helper T cells), CD8 (cytotoxic T cells), and B220/CD45R (B cells), are routinely used to differentiate lymphocyte populations [1–2]. Advances in flow cytometry labeling techniques led to the characterization of CD4 subtypes (Th1, Th2, Th17 and Treg cells) based on the detection of lineage-specific transcription factors [3]. The discovery of regulatory ‘B10 cells’ was based on the identification of a small subset of B cells that are CD1dhiCD5+ and secrete IL-10 [4–6]. In addition, lineage specific surface markers (such as the B cell marker CD20), represent useful targets for the development of therapeutic mAbs that have proven effective against various lymphomas as well as autoimmune diseases like Rheumatoid Arthritis (RA1) through their ability to delete pathogenic B cells [7–8].
1.1 TSPAN33 is a novel B cell activation marker
We sought to identify novel markers of human leukocytes. To this end, we analyzed a comprehensive database of human gene expression from 105 different human tissues including cells of the immune system (known as the Body Index of Gene Expression (BIGE) database) [9–10]. This database is useful for the identification of novel genes associated with specific organs or cells [11]. We identified a gene (Tspan33) that encodes a transmembrane protein not previously associated with B cells. The tetraspanin superfamily is defined by a conserved domain structure (Pfam00335) with a cysteine-rich long extracellular loop (LEL) containing a highly conserved cysteine-cysteine-glycine (CCG) motif [12]. These features facilitate the formation of large molecular complexes with other proteins, such as integrins or other tetraspanins and mediate diverse functions including proliferation, adhesion, motility, and differentiation. Some tetraspanins are widely expressed in adult tissues while others, (including CD82, CD151 and CD37), exhibit a more limited expression profile and are highly expressed in specific cell lineages of the immune system [13].
1.2 Previous reports on TSPAN33
TSPAN33 has been previously reported as Penumbra (proerythroblast nu membrane), since it was originally detected in a subpopulation of erythrocyte progenitors in murine bone marrow suggesting that it was involved in hematopoiesis [14]. Tspan33 expression in the mouse bone marrow was detected in the TER 119+ fraction of bone marrow cells (erythroblasts), but not in neutrophils, T cells, monocytes, NK cells, or (resting) B cells [14]. Indeed, it is expressed in mouse pre-CFU erythroid cells and in mouse bone marrow [15]. These results may be explained by the small contribution that these Tspan33+ erythrocyte progenitors make to total bone marrow RNA. Interestingly, Heikens et al. [14] generated a Tspan33−/− mouse, and some of these mice displayed abnormal erythropoiesis within 3 months and splenomegaly at 1 year of age. However, as we show here, the expression of TSPAN33 in normal human bone marrow is very low (Figure 1) and is instead specifically and strongly expressed by activated B lymphocytes.
Figure 1. TSPAN33 expression is restricted to activated B cells in normal human tissues.
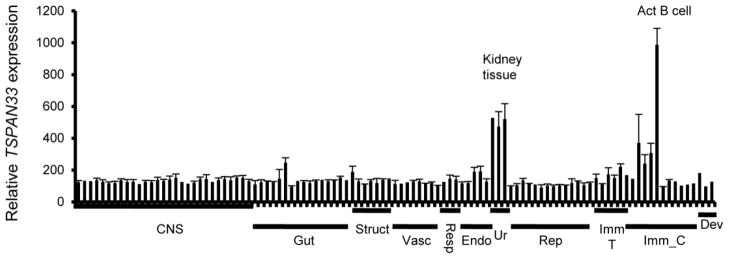
Affymetrix gene array (U133 plus 2.0) data compiled from the human body index of gene expression database observing TSPAN33 expression in normal human tissue (n= 8) and immune cells. X axis is organized by organ systems: CNS (central nervous system), Gut (gastrointestinal), Struct (structural), Vasc (vasculature), Resp (respiratory), Endo (endocrine), Ur (urinary), Rep (reproductive), Imm_T (immune tissue), Imm_C (immune cells), and Dev (developmental).
2.3 Approach
We have confirmed the expression of TSPAN33 in both mouse and human B cells. Taken together, these results indicate that TSPAN33 is a novel marker of activated B cells. In contrast to other B cell specific antigens (i.e. CD20, CD19) that are present on both resting and activated B cells, TSPAN33 is only expressed by activated B cells. We next sought to determine if TSPAN33 was also expressed in human diseases that involved activated malignant B cells. To this end we measured TSPAN33 expression in Hodgkin’s lymphoma (HL), various types of non-Hodgkin’s lymphoma (NHL), and in two autoimmune diseases, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
2. Materials and Methods
2.1 Microarray analyses
The generation of the Body Index of Gene Expression database (BIGE) has been described [9–10]. Briefly, total RNAs were obtained from 4 male and 4 female human donors, between 3–5 hours postmortem or augmented with commercially available human tissue RNAs (Clontech, Palo Alto, CA). Genome-wide gene expression data was obtained using Affymetrix Human Genome U133 Plus 2.0 gene arrays (Affymetrix, Santa Clara, CA) and data normalization, and summarization were done in ArrayAssist software (Iobion Labs, La Jolla, CA).
2.2 qRT-PCR
RNA was isolated from human cell lines/cells or tissue using the QiagenRNeasy® kit according to the manufacturer’s instructions (Qiagen, CA). The RNA was converted to cDNA using the QuantiTect® Reverse Transcription (Qiagen, CA). qPCR was performed using the Roche LightCycler® 480 Real-Time PCR system with probes designed to detect TSPAN33, CD19, CD20, CD138 and GAPDH (Roche, Pleasanton, CA).
2.3 Detection of TSPAN33 protein
Polyclonal rabbit antibodies against human beta actin (Santa Cruz biotech, Santa Cruz, CA), beta tubulin (MP Biomedicals, Santa Ana, CA) and Tspan33/TSPAN33 (Abcam, Cambridge, MA) were used for western blotting.
2.4 Cell lines
The human B cell line 2E2 has been described [16]. The human T cell line Jurkat, was obtained from the ATCC (American Type Culture Collection, Manassas, VA). The murine cell line A20-2J has been described [17]. All DLBCL lines were a kind gift of David Fruman (UC Irvine Institute for Immunology). PBMCs from human donors were isolated by Ficoll density gradient. Mouse spleen B cells were enriched using Ficoll density gradient separation followed by panning with anti-CD3 mAb (Biolegend, San Diego, CA) and anti-CD11c mAb (Biolegend) coated plates. Briefly, 10cm tissue culture plates were coated with anti-CD3 and anti-CD11c for 2 hours at 37°C. Splenocytes isolated by Ficoll density gradient separation were incubated on the coated plates for 2 hours and the non-adherent cells were collected and passed through a second round of enrichment.
2.5 Reagents
B cells were stimulated using either LPS (Sigma Aldrich, St Louis, MO) + mouse or human rIL-4 (Sigma), anti-CD40 mAb clone G38.5 (Invitrogen, Carlsbad, CA) + rIL-4 or CpG + pokeweed mitogen (PWM) + pansorbin (Sigma). T cells were stimulated using anti-CD3 mAb + anti-CD28 mAb (Biolegend) or phorbol 12-myristate 13-acetate (PMA) + ionomycin (Sigma).
2.6 Mice
C57Bl/6j (stock number 000664) and MRL/faslpr/lpr mice (stock number 000485) were obtained from the Jackson Laboratory (Bar Harbor, ME). All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Irvine.
2.7 Human samples
Human PBMC’s were obtained from peripheral blood by venipucture from Lupus patients or normal subjects. This protocol was approved by the lnstitutional Review Board (IRB) of the INNCMSZ and the samples were obtained following informed consent. Lupus patients fulfilled at least four 1982 American Rheumatism Association revised criteria for SLE [18]. Clinical disease activity was scored using the SLE Disease Activity Index or SLEDAI [19]. Controls had inactive disease (SLEDAI<3) and patients with active disease with indices above 3 were considered as having active disease. cDNA was prepared using the M-MLV reverse transcriptase according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA).
2.8 Tissue Array
Human tissue samples for immunohistochemistry were obtained from autopsies and represent archival samples from the Anatomy and Pathology Service of the University Hospital of the UANL. Tissue arrays were performed on normal human kidney or human lymphoma biopsies, including 6 HL patients, 6 Follicular lymphoma patients, 6 DLBCL patients, and 2 mantle cell lymphoma, following antigen retrieval (demasking) using protease and/or heat treatment as described [20]. Sections were then stained using anti-TSPAN33 antibodies followed by secondary donkey anti-rabbit IgG enzyme conjugates (Abcam).
2.9 Statistical analyses
The statistical significance was calculated using the student’s T-test. Values of p<0.05 were considered statistically significant. Error bars indicate standard deviation (SD).
3. Results
3.1 TSPAN33 is highly expressed in activated B cells
We identified TSPAN33 as a B cell activation-specific marker through the analysis of its expression in the BIGE database (Figure 1). Its expression profile indicates specific and restricted expression, with the highest levels observed in peripheral blood B cells activated with anti-CD40 and IL-4, followed by kidney (Table I lists the top ten sites of Tspan33 expression; the complete list is shown in supplementary information (SI 1)). The Tspan33 expression pattern from the BIGE database was confirmed using qRT-PCR on human RNAs (SI 2A) with low or undetectable expression in most other tissues including bone marrow, thymus and spleen.
Table 1.
Top ten sites of TSPAN33 expression in humans.
| Sample | Average Intensity |
|---|---|
| B cells, Activated | 985.4 |
| Kidney | 526 |
| Kidney Medulla | 519.1 |
| Kidney Cortex | 471.3 |
| B cells, Resting | 305.1 |
| Salivary Gland | 244.1 |
| Monocytes, Activated (LPS+IFNγ) | 238.5 |
| Tonsil | 218 |
| Pituitary Gland | 189.3 |
Table shows the top ten sites of TSPAN33 expression ranking from highest to lowest average intensity. The data is derived from the BIGE database shown in Figure 1.
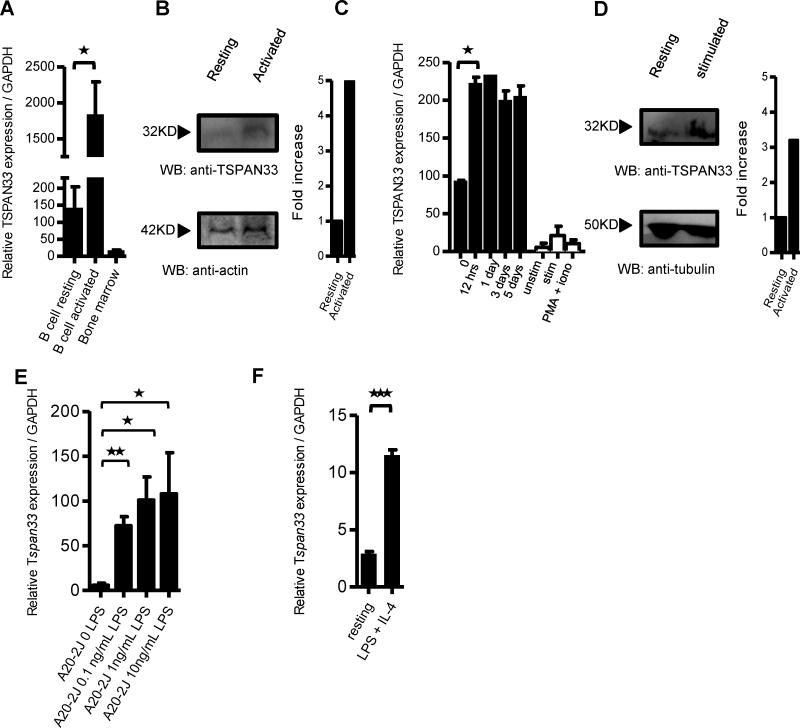
To confirm the microarray data, we performed qRT-PCR for Tspan33 mRNA on human B cells isolated from PBMCs, under resting or activating conditions (anti-CD40 + IL-4) as well as human bone marrow (Figure 2A). Although Tspan33 was initially identified as expressed in a subset of erythrocyte progenitors in mouse bone marrow [14], we did not detect significant Tspan33 expression in human bone marrow (Figure 2A). Tspan33 levels in activated B cells are over 40-fold higher than either resting B cells (p=0.0204) or whole bone marrow. Since Tspan33 has only recently been studied, there are not many reagents available (including antibodies). However, we obtained an anti-TSPAN33 polyclonal antibody (Abcam) that worked inWestern blot and immunohistochemistry (IHC)(following epitope retrieval) but not for FACS analyses (data not shown). Using this antibody, we observed a significant increase in TSPAN33 protein expression in activated human PBMCs (Figure 2B). Densitometric analyses revealed a ~5 fold increase in TSPAN33 protein expression in stimulated versus unstimulated PBMC samples.
Figure 2. TSPAN33 expression is restricted to activated B cells in mice and humans.
A) qRT-PCR of TSPAN33 expression in resting and activated (anti-CD40 + IL-4) human B lymphocytes purified from human blood compared to human bone marrow, n=3. B) Western blot of PBMC’s for TSPAN33 expression under resting and activating conditions with CpG + pokeweed mitogen (PWM) + pansorbin using actin as a loading control. Also shown are densitometric analyses. C) qRT-PCR of TSPAN33 expression over time in human 2E2 B cells (Black bars) with anti-CD40 mAb + IL-4 stimulation and human Jurkat T cells (White bars) under unstimulated, anti-CD3 + anti-CD28 mAb, or PMA + ionomycin stimulation for 12 hrs, n=3. D) Western blot of TSPAN33 expression in resting vs. activated human 2E2 B cells with anti-CD40 mAb + IL-4. Also shown are densitometric analyses. E) qRT-PCR of Tspan33 A20-2J B cells under resting and activating conditions with 0.1, 1, or 10 ng/mL of LPS + IL-4. F) qRT-PCR of resting or stimulated B cells enriched from C57BL/6 spleens with 10 ng/mL LPS + IL-4 for 12 hours, n=3,* p≤0.05, ** p≤0.01 and *** p≤0.001 indicate statistical significance according to Student’s t test. Data are representative of three independent experiments. Error bars indicate standard deviation (SD).
The human 2E2 B cell line is a model for inducible B cell activation and differentiation [16]. It expresses IgM and IgD in a non-stimulated state and it readily upregulates activation-induced cytidine deaminase (Aicda) to induce class switching to downstream isotypes (a measure of activation) [21–22] following stimulation with anti-CD40 mAb + IL-4. Using qRT-PCR we observed a significant increase in Tspan33 mRNA levels following stimulation with anti-CD40 + IL-4 for 12 hours compared with unstimulated 2E2 cells (p=0.013) (Figure 2C), and the elevated Tspan33 transcript levels remained high for up to 120 hours after stimulation. Conversely, Tspan33 expression was not detectable in resting, anti-CD3+ anti-CD28 or PMA + ionomycin-stimulated Jurkat cells (human T cell leukemia). The increased expression of Tspan33 in 2E2 cells was confirmed by western blot, with a >3 fold increase (by densitometry) observed when using a polyclonal anti-Tspan33 antibody (Figure 2D). Tspan33 expression was also measured in mouse tissue using qRT-PCR (SI 2B) and the results confirmed the human expression profile. We also observed a dose-dependent increase in Tspan33 mRNA expression in the murine B cell line A20-2J upon stimulation with increasing concentrations of LPS + IL-4 and measured by qRT-PCR (Figure 2E). Tspan33 transcription increased in A20-2J over 50 fold (p= 0.0014) with 0.1 ng/mL LPS + IL-4 stimulation and over 100 fold with 1 ng/mL or 10 ng/ml LPS + IL-4 (p= 0.011 and p=0.045). Additionally, mouse spleen B cells were isolated by Ficoll density gradient separation and enriched by panning with anti-CD3 and anti-CD11c [23]. The enriched B cells were stimulated with 10 ng/mL of LPS + IL-4 for 12 hours and analyzed for Tspan33 expression by qRT-PCR. As shown in Figure 2F, there was a ~4 fold increase in Tspan33 transcription following LPS stimulation compared to resting conditions (p= 0.00003). We should note that we also performed qRT-PCR on total mouse splenocytes under various stimulation conditions and significant upregulation of Tspan33 expression was observed when splenocytes were stimulated with CD40L +IL-4 or with anti-IgD+IL-4, but not with anti-CD3 + anti-CD28 (which stimulates T cells)(data not shown). Taken together, these results indicate that TSPAN33 is a novel marker of activated B cells in both mouse and human.
3.2 TSPAN33 is expressed by malignant B cells
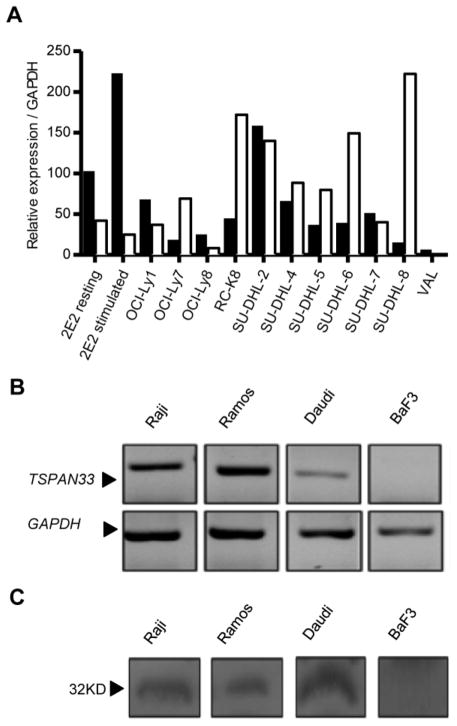
B cell activation markers are important as diagnostic tools, since elevated levels of some of these molecules, such as serum levels of sCD23, sCD27, sCD30, sCD44, CXCL13, IL-6 and IL-10 [24–25] have been reported to be associated with cancer (for example, NHL). Other known B cell antigens (i.e. CD19 and CD20) are also highly expressed in NHL [26]. We therefore hypothesized that TSPAN33 would also be expressed in human lymphomas. To test this, we performed qRT-PCR for Tspan33 expression and compared it to ms4a1 (CD20) in 11 lines including NHL cell lines characterized as DLBCL (OCI-LY1, OCI-LY7, OCI-LY8, RC-K8, SU-DHL-2, SU-DHL4, SU-DHL-5, SU-DHL-6, SU-DHL-7, and SU-DHL-8 and VAL), along with non-stimulated or stimulated (anti-CD40 mAb + IL-4) 2E2 cells (Figure 3A). DLBCL is the most common type of aggressive NHL and represents a heterogeneous group of lymphomas with a common characteristic of diffuse proliferation of large B cells with nuclei at least twice the size of normal lymphocytes [27]. DLBCL may include centroblast, immunoblast, or anaplastic variants (similar to highly activated Reed Sternberg cells of HL) and have a proliferative index of >90% [28]. ms4a1/CD20 mRNA levels were also measured to compare its expression with Tspan33 in these lymphoma cell lines. Both ms4a1/CD20 and Tspan33 were detected in all DLBCL lines. In fact, Tspan33 expression levels were comparable to CD20 in DLBCL.
Figure 3. TSPAN33 is expressed in human Hodgkin’s and non-Hodgkin’s lymphoma.
A) qRT-PCR was performed on several human NHL lines and measured for TSPAN33 (Black bars) vs. MS4A1/CD20 (White bars) expression. Samples were normalized to GAPDH. B) RT-PCR expression analysis corresponding to the large extracellular loop 33 (LEL) of TSPAN33 in human Burkitt’s lymphoma lines Raji, Ramos, and Daudi against BaF3 (a mouse pro-B cell line) compared to GAPDH. C) Western blot analysis of TSPAN33 expression of Raji, Ramos, Daudi, and BaF3 cells using a rabbit anti-TSPAN33 polyclonal antibody. Data are representative of three independent experiments.
In contrast to DLBCL, Burkitt’s lymphoma (BL) has a germinal center phenotype [21], including a CD10+, BCL6+ and BCL2+ distinct phenotype with round, medium-sized morphology, with a proliferative index of 100% [29] and may express CD20 [28]. To explore the expression of TSPAN33 in Burkitt’s lymphoma, we performed RT-PCR and western blotting on several Burkitt’s lymphoma lines including Raji, Ramos, and Daudi, as well as in mouse Baf3 cells (Pro-B cell line) as a control (Figures 3B and 3C). TSPAN33 expression was detected at both the mRNA and protein levels in all Burkitt’s lymphoma lines, but not in BaF3 cells. We conclude that TSPAN33 is also expressed in human Burkitt’s lymphoma.
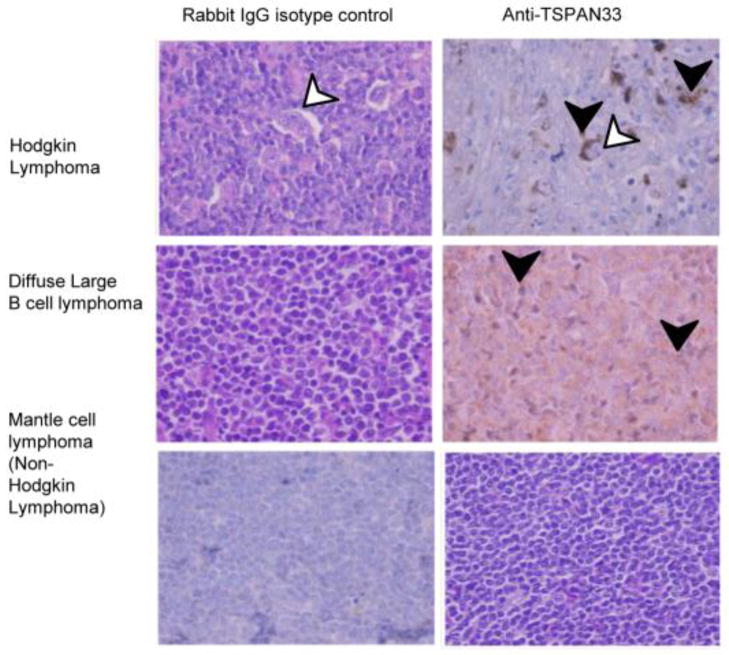
To further characterize TSPAN33 expression in other B cell lymphomas, we sought to perform immunohistochemistry (IHC) on tissue arrays prepared from biopsies of patients diagnosed with DLBCL (n=6), mantle cell lymphoma (another type of NHL, n=2), Follicular lymphoma (second most common type of indolent NHL, n=6) and HL (n=6). Table 2 and Figure 4 show representative images of lymph nodes from patients with HL, DLBCL, or mantle cell lymphomas. Tspan33 was highly expressed in Reed-Sternberg cells (a cell characteristic of Hodgkin’s Lymphoma) in HL, while DLBCL also stained positive for TSPAN33 uniformly, consistent with the qPCR data shown in Figure 3. Mantle cell lymphoma was negative for TSPAN33 staining. Reed-Sternberg cells are thought to be derived from germinal center B cells that have undergone somatic hypermutation and failed to undergo apoptosis, and therefore may represent an activated form of lymphoma [30]. DLBCL has been described above. Mantle cell lymphoma, on the other hand, is a type of mature CD5+ B cell lymphoma believed to originate from naïve, pre-germinal center lymphocytes, and may represent a form of non-activated B lymphocyte [31]. These differences in TSPAN33 levels may reflect the activation or differentiation state of each B cell lymphoma. On the other hand, the expression of TSPAN33 in each lymphoma suggests that it may represent another biomarker that could reflect the aggressiveness of each lymphoma or could be used as a prognostic factor [32–33].
Table 2.
TSPAN33 expression in human lymphomas.
| Case | TSPAN33 positive samples | Pattern of staining |
|---|---|---|
| HL | 6/6 | Localized to Reed Sternberg cells |
| DLBCL | 6/6 | uniform |
| Mantle cell lymphoma | 0/2 | negative |
Table shows the results from the IHC staining of TSPAN33 expression on tissue arrays taken human biopsies from individual patients diagnosed with HL (n=6), DLBCL (n=6), and mantle cell lymphoma (n=2). The total number of patients and staining pattern are also indicated.
Figure 4. TSPAN33 is expressed in human lymphomas.
Lymphoma biopsies were sectioned and stained with Hematoxilin/Eosin and anti-TSPAN33, followed by isotype or anti-rabbit IgG-HRP. White arrows indicate Reed-Stenberg cells and black arrows indicate positive TSPAN33-stained cells. Representative images from biopsies taken from patients diagnosed HL (n=6), DLBCL (n=6), and mantle cell lymphoma (n=2).
3.3 TSPAN33 is expressed in Systemic Lupus Erythematosus and Rheumatoid Arthritis lesions
Markers of B cell activation are also associated with certain autoimmune diseases. For example, CD25, HLA-DR, CD38, and BLyS are all elevated and associated with autoantibody production in clinical SLE [34–35]. Serum immunoglobulin levels and the B cell-associated cytokines IL-6, IL-21 and BLyS are all significantly elevated in patients with newly diagnosed RA [36–38]. Blocking BLyS reduces disease symptoms in MRL/faslpr/lpr mice (soluble TACI) [39] and also provides therapeutic benefit in humans (anti-BLyS mAb:Benlysta) [40]. To address the role of Tspan33 in autoimmune diseases, we measured Tspan33 mRNA expression in PBMCs from SLE patients, in RA synovial lesions or in a mouse model of SLE.
MRL/faslpr/lpr mice develop a spontaneous and progressive systemic autoimmune syndrome sharing many features with human SLE and RA, including dysregulated B cell activation, elevated antibody and autoantibody production, inflammation, and immune complex deposition in the kidney, which results in fatal glomerulonephritis [39–40]. The abnormal activation of B cells in MRL/faslpr/lpr mice and human SLE leads to elevated Aicda expression, resulting in pathogenic class-switched and hypermutated antibodies, which mediate tissue and organ damage [39, 41]. MRL/faslpr/lpr mice develop high titers of autoantibodies and severe kidney damage by 16 weeks of age [42]. Thus, B cells play important roles in lupus pathogenesis, through both antibody-dependent and antibody-independent mechanisms [43].
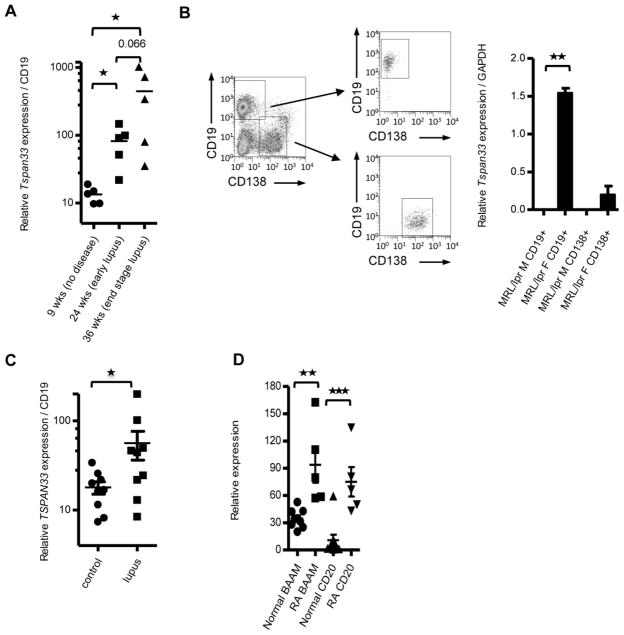
We measured Tspan33 mRNA expression in splenocytes from MRL/faslpr/lpr mice at 9, 24 and 36 weeks of age and normalized it to CD19 in order to explore the B cell contribution (Figure 5A). We found that 24-week-old MRL/faslpr/lpr mice, which already exhibit extensive Lupus symptoms including skin lesions, autoantibodies, and renal pathology, had a ~10-fold increase in Tspan33 mRNA expression when compared to their 9-week-old counterparts (p= 0.016), which did not yet show overt signs of pathology (although some B cells may already be activated at 9 weeks, MRL/faslpr/lpr display 90% mortality by 30 weeks of age, with the few surviving mice displaying particularly dysregulated levels of cytokines and chemokines) [42]. Tspan33 transcript expression in 36-week-old MRL/faslpr/lpr mice increased further (compared to 24-week-old mice), although this increase was not statistically significant (p= 0.062). Taken together, these observations strongly suggest an important role for TSPAN33 in the pathogenesis of SLE.
Figure 5. TSPAN33 is upregulated in B cell-associated autoimmunity.
A) qRT-PCR of Tspan33 expression of total splenocytes taken from MRL/faslpr/lpr mice normalized to CD19 expression. Mice ages 9 weeks old (no detectable pathology), 24 weeks old (lymphadenopathy with or without mild ear lesions) and 36 weeks old (lymphadenopathy with ear and face lesions) were compared for Tspan33 expression, n=5. B) qRT-PCR of Tspan33 expression in CD19+CD138− and CD19−CD138+ splenocytes from 11.5 week old female (lymphadenopathy) and 12.5 week old male (no pathology) MRL/faslpr/lpr mice, n=2. C) qRT-PCR of TSPAN33 expression analysis of PBMCs from human SLE patients or healthy controls, n=9. D) Microarray analysis of TSPAN33 vs MS4A1/CD20 expression in synovial membranes from healthy and RA patients. Synovial membranes were isolated from controls or RA patients as described [48]. The RNA was isolated from the membranes and analyzed for MS4A1/CD20 and TSPAN33 expression using the Affymetrix gene array U133 plus 2.0, n=9 healthy and n=5 RA patients, * p≤0.05, ** p≤0.01, ***p≤0.001 (Student’s t test). Data are representative of at least three independent experiments (A–C). Error bars indicate standard deviation (SD).
As B cells are not exclusively responsible for Lupus pathogenesis, we sought to determine whether TSPAN33 upregulation during Lupus disease in MRL/faslpr/lpr mice was associated with plasma cells. To address this, we FACS-sorted splenocytes from 12 week old male and female MRL/faslpr/lpr mice for CD19+ 138− B cells and CD19− CD138+ plasma cells and analyzed Tspan33 expression by qRT-PCR (Figure 5B). Tspan33 expression was significantly upregulated in CD19+ B cells from 12-week-old female MRL/faslpr/lpr mice (p= 0.004) over their male counterparts (similar to the human disease, females are more prone to lupus-like disease with an earlier onset than males in MRL/faslpr/lpr mice). Furthermore, Tspan33 was not expressed in CD138+ cells, indicating that its expression is restricted to activated B cells but does not extend to terminally differentiated B cells (plasma cells). Further support for this conclusion comes from the expression of plasma cell specific markers in the BIGE database. For example, B cell maturation antigen (BCMA) is a receptor for BLyS and APRIL expressed by plasma cells [44]. In the BIGE database, BCMA is strongly expressed in human tonsil, bronchus and trachea, indicating that these tissues contain significant numbers of plasma cells (data not shown); in contrast, TSPAN33 expression is low or absent in these tissues (Figure 1 and SI 1). We conclude that TSPAN33 is unlikely to be expressed by plasma cells. This is consistent with other markers of B cell activation that decrease upon differentiation into plasma/memory cells [45–47].
To confirm a possible role of TSPAN33 activation in human SLE, we measured the expression of Tspan33 mRNA by qRT-PCR in PBMCs from 9 healthy subjects or 9 SLE patients (Figure 5C). PBMCs from SLE patients had a >3 fold increase in Tspan33 mRNA expression (p= 0.038). These results indicate that TSPAN33 is elevated in human SLE.
We next sought to explore a possible role of activated B cells in RA. To this end, we analyzed TSPAN33 mRNA expression in a RA microarray database produced from synovial membranes of patients with this disease [48]. Levels of both TSPAN33 (p= 0.0019) and CD20 (p= 0.0008) transcripts were elevated in RA patients (Figure 5D). It has been reported that the top genes elevated in the RA synovial joint membranes include multiple markers of B cell activation, including immunoglobulin light and heavy chain genes, as well as genes that target B cells like BLyS and CXCL13 [48]. These observations are consistent with previous reports that have documented the role of activated B cells in RA lesions [49–50] as well as the fact that anti-CD20 (rituxan) is an effective treatment in RA [51–52].
3.4 TSPAN33 expression in the kidney
As shown in Figure 1A and SI 2 A, TSPAN33 mRNA is also detectable in the kidney by both microarray and qPCR. Given the important physiologic role of the kidney, we sought to determine the location of TSPAN33 expression within the kidneys. To this end, we performed immunohistochemistry to detect TSPAN33 in normal human kidney sections (Figure 6 A–D) including a section of renal tissue where lymphoid infiltrates are present (Figure 6A). TSPAN33 staining was detected in the proximal convoluted tubules, distal convoluted tubules and collecting ducts (Figure 6B–C) but not in infiltrating lymphocytes (a result consistent with previous experiments) or in the glomeruli. Higher magnification revealed that TSPAN33 is expressed at the apical membrane and granules of epithelial brush border cells of the proximal convoluted tubules (Figure 6D). These results support TSPAN33 as a target for therapeutic antibody development, because these sites are normally not accessible to antibodies.
Figure 6. TSPAN33 is expressed in the proximal, distal convoluted tubules and collecting duct but not in the kidney glomerulus.
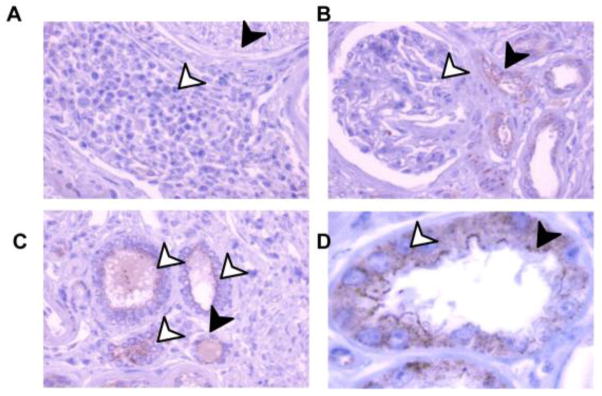
Kidney biopsies were stained for IHC in a tissue array. Samples were stained with H&E and anti-TSPAN33 or rabbit IgG isotype control, followed by anti-rabbit IgG-HRP. A) 40X magnification showing Lymphocytes (black arrows) and nerves (white arrows). B) 40X magnification showing proximal convoluted tubules (black arrows) and kidney glomeruli (white arrows). C) 40X magnification showing distal convoluted tubule (black arrows) and collecting duct (white arrows). D) 100X magnification of proximal convoluted tubule showing the apical surface (black arrow) and granules (white arrows).
4. Discussion
4.1 Overview
We have found that a member of the tetraspanin family (TSPAN 33) is a B cell activation marker because it is strongly expressed in activated B cells, and is also expressed in several lymphomas and in autoimmune diseases where pathogenic B cells are involved (including SLE and RA).
4.2 TSPAN33 as a novel B cell activation biomarker
A number of markers, including CD72, CD20, CD19, and CD24 are currently used to identify and track B cells [53]. Activated germinal center B cells have been reported to express a variety of genes, including GL7 [54], CD10 and BCL6 [55]. Other B cell activation markers such as MUM1/IRF4 and FOXP1, as well as CD23, CD69 and the systemic B cell activation markers CXCL13, sCD23, sCD27, sCD30, sCD44 have been used as markers in the diagnosis and risk assessment of NHL and RA [25, 32, 56]. Importantly, none of these activation markers are exclusively expressed on activated B cells, as they have also been associated with other immune cell types in the periphery. Therefore, TSPAN33 represents a B cell specific activation marker that may be useful as a diagnostic tool for diseases involving B cell activation. The likelihood of using TSPAN33 expression as a potential prognostic biomarker in both lymphoma and autoimmune diseases deserves further study [57–59].
4.3 TSPAN33 is a possible target for therapeutic mAbs against malignant B cells
In addition to a possible use of TSPAN33 as a B cell activation marker, TSPAN33 is the 33th member of the tetraspanin family (TSPAN33), and therefore a transmembrane protein. This makes TSPAN33 a suitable candidate for the production of anti-TSPAN33 mAbs for therapeutic purposes. CD20, a closely related protein now assigned to the membrane-spanning 4-domains superfamily (MS4A1), is an example of an important target for the production of therapeutic monoclonal antibodies that have proven effective for the treatment of B cell malignancies such as NHL, chronic lymphocytic leukemia (CLL) and also for certain autoimmune diseases including RA [51–52, 60]. However, since CD20 is expressed on both resting and activated B cells, anti-CD20 mAb therapy results in depletion of all B cells in the peripheral blood as well as 70% of B cells in the bone marrow [25, 36, 61]. Therefore the identification of a B cell marker restricted to activated B cells, such as TSPAN33, could represent an alternative strategy for the development of a “second generation” of mAbs for the treatment of B cell-associated pathologies [32]. Other tetraspanins (CD151) are being explored as possible therapeutic antibody targets [62]. Our data strongly suggest that anti-TSPAN33 therapeutic mAbs would have the important advantage of avoiding depletion of most resting B cells in the treated patients.
4.4 Other sites of TSPAN33 expression
TSPAN33 has been previously reported as Penumbra (Pro Erythroblast nu membrane) because it was originally identified as a molecule expressed in a small erythrocyte progenitor population in the bone marrow [14]. Given this expression pattern, it was described to play a role in hematopoiesis. Tspan33 −/− mice have been described [14] and some of them developed abnormal erythrocytes at 3 months of age. Acquired pure red cell aplasia is a related condition in humans where patients lack erythroblasts and depending on the cause may be self limiting [63]. These observations suggest that temporary inhibition of TSPAN33 in humans may have limited or manageable side effects. Another possible complication in the use of anti-TSPAN33 mAbs as human therapeutics is its expression in the kidney. Its expression pattern there, however, suggests that this will not represent a significant obstacle because Tspan33 is not expressed in the glomeruli (Figure 6B) but is instead expressed by epithelial cells in the proximal and distal convoluted tubules (Figures 6B–C). Access of antibodies to these sites is normally prevented by size exclusion, since only smaller molecular weight proteins (like albumin ~67KD or hemoglobin ~68KD) are permeable through the glomerular barrier [64]. TSPAN33 protein expression was observed in the apical surface and granules of the epithelial cells of the kidney and these cells are involved in secretion and absorption of small proteins, ions, and organic solutes (glucose and amino acids), suggesting that TSPAN33 may participate in vesicular trafficking and/or signaling during urine filtration [12]. Moreover, kidney epithelial cells have been reported to be refractory to biologically-based cytotoxic agents and kidney cell carcinomas are also resistant to ADCC (antibody dependent cellular cytotoxicity) [65]. Finally, a Tspan33 −/− mouse has been reported to be viable and fertile [14], indicating that absence of Tspan33 has limited physiological impact in kidney function.
4.5 The function of Tspan33 in B cell activation
Although the function of Tspan33 in B cells is currently unknown, the strong induction of Tspan33 expression upon B cell activation strongly suggests that it may be involved in B cell signaling/activation (i.e. CD9 and CD81), maturation/survival (i.e. CD37), or antigen presentation (i.e. CD63), since other B cell-expressed tetraspanins are known to participate in these processes [66–69].
4.6 Summary
We conclude that TSPAN33 represents a potentially important biomarker of activated and malignant B cells, as well as a potential target for the development of therapeutic mAbs for the treatment of several types of B cell lymphoma (DLBCL, BL, HL) as well as some autoimmune diseases associated with pathogenic B cells showing an activated B cell phenotype (SLE and RA).
Supplementary Material
Highlights.
TSPAN33 is a biomarker for activated B cells
TSPAN33 is expressed in certain Lymphomas
TSPAN33 is expressed in some autoimmune diseases (SLE and RA)
TSPAN33 is a potential diagnostic and therapeutic target of malignant B cells.
Acknowledgments
We thank David Fruman for his kind gift of B cell lymphoma lines. We also thank Janette Furuzawa-Carballeda and Carlos A Rodríguez-Osorio for their help with the human lupus samples. This work was funded in part by NSF IGERT LifeChips award #DGE-0549479, a collaborative grant from UCMEXUS, and an NIH NIAID grant R21096278 (to AZ).
The online version contains supplementary information
Footnotes
Abbreviations: BCMA, B cell Maturation Antigen; BIGE, Body Index of Gene Expression (database); TSPAN33, tetraspanin 33; BL, Burkitt’s lymphoma; RA, Rheumatoid arthritis; NHL, non-Hodgkin’s lymphoma; DLBCL, Diffuse large B cell lymphoma; HL, Hodgkin’s lymphoma; SLE, systemic lupus erythematosus.
Conflict of interest statement
The author(s) declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat Rev Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suda T, O’Garra A, MacNeil I, Fischer M, Bond MW, Zlotnik A. Identification of a novel thymocyte growth-promoting factor derived from B cell lymphomas. Cell Immunol. 1990;129:228–240. doi: 10.1016/0008-8749(90)90200-b. [DOI] [PubMed] [Google Scholar]
- 5.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 6.Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251:550–551. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- 7.Korhonen R, Moilanen E. Anti-CD20 antibody rituximab in the treatment of rheumatoid arthritis. Basic Clin Pharmacol Toxicol. 2010;106:13–21. doi: 10.1111/j.1742-7843.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 8.Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6:859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Hever A, Willhite D, Zlotnik A, Hevezi P. Effects of RNA degradation on gene expression analysis of human postmortem tissues. FASEB J. 2005;19:1356–1358. doi: 10.1096/fj.04-3552fje. [DOI] [PubMed] [Google Scholar]
- 10.Roth RB, Hevezi P, Lee J, Willhite D, Lechner SM, Foster AC, Zlotnik A. Gene expression analyses reveal molecular relationships among 20 regions of the human CNS. Neurogenetics. 2006;7:67–80. doi: 10.1007/s10048-006-0032-6. [DOI] [PubMed] [Google Scholar]
- 11.Gerber PA, Hevezi PA, Buhren BA, Martinez C, Schrumpf H, Gasis M, Grether-Beck S, Krutmann J, Homey B, Zlotnik A. Systematic identification and characterization of novel human skin-associated genes encoding membrane and secreted proteins. PlOS ONE. 2013 doi: 10.1371/journal.pone.0063949. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 13.van Spriel AB, Puls KL, Sofi M, Pouniotis D, Hochrein H, Orinska Z, Knobeloch KP, Plebanski M, Wright MD. A regulatory role for CD37 in T cell proliferation. J Immunol. 2004;172:2953–2961. doi: 10.4049/jimmunol.172.5.2953. [DOI] [PubMed] [Google Scholar]
- 14.Heikens MJ, Cao TM, Morita C, Dehart SL, Tsai S. Penumbra encodes a novel tetraspanin that is highly expressed in erythroid progenitors and promotes effective erythropoiesis. Blood. 2007;109:3244–3252. doi: 10.1182/blood-2006-09-046672. [DOI] [PubMed] [Google Scholar]
- 15.Seita J, Sahoo D, Rossi DJ, Bhattacharya D, Serwold T, Inlay MA, Ehrlich LI, Fathman JW, Dill DL, Weissman IL. Gene Expression Commons: an open platform for absolute gene expression profiling. PlOS ONE. 2012;7:e40321. doi: 10.1371/journal.pone.0040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Fulop Z, Wu G, Pone EJ, Zhang J, Mai T, Thomas LM, Al-Qahtani A, White CA, Park SR, Steinacker P, Li Z, Yates J, 3rd, Herron B, Otto M, Zan H, Fu H, Casali P. 14-3-3 adaptor proteins recruit AID to 5′-AGCT-3′-rich switch regions for class switch recombination. Nat Struct Mol Biol. 2010;17:1124–1135. doi: 10.1038/nsmb.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 18.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 19.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 20.Burkhardt AM, Tai KP, Flores-Guiterrez JP, Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Zuniga J, Selman M, Ouellette AJ, Zlotnik A. CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity. J Immunol. 2012;188:6399–6406. doi: 10.4049/jimmunol.1102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaffer A, Cerutti A, Shah S, Zan H, Casali P. The evolutionarily conserved sequence upstream of the human Ig heavy chain S gamma 3 region is an inducible promoter: synergistic activation by CD40 ligand and IL-4 via cooperative NF-kappa B and STAT-6 binding sites. J Immunol. 1999;162:5327–5336. [PubMed] [Google Scholar]
- 22.Park SR, Zan H, Pal Z, Zhang J, Al-Qahtani A, Pone EJ, Xu Z, Mai T, Casali P. HoxC4 binds to the promoter of the cytidine deaminase AID gene to induce AID expression, class-switch DNA recombination and somatic hypermutation. Nat Immunol. 2009;10:540–550. doi: 10.1038/ni.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maravillas-Montero JL, Gillespie PG, Patino-Lopez G, Shaw S, Santos-Argumedo L. Myosin 1c participates in B cell cytoskeleton rearrangements, is recruited to the immunologic synapse, and contributes to antigen presentation. J Immunol. 2011;187:3053–3063. doi: 10.4049/jimmunol.1004018. [DOI] [PubMed] [Google Scholar]
- 24.Breen EC, Hussain SK, Magpantay L, Jacobson LP, Detels R, Rabkin CS, Kaslow RA, Variakojis D, Bream JH, Rinaldo CR, Ambinder RF, Martinez-Maza O. B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Roos AJ, Mirick DK, Edlefsen KL, LaCroix AZ, Kopecky KJ, Madeleine MM, Magpantay L, Martinez-Maza O. Markers of B-cell activation in relation to risk of non-Hodgkin lymphoma. Cancer Res. 2012;72:4733–4743. doi: 10.1158/0008-5472.CAN-12-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson KC, Bates MP, Slaughenhoupt BL, Pinkus GS, Schlossman SF, Nadler LM. Expression of human B cell-associated antigens on leukemias and lymphomas: a model of human B cell differentiation. Blood. 1984;63:1424–1433. [PubMed] [Google Scholar]
- 27.Gurbuxani S, Anastasi J, Hyjek E. Diffuse large B-cell lymphoma--more than a diffuse collection of large B cells: an entity in search of a meaningful classification. Arch Pathol Lab Med. 2009;133:1121–1134. doi: 10.5858/133.7.1121. [DOI] [PubMed] [Google Scholar]
- 28.McGowan P, Nelles N, Wimmer J, Williams D, Wen J, Li M, Ewton A, Curry C, Zu Y, Sheehan A, Chang CC. Differentiating between Burkitt lymphoma and CD10+ diffuse large B-cell lymphoma: the role of commonly used flow cytometry cell markers and the application of a multiparameter scoring system. Am J Clin Pathol. 2012;137:665–670. doi: 10.1309/AJCP3FEPX5BEEKGX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura N, Nakamine H, Tamaru J, Nakamura S, Yoshino T, Ohshima K, Abe M. The distinction between Burkitt lymphoma and diffuse large B-Cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771–776. doi: 10.1097/01.MP.0000019577.73786.64. [DOI] [PubMed] [Google Scholar]
- 30.Kuppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyman H, Jerkeman M, Karjalainen-Lindsberg ML, Banham AH, Leppa S. Prognostic impact of activated B-cell focused classification in diffuse large B-cell lymphoma patients treated with R-CHOP. Mod Pathol. 2009;22:1094–1101. doi: 10.1038/modpathol.2009.73. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y, Yoshida T, Wang G, Togano T, Miyamoto S, Miyazaki K, Iwabuchi K, Nakayama M, Horie R, Niitsu N, Sato Y, Nakamura N. Association of CD20 levels with clinicopathological parameters and its prognostic significance for patients with DLBCL. Ann Hematol. 2012;91:997–1005. doi: 10.1007/s00277-012-1407-4. [DOI] [PubMed] [Google Scholar]
- 34.Dorner T, Giesecke C, Lipsky PE. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther. 2011;13:243. doi: 10.1186/ar3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spronk PE, vd Gun BT, Limburg PC, Kallenberg CG. B cell activation in clinically quiescent systemic lupus erythematosus (SLE) is related to immunoglobulin levels, but not to levels of anti-dsDNA, nor to concurrent T cell activation. Clin Exp Immunol. 1993;93:39–44. doi: 10.1111/j.1365-2249.1993.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottenberg JE, Miceli-Richard C, Ducot B, Goupille P, Combe B, Mariette X. Markers of B-lymphocyte activation are elevated in patients with early rheumatoid arthritis and correlated with disease activity in the ESPOIR cohort. Arthritis Res Ther. 2009;11:R114. doi: 10.1186/ar2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottenberg JE, Dayer JM, Lukas C, Ducot B, Chiocchia G, Cantagrel A, Saraux A, Roux-Lombard P, Mariette X. Serum IL-6 and IL-21 are associated with markers of B cell activation and structural progression in early rheumatoid arthritis: results from the ESPOIR cohort. Ann Rheum Dis. 2012;71:1243–1248. doi: 10.1136/annrheumdis-2011-200975. [DOI] [PubMed] [Google Scholar]
- 38.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, Weyand CM. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–3092. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White CA, Seth Hawkins J, Pone EJ, Yu ES, Al-Qahtani A, Mai T, Zan H, Casali P. AID dysregulation in lupus-prone MRL/Fas(lpr/lpr) mice increases class switch DNA recombination and promotes interchromosomal c-Myc/IgH loci translocations: modulation by HoxC4. Autoimmunity. 2011;44:585–598. doi: 10.3109/08916934.2011.577128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 41.Zan H, Zhang J, Ardeshna S, Xu Z, Park SR, Casali P. Lupus-prone MRL/faslpr/lpr mice display increased AID expression and extensive DNA lesions, comprising deletions and insertions, in the immunoglobulin locus: concurrent upregulation of somatic hypermutation and class switch DNA recombination. Autoimmunity. 2009;42:89–103. doi: 10.1080/08916930802629554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Karypis G, Hippen KL, Vegoe AL, Ruiz P, Gilkeson GS, Behrens TW. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 2006;7:156–168. doi: 10.1038/sj.gene.6364286. [DOI] [PubMed] [Google Scholar]
- 43.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coquery CM, Erickson LD. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol. 2012;32:287–305. doi: 10.1615/critrevimmunol.v32.i4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 46.Jourdan M, Caraux A, De Vos J, Fiol G, Larroque M, Cognot C, Bret C, Duperray C, Hose D, Klein B. An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization. Blood. 2009;114:5173–5181. doi: 10.1182/blood-2009-07-235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol. 2008;20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Soto H, Hevezi P, Roth RB, Pahuja A, Alleva D, Acosta HM, Martinez C, Ortega A, Lopez A, Araiza-Casillas R, Zlotnik A. Gene array analysis comparison between rat collagen-induced arthritis and human rheumatoid arthritis. Scand J Immunol. 2008;68:43–57. doi: 10.1111/j.1365-3083.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- 49.Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther. 2003;5(Suppl 4):S1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Gamboa L, Brezinschek HP, Burmester GR, Dorner T. Immunopathologic role of B lymphocytes in rheumatoid arthritis: rationale of B cell-directed therapy. Autoimmun Rev. 2006;5:437–442. doi: 10.1016/j.autrev.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Bluml S, McKeever K, Ettinger R, Smolen J, Herbst R. B-cell targeted therapeutics in clinical development. Arthritis Res Ther. 2013;15(Suppl 1):S4. doi: 10.1186/ar3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vancsa A, Szabo Z, Szamosi S, Bodnar N, Vegh E, Gergely L, Szucs G, Szanto S, Szekanecz Z. Longterm Effects of Rituximab on B Cell Counts and Autoantibody Production in Rheumatoid Arthritis: Use of High-sensitivity Flow Cytometry for More Sensitive Assessment of B Cell Depletion. J Rheumatol. 2013;40:565–571. doi: 10.3899/jrheum.111488. [DOI] [PubMed] [Google Scholar]
- 53.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naito Y, Takematsu H, Koyama S, Miyake S, Yamamoto H, Fujinawa R, Sugai M, Okuno Y, Tsujimoto G, Yamaji T, Hashimoto Y, Itohara S, Kawasaki T, Suzuki A, Kozutsumi Y. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. 2007;27:3008–3022. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goteri G, Lucarini G, Zizzi A, Costagliola A, Giantomassi F, Stramazzotti D, Rubini C, Leoni P. Comparison of germinal center markers CD10, BCL6 and human germinal center-associated lymphoma (HGAL) in follicular lymphomas. Diagn Pathol. 2011;6:97. doi: 10.1186/1746-1596-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erlanson M, Gronlund E, Lofvenberg E, Roos G, Lindh J. Expression of activation markers CD23 and CD69 in B-cell non-Hodgkin’s lymphoma. Eur J Haematol. 1998;60:125–132. doi: 10.1111/j.1600-0609.1998.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 57.Gregersen JW, Jayne DR. B-cell depletion in the treatment of lupus nephritis. Nat Rev Nephrol. 2012;8:505–514. doi: 10.1038/nrneph.2012.141. [DOI] [PubMed] [Google Scholar]
- 58.Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2012;51(Suppl 5):v3–11. doi: 10.1093/rheumatology/kes113. [DOI] [PubMed] [Google Scholar]
- 59.Cornec D, Devauchelle-Pensec V, Tobon GJ, Pers JO, Jousse-Joulin S, Saraux A. B cells in Sjogren’s syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun. 2012;39:161–167. doi: 10.1016/j.jaut.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 61.Rehnberg M, Amu S, Tarkowski A, Bokarewa MI, Brisslert M. Short- and long-term effects of anti-CD20 treatment on B cell ontogeny in bone marrow of patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11:R123. doi: 10.1186/ar2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haeuw JF, Goetsch L, Bailly C, Corvaia N. Tetraspanin CD151 as a target for antibody-based cancer immunotherapy. Biochem Soc Trans. 2011;39:553–558. doi: 10.1042/BST0390553. [DOI] [PubMed] [Google Scholar]
- 63.Sawada K, Hirokawa M, Fujishima N. Diagnosis and management of acquired pure red cell aplasia. Hematol Oncol Clin North Am. 2009;23:249–259. doi: 10.1016/j.hoc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 65.Surfus JE, Hank JA, Oosterwijk E, Welt S, Lindstrom MJ, Albertini MR, Schiller JH, Sondel PM. Anti-renal-cell carcinoma chimeric antibody G250 facilitates antibody-dependent cellular cytotoxicity with in vitro and in vivo interleukin-2-activated effectors. J Immunother Emphasis Tumor Immunol. 1996;19:184–191. doi: 10.1097/00002371-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 66.van Spriel AB. Tetraspanins in the humoral immune response. Biochem Soc Trans. 2011;39:512–517. doi: 10.1042/BST0390512. [DOI] [PubMed] [Google Scholar]
- 67.Mattila PK, Feest C, Depoil D, Treanor B, Montaner B, Otipoby KL, Carter R, Justement LB, Bruckbauer A, Batista FD. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity. 2013;38:461–474. doi: 10.1016/j.immuni.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 68.van Spriel AB, de Keijzer S, van der Schaaf A, Gartlan KH, Sofi M, Light A, Linssen PC, Boezeman JB, Zuidscherwoude M, Reinieren-Beeren I, Cambi A, Mackay F, Tarlinton DM, Figdor CG, Wright MD. The tetraspanin CD37 orchestrates the alpha(4)beta(1) integrin-Akt signaling axis and supports long-lived plasma cell survival. Sci Signal. 2012;5:ra82. doi: 10.1126/scisignal.2003113. [DOI] [PubMed] [Google Scholar]
- 69.Petersen SH, Odintsova E, Haigh TA, Rickinson AB, Taylor GS, Berditchevski F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur J Immunol. 2011;41:2556–2561. doi: 10.1002/eji.201141438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.