Abstract
Twenty years ago, the prevalent view in psychology was that although learning and the formation of new memories are lifelong occurrences, the neural changes associated with these events were all in the existing receptors. No new neural hardware, from synapses to neurons, was thought to appear after a protracted period early in life. In the past 20 years, another view has supplanted this one, showing that although the juvenile period is especially suited to neuroplastic adaptation, there is hard neuroplastic change later in life as well. We review a selection of evidence for this view from both animal and human models, showing how it reflects three principles of neuroplasticity: 1) earlier and later experience-induced changes to neuroarchitecture differ in degree more so than in type; 2) the types of experiences that lead to neuroplastic change narrow with age; and 3) differences in the amenability of neural circuitry to change result from basic differences in neuroarchitecture and neuroenvironment in different phases of development.
Keywords: Neuroplasticity, Critical Period, Sensitive Period, Development
An age-old doctrine in Psychology is that although the brain can change during the juvenile period, once that period is over, its structures are fixed (Nelson, de Haan, & Thomas, 2006). Recently the commonly recognized period for malleability of the human brain has moved from toddlerhood to puberty to around age 20 (Gogtay et al., 2004). However, it has become increasingly clear that even this period is too short, and that neuroplastic changes continue to occur in the human brain throughout life. What are these later changes, and how are they similar to and different from changes that occur during the juvenile period?
In this paper, after a brief introduction to neuroplasticity and brain development, we review evidence of that helped to cement the fixed-brain idea into the accumulated wisdom of Psychology. We then examine counterevidence: post-juvenile neuroplastic changes associated both with learning and with drastic changes in sensory and motor experience. The vast literature from developmental and cognitive psychology as well as from biological neuroscience points to three essential principles governing neuroplastic change:
Early life changes include large-scale axonal and dendritic extensions and reorganization; neuroplastic changes in adulthood typically are at a smaller scale, involving changes to the synapse, unmasking, the addition and subtraction of dendritic spines or axon terminal boutons, and adjustments to myelin, changing the speed of neural conductance. There are three exceptions to the principle of smaller scale adult changes: a) widespread changes in cortical fields, which result from the combined effect of many smaller scale changes; b) adult neurogenesis, which also brings new axon and dendrites; and c) axon growth in response to extreme changes in sensory and motor input.
Changes early in life can result from passive exposure, although attention and vigilance may facilitate neuroplastic change; later in life, the organism’s attention to external stimuli, and the meaning of the stimuli to the organism, are key to neuroplastic change. Later changes also often require small sequenced changes in input.
The differences in the neural circuitry’s amenability to change in youth and adulthood may be due to basic differences in neural structure, including packing of cells and dendrites, myelination, cell adhesion molecules, and glia. In addition, the molecular environment of the young brain is different: as organisms age, enzymes that once supported neural change are replaced with enzymes that support neural stability.
What is Neuroplasticity?
At base, “neuroplasticity” simply refers to the malleability of the brain, observable as changes in neuronal structure and connectivity, typically as a consequence of influences outside of the brain. The existence of neuroplasticity was identified in the seminal work of William James (1890), when he claimed that “the phenomena of habit in living beings are due to plasticity of the organic material of which their bodies are composed” (p. 105). (See also Bach-y-Rita & Kercel, 2003; Pascual-Leone, Amedi, Fregni, & Merabet, 2005). The concept of neuroplasticity gained momentum in the 1950s with research on enriched environments and the development of Hebbian theory (Rosenzweig, 2007), and it is currently a “hot topic” in psychology and neuroscience (e.g., Bavelier, Levi, Li, Dan, & Hensch, 2010; Buonomano & Merzenich, 1998; Feldman, 2009; Holtmaat & Svoboda, 2009; Nelson, 1999). The Appendix provides a reference to the many different methods for observing neuroplastic change discussed in this article.
Our definition of neuroplasticity hints at two crucial properties: activity-dependence and changes in neural circuits. Neuroplasticity is activity-dependent because sensory activity is the means through which the outside world influences the brain. Repeated motor or cognitive activity can also drive neuroplastic changes. Regardless of the source, a sustained change in a pattern of neural activity is a necessary trigger for neuroplasticity. The change in neural activity pattern leads to a reorganization in neural circuits, which produces long lasting functional change. Thus, the capacity of neural circuits to reorganize (neural malleability or neuroplasticity) enables the brain to use its internal resources more efficiently to respond to external information as a new repertoire of behaviors (see also Lovden, Backman, Lindenberger, Schaefer, & Schmiedek, 2010).
Structural changes that reflect neuroplasticity also occur in the stage of neural development, and these two phenomen are important to delineate.
Neuroplasticity versus Neural Development
The period of neural development necessarily involves enormous structural changes in the brain, including the birth, differentiation and migration of new neurons followed by elaboration of their dendritic structure and the formation of synapses. During neural development these events are orchestrated mainly by genetic information, which specifies an intricate sequence of guidance molecules that instruct newly born cells in what type of neurons to be and where to go. Genetically-determined intrinsic neural activity instructs the refinement of axonal projections which are guided by molecular cues to their approximate target area (Chalupa, 2009; Huberman, 2009; Katz & Shatz, 1996). In contrast, with neuroplasticity, one is only concerned about structural changes that are consequences of neural activity evoked by external stimulation or internal reverberation, and that lead to modification of neural responses. Neuroplastic and neurodevelopmental changes can occur contemporaneously.
Prenatally, neuroplastic changes are often negative: typically teratogenic factors that hamper neurogenesis or the action of guidance molecules, thereby interfering with normal development. Prenatal alcohol exposure, for example, interferes with glutamatergic and GABAergic neurotransmitter function at circuits that require spontaneously generated neural activity to stabilize previously tentative synapses (Medina & Krahe, 2008). Interruption of the molecular guides for development can lead to structurally different yet functionally viable circuits. An example of positive neuroplastic change in development is when auditory stimulation of the fetal ear receptors determines how auditory stimuli will be processed postnatally (DeCasper & Spence, 1986). These changes agree with our definition of neuroplasticity because the normal developmental sequence was altered or guided in an experience-dependent way (Greenough, Black, & Wallace, 1987).
Although neuroplastic changes can occur before birth, most neuroplastic change occurs postnatally, after the sensory organs have matured enough to bring the environment to the brain. Sensory activity evoked by the native environment is the dominant instructive factor for the formation and stabilization of neural circuits that are adapted to that environment. Thus a child raised in an environment in which French is the dominant language forms neural circuits that specialize in the sounds and syntax of French.
Different types of structural changes to the brain that can occur as part of normal neurodevelopment and as instances of neuroplastic change are reviewed next, primarily with reference to normal neurodevelopment.
Neurogenesis
The birth of neurons is the paramount feature of brain development. The brain develops when, from about 6 to 24 weeks post-conception, an astonishing ~4000 neurons per second (Brown, Keynes, & Lumsden, 2001) emanate from dividing neural stem cells in the ventricles, which were formed when the emerging spinal cord folded. In the cerebral cortex—about the outer centimeter in the human brain-- neurons are arranged in layers that are formed from the innermost layer (layer VI) out. Later-born neurons migrate along radial glial tracts through existing neurons to their destinations. At birth, there are thought to be 100 billion neurons in the human brain (Naegele & Lombroso, 2001). Although many of these neurons die off early in development, grey matter (glia, neurons, unmyelinated axons, and dendrites) continues to increase. Peak grey matter volume differs by brain area and gender. For example, in the sensory and motor areas it peaks in the first two years (Huttenlocher, 2002), in the frontal lobes it peaks at age 11 (girls) or 12 (boys), and in the temporal lobes it peaks at 16 for both genders (Giedd, 2004).
Axons
Neurons without connections are no different than any other cell in the body. What makes neurons unique is their axons and dendrites, which allow neurons to communicate synaptically with other neurons, forming unique circuits. After neurons migrate to their destinations, one part of the neural cell begins to protrude, creating the beginning form of an axon. At the tip of the developing axon is a swelling called the growth cone, which leads the axon to its destination on a dendrite or dendritic spine or in some cases directly on to another neuron. Many different molecules guide axons to their destinations. During development, vast open spaces in the extracellular matrix allow for the long-range travel of growth cones.
Axons make synaptic connections onto other cells via specialized swellings, or boutons, which encase neurotransmitter-containing vesicles. Although axons can grow new branches and make new boutons throughout life, they are largely stable, with just a small percentage (less than 10%) of boutons turning over in a given period (De Paola et al., 2006; Stettler, Yamahachi, Li, Denk, & Gilbert, 2006); in some cases, their shape may change with learning (Geinisman, Berry, Disterhoft, Power, & Van der Zee, 2001).
Dendrites and spines
In addition to the solitary axon and its branches and boutons, each neuron extends several dendritic branches, on to which the axon boutons typically synapse. In most neurons, very small protrusions called spines emanate from dendrites and the spines receive synapses. Increases in spine numbers are also considered an indication that new synapses have formed on the neuron.
As with axons, dendrites are laid down early in development and their basic structure is fixed (Nimchinsky, Sabatini, & Svoboda, 2002). However, like axon boutons, dendritic spines do change, and so rapidly that their changes are visible on a minute-by-minute basis. This is particularly the case in younger animals (Lendvai, Stern, Chen, & Svoboda, 2000; Wilbrecht, Holtmaat, Wright, Fox, & Svoboda, 2010), but even in mature brains, new spines rapidly appear and disappear. There are several different types of dendritic spines, often categorized by shape into thin, stubby, and mushroom, and some types (namely smaller ones) are more apt to change than others (Bourne & Harris, 2007; Nimchinsky et al., 2002).
Synapses
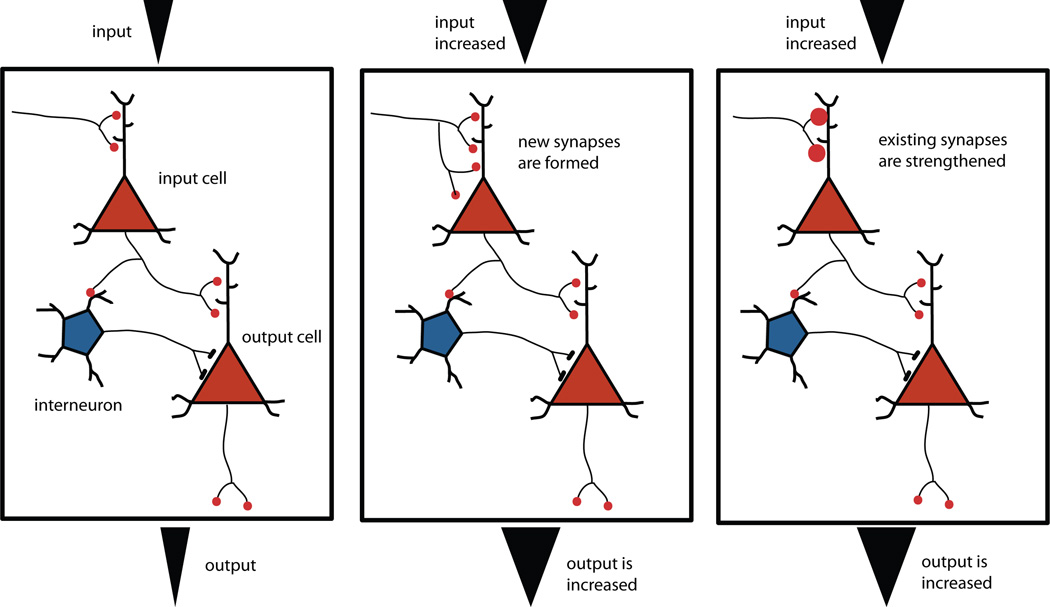
The space between the axon’s terminal bouton and the dendrite is the synaptic cleft, across which the neurotransmitters carry a signal from one neuron to the next. Binding of the neurotransmitter to a receptor (a protein inserted in the postsynaptic membrane) opens ion channels, changing the membrane potential of the postsynaptic cell to create a postsynaptic response. Strengthening of a synapse is achieved either by making more synaptic contacts between the pre and postsynaptic cell, or by changing the efficacy of the existing synapse, either by increasing the amount of neurotransmitter released or by increasing the number of receptor molecules at the postsynaptic site (Cowan, Sdhof, & Stevens, 2003).
The concept of synapse strengthening was proposed by Donald Hebb in the 1940s and 50s. He postulated that, “When an axon of cell A is near enough to excite B and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that A’s efficiency, as one of the cells firing B, is increased” (Hebb, 1949, p. 62). This was a phenomenal idea because it seemed to suggest that activity could influence circuit formation. This idea explains many developmental and neuroplastic phenomena unveiled in the ensuing decades. For example, developing sensory neurons that fire in synchrony tend to strengthen their synapses on similar postsynaptic cells–an outcome that solidifies their receptive field maps (Dinse, Recanzone, & Merzenich, 1993); nonsynchronous firing is more likely to result in dendritic and axonal atrophy or synapse loss (Fitzsimonds, Song, & Poo, 1997). In addition, Hebbian mechanisms explain how repetitive stimulation of a pathway leads to enhanced responsiveness in the postsynaptic cells in the face of subsequent stimulation (Lisman, Lichtman, & Sanes, 2003; Lynch, 2004), resulting in LTP (long term potentiation) or learning.
Myelin
Axon myelination occurs when oligiodendrocytes issue 10 to 150 layers of fatty membrane that they wrap around axons, in short segments separated by junctions termed “the nodes of Ranvier”. The fatty sheaths function as electrical insulators and improve conductance of the action potential down the axon, changing the speed with which a signal is conducted. As with neurogenesis and synaptogenesis, myelination occurs area by area, beginning with the sensory and motor areas early in development and continuing with the prefrontal cortex much later. The corpus callosum that connects the hemispheres also myelinates late. Myelin increases in the brain throughout childhood and adolescence (Gogolla, Galimberti, & Caroni, 2007), and even into one’s 40s (Bartzokis, Beckson, Nuechterlein, Edwards, & Mintz, 2001). Myelin also works against axonal plasticity and for neural stability by inhibiting growth cones in the CNS, making for an inverse relationship between axonal plasticity and myelinization. The degree of myelinization has been correlated with IQ scores in children from 5 to 18 years of age (Schmithorst, Wilke, Dardzinski, & Holland, 2005).
Neural Circuits and Cortical Maps
Neurons are organized into neural circuits through which neural signals (excitatory, inhibitory, or modulatory) are transferred via the release of a neurotransmitter at each synaptic contact. Multiples of these circuits create different “cortical maps” of organized receptive (or motor) fields for given functions. For example, the primary visual cortex is organized such that groups of neurons located adjacent to each other encode adjacent locations in the visual field. All of the functional divisions of the sensorimotor cortex, including the somatosensory and auditory sensory areas, the motor areas that control every muscle in the body, and even the association areas that integrate multi sensory information, are organized in topographic maps.
Unmasking
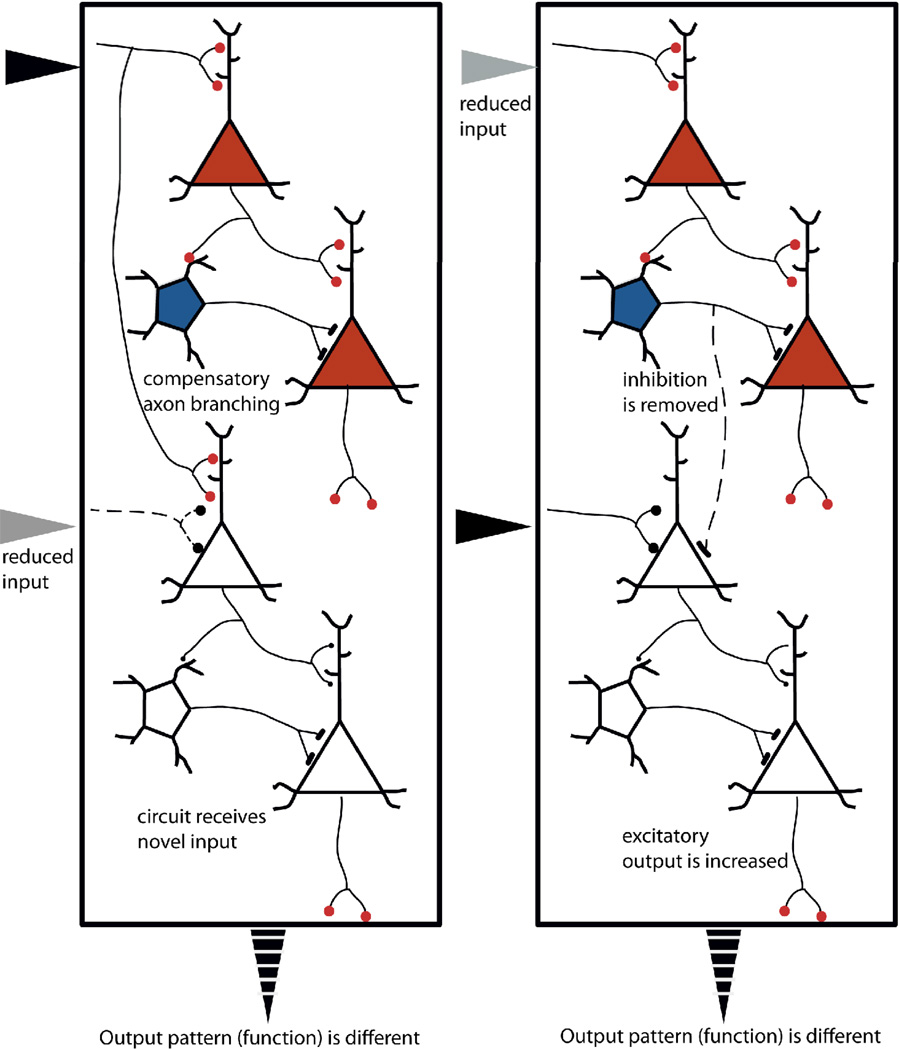
A special case of changes to existing circuits occurs with unmasking, when excitation of a neuron is reduced, mitigating its inhibitory influence onto other neurons within the same sensory map. The result can be an instant increase of activity in those other neurons. Unmasking can be detected as a widening of the functional response area. This functional change is immediate, and it is sustained as long as the responsible stimulus is maintained; however no hard structural change occurs in neural circuits, making unmasking a special case of neuroplasticity. Unmasking is an extreme instance of a normal function of neural circuits adapting to fluctuations in sensory input. Unlike the other neural changes just reviewed it is pertinent only to neuroplasticity, not neurodevelopment.
Cortical Layers
As a useful aside regarding the structure of the brain, note that most cortical areas have 6 layers of neurons covering the outer ~1 cm of the human brain. Below this is an area of axons connecting the cortex to the more primitive subcortical structures like the basal ganglia and the thalamus. The six cortical layers are rarely mentioned in human brain research because imaging methods are not refined enough to discriminate between them, but the precise involvement of neural circuits located in individual cortical layers become evident in animal models that approximate behavioral outcomes in human neuroplasticity. Each layer has particular types of cells, for example layer II has small pyramidal cells, layers III and V have large pyramidal cells, and layer IV has small multipolar cells (Callaway, 1998; Huttenlocher, 2002). Different layers also constitute distinct levels in hierarchical circuits, displaying unique input-output properties to which their different cells are suited. For example, in the sensory cortical areas, layer IV’s small multipolar cells receive primary inputs from the thalamus. The layer IV neurons (“multi-polar” as they are) in turn project to the pyramidal cells of layers III and II, which generate lateral projections within and among cortical areas. Layers VI and V contain neurons that project back to the thalamus and other subcortical areas, sometimes over very long distances. Layer I, the outermost layer of cortex, is composed of apical tufts of boutons stemming from pyramidal cells located in layer V, connections from the thalamus, and intra-layer connections.
Despite these difference in inputs and outputs across the layers, all cortical neurons use the same excitatory and inhibitory neurotransmitters (glutamate and GABA, respectively), and are arranged in columns, which in the sensorimotor areas form topographic or tonotopic maps (Douglas & Martin, 2004; Gilbert, 1993). All neurons are also supported by glial cells, and recently neural signaling properties have been attributed to glia as well (Haydon, 2001).
The different structural changes that can occur in the brain during development, as a result of genetic instructions and their sequelae, and with neuroplasticity, in response to changes in the input and internal reverberations, fall into seven levels of neuroplastic change, shown in Table 2. These levels are: 1) neurogenesis, 2) axon and dendrite growth, 3) the appearance and disappearance of axon boutons and dendritic spines, 3) changes to existing synapses; 5) myelination of axons, 6) changes to existing neural circuits including unmasking, and 7) establishment and modification of cortical fields. All of these except unmasking occur routinely in neurodevelopment, and one of the reasons for the old fixed brain adage were findings that for some behaviors there is a period early in life when the behavior must be given certain types of experience in order for normal development to occur, and normal behavior to ensue. A few of the classic examples of this are reviewed next.
Table 2.
Seven Levels of Neuroplastic Change
| Neurogenesis | New Axon/Dendrite Growth or Distant Movement |
Axon Boutons/ Dendritic Spines |
Changes to Existing Synapses |
Myelination | Changes to Existing Circuits, Including Unmasking |
Cortical Map Changes |
|
|---|---|---|---|---|---|---|---|
| Juvenile Period | Massive | Massive | Massive | Massive | Protracted | Established; occurs even with passive exposure for audition | |
| Adult Period | Limited to Hippocampus and Olfactory Bulb; Associated with learning (EE, Exercise) | Limited to cases of extreme sensory change (Silver Spring Monkeys) | Plentiful in adulthood, with learning and extreme changes in input (whisker removal). | Plentiful in adulthood, with learning and extreme changes in input. | Peaks as late as mid-40s in some areas, like corpus callosum. Responsive to learning. | Plentiful with learning and extreme changes to input. | Plentiful in adulthood with learning and extreme changes to input. |
| Time Course | Days | Years in adults | Minutes | Days | Immediate | Days | |
| Model | Animal and Human | Animal | Animal | Animal and Human | Human | Animal and Human |
Plasticity in Critical and Sensitive Periods
Sensitive and critical periods are times when a change in environmental input from what is normal for the organism leads to consequential changes in the brain and behavior, when that same input during a different developmental period would likely lead to much less or even no change at all. Such periods are critical when a specific development must occur or it never would occur at all, and sensitive when it simply requires much more input later to change. More recent research has shown that under the right conditions, such as when the input is especially intense or when the neural environment is changed by the introduction of molecules normally only present during critical or sensitive periods (Bavelier et al., 2010), these periods can be sensitive rather than critical (Keuroghlian & Knudsen, 2007; M. Thomas & Johnson, 2008). (The recasting of critical as sensitive periods in itself indicates an increasingly plastic view of the brain.) Examples of sensitive periods in development encompass both behavioral and underlying neural findings; outcomes for which the research has had a more behavioral emphasis are reviewed first, followed by outcomes from which the research has had a more neural emphasis.
Sensitive Periods in Behavior: Imprinting and Attachment
One of the first clear cases of critical period plasticity in behavioral science was Konrad Lorenz’s (1965) observations of imprinting in geese and other fowl. In what has become a classic body of research, Lorenz found that only in a specific period in the days or weeks after birth (it varies by species) do birds become “imprinted” on a moving target, which they immediately proceed to follow and with which later in life they even attempt to mate. Closing such a period early in life makes good adaptive sense, since young organisms are likely to be near an often-moving mother who is naturally a conspecific. Neural circuits underlying imprinting have been identified (Nakamori et al., 2010). Research on social isolation in monkeys suggests that for primates as well, the juvenile period is a sensitive one for subsequent normal behavior with conspecifics. Peer- and isolate-reared monkeys show behavioral disturbances not seen in monkeys raised by their mothers, whereas being alone in adulthood does not have negative consequences for social behavior (Suomi, 2006).
These animal findings have served as a model for early attachment in humans. Many children whose early years were spent in extreme social deprivation in Romanian orphanages under Ceauşescu later suffered from attachment disorder (Nelson, 2007; Rutter & O'Connor, 2004). Cognitive impairments were also observed in some of these children, and the later children were adopted, the worse they fared, even controlling for years in the adoptive homes. Thus in fowl, primates, and humans, there appear to be sensitive periods during which normal (“experience-expectant”) social input is necessary for normal social and cognitive development to occur. Without that normal input, the neural system develops awry, resulting later in socially deviant behaviors.
Recent research with animal models has begun to explore possible biological underpinnings of a sensitive period for social input. In particular, Meaney (2010) and his colleagues have been examining the physiological changes in mice that result from variations in the early social environment, via their influence on gene expression and subsequent behavior. For example, mice who are licked more by their mothers during the first week of life themselves later show reduced cortiosteroid surges in response to stress, as compared with mice who were licked less (Kaffman & Meaney, 2007), and the reason for this is that DNA methylation occurs subsequent to the licking and specifies the corticosteroid response. Experimenters can reproduce the licking effect artificially by stroking neonatal mice with a paintbrush. In addition to hormonal change, maternal care is also associated with neural change, particularly in dendritic morphology in the hippocampus (Champagne et al., 2008); these neural changes are accompanied by differences in learning aptitude (Bagot et al., 2009). It is possible that similar kinds of change undergird imprinting and attachment. Epigenetic influences on neural plasticity during sensitive periods will clearly be a hot area in coming years (Fagiolini, Jensen, & Champagne, 2009), with animal models more directly extended to humans.
Sensitive Periods in Behavior: Language
Another behavior that has clear sensitive periods is language. Birdsong has sometimes been viewed as a distant analog here (Abe & Watanabe, 2011; Jarvis, 2004), given some species need to hear their species’ song when young, and language and birdsong (although at very different levels) are both forms of vocal communication (Ball & Hulse, 1998). Different facets of language, like phonetics and syntax, have different sensitive periods (M. Thomas & Johnson, 2008). Patricia Kuhl and Janet Werker have shown that young infants are able to distinguish the sounds (phonemes) of every language tested, but that by the end of the first year, this ability diminishes and discrimination is limited to the languages spoken by live human beings (not televised ones) around them (Kuhl, 1987; Kuhl, Tsao, & Liu, 2003; Werker & Lalonde, 1988; Werker & Tees, 2002). Extensive training is usually required to make the phonetic distinctions later (Bradlow, Akahane-Yamada, Pisoni, & Tohkura, 1999). In addition, infants who begin to specialize in their native tongue earlier, as indicated by their losing the ability to distinguish foreign phonemes, also have more advanced language two years later (Tsao, Liu, Kuhl, & Tsao, 2004). Thus a benefit of closing the opportunity to detect phonetic distinctions in all languages could be that phonetic specialization in one’s own language facilitates syntactic processing. The specialization appears to be adaptive for the individual.
Johnson and Newport (1989) suggested a critical period for the acquisition of syntax. They showed that adults who had learned a second language before age 7 were proficient as native speakers regardless of when in those years they learned it; for languages learned after age 7, syntactic proficiency dropped at each year learned until puberty, when it leveled off. This finding is corroborated by studies of children learning sign language: The syntax of those who learned sign language later in life is much less regular than is that of people who learned it when young (Senghas, Kita, & Ozyurek, 2004). If people learn a second language before age seven, hearing the language activates the same brain areas as are typically activated by native languages; if the second language is learned later, it activates adjacent areas (Kim, Relkin, Lee, & Hirsch, 1997; Neville & Bavelier, 1998; Perani et al., 1996). Recovery of stroke in language areas is also fullest before age eight and drops gradually through puberty, and there is a corresponding drop in synaptic density in areas of the brain relevant to language across these ages (Huttenlocher, 2002).
Thus the first 8–10 months appears to be a sensitive period for making phonological distinctions, and the first 7 or 8 years appears to be a sensitive period for syntax. In both, changes in the brain correspond to the behavioral findings, with synaptic pruning in language areas coincides with the end of the sensitive period. The neural connection for attachment is much more distant, but different types of early maternal/offspring relations do result in changes in brain architecture.
The Neuroscience of Critical Periods: Vision
Research on sensitive period plasticity involving attachment and language is complemented by research on sensorimotor neuroplasticity, including research on vision and the development of ocular dominance columns. In the pediatric literature, examples of sensorimotor critical or sensitive periods for vision (as well as audition) are plentiful. For example, if a neonatal unilateral cataract (the clouding of the lens of one eye at birth) is not corrected by 6 months of age, poor binocular depth perception results. However, if the cataract is corrected before 3 months of age, or does not develop until adulthood, there is no permanent impact on binocular vision. This is just one of many critical periods of vulnerability to abnormal sensory activity in the visual system (Harwerth, Smith, Duncan, Crawford, & von Noorden, 1986; Lewis & Maurer, 2005). Unlike language and attachment, where behavioral research predominates, there has been extensive research at the neural level on critical periods in visual development.
Hubel and Weisel’s Nobel-winning research on the development of the visual cortex in kittens is the classic case of critical period plasticity at the neural level. Input from the right or left eye goes to thalamic cells, which then relay signals on to layer IV of the primary visual cortex. There, alternating patches of neurons receiving input from the right or left eye form ocular dominance columns. The monocular layer IV cells project up to layers III and II, fanning their own axons out just enough that the cells located directly above the borders of the monocular IV columns receive convergent input from both eyes, thus become binocular. As a result, within a population of cortical cells, spatially distinct groups of neurons are either right-eye monocular, left-eye monocular, or binocular.
Mimicking the circumstances of a neonatal unilateral cataract by patching just one of a kitten’s eyes, Hubel and Wiesel revealed a critical period for visual cortex plasticity, from about 3 to 8 weeks of age. One major finding of this research was that if a kitten was deprived of visual input during this critical period, the cortical neurons (in all layers) lost responsiveness to the deprived eye and started to respond primarily to the non-deprived eye (Wiesel & Hubel, 1965a). These electrophysiological outcomes had an anatomical underpinning: thalamic axons coming from the deprived eye retracted their branches from “deprived” columns, and those from the nondeprived eye invaded the space vacated by deprived axons. Even a year after the eye was opened, expanded axons prevailed, thus recovery was severely limited (Wiesel & Hubel, 1965b). Depriving a mature cat of input in one eye for a similar length of time had no such influence. Thus there is a critical period for the reorganization of ocular dominance columns early in development, and this neuroplasticity depends crucially on environmental input.
Since these findings in 1960s, a large body of research has examined the biological basis of this axonal reorganization plasticity, reviewed in (Hensch, 2004), and the ocular dominance column plasticity model has been studied extensively. It is now known that many things can block neuroplastic changes, including blockade of NMDA type glutamate receptors, intrinsic inhibition, neurotrophic factors, and modulatory neurotransmitters (acetylecholine and norepinephrine), and premature induction of cell surface adhesion molecules, to name a few. It is also known that different neuroplastic changes, which occur in different cortical layers at different timeframes, contribute in concert to the functional outcome of sensory perturbations. For example, electrophysiological plasticity encountered in layers II-III or V of the cortex outlasts the end of critical period for thalamocortical axon plasticity in layer IV by several weeks. Similarly, in addition to the axonal neuroplasticity just described, ocular dominance column changes are also associated with synaptic plasticity: Within a few days after the closing of one eye, the cells in layer IV of the visual cortex display LTD for stimulation from the deprived eye, indicating that the unused synapses are weakened. A few more days of deprivation elicits LTP of cells coming from the non-deprived eye, indicating that nondeprived synapses are strengthened (Smith, Heynen, & Bear, 2009). These synaptic changes, detected electrophysiologically, parallel an initial reduction followed by an increase in the number of synapses formed by thalamocortical axons in layer IV (Coleman et al., 2010).
Earlier models of ocular dominance plasticity hypothesized that the initially exuberant innervation of layer IV by thalamocortical axons was pruned back to form ocular dominance columns based on a Hebbian-type competition of each eye for synaptic space, and that the effect of monocular deprivation was prevention of that normal segregation process (Katz & Shatz, 1996; Luo & O'Leary, 2005; Penn & Shatz, 1999). This mechanism was similar to the prevailing retinogeniculate segregation models of the time. However, more recent work has indicated that the formation of ocular dominance columns and subsequent critical period plasticity are distinct developmental phases with different mechanisms (Crowley & Katz, 2000; Katz & Crowley, 2002), and only the axonal neuroplasticity, not the initial formation of the ocular dominance columns, is dependent on sensory activity. (For discussion see Huberman, Feller, & Chapman, 2008). The cortical dominance columns are now known to self-organize. Regardless, the fact remains that there is a critical period, such that depriving an eye of input before or after a certain period in development has no effect, whereas deprivation during that period has lasting structural and functional consequences.
The role of neuromodulators, particularly acetylcholine and norepinephrine which are involved in attention and vigilance in juvenile plasticity, is important to note, as it suggests that experience-dependent plasticity requires that animals be awake, alert, and paying attention to sensory stimuli (Singer, 1995). Deletion of these neurotransmitters by toxins or surgical cuts prevents ocular dominance plasticity (Bear & Singer, 1986) and their infusion onto neural circuits facilitates synaptic strengthening (Kirkwood, Rozas, Kirkwood, Perez, & Bear, 1999). The influence of neuromodulators on juvenile and adult neuroplasticity is likely to be a growing field as more cholinergic and catecholaminergic factors are identified as the brakes for juvenile plasticity (Morishita, Miwa, Heintz, & Hensch, 2010). It is because of the identification of brakes like these that one might even consider supposed critical periods to be merely sensitive ones.
The Neuroscience of Critical Periods: Visual-Auditory Maps
Alignment of sensory maps in barn owl tectum is another important model of critical period neuroplasticity that has been studied at the neural level. The tectum in birds contains overlapping maps of auditory and visual space, which are interconnected to mediate multisensory behavior. When an owl hears the flicker of prey in stark darkness, it executes a visually guided flight to the location of its nighttime snack. Eric Knudsen and his colleagues have revealed that the alignment of auditory and visual tectal maps requires a precise orchestration of early postnatal sensory activity: When neonatal barn owls were fitted with prisms that shifted their visual maps during a critical period, visual axons spread out to make new synapses that were realigned on the auditory map, thus adapting to the new ‘shifted’ environment (Knudsen, 1999). When the prism was removed, the young owls quickly adapted back to the natural environment. Interestingly, although not functional, the “shifted” connections are retained into adulthood in prism-experienced owls: When previously shifted owls wore the prism again in adult ages, they were able to hunt with considerable precision, suggesting that the circuits that enable the juvenile remapping remained in place to be functionally unmasked in adulthood (Knudsen, 1998; Linkenhoker, von der Ohe, & Knudsen, 2004), an apparent manifestation of the savings-in-relearning phenomena discussed in memory research. Knudsen’s naïve adult owls did not adapt to the prisms, nor did they display the wide scale anatomical reorganization in their visual pathways seen in juvenile owls. However, if provided with small prism increments over time, it has recently been shown that adult owls can adapt gradually, providing evidence that adult brain also had a capacity, albeit greatly subdued, for neuroplasticity when the input changes are gradual (Linkenhoker & Knudsen, 2002), a manifestation of our second principle.
As a brief behavioral-level aside, human children can also adapt to unusual visual input. The Moken are a group of sea gypsies living along the coast of Western Thailand and Burma, whose children dive for food; these children have far better underwater acuity than European children (Gislen et al., 2003). However, European children ages 9 and 13 were trained (in just 11 sessions over 4 months) to see underwater as well as the Moken children, and the improved underwater vision was still in evidence 8 months later (Gislen, Warrant, Dacke, & Kroger, 2006). It would be interesting to see the degree of plasticity human adults have in this domain.
The Extent of Plasticity in Sensory Cortices
In a radical demonstration of sensory system plasticity in an animal model, Sur and colleagues rewired the brains of neonatal ferrets so that axons normally projecting into the visual cortex were surgically connected to the auditory cortex, and vice-versa (Sur & Learney, 2001). Following this, neurons in the auditory cortex appeared to “see”, and even took on, to some degree, properties normally seen in neurons in the visual cortex, such as orientation selectivity. This research sheds light on the importance of the input for how the neural system develops. As will be considered later, in humans who are congenitally blind and deaf there is also neuroplastic change relative to what develops normally. For example, the auditory cortex of congenitally deaf subjects processes visual information. These differences are especially pronounced in secondary and multi-sensory processing areas (Bavelier & Neville, 2002).
Summary: Juvenile Period Plasticity
Much fascinating research in psychology and neuroscience over the past 50 years of has supported the idea that the brain is plastic during the juvenile period, and that certain functions are “laid down” during that time and become fixed. A child who does not have vision problems corrected early will never develop depth perception because this aspect of the visual system becomes fixed; a child who does not hear the phonemic distinctions of a certain language during the first year will normally never hear those distinctions; early sensorimotor experiences result in specific neural maps and response patterns that last for life. These examples are among the classics that cemented into Psychology the idea that the neural hardware is fixed after the juvenile period. (For another recent discussion of neuroplasticity in the juvenile period, see Fox, Levitt, & Nelson, 2010.) However, just as one can to a great degree renovate a house, moving walls and rooms and adding windows, a wealth of evidence from animal and humans suggests that the brain retains the possibility of structural change in response to experiences throughout life.
Neuroplasticity After the Juvenile Period
Next we discuss neuroplastic changes observed after the juvenile period, particularly from learning and from extreme changes in sensorimotor input, to illustrate the type and breadth of neural changes that can occur in adulthood. One of the most important areas we neglect—for reasons of space--is neuroplastic change after strokes. Excellent reviews of this topic exist (Dimyan & Cohen, 2011; Murphy & Corbett, 2009; Robertson & Murre, 1999).
Our three principles pervade this discussion: as was just seen, early life changes involve large-scale changes to axonal and dendritic projections, whereas the later changes we are about to review typically occur at smaller scales; in early life, even passive exposure can lead to neuroplastic change, whereas in mature organisms, attention and small increments are often necessary conditions for neuroplastic change; and these differences between adult and juvenile period neuroplasticity are due to structural and molecular differences in the brain in these different life periods.
Learning
There is a large body of research showing neuroplastic change associated with learning and memory formation in adult animals including humans. We begin with basic animal models, including research on enriched environments, before turning to recent examples of neuroplastic change in adult humans.
Sensitization, Habituation, and Long-Term Potentiation (LTP). Of course organisms can learn throughout life, and clearly there must be underlying neural substrate. Cajal speculated as much, and Kandel’s Nobel-prize winning research first showed the physical changes associated with formation of short- and long-term memories in adult Aplysia sea slugs, whose very simple neural systems and large cells make it easier to see changes (Kandel & Schwartz, 1982). The two types of simple memory Kandel elicited were habituation responses, in which the slugs stopped responding to stimuli that were presented repeatedly, and sensitization, in which the slugs started responding to a second stimulus that was repeatedly paired with a primary one. In both cases, a prior experience influenced a later response, and thus is a type of learning and memory. Habituation and sensitization can each occur over the short or long term; when the changes are long-term, they are considered long-term potentiation or depression (LTP or LTD).
The discovery of LTP (Bliss & Lomo, 1973) has led to literally thousands of research articles, making it the most popular topic of 20th century neuroscience (Lisman et al., 2003; Lynch, 2004; Malenka, 2003). LTP is now accepted as the electrophysiological manifestation of synaptic plasticity that occurs at the time of learning (Gruart, Munoz, & Delgado-Garcia, 2006; Whitlock, Heynen, Shuler, & Bear, 2006). Although LTP can be nonstructural, involving changes in the receptor activation only, structural changes in the synapse stemming from changes in gene expression and the synthesis of new proteins can also occur. Examining hippocampal slices after LTP has shown that LTP can be associated with a change in the structure of the postsynaptic spines (Engert & Bonhoeffer, 1991; Matsuzaki, Honkura, Ellis-Davies, & Kasai, 2004; Park et al., 2006). Conditions that induce LTP also lead to enlargement of the existing dendritic spines and formation of new ones (De Roo, Klauser, Garcia, Poglia, & Muller, 2008), although in some research the number of synapses has remained constant (Sorra & Harris, 1998). New spines are not immediately functional and many disappear, but some over the course of many hours or days do become functional (Knott, Holtmaat, Wilbrecht, Welker, & Svoboda, 2006). These changes at the level of dendritic spines occur across the lifespan and characterize learning. Although studied in animal models, evolution is conservative, and this type of change surely characterizes the physical substrate of lifelong learning in humans as well. Consistent with our first principle, adult organisms do not make large-scale changes to their basic axon-dendrite architecture in response to environmental novelty they represent; instead they make small adjustments to the existing framework architecture, changing synapses, myelin, axon boutons, and dendritic spines.
Enriched Environments
Adult animals learn, especially when placed in environments where there is a lot to learn. This makes enriched environments, replete with conspecifics and ladders and bridges and burrows, a great paradigm in which to study learning-dependent neuroplastic changes. (Contrast these with the standard plexiglass lab rat cage, holding only a water bottle, food dish, and shavings, and often housing just one rodent.) Rosenzweig and colleagues initially used enriched environments as a means to study individual differences in rat learning (Rosenzweig, 2007). They examined rats’ performance in different mazes, and found that performance correlated with levels of acetylcholine (AChE), a neurotransmitter that modulates glutamate (the excitatory neurotransmitter) in the central nervous system and thereby underpins attention and learning. What was really surprising was that the complexity of the maze in which a rat was run was linearly related to AChE levels. Rather than pursue the more expensive option of continuing to test in different mazes, Rosenzweig opted to create environments that were more enriched (10 to 12 rats with a lot of stuff), impoverished (a single rat, no stuff) or their standard (3 rats per cage, no stuff), building on earlier similar research by Hebb (1947). Over the years and across many experiments, an interesting body of evidence on the effects of “enriched” environments (although, we would argue, still simple as compared to the natural world) has accrued (Comery, Shah, & Greenough, 1995; Rosenzweig, 2007; Rosenzweig, Bennett, & Diamond, 1972).
Many neuroplastic changes have been associated with placement of mammals in enriched laboratory environments, even in adulthood. In rats, the size of synapses and the number of dendritic spines is significantly increased, as is the number of glia cells, and (as might be expected from these other changes) the cortex is thicker overall (Rosenzweig, 2007). Other research has shown that enrichment for 3 months both as a young adult (4 months) and in old age (24 months) led to neuroplastic changes in the parietal and occipital cortices, particularly an increase in dendrite length and dendritic spine density (Kolb, Gibb, & Gorny, 2003), suggesting changes aimed at processing visual and sensorimotor information. In contrast, for neonatal rats, early enrichment was associated with a decrease in dendritic spine density, perhaps suggesting early specialization for efficiently processing the environment. A gender difference also emerged for young rats: males had an increase in dendrite length, but females did not (Kolb et al., 2003). Others have also found sex differences in responses to enriched environments, which might be related to differences in male and female rat spatial learning and exploratory behavior (Cavigelli, Michael, West, & Klein, 2011).
Fascinatingly, the offspring of female rats that lived in enriched environments during pregnancy also had increased dendritic spine density (like those who experience enriched environments in adulthood, but not childhood), but shorter dendrite length, than had offspring of rats who had been in normal lab cages during pregnancy (Gibb, Gonzales, & Kolb, 2010). An increase in density with a decrease in length might indicate a similar number of synapses overall, but the mechanism by which the enriched environment experienced by the mother rat during pregnancy resulted in differences in offspring brains is unknown. More research will shed light on the mechanisms and meaning of these various findings but clearly the effects of enrichment vary by period of development, and neuroplastic changes at least at the level of the synapse and dendritic spines occur in response to enriched environments in both young and old (later we will also discuss neurogenesis in response to enriched environments).
Some questioned whether the neural changes associated with living in enriched environments stemmed from mere exercise, since animals move around in enriched environments more than they move in standard laboratory cages. To examine this, researchers gave adult rats a month of either acrobatic experience (frequently found in enriched environments) without increased exercise, forced exercise (a running wheel, gradually increased to one hour per day), voluntary exercise (the running wheel was always available), or standard conditions (Black, Isaacs, Anderson, Alcantara, & Greenough, 1990). The acrobatic rats were trained to obtain treats by crossing bridges that included see-saws, rope sections, and narrow board sections that became progressively more difficult across the month. (Rats in the other conditions got the same treats for free.) Investigation was limited to cells in the cerebellum, because of its role in spatial learning. As compared with the controls and the exercised rats, the acrobatic ones had more synapses per Purkinje cell (cells that modulate motor activity and are exclusive to the cerebellum and). Needing to learn specific skilled movements thus resulted in increased synaptic connectivity in the cerebellum. In contrast, rats in the two exercise groups had significantly more capillaries than rats in the other two groups, presumably to feed the repetitive neuronal activity required for exercise. Thus two different patterns of adaptation resulted from the different environmental demands placed on the different groups of adult rats, and there was adult neuroplasticity at the level of the synapse in the cerebellum in response to the challenging enriched environment. Exercise alone did not result in such change. Although this study only examined the cerebellum, other research discussed later found exercise alone did result in neuroplastic change in the hippocampus.
The Importance of Attention
Rodents in enriched environments surely pay attention to learn; one would have to pay attention to a wobbly obstacle-laden bridge to learn to cross it. This raises important issue of attention, manifested by top-down as opposed to bottom-up processing. Bottom-up processes are driven by sensory receptors, and have been observed as a matter of course early in life; for this discussion then, we return briefly to the issue of neuroplastic change in the juvenile period. Merzenich and his colleagues found that exposing rat pups to specific tones early in development resulted in changes in the receptive fields of whole clusters of neurons that were tuned to those tones, such that larger areas of cortex responded more to them than to other tones that had not been repeatedly experienced (Zhang, Bao, & Merzenich, 2001). In addition, the cells fired more synchronously, and were more finely tuned to the stimulus. It was not necessary that there be any consequence to the tones; simply hearing the sounds was associated with neural specialization. However, this only occurred in immature animals; adults’ cortical maps did not change in response to the specific auditory environment. Thus, as reflected in our second principle, a distinguishing feature of development in the juvenile period appears to be that passively experienced stimuli can shape the brain so it is specially tuned to those stimuli.
In contrast, top-down attentional processes can influence cortical organization even later in life. In a clever experiment examining this, adult rats (14–18 weeks old) were trained to seek a reward in response to either tone intensity or tone frequency (Polley, Steinberg, & Merzenich, 2006). All the rats in fact heard the same range of intensity and frequency, but each rat was trained to respond just to one or the other; additional control rats were not trained at all but were exposed to the same tones. If cortical organization were driven from the bottom up, the cortical maps of all the rats following training should be the same. If it were instead driven from by top down processes—by what each rat paid attention to—the cortical maps would be different and specific to the type of stimulus to which they were trained to attend. The latter result was obtained. Rats who could get a reward when a specific frequency of tone was heard had twice as many neurons in A1 and suprarhinal auditory field that responded to that tone frequency as compared to the control rats and those trained to intensity. In contrast, rats that could get a reward by responding when a specific intensity of tone was heard had more neurons in A1 and suprarhinal auditory field that responded to that degree of amplitude. In addition, for any given rat, the extent of reorganization in the auditory cortex was related to the degree of learning. Thus after the juvenile period, attention was necessary for modification of brain structure. During the juvenile period, exposure alone was sufficient.
A second study showing this used adult owl monkeys and permanently implanted electrodes in A1 to compare the neural responses of a trained animal with those of a yoked control that was rewarded for the same stimuli but was not required to make any behavioral response to get that reward (Blake, Heiser, Caywood, & Merzenich, 2006). The target monkey moved its head to a forward position breaking an infrared light beam, which resulted in the presentation of 2–6 standard frequency stimuli followed by higher-frequency target tones. If the monkey moved its head back out of the light beam after the first and before the third target sound, it was rewarded with a sip of orange-flavored drink. Initial testing showed both monkeys were classically conditioned—based presumably on underlying Hebbian processes: over time, both showed reward-anticipatory behaviors in response to the stimulus. This led to increases in excitability to the stimulus in A1 neurons. In contrast, operant conditioning, experienced only by the target monkey, led to increases in selectivity of responses. This probably stemmed from its needing to inhibit responses to non-target stimuli.
These studies exemplify our second principle in animal models: paying attention and acting in response to the attended stimulus leads to neuroplastic changes even in adulthood (for more discussion of the importance of attention for later neuroplasticiy, see Keuroghlian & Knudsen, 2007). It is difficult to specify what “paying attention” means for an owl monkey or a rat, but very young mammals have been shown to undergo neuroplastic changes in response to passively experienced auditory stimuli, whereas adult mammals only showed such change when they needed to pay attention to learn. These changes were to cortical fields, undergirded by unspecified changes at lower levels.
These changes were observed in animal models. Although today’s imaging methods cannot reveal changes at the synapse level in humans, differences in cortical fields as well as in cortical thickness have been observed with human learning; indeed one study has shown that imaging of maze-learning mice reveals changes reflected also in microscopic investigation of hippocampal neuron morphology (Lerch et al., 2010). Several examples of human neuroplastic change that presumably rest on the microchanges observed in animal models are discussed next. In all of these cases, people were paying attention, consciously trying to learn (for example) the streets of London, or how to juggle. We know of no cases of structural change in adult human brains in response to passive experience, underscoring our second principle. Further research should examine the developmental mechanism behind this difference in the attentional requirements for learning across these periods.
Visuo-Motor-Spatial Learning in Humans
The most extensively studied example of neuroplasticity associated with learning in humans comes from London cabbies, who have been shown in a series of studies to have larger posterior hippocampi and smaller anterior hippocampi than control subjects, including even London bus drivers (Maguire, Gadian, & al, 2000; Maguire, Woollett, & Spiers, 2006; Woollett & Maguire, 2009). London cabbies are a particularly interesting study because as adults they undergo intensive training to learn 25,000 streets in a 1200-mile radius of Charing Cross. Only 3 of 10 people beginning this training finish it, and it frequently takes 5 years. The size of the posterior hippocampus is correlated with the number of years driving the cabs, and is also correlated with the proficiency of a driver in finding London landmarks in a Virtual Reality (VR) environment. In addition, after retirement, the cabbies’ hippocampi shrink to midway between the size of current drivers and others, including those of bus drivers (Woollett, Spiers, & Maguire, 2009). This shrinking of the hippocampus corresponds to a loss of the knowledge base, as revealed in the VR environment and simple tests. Research in the VR environment also confirms that the hippocampus is very active when the cabbies are navigating the streets of London. The underlying causes of this change are uncertain. It will be seen later that the hippocampus is a site of neurogenesis, and this is a possible contributor to the change in size, with new neurons going to the part of the hippocampus where they are needed to support learning the spatial configuration. Changes to dendritic spines and axon boutons are clearly also good possibilities. DTI (Diffusion Tensor Imagining, see Appendix) can be used to examine whether myelination in this regions also differs in cabbies.
Interestingly, the cabbies were also worse than controls at acquiring new spatial information (Maguire et al., 2006; Woollett & Maguire, 2009), suggesting that committing the brain region to one space (London) might impede its use for other spaces. The decrement extended to memorizing complex figures, placement of photos of unrelated objects, and even word-pair associates in which the words were concrete image-able nouns. The cabbies were however better than controls at placing London landmarks and judging their relative distances, and equal on a range of other cognitive tests including verbal memory (Woollett & Maguire, 2009). Learning new visuo-spatial information was particularly impaired. Expertise had a clear cost, and that cost was manifest in neuroplastic changes as well as behaviors. This cost—physically manifesting what Huttenlocher (2002) referred to as “crowding” of the neural space—does not seem to occur in juveniles for spatial learning. For example, Austrailian aborigine children learn to map the natural space of the desert very well, and this transfers to their learning other spatial information better than city-raised children (Kearins, 1981). This is another possible candidate difference in early and later neuroplasticity. However, it is also possible that learning in other realms in the juvenile period learning does impair other learning. For example, a person whose motor systems were given over to skiing in youth might not be able to play tennis as well as an adult than they would have they not wired their motor system for skiing. More research on this issue is needed on the issue of when skills interfere with later learning, and when they can transfer thereby facilitating later learning.
One concern with the London cabbie studies is that people with larger hippocampi to begin with might be particularly able to complete the training. The correlation between years spent driving and size of the posterior hippocampus suggests the growth occurs over time, but results from a new longitudinal study will shed more definitive light on this issue (Woollet, in preparation).
The learning of complex manual visuo-spatial skills, like juggling and playing a musical instrument, are also associated with neuroplastic changes in adulthood; these studies have looked longitudinally at before and after images. Adults who learned to juggle over a 3-month period showed 3% increases in grey matter in brain areas that process complex visual information, such as the mid-temporal and left posterior inferior parietal sulcus; a 3-month break from juggling reduced the size of the areas to baseline levels (B Draganski et al., 2004). The size of the increase, moreover, was correlated at least in this one study with juggling performance—better jugglers showed more increase (c.f., Scholz, Klein, Behrens, & Johansen-Berg, 2009). The changes were observed just 7 days after commencing juggling (Driemeyer, Boyke, Gaser, Buchel, & May, 2008). Even senior citizens who began to practice juggling experienced a similar degree of increases in grey matter in the same areas of the brain—although their level of achieved proficiency did not approach that of the younger subjects (Boyke, Driemeyer, Gaser, Buchel, & May, 2008). A 3% increase seems quite small, but then juggling constitutes only one of many activities supported by these brain areas, so perhaps one should not expect more. As with the cabbies, the underlying cause of the changes is unclear. Their location makes it unlikely they are due to neurogenesis (discussed later); animal models suggest they are likely due to increases in dendritic spines and synapses.
Similarly, musicians’ motor representations of the fingers they use when playing their instrument are larger than those of non-musicians (Elbert, Pantev, Wienbruch, & Rockstroh, 1995). This was particularly true of musicians who started playing their instrument at a young age, showing that there is a sensitive period early in life; but the adult findings show that structural change still occurs after that early period. This reinforces the idea that the juvenile period is one of greater plasticity, yet that plasticity exists later in life as well.
Not only have neuroplastic changes been observed in adults learning new spatio-motor skills, but they have also been reported with more abstract learning. Medical students’ posterior and lateral parietal cortex enlarged during exam study time, and their hippocampi increased in size during the study period and remained larger even 3 months after the exam (Bogdan Draganski et al., 2006). These results are novel and controversial (A. Thomas et al., 2009) but intriguing grounds for further study.
There is also a growing literature on neural changes associated with the acquired skill of meditation: sitting still and focusing attention on the present moment, one’s breath, or some other object. For example, long-time meditators have greater thickness in brain areas related to bodily and visceral attention, especially in the right hemisphere (Lazar et al., 2005). One concern with this research—as with the London cabbies--is whether neurophysiological and behavioral differences existed prior to meditation, and were part of what led to the meditation practice. Against this, and like the cabbies, within the meditation group meditation experience was also correlated with changes in cortical thickness, particularly in the inferior occipital-temporal cortex but also in other areas when controlling for age (there are typically age-related declines in cortical thickness; several of the most experienced meditators were also the oldest). It is interesting that this brain area is implicated in higher-level visual processing (Knudsen, 2007), given that meditation is not a visually-intensive process. What underlies the increased thickness is again unknown; the authors speculate that dendritic branching, more glia, and larger vasculature are all possibilities. In another study, increases in white matter (discussed later) were found in the anterior cingulate cortex of people after just 11 hours of meditation training (Tang et al., 2010). This makes good sense, given that meditation is a form of attention training and the anterior cingulate cortex is activated by attention-consuming activities (Westlye, Grydeland, Walhovd, & Fjell, 2011). All in all, these studies support that attention training results in neuroplastic changes in adults.
Whereas these human studies have correlated learning of skills and information with the size of particular brain areas, other research has looked more specifically at changes to cortical maps with human learning. In one illustration of this, people were taught a new 5-finger piano exercise, which they practiced 2 hours a day for 5 days (Pascual-Leone, Dang, Cohen, & Brasil-Neto, 1995). Some participants went on with this 5-day-per-week practice regime for 5 weeks (Pascual-Leone et al., 2005). TMS (see Appendix) targeting the cortical output maps for the finger flexors of the trained and untrained hand indicated the impact of the practice on the motor representation.
The researchers found that 20–30 minutes after each daily practice, the motor representation of the trained hand was significantly more activated than that of the untrained hand, and the amount of post-practice activation of the trained hand increased over the first five days of practice, suggesting an increase in the number of motor synapses involved in producing the piano exercise. The rapid increase in the size and intensity of the motor output representation in the initial 5 days could suggests recruitment and unmasking of existing synaptic connections, rather than formation of new ones, because new connections require a longer time course (Jacobs & Donoghue, 1991). Also, in the minutes before daily practice there was no difference in the cortical maps of the trained and untrained hands, even after five days of practice. Thus the increases were not yet permanent but were only reflecting temporary activations.
When examined week-by-week in a subsample that continued the 5-day-a-week practice for five weeks, a different pattern emerged. By week 5, the before-practice cortical map of the trained hand had expanded significantly, showing a more permanent increase in the cortical field driving the practice. In contrast, by week 5, the after-practice activation of the trained hand was reduced relative to what it had been after practice in the first week. The authors interpreted this as showing that other brain areas had taken over.
A recurrent theme in neuroplasticity research is that the brain is a great symphony of action, with different parts compensating for each other, taking over with practice, resulting in both increased and reduced activations across areas (Karni et al., 1995; Sanes & Donoghue, 2000). This is particularly the case with more complex functions, which tend to be more widespread in their activations (Huttenlocher, 2002). Another (non-exclusive) possibility is that a smaller number of connections had become stronger (perhaps reflecting more synaptic vesicles or receptor sites), reflecting increased efficiency, which also occurs over time: adults in general activate a smaller and more discrete circuitry than children when performing any given task (Huttenlocher, 2002).
Changes in cortical fields occur even when one simply imagines. In the Pascal-Leone study just described, people in another condition imagined doing the 5-finger exercise on the piano for five days. As with the actual players, for the imaginers the motor map representing the “playing” hand increased in size after practice each day. A difference from the actual players was that despite the parallel changes in the motor map, when the imaginers actually played the piece at the end of the five days, they did not do it as well as those who had actually practiced each day. However, after just one day of physical practice they did play it as well, so that several days of imagining followed by one real practice was as good for one’s playing as five days of real practice. In addition, five days of mental practice led to the same level of playing as did three days of actual practice. Controls who had not imagined playing still had a very high error rate at day 5. Many other studies show similar neural activity occurs when one imagines doing something as when one actually does it; a recent review concludes that imagined activity occurs in the same area of the brain but is subdued relative to real activity (Munzert, Lorey, & Zentgraf, 2009).
In sum, many studies with human subjects have shown with imaging or TMS that learning involves changes in the size of brain regions that undergird that learning. Studies with London cabbies, jugglers, musicians, medical students, meditators, and people asked to learn finger exercises for the study all showed such changes. But with humans, imaging methods do not allow a close look at what structural changes are responsible for the changes. Animal studies suggest they are undergirded not only by changes in receptivity at the membrane (thus synapse level), but also by “harder” structural changes like the appearance and disappearance of dendritic spines as well as shape changes in existing spines.
Thus far, most of the changes we have reviewed support the principles outlined earlier: neuroplastic change is most dramatic in the juvenile period, and when later change occurs, it tends to be at the level of the synapses, axon boutons and dendritritic spines; and change in adulthood requires attention on the part of the organism, or that changes are made incrementally as with Knudsen’s prisms (a claim supported by stroke recovery research as well). The topics of the next section presents an exception to the first principle.
Neurogenesis
Recently a radical exception to the idea that later changes are at the level of the synapse, including changes to dendritic spines and unmasking, has become widely accepted: neurogenesis occurs in adulthood (Bruel-Jungerman, Laroche, & Rampon, 2005). Importantly, it appears to occur only in two areas in the mammalian brain, the hippocampus and the olfactory bulb. Still, it upends the centuries-old doctrine (Gall & Spurzheim, 1835, cited in Pascual-Leone, 2001) that after birth, no new neurons would ever be produced. That doctrine made sense: if new neurons kept coming along, what would happen to sense of self, memories, and representations of place? A well-functioning organism, it would seem, requires stable neural circuitry, which by definition is disrupted when new neurons join.
The first challenge to the doctrine of no-new-neurons came in reports of neurogenesis in the adult rat hippocampus (Altman, 1962; Altman & Das, 1965). However the findings were disputed and largely disregarded (see Gould & Gross, 2002 for a more thorough discussion of this history.) The first widely-accepted challenge came in the 1980s, when impressive neurogenesis was revealed in the high vocal center (HVC) of male adult canaries learning new song elements during mating season (Goldman & Nottebohm, 1983); the cells died off when the season was over. Then in the 1990s, extending beyond song learning, new neurons were observed in the hippocampi of adult chickadees during the fall seed-hiding season (Goldman & Nottebohm, 1983). However, neurogenesis in adulthood was typically interpreted to be a special adaptation for birds, since birds need to be light in order to fly and thus could not afford relatively heavy brains like mammals have. By generating new neurons when needed and deleting them when their usefulness subsided, birds could better maintain the weight necessary for flight.
Resurrecting Altman, technical advances in the 1990s brought on a mushrooming of reports of neurogenesis in mammals. In 1992, Reynold and Weiss induced in vitro neural proliferation from progenitor cells collected from mouse striatum, showing that the cells could divide, but not necessarily that they did so in vivo. In 1994, Lois and Alvarez-Buylla found that newborn cells could migrate to the olfactory bulb and become neurons. Then Gould and colleagues found neurogenesis in the tree shrew (Gould, McEwen, Tanapat, & Galea, 1997), and a year later, she revealed the same in marmoset monkeys (Gould, Tanapat, McEwen, Flügge, & Fuchs, 1998). Recent years have seen a plethora of evidence for later-born neurons in a variety of mammals, including cats, rats, and mice (Gould, 2007; Gould, Beylin, Tanapat, Reeves, & Shors, 1999), rabbits (Luzzati, De Marchis, Fasolo, & Peretto, 2006), nonhuman primates (Bernier, Bedard, Vinet, Levesque, & Parent, 2002; Eriksson et al., 1998; Gould, Reeves, Graziano, & Gross, 1999; Kornack & Rakic, 1999). These findings and the others cited earlier overturned the no-neurogenesis doctrine in adult mammals generally. Breaking the human barrier, Peter Eriksson serendipitously discovered that a substance (BrdU) allowing for identification of newly born neurons by tagging DNA synthesis had been injected into some cancer patients for diagnostic purposes in Sweden. Eriksson got permission from five patients for post-mortem examination of their brains, and revealed that neurogenesis occurs in the adult human hippocampus as well (Eriksson et al., 1998). The proposal for human adult neurogenesis is not without controversy (Rakic, 2006), but its occurrence in many other animals on top of the Eriksson data has led to widespread acceptance that it must occur in humans as well.
Estimates of neuron birthrates were initially 1000/day in the rat (Gould & Gross, 2002); more recent estimates are 9000/day, or 270,000 month in the dentate gyrus of the rat, which is an impressive number given that the total number of neurons in that region is estimated to be just 4–8 times that (Cameron & Mckay, 2001). Thus neurogenesis does occur after the juvenile period, and the number of neurons born is not insubstantial. What role might these new neurons play?
First, they do appear to be functional (Leuner & Gould, 2010; Schwartz & Begley, 2002). They grow axons and dendrites (Markakis & Gage, 1999; Stanfield & Trice, 1988), and they become integrated into the neural circuitry (van Praag, Shubert, Zhao, & Gage, 2005). They are most often reported in the olfactory bulb and the hippocampus (Gage, 2003)1. In the olfactory bulb, they appear in response to smell-rich environments (Livneh, Feinstein, Klein, & Mizrahi, 2009; Rochefort, Gheusi, Vincent, & Lledo, 2002), and are thought to be a special adaptation to the complexity and dynamism of odor (Lledo & Saghatelyan, 2005). In the hippocampus, they appear with placement in enriched environments (Kempermann, Kuhn, & Gage, 1998), and with exercise (van Praag, 2008). Tashiro and colleagues showed that rats’ exposure to an enriched environment 2–3 weeks after the birth of new neurons in the dentate gyrus was associated with increased neural response to that environment (but not to other environments) later, raising the interesting possibility of critical periods for neurons as opposed to for entire organisms (Bruel-Jungerman et al., 2005; Tashiro, Makino, & Gage, 2007). Furthermore, learning appears to induce both neurogenesis and apoptotic processes that depend on the age of newborn cells at the time of learning (Dupret et al., 2007). In a series of experiments, rats were trained to find a platform in a Morris water maze or put into a variety of control conditions. They found that in the early stages of learning, all newborn cells survived. But once learning stabilized—at the 5th day of maze training—then the older newborn cells (12 days old), which had formed more axonal and dendritic connections than the younger ones, were more apt to survive, and the younger newborn cells (8 days old) were more apt to die, than in untrained rats. These findings were specific to the dentate gyrus, the site of all adult neurogenesis in the hippocampus, and were not found in areas CA1 and CA3 in the hippocampus. Furthermore, the survival of older cells was associated with production of more newborn cells. The authors compared the process to sculpting in immature organisms, in which functional connections are retained and nonfunctioning ones retract. In addition, the learning system seems to be setting itself up for more learning by generating more newborn cells.
One could argue that neurogenesis after critical periods goes along with having something new to register (for thoughtful discussion see Leuner, Gould, & Shors, 2006). In the natural world there are no running wheels, and when one exercises one is typically moving through the environment (Kempermann, 2008). There are sights, sounds, and smells to register and locate. In favor of the idea that neurogenesis after juvenile periods is to help with learning is the fact that elderly mice who exercised, and thus had a 25% increase in hippocampal neurons (a sedentary elderly group a quarter as many new neurons), also were better at learning a Morris water maze (van Praag et al., 2005)2. In addition, preventing neurogenesis in the hippocampus of adult rats is related to decreases in performance on a task in which they must associate stimuli across time (Shors et al., 2001). Restoring neurogenesis leads to improvements in performance. This research supports the second principle: neuroplasticity arises from situations in which organisms pay attention, as they do to learn mazes to get rewards.
Neurogenesis is also downregulated by certain conditions: Stress, both in adulthood and prenatally (Mirescu, Peters, Noiman, & Gould, 2006; Tanapat, Galea, & Gould, 1998), sleep deprivation (Mirescu et al., 2006), depression (Santarelli et al., 2003), and period of the estrous cycle (Tanapat, Hastings, Reeves, & Gould, 1999) all influence the rate of neurogenesis. Indeed, some pharmacological treatments for depression upregulate neurogenesis (Schwartz & Begley, 2002). In evolutionary terms, organisms that are stressed seem to be programmed to stay put, to hunker down and conserve their resources (Hobfoll, 1989).
In sum, neurogenesis after critical periods is established, with new neurons in the olfactory bulb and the hippocampus, and possibly other areas, which become integrated in the circuitry. Exponentially more new neurons appear early in life, however the fact of whole new neurons mitigates our first principle. Neurons born in adulthood spawn axons and dendrites that support new synaptic connections that appear to undergird learning, and learning in turn influences the production of new neurons. Although an except to the first principle, the evidence is in solid accord with the second principle, in that neurogenesis particularly seems to occur when organisms are actually or by implication (movement), paying attention, and learning in new environments.
Myelination
Myelin--the fatty sheath that forms around an axon and permits much faster transmission of the neural signal—is another type of lifelong neuroplasticity, and in this case it occurs throughout the brain. In one study of 70 men ages 19 to 76, myelin was found to peak at age 44 in the frontal lobes and 47 in the temporal lobes (Bartzokis et al., 2001). It begins to decline by middle age (Giorgio et al., 2010; Johansen-Berg, 2010), and even more so with appearance of mild Alzheimers (Salat et al., 2009). In contrast to the increase to mid-life in white matter, grey matter decreases with neural shrinkage throughout adulthood (Terry, DeTeresa, & Hansen, 1987), although as seen previously learning specific tasks is associated with less loss or even gain in targeted areas. White matter increase is probably not due to increases in the number or length of axons: inhibiting factors that even come from the myelin itself largely prevent axonal growth in the CNS after early development. Instead, it appears to be that more existing axons become myelinated (or get thicker layers of myelin for even faster conductance) over time3.
Although twin studies show the amount of myelin in one’s brain is strongly influenced by genes (Chiang et al., 2009), recent research suggests that like neurogenesis, myelinization is influenced by learning and experience. One piece of evidence for this is that adult rats placed in enriched environments have more myelin (Markham & Greenough, 2004). In humans, Ullen and colleagues showed that professional piano players have more myelin than non-players in a brain region associated with playing, and that amount of practice during different life periods (childhood, adolescence, and adulthood) is correlated with amount of myelin in specific regions, probably ones in which myelination matured during the time of intense practicing (Bengtsson et al., 2005).
DTI has led to a surge of research on the effects of experience on white matter. Following on the Dragnaski (2004) juggling studies mentioned earlier, a later study found that juggling also increased white matter in regions of interest (Scholz et al., 2009). Keller and Just (2009) found that 100 hours of intensive reading practice over 6 months increased white matter in the left anterior centrum semiovale, under the frontal and parietal lobes, for poor readers, who had impoverished white matter in that region prior to training. Within participants, the degree of increase was correlated with improvement in reading. In a study mentioned earlier, Chinese participants randomly assigned to just 11 hours of meditation training showed significant increases in white matter in the anterior cingulate cortex, a change not seen in those randomly assigned to relaxation training (Tang et al., 2010). Long-term practice at the game Baduk (somewhat like chess) was also associated with changes in white matter in areas mediating attention (Jang, Kang, & Kwon, 2010). Two studies have shown that working memory training impacts white matter. One had 11 undergraduates train for 2 months, 30 min/day, on working memory training tasks. Increases in white matter in the anterior corpus callosum occurred in the training group and degree of change was correlated with degree of memory improvement. Lovden et al (2010) extended this to study an elderly (65–75 years) as well as a younger (21–30 years) sample, with 100 hours of training. They also found an impact on the anterior corpus callosum, and the degree of change was the same in older as in younger participants. Johansen-Berg (2010) notes that white matter change is particularly apt to be seen in the corpus callosum.
Thus a great deal of recent research shows that white matter increases at least until midlife and that with experience it can even increase in old age. The later changes shown here are all in response to tasks involving focused attention: reading, juggling, memory training, playing musical instrument and games. But the degree of change that can be induced by experience was no greater in one’s 20s than in old age, suggesting that for myelination, the molecular environment hosts plasticity later in life.
Summary
In sum, learning is associated with structural changes in the brain that go beyond the changes in synaptic sensitivity discovered by Kandel. When humans learn, areas involved in the learning change size, presumably due to underlying neuroplastic change. This can even occur in response to imagining. Some of this learning involves unmasking of existing connections, but growth of dendritic spines and axon boutons—and with them new synapses—and more glia and vascular changes also support the increased activity. Most radically, these changes appear to extend to the birth of new neurons, although much remains to be learned about neurogenesis in adulthood in humans and other mammals. Myelinization appears to be a dynamic process well into mid-life and will likely prove to be an important source of neuroplastic change in response to learning. By changing the speed of neural transmission, myelin can modulate neural synchrony and thus efficiency in adaptation to environmental change.
Neuroplasticity after Extreme Changes in Sensorimotor Experience
More extreme changes in neural function can result from a change to the sensory or motor interface. Below we first consider human imaging studies before turning to animal models in which the activity of single cells has been recorded. We do this first for perceptual systems (vision, barrel cortex) and then we examine the results of extreme changes resulting from amputation or deafferentiation. In both cases, an initial period of change is thought to be induced by unmasking, and is followed by harder structural change.
Perceptual Input Changes
Neuroplasticity is observed in individuals with atypical sensory input. For example, in the congenitally blind, the visual cortex is activated by reading Braille and hearing speech (Buchel, Price, Frackowiak, & Friston, 1998; Roder, Stock, Bien, Neville, & Risler, 2002), and, in the congenitally deaf, the auditory cortex is activated by visual input (Finney, Fine, & Dobkins, 2001). An extreme difference in experience therefore changes the organization of the brain from what would be prescribed by genes to adapt to the differences in normal sensory experience. These kinds of neuroplastic changes can result from an extreme difference in sensory input at any point in life.
Sensory substitution devices reveal functional plasticity in adulthood. Bach-y-Rita (2003), who pioneered the use of such devices, opens one article with the startling statement, “Persons who become blind do not lose the capacity to see” (p. 541). Even congenitally blind adults have been trained to detect visual stimuli (for example, the orientation of the letter “T”) via a device placed on tongue with a grid on sensors that reflect a spatial array. Underlying neuroplastic change accompanies this functional change. Thus after 7 one-hour training sessions with the tongue device, congenitally blind adults—but not sighted blindfolded controls—had increased blood flow to the occipital cortex (Ptito, Moesgaard, Gjedde, & Kupers, 2005), supporting increased neural activity. Blindfolded controls performed equally well on the behavioral task of detecting objects through the tongue device, but not by recruiting visual areas. Further, the location of the increased blood flow in blind subjects matched that observed in sighted subjects in a visual discrimination task (see Ptito et al, 2005). Thus although the subjects had been blind since birth, and the sensory modality involved was receptors on the tongue, over just a week of training the visual cortex began to take part in the act of “seeing” when a sensory substitution device was employed. This is neuroplastic change in response to an extreme change in sensory experience in adulthood, in concert with our second principle.
These changes are so rapid that they are thought to involve unmasking of already-existing connections. For example, communication from one cell (A) to another (B), often from a deeper to a more surface brain layer, might typically be masked or silenced at the higher layer by inhibitory signals. When the inhibiting cells at the higher level die or are themselves silenced by inhibition, then the signals from the deeper-layer cell (A) are no longer inhibited: they are unmasked. Structurally nothing has changed within A or B, but the neural signal is now given voice. Unmasking can also occur within layer. Typically activation of a neuron is accompanied by inhibition of the surrounding neurons. When activation of that one neuron ceases, so does the local inhibition, allowing for unmasking of other local connections.
Unmasking is also invoked to explain what happens when sighted adults wear a blindfold. After just five days of blindfolding, TMS to the visual cortex of sighted Braille readers disrupted ability to read Braille, showing that five days of blindfolding was sufficient to make the visual cortex of sighted people begin to process the tactile input of Braille (as it does for congenitally blind people). No such disruption from TMS was seen in unblindfolded Braille-trained controls (Merabet et al., 2008). The 5-day time course is thought to be too fast to reflect the structural changes that would be required to have tactile inputs newly routed the visual cortex, and thus the effects are presumed to be due to unmasking.
Animal models have revealed at a single cell level the kinds of changes that could underlie unmasking events. In one study, a small imaging window was placed over the barrel cortex in adult mice that expressed a florescent protein in some pyramidal neurons (Trachtenberg et al., 2002). Revealing the normal rate of change, 20% of imaged dendritic spines (particularly the smaller ones) turned over in a 24-hour period, which is fewer than has been observed in juveniles (Lendvai et al., 2000), but is still substantive. To examine the influence of sensory deprivation on synapse construction in the barrel cortex, they then performed a “chessboard” operation by removing every other whisker on one side of the face. At first, this change in input did not impact the rate of spine turnover, but from day 2 to 4, there was a significant increase in turnover in the side of the cortex receiving input from the chessboard cheek. In keeping with the first principle, no new axons or dendrites were observed over the 8 days. Nor was there change in the number of synapses overall. Whether longer periods of observation would reveal changes in the number of synapses is not known.
In another study with animal models, immediate unmasking-like changes appeared followed by the apparent addition of synapses. Gilbert and colleagues injured the retina of adult cats with small laser beams, creating a visual blind spot. Minutes after the injury, they recorded from cortical cells that had receptive fields at that visual location, and found that the cells that were located at the edge of the scotoma increased their receptive field size to respond to the visual field locations that were closer to the center (Gilbert & Wiesel, 1992). The plastic response was so rapid (minutes) that it was explained as unmasking of existing circuitry, normally subdued by lateral inhibition. Strikingly, a few months after the injury, they recorded from the same cortical region, to find that even the areas that were initially silenced recovered visual function, even though the corresponding region in the LGN that supplied the visual input to cortex was still silent. This could only explained by formation of new synapses between intrinsic cortical cells located at the edge and the center of the scotoma. Thus, a single change in sensory activity pattern first led to unmasking, and later to structural plasticity (Gilbert, 1998), in particular the sprouting of axon boutons and thus presumably new synapses (Darian-Smith & Gilbert, 1994). Note the parallel to Pascal-Leone’s human model study involving the five-finger exercises, where initial unmasking was apparently followed by structural change.
Phantom Limbs
Returning to human models, phantom limbs are a special case of somatosensory plasticity in response to extreme changes in the input. A phantom limb is the sensation that a limb is still there following its loss. Sensory stimulation to other body parts after limb loss—typically ones near the limb on the Penfield map (‘motor homunculus”)—can result in the phantom sensation. For example, stroking the face can result in the sensation that one’s missing hand is being stroked (Ramachandran & Hirstein, 1998).
Phantom limbs occur in over 90% of adults who lose a limb; one study showed that before age 8, the younger one was when one lost a limb, the less chance of experiencing a phantom, suggesting the central representation of the limbs is built up over the first eight years of life (Simmel, 1962). Interestingly, phantom limbs are sometimes reported even among people who were born without the limb, and even when they are 20 years old, suggesting that to some degree the neural circuitry for representation of body parts is hardwired—as is hinted at by Meltzoff and Moore’s (1979) seminal research on newborn imitation--and that it is maintained over a long period even without feedback (Ramachandran & Hirstein, 1998). Indeed, one girl who was born without arms reportedly used her imagined fingers to count (Poeck, 1969, as cited in Ramachandran & Hirstein, 1998)!
Neuroplastic reorganization can occur even within 24 hours following limb loss (Borsook et al., 1998) and across astounding distances. MEG (see Appendix) shows that reorganization following limb removal involves lateral movements of synapses by as much as 2–3 cm of cortex (Ramachandran, 1993). The fast time course coupled with these large distances suggest that the plasticity evidenced at least initially likely involves unmasking, whereby inputs from the lost limb silenced neurons that received voice when the limb was lost, and the inputs were thus removed. In concert with this, other research has shown that in the absence of sensory activity as would occur with limb loss, production of the inhibitory neurotransmitter GABA is reduced (Garraghty, LaChica, & Kaas, 1991).
Phantom limbs are often very painful
One explanation for this is that the motor cortex is sending signals to move the hand, but no sensory feedback confirms the success of those signals, and this confusion is perceived as pain (Ramachandran & Blakeslee, 1998). “Mirror therapy” can attenuate the pain (Ramachandran & Blakeslee, 1998). Patients are seated in front of a mirror that reflects the movement of their intact hand to a physical space where the patient would see the limb were it there. The patient is then asked to move the phantom and the intact hand in particular ways. The visual feedback often fools the patient’s brain and the phantom pain subsides, presumably because sensory feedback, albeit through the wrong hand, tricks the visual system so it feels as if the phantom is present and giving somatosensory feedback. Giraux and Sirigu (2003) suggest that phantom limb pain is caused by motor reorganization—a loss of signal coherence--that occurs following limb loss, and that the reason mirror therapy helps is because it attenuates the take-over of the motor cortex region. Supporting this, others have found that the degree of pain is very strongly related (r = .93) to the degree of cortical reorganization (Flor et al., 1995). In this study 13 amputees were selected to represent a wide range of phantom pain. In those with more phantom pain, the degree to which the neural representation of the face had shifted was almost 5 times larger—over 2 cm of shift on average (as measured by MEG)—as compared to the shift observed in those not experiencing pain. Thus neuroplastic change begins immediately the loss of the limb, and the extent of shift in signals to parts of the motor homunculus that normally respond to other input predicts the extent of phantom limb pain.
Another therapy that results in substantive pain reduction after limb loss is imagining the body part and intentionally moving it (MacIver, Lloyd, Kelly, Nurmikko, & Roberts, 2008). Because activation in the motor cortex occurs even in response to imagining movement (Glenberg et al., 2008; Sato, Cattaneo, Rizzolatti, & Gallese, 2007), this is presumably working in a manner similar to mirror therapy. In this study as well, the less the motor hand area responded to a lip purse, the less phantom pain was experienced by hand amputees (MacIver et al, 2008).
Structural plasticity also appears to result over extended time after limb loss; here we have the second exception to the first principle, because axonal growth occurs in adulthood. This was observed in the famous Silver Spring monkeys who had lived over 12 years with deafferented limbs—essentially amputation effected by cutting of the nerves (Pons et al., 1991). Whereas a few months of deafferentation is sufficient to generate cortical reorganization of 1–2mm (Merzenich et al., 1983), extensive reorganizations (10–14mm of cortical surface, thalamus and brainstem reorganizations) were observed after years of deprivation (Jain, Florence, Qi, & Kaas, 2000; Jones & Pons, 1998; Kaas, Florence, & Jain, 1999; Pons et al., 1991). Although human neuroimaging methods have not allowed investigation at this level, it seems likely that this degree of reorganization must eventually occur in humans as well.
Summary
Cases of severe sensorimotor change, as when one becomes blind or loses a limb, can result in neuroplastic changes even after the juvenile period. These changes are most readily explained as unmasking of existing connections, undergirded by synapse-level changes like the amount of GABA or dendritic spine turnover, although even long-distance axonal reorganization has been observed after long periods of sustained deprivation.
Neuroplasticity Before vs. After the Juvenille Period
The evidence just reviewed samples from a larger literature showing structural changes in adult brains. How do these compare with the changes observed in youth? First, consider three basic groups of neuroplastic changes, using Figure 1, A, as a reference neural circuit: a dendrite from an input cell fires onto the axon of an output cell, modulated by an interneuron. In the first group of neuroplastic changes, all the hard structures in the brain are stable, but functionality is plastic, either via mechanisms of unmasking (Figure 1, E), or via up- or down-regulation of synaptic proteins with LTP/LTD Figure 1, C). These kinds of changes in circuitry function are seen frequently in adult learning. When many of these functional changes occur together, they can result in neuroplastic change at the level of cortical maps. In the second group, the basic neuron, axon, and dendrite structures are fixed, but new synapses are formed, often on new dendritic spines or axon boutons, as another outcome of LTP, as well as under conditions that increase brain growth factors (Figure 1, B). These kinds of changes are most prominent in the juvenile brain and occur even in response to passive experience, but they are also encountered in the adult brain, particularly under conditions of focused attention (as in learning to negotiate an enriched environment), gradual change (as with prism goggles), and in cases of extreme sensorimotor change (as with removal of whiskers, and injury to the retina). In the third group, new structures—new axon branches, dendrites and neurons are formed, or axonal branches move into new territory, leading to major changes in neuronal circuits (Figure 1, D). Whereas juvenile brains generate this third group of changes as a matter of course, adult brains are capable of them under particular circumstances, including new learning challenges (neurogenesis in the hippocampus in enriched environments) and extreme sensory changes (axonal growth following limb loss). All three types of neuroplastic changes can bring on new tricks in old dogs.
Figure 1.
From the perspective of neural circuits, neuroplasticity is what changes the output of neural circuits. There are many different ways in which the output of a neural circuit can change as a result of changes in the pattern of incoming activity.
A. In the simplest form, neural circuits are driven by inputs that come onto them, and their gain is modified by intrinsic inputs (GABA, ACh and other modulatory inputs). Neural circuits in close proximity (in different columns, or processing different features of sensory input) also modulate each other (such as by lateral inhibition, not depicted here) to produce a unified output.
B. If a cell is induced to make new synapses onto existing targets (in the case of axon sprouting, new spines, or new dendrites), the individual output of the affected neuron changes, altering the output of the neural circuit. The output changes in strength.
C. As in B, but instead of new synapses, the existing synapse becomes more efficient in activating the postsynaptic neuron, by for example upregulating the number of receptors at the synapse.
D. If the axon is able to sprout more to extend farther distances, and to form synapses onto a neuron to which it was previously unconnected, then the affected new neuron receives a novel stimulus, changing the character of the output.
E. The output of the neural circuit can also change without any structural change: If one of the inputs coming to the neural circuit is weakened or cut, thus depriving the circuit of its drive, then the interactions between local neural circuits will be influenced, particularly by reducing the lateral inhibition it exerts onto neighboring circuits. Without the local inhibition, the neighboring neuronal circuit will now be more sensitive to its own input, thereby leading to a ‘novel’ output. This “unmasking” change is immediate, since it does not require structural changes that need time.
In considering these changes, it is useful to note the paradigms in which they occur. One of the most useful paradigms for observing neuroplastic change has been the enriched environments used mainly with rodents and sometimes primates, which show changes at all three levels in adults and juvenilles. Enriched environment exposure leads to more neurons, which bring with them more axons and more dendrites. The dendrites are longer and more arborized, and have more densely packed spines. In keeping with this, there are more synapses per cell, and the synapses are larger. There is more myelin covering the axon, generally to speed signals but also to modulate the timing of signals, and there are more glia and vascularization to support the additional hardware. Cortical thickness is increased as a necessary result of all these other changes. There are also some specific differences in juvenile and adult changes in response to enriched environments, although the reason for these differences is not yet known.
In research with humans, the nearest analog to the enriched environment might be studies of focused learning, as with the London cabbies, jugglers, musicians, meditators, and the 5-finger exercises. This analog is only in the comparative sense: the enriched environments used in animal experiments are still impoverished relative to the real world, whereas the humans in learning studies are living in the real world and the focus is on how particular brain regions change in response to learning particular skills or information. In both, at issue is how brains change relative to the brains of others who are lacking in that particular experience. Whereas in humans the neural changes are observed as cortical thickening or changes the size of a cortical field, any or all of the underlying changes observed in rodents in enriched environments might be implicated. Changes in adulthood are sometimes not as extreme because for some types of change the adult brain is less plastic—for example, in musicians the cortical area representing the fingers that play their instrument is always larger, but more so in those who began to play as children. The extent of cortical change has often been associated with behavioral outcomes, for example the most knowledgeable London cabbies have the largest posterior hippocampi.
The studies of extreme changes in sensory input in both humans and animals are close analogs, and in this case juvenile and adult differences are clearly apparent. The paradigmatic example of a critical period is the research of Hubel and Weisel, showing that adult cats do not experience long-term permanent changes based on extreme alternations in sensory experience, whereas young cats do—at least over the durations of these experiments. The axons and dendrites that carry signals for visual perception including depth are laid down in a particular period pre and post-natally, and environmental feedback is necessary to their sustained development. Thus when a young eye is sutured shut or blocked by a cataract, abnormal development ensues. In adults, a cataract impacts vision for the moment, but not permanently. Yet closing off vision in adult humans with blindfolds does lead to changes in the locations of neural activity, and this is typically interpreted as unmasking of existing connections. A model of unmasking is also observed in adult owls that wore prisms in their youth: they can rapidly relearn object locations in the shifted environment. Yet research on adult animal models suggests than after very immediate unmasking-type changes, dendritic spine and axon bouton growths lead to the formation of new synapses as well. Over the very long haul, longer than the visual cortex studies of cats endured, even longer-range axonal change seems possible, judging from the motor plasticity observed after limb loss.
Studies of cortical map plasticity in both young and older animals have provided a view that might apply to neural change more generally. In adults, the immediate response to sensory change is frequently the unmasking of existing connections, whereas in the juvenile it is a rapid cascade of events that may involve synaptic plasticity first, followed by axonal reorganization. In cortical map plasticity, rapid electrophysiological changes always precede detectable axonal changes by a few days, and restoring a normal sensory environment restores electrophysiological plasticity within minutes. Axonal reorganization is what makes the brain respond in a different pattern efficiently for a long period of time. In the juvenile brain, the role of unmasking may be too fleeting to observe and it is often preparatory for the ultimate aim: reorganizing the circuit. The response of the adult brain to sensory change is protracted unmasking accompanied by synaptic changes, until axonal reorganization eventually takes place.
Unmasking and synaptic plasticity are rapid responses of the brain, yet they are not stable enough to sustain a reorganized response pattern to long term, even permanent, change in the sensory environment. Yet these early plastic responses prepare the brain for more hard-wired changes. In the juvenile, the lag between the early and hard-wired plastic changes is very brief (3–5 days of suture leads to the axonal reorganization underlying ocular dominance plasticity) whereas in the adult, it is very long (months in the case of the adult cat visual cortex as with Gilbert, or years in the case of the deafferented monkeys studied by Pons). Attention, motivation, trophic factors, and hormones contribute to influence the different early and late responses.
Finally, neurogenesis occurs vastly more in young animals—largely prenatally—than older ones. For the most part, then, it is neural development, not neuroplasticity by our definition. But new neurons in mature animals have been observed, mainly in the hippocampus and the olfactory bulb. In both young and old, exercise increases neurogenesis, and stress decreases it. In birds, neurogenesis is associated with learning songs and hiding seeds in season. Research on neurogenesis later in life and its implications is in its infancy, but it will surely be a burgeoning area in the coming years.
A limitation to our knowledge stems from the complexity of technical equipment and knowledge required for imaging different neural structures at different levels in different species. Laboratories need to specialize in the types of neuroplasticity they observe, and the models in which they observe it. Over time, for example, we might learn how myelin changes after amputation, but at this point our knowledge is limited by the technology used in the laboratories that use different models. The fullest picture of neuroplasticity before and after critical periods has stemmed from enriched environment research with rodent and primate models, but the reasons for the changes observed—spine density at one time, spine length at another—are not yet understood.
We conclude with restating our three essential principles regarding neuroplastic change in the juvenile and adult periods:
Changes in the brain in early and later life occur at different levels and scales, with later changes only rarely involving new axons and dendrites, and instead involving changes to the synapse, new dendritic spines and axon buttons, myelination, and unmasking. When many such changes occur in concert, larger scale changes to cortical maps can occur in adulthood. The other exceptions to this principle are neurogenesis, which is particular to the hippocampus and olfactory bulb, and axonal change in response to extreme changes in sensorimotor input.
Passive exposure can bring neuroplastic change early in life, but in older organisms, paying attention is key to neuroplastic change. In addition, neuroplastic change later in life is more apt to appear if the stimulus gradient changes in small increments.
The reason for the differences in levels of plasticity in the juvenile and adult periods is the molecular and neural environments that make them more amenable to structural modifications in young brains. Periods of neuroplasticity can be extended or replicated later in life with methods that somehow restore the early molecular environment.
In sum, if one wants to teach new tricks, one can do so most easily with young dogs. But an old cur can learn new tricks when there is a reason. Treats to inspire it to pay attention are important, but gradual changes in the input, or unmasking, also lead to change. Whether those changes are very temporary, involving mainly synaptic strength and temporary facilitation or inhibition, or entail longer-term changes in the numbers of synapses in a cortical field, has importance for how those connections will be used. If one wants only a temporary trick, it can be induced quickly; if one wants it to stay, it must be induced gradually, allowing for harder neuroplastic change.
Acknowledgements
During the writing of this manuscript the authors were supported by NIH grants R01 EY12138 and DC010183 to AE and NSF grant 1024293 and a Brady Education Foundation grant to AL.
Appendix
Methods for Observing Neuroplasticity
How can we tell that a neural circuit has changed? A handful of techniques (summarized in Table 1) are used to provide evidence for brain plasticity. Neuroanatomical techniques, used mainly with animal models, can reveal changes in cell numbers, indicating cell death or neurogenesis. Techniques such as tract tracing in which a soluble dye is incorporated into the neuron and fill the processes to reveal changes in axonal branching patterns provide the most direct evidence that neurons have formed new synapses, creating new circuits. Changes in dendritic morphology are not very common after early developmental stages. However, in recent years it has become clear that dendritic spines are in a near-continual state of flux, and even more so when there is a change in the sensory environment that may induce neuroplasticity. Increasing numbers of spines are taken as an evidence for new synapse formation, whereas changes in the shape of the spine, such as elongated vs. mushroom or V-shaped, are considered evidence of the pursuit of new presynaptic partners or addition of new receptor protein into the postsynaptic membrane (Bourne & Harris, 2007; Nimchinsky et al., 2002). Techniques for viewing these changes in dendritic spine morphonology range from live optical imaging of transgenic-tagged neurons in awake-behaving animal models to ultrastuctural quantifications of in fixed brain slices from animals that underwent plastic experiences.
Table 1.
Methods for Observing Neuroplastic Changes in the Brain.
| Neuroanatomical Techniques | |
| Light/Confocal/Two-photon Microscopy | Changes in cell numbers, axonal branching patterns, dendritic morphology, spine motility; changes in sensory maps. Resolution: micrometers; individual neuron structure. |
| Electron Microscopy | Spine numbers, shape and organelles, receptor quantification. Resolution: nanometers; synapses, postsynaptic density, organelles. |
| Electrophysiological Techniques | |
| in vivo and in vitro Electrophysiology | Changes in sensory maps; changes in synaptic strength, i.e., LTP, LTD. Resolution: activity of single cells or group of cells in close proximity (spatial), nanoseconds (temporal). |
| Neuroimaging Techniques | |
| Magnetic Resonance Imaging (MRI) | Changes in gray and white matter volumes; cellular degeneration, myelinization. Resolution: micrometers. |
| Functional MRI (fMRI) | Changes in cerebral blood flow during activity; unmasking or recruitment of brain areas via rewiring. Resolution: millimeters (spatial), seconds to minutes (temporal). |
| Voxel-Based Morphometry (VBM) | A statistical treatment of MRI data, which allows population analysis. |
| Diffusion Tensor Imagining (DTI) | Visualizes white matter fiber arrangement and volume. White matter volume and myelinization changes |
| Transcranial Magnetic Stimulation (TMS) | Stimulation applied to the outer layers of cortex, which can boost signal strength or, with repetitive stimulation (rTMS) interfere with signals. |
| Positron Emission Tomography (PET) | Metabolic activity or receptor binding. Rarely used in studies of neuroplasticity. |
| Electroencephalogram (EEG) | Detects changes in neuron field potential patterns, recorded from over the skull. MEG has better spatial (mm to several cm) and temporal resolutions (1msec). |
| Magnetoencephalography (MEG) | |
| Evoked response potential (ERP) | Changes in EEG in response to a specific stimulus. Pertinent for looking at timing of activity, but lacks precision on location. Changes in ERP when the stimulus remains constant would reflect neuroplastic change. |
Electrophysiological techniques provide the largest body of literature concerning synaptic plasticity and LTP/LTD (a comprehensive review can be found in Cowan et al., 2003). With these techniques one can document activity changes in single cells within experimentally defined stimulation paradigms. Stimulating input axons repetitively and strongly for a short period of time leads to changes in receptivity, such that the postsynaptic cell responds to a subsequent single stimulation more (long term potentiation or LTP) or less (long term depression or LTD) than it did before the repetitive stimulation. These phenomena, which happen in a matter of seconds and last for hours, are considered indications that neurons have modified their responses based on prior activity. LTP and LTD are ideal mechanisms for detecting significant changes in the sensory environment, such as when vision is reduced from one eye, or when one finger is moved repetitively for days. Electrophysiological techniques are also at the crux of a large literature that examines the biological bases of learning. LTP is the result of either presynaptic changes, in which the axon terminal releases more neurotransmitter, or postsynaptic changes, in which the synapse increases the number of neurotransmitter receptors. Biochemical, genetic and anatomical techniques, which are used to quantify the number of receptor molecules at synapses, also provide evidence to support postsynaptic receptor changes in LTP/LTD.
NeuroImaging techniques are the most commonly used in humans. These indicators of neuroplasticity unavoidably have lower resolution than the techniques we use with animals. Table 1 describes these techniques. Gray and white matter volume (observed with magnetic resonance imaging (MRI) and differential tensor imaging (DTI)), task-dependent differential activation of neurons and blood flow (functional FMRI or fMRI, evoked potentials or EP in which brain wave changes in response to a specific stimulus are observed, EEG in which resting brain waves are observed, transcranial magnetic stimulation or TMS, receptor binding techniques like Positon Emission Tomography (PET), and so on, all provide a window into experience dependent changes that occur in the human brain.
When using neuroimaging techniques it is important to remember that not all gross volume changes in the brain are indicative of neuroplasticity, and of those that are, different sources of neuroplasticity can result in the same images using these techniques. Grey matter size fluctuates mainly with cell appearance and death, but inflammatory reactions, changes in extracellular fluids, and changes in the dendritic structure of neurons may also contribute to grey matter changes. Not all of these changes are neuroplastic. White matter mostly consists of myelinated axon bundles. A decrease in white matter volume can indicate neuronal death or de-myelinization (loss of the glial sheet that surrounds the axons, as seen in some pathological conditions like myestania gravis), whereas an increase in white matter volume indicates myelinization, which increases the speed of neuronal transmission. With functional imaging techniques, seeing new areas of activity after an experience might indicate that novel circuits have formed or that existing circuits are responding in a different pattern.
Behavioral techniques are also widely used to characterize the outcomes of neuroplastic changes, and to provide noninvasive assays that can be used both in human and animal models of plasticity.
Footnotes
New neurons have also been reported in human olfactory bulb (Bedard & Parent, 2004), the parietal lobes and the cyngulate gyrus of adult macaques (Gould, Reeves, et al., 1999) and in the temporal lobe of adult primates (Bernier et al., 2002).
The voluntary aspect to the exercise might be important: a previous study had found that forced exercise (on a treadmill) was not associated with improved memory in elderly rats on a less physically-demanding circular platform water maze task (Barnes, Forster, Fleshner, & Ahanotu, 1991); however, another study found forced-exercising rats (age unspecified) learned a circular platform water maze task better than no-exercise controls (Ang, Dawe, Wong, Moochhala, & Ng, 2006). Different ages and strain of rats and/or different behavioral tasks could be responsible.
Other possible reasons for increased fractional anistrophy are discussed elsewhere (Johansen-Berg, 2010). Here we use “increased white matter” as shorthand for such changes.
References
- Abe K, Watanabe D. Songbirds possess the spontaneous ability to discriminate syntactic rules. Nature neuroscience. 2011;14:1067–1074. doi: 10.1038/nn.2869. [DOI] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135(3509):1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das G. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of Comparative Neurology. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Ang E, Dawe G, Wong P, Moochhala S, Ng Y. Alterations in spatial learning and memory after forced exercise. Brain research. 2006;1113(1):186–193. doi: 10.1016/j.brainres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P, Kercel SW. Sensory substitution and the human-machine interface. Trends in Cognitive Sciences. 2003;7(12):541–546. doi: 10.1016/j.tics.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Bagot R, van Hasselt F, Champagne D, Meaney M, Krugers H, Jo ls M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiology of Learning and Memory. 2009;92(3):292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Ball G, Hulse S. Birdsong. American Psychologist. 1998;53:37–58. doi: 10.1037//0003-066x.53.1.37. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Forster MJ, Fleshner M, Ahanotu EN. Exercise does not modify spatial memory, brain autoimmunity, or antibody response in aged F-344 rats. Neurobiology of Aging. 1991;12(1):47–53. doi: 10.1016/0197-4580(91)90038-l. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Archives of General Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing Brakes on Adult Brain Plasticity: From Molecular to Behavioral Interventions. The Journal of Neuroscience. 2010;30(45):14964. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ. Cross-modal plasticity: Where and how? Nature Reviews Neuroscience. 2002;3(443–452) doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320(6058):172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Bedard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Developmental brain research. 2004;151(1–2):159–168. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Bengtsson S, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nature Neuroscience. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bernier P, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proceedings of the National Academy of Sciences. 2002;99(17):11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J, Isaacs K, Anderson B, Alcantara A, Greenough W. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(14):5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Heiser M, Caywood M, Merzenich M. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52(2):371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Fishman S, Edwards A, Jennings C, Stojanovic M, Breiter H. Acute plasticity in the human somatosensory cortex following amputation. Neuroreport. 1998;9(6):1013–1017. doi: 10.1097/00001756-199804200-00011. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris K. Do thin spines learn to be mushroom spines that remember? Current Opinion in Neurobiology. 2007;17(3):381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. Journal of Neuroscience. 2008;28(28):7031. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlow A, Akahane-Yamada R, Pisoni D, Tohkura Y. Training Japanese listeners to identify English/r/and/l: Long-term retention of learning in perception and production. Perception and Psychophysics. 1999;61(5):977–985. doi: 10.3758/bf03206911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Keynes R, Lumsden A. The developing brain. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. European Journal of Neuroscience. 2005;21(2):513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Buchel C, Price C, Frackowiak RSJ, Friston K. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain: A Journal of Neurology. 1998;121(3):409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- Buonomano D, Merzenich M. Cortical plasticity: from synapses to maps. Annual Review of Neuroscience. 1998;21(1):149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Callaway E. Local circuits in primary visual cortex of the macaque monkey. Annual Review of Neuroscience. 1998;21(1):47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- Cameron H, Mckay R. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of Comparative Neurology. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Michael KC, West SG, Klein LC. Behavioral responses to physical vs. social novelty in male and female laboratory rats. Behavioural Processes. 2011 doi: 10.1016/j.beproc.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa L. Retinal waves are unlikely to instruct the formation of eye-specific retinogeniculate projections. Neural Development. 2009;4:25. doi: 10.1186/1749-8104-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne D, Bagot R, van Hasselt F, Ramakers G, Meaney M, de Kloet E, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. Journal of Neuroscience. 2008;28(23):6037. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, De Zubicaray GI. Genetics of brain fiber architecture and intellectual performance. The Journal of Neuroscience. 2009;29(7):2212. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Nahmani M, Gavornik JP, Haslinger RH, Heynen AJ, Erisir A, Bear MF. Rapid Structural Remodeling of Thalamocortical Synapses Parallels Experience-Dependent Functional Plasticity in Mouse Primary Visual Cortex. Journal of Neuroscience. 2010 doi: 10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery T, Shah R, Greenough W. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiology of Learning and Memory. 1995;63(3):217–219. doi: 10.1006/nlme.1995.1025. [DOI] [PubMed] [Google Scholar]
- Cowan W, Sdhof T, Stevens C. Synapses. Baltimore: Johns Hopkins; 2003. [Google Scholar]
- Crowley JC, Katz LC. Early development of ocular dominance columns. Science. 2000;290:1321–1324. doi: 10.1126/science.290.5495.1321. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert C. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368(6473):737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49(6):861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia P, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. In: Sossin WS, Lacaille J-C, Castellucci VF, Belleville S, editors. Progress in Brain Research. Vol. 169. Elsevier; 2008. pp. 199–207. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborn's perception of speech sounds. 1986;9:133–150. [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nature Reviews Neurology. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse H, Recanzone G, Merzenich M. Alterations in correlated activity parallel ICMS-induced representational plasticity. Neuroreport. 1993;5(2):173. doi: 10.1097/00001756-199311180-00020. [DOI] [PubMed] [Google Scholar]
- Douglas R, Martin K. Neuronal circuits of the neocortex. Annual Reviews of Neuroscience. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and Spatial Dynamics of Brain Structure Changes during Extensive Learning. Journal of Neuroscience. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Buchel C, May A. Changes in gray matter induced by learning-revisited. PLoS ONE. 2008;3(7) doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Döbrössy MD, Panatier A, RodrÌguez JJ, Lamarque S, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biology. 2007;5(8):e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270(5234):305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. J. Physiol. 1991;260:C917–C925. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn A-M, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen C, Champagne F. Epigenetic influences on brain development and plasticity. Current Opinion in Neurobiology. 2009;19(2):207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. Synaptic mechanisms for plasticity in neocortex. Annual Review of Neuroscience. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney E, Fine I, Dobkins K. Visual stimuli activate auditory cortex in the deaf. Nature neuroscience. 2001;4(12):1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Fitzsimonds R, Song H, Poo M. Propagation of activity-dependent synaptic depression in simple neural networks. Nature. 1997;388(6641):439–448. doi: 10.1038/41267. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375(6531):482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Fox S, Levitt P, Nelson C. How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2003;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gall FJ, Spurzheim JC. On the function of the brain and each of its parts: with observations on the possiblity of determining the instrincts, propensities, and talkents, or the moral and intellectuion dispositions of men and animals, and by the configuration of the brain and head. Boston: Marsh, Capen, and Lyon; 1835. [Google Scholar]
- Garraghty P, LaChica E, Kaas J. Injury-induced reorganization of somatosensory cortex is accompanied by reductions in GABA staining. Somatosensory Motor Research. 1991;8:347–354. doi: 10.3109/08990229109144757. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Berry R, Disterhoft J, Power J, Van der Zee E. Associative learning elicits the formation of multiple-synapse boutons. Journal of Neuroscience. 2001;21(15):5568. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Gonzales CLR, Kolb B. Prenatal enrichment and recovery from perinatal cortical damage: Effects of maternal complex housing. 2010 doi: 10.3389/fnbeh.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Science. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Circuitry, architecture, and functional dynamics of visual cortex. Cerebral Cortex. 1993;3(5):373–386. doi: 10.1093/cercor/3.5.373. [DOI] [PubMed] [Google Scholar]
- Gilbert C. Adult cortical dynamics. Physiological Reviews. 1998;78(2):467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- Gilbert C, Wiesel T. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraux P, Sirigu A. Illusory movements of the paralyzed limb restore motor cortex activity. NeuroImage. 2003;20:S107–S111. doi: 10.1016/j.neuroimage.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Gislen A, Dacke M, Kröger R, Abrahamsson M, Nilsson D, Warrant E. Superior underwater vision in a human population of sea gypsies. Current Biology. 2003;13(10):833–836. doi: 10.1016/s0960-9822(03)00290-2. [DOI] [PubMed] [Google Scholar]
- Gislen A, Warrant E, Dacke M, Kroger R. Visual training improves underwater vision in children. Vision Research. 2006;46(20):3443–3450. doi: 10.1016/j.visres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Sato M, Cattaneo L, Riggio L, Palumbo D, Buccino G. Processing abstract language modulates motor system activity. THE QUARTERLY JOURNAL OF EXPERIMENTAL PSYCHOLOGY. 2008;61(6):905–919. doi: 10.1080/17470210701625550. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Current Opinion in Neurobiology. 2007;17(5):516–524. doi: 10.1016/j.conb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migtation, and differentiation in a vocal control nucleus of the adult female canary bird. Proceedings of the National Academy of Science. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nature Reviews Neuroscience. 2007;8(6):481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Gross C. Neurogenesis in adult mammals: some progress and problems. Journal of Neuroscience. 2002;22(3):619. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LAM. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. Journal of Neuroscience. 1997;17(7):2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MSA, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286(5439):548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proceedings of the National Academy of Science. 1998;95(6):3618–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W, Black J, Wallace C. Experience and brain development. Child Development. 1987;58(3):539–559. [PubMed] [Google Scholar]
- Gruart A, Munoz M, Delgado-Garcia J. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. Journal of Neuroscience. 2006;26(4):1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth R, Smith E, Duncan G, Crawford M, von Noorden G. Multiple sensitive periods in the development of the primate visual system. Science. 1986;232(4747):235–238. doi: 10.1126/science.3952507. [DOI] [PubMed] [Google Scholar]
- Haydon P. GLIA: listening and talking to the synapse. Nature Reviews Neuroscience. 2001;2(3):185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hebb D. The effects of early experience on problem solving at maturity. American Psychologist. 1947;2(2):306. [Google Scholar]
- Hebb D. The organization of behavior: A neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Hensch T. Critical period regulation. Annual Reviews of Neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hobfoll SE. Conservation of resources: A new attempt at conceptualizing stress. American Psychologist. 1989;44(3):513. doi: 10.1037//0003-066x.44.3.513. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent strutural synaptic plasticity in the mammalian brain. Nature reviews: Neuroscience. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Huberman A. Mammalian DSCAMs: They Won't Help You Find a Partner, but They'll Guarantee You Some Personal Space. Neuron. 2009;64(4):441–443. doi: 10.1016/j.neuron.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Huberman A, Feller M, Chapman B. Mechanisms underlying development of visual maps and receptive fields. 2008 doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Neural Plasticity. Cambridge MA: Harvard University Press; 2002. [Google Scholar]
- Jacobs K, Donoghue J. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251(4996):944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jain N, Florence S, Qi H, Kaas J. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(10):5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The principles of psychology (Vol. 1) Vol. 1. New York: Henry Holt; 1890. [Google Scholar]
- Jang JH, Kang DH, Kwon JS. White matter neuroplastic changes in long-term trained players of the game of Baduk: A voxel-based diffusion-tensor imaging study. Neuroimage. 2010;52:9–19. doi: 10.1016/j.neuroimage.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Annals of the New York Academy of Sciences. 2004;1016:749. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H. Behavioural relevance of variation in white matter microstructure. Current opinion in neurology. 2010;23(4):351–358. doi: 10.1097/WCO.0b013e32833b7631. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: The influence of maturational state on the acquisition of English language. Cognitive Psychology. 1989;21:60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Jones E, Pons T. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282(5391):1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- Kaas J, Florence S, Jain N. Subcortical contributions to massive cortical reorganizations. NEURON-CAMBRIDGE MA- 1999;22:657–660. doi: 10.1016/s0896-6273(00)80725-4. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. Journal of Child Psychology and Psychiatry. 2007;48(3–4):224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218(4571):433. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams M, Turner R, Ungerleider L. Functional MRI evidence for adult motor cortex plasticity associated with skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Katz L, Crowley J. Development of cortical circuits: lessons from ocular dominance columns. Nature Reviews Neuroscience. 2002;3(1):34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- Katz L, Shatz C. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kearins JM. Visual spatial memory in Australian Aboriginal children of desert regions. Cognitive Psychology. 1981;13(3):434–460. doi: 10.1016/0010-0285(81)90017-7. [DOI] [PubMed] [Google Scholar]
- Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends in Neurosciences. 2008;31(4):163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. Journal of Neuroscience. 1998;18(9):3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuroghlian A, Knudsen E. Adaptive auditory plasticity in developing and adult animals. Progress in neurobiology. 2007;82(3):109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kim K, Relkin N, Lee K, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997;388(6638):171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF. Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. The Journal of Neuroscience. 1999;19(5):1599. doi: 10.1523/JNEUROSCI.19-05-01599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott G, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nature neuroscience. 2006;9(9):1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- Knudsen E. Capacity for plasticity in the adult owl auditory system expanded by juvenile experience. Science. 1998;279(5356):1531. doi: 10.1126/science.279.5356.1531. [DOI] [PubMed] [Google Scholar]
- Knudsen E. Mechanisms of experience-dependent plasticity in the auditory localization pathway of the barn owl. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1999;185(4):305–321. doi: 10.1007/s003590050391. [DOI] [PubMed] [Google Scholar]
- Knudsen E. Fundamental components of attention. Annual Review of Neuroscience. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiology of Learning and Memory. 2003;79(1):1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Kornack D, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(10):5768. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P. Perception of speech and sound in early infancy. In: Salapatek P, Cohen L, editors. Handbook of infant perception: Vol. 2. From perception to cognition. Orlando, FL: Academic Press; 1987. [Google Scholar]
- Kuhl P, Tsao F, Liu H. Foreign-language experience in infancy: Effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences. 2003;100(15):9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, Fischl B. Meditation experience is associated with increased cortical thickness. Neuroreport: For Rapid Communication of Neuroscience Research. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern E, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404(6780):876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW, Sled JG. Maze training in mice induces MRI detectable brain shape changes specific to the type of learning. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annual Review of Psychology. 2010;61:111. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is There A Link Between Adult Neurogenesis and Learning? Hippocampus. 2006;16(3):216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lewis T, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Developmental psychobiology. 2005;46(3):163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- Linkenhoker B, Knudsen E. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature. 2002;419(6904):293–296. doi: 10.1038/nature01002. [DOI] [PubMed] [Google Scholar]
- Linkenhoker B, von der Ohe C, Knudsen E. Anatomical traces of juvenile learning in the auditory system of adult barn owls. Nature neuroscience. 2004;8(1):93–98. doi: 10.1038/nn1367. [DOI] [PubMed] [Google Scholar]
- Lisman J, Lichtman J, Sanes J. LTP: perils and progress. Nature Reviews Neuroscience. 2003;4(11):926–929. doi: 10.1038/nrn1259. [DOI] [PubMed] [Google Scholar]
- Livneh Y, Feinstein N, Klein M, Mizrahi A. Sensory input enhances synaptogenesis of adult-born neurons. Journal of Neuroscience. 2009;29(1):86–97. doi: 10.1523/JNEUROSCI.4105-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends in Neurosciences. 2005;28(5):248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lorenz K. Evolution and the modification of behavior. Chicago: University of Chicago Press; 1965. Critique of modern ethologists' attitude; pp. 29–48. [Google Scholar]
- Lovden M, Backman L, Lindenberger U, Schaefer S, Schmiedek F. A theoretical framework for the study of adult cognitive plasticity. Psychological Bulletin. 2010;136(4):659–676. doi: 10.1037/a0020080. [DOI] [PubMed] [Google Scholar]
- Lovden M, Bodammer NC, Kuhn S, Kaufmann J, Schutze H, Tempelmann C, Schmiedek F. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48(13):3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Luo L, O'Leary D. Axon retraction and degeneration in development and disease. Annual Reviews of Neuroscience. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Luzzati F, De Marchis S, Fasolo A, Peretto P. Neurogenesis in the caudate nucleus of the adult rabbit. Journal of Neuroscience. 2006;26(2):609. doi: 10.1523/JNEUROSCI.4371-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. Long-term potentiation and memory. Physiological reviews. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- MacIver K, Lloyd DM, Kelly S, Nurmikko T, Roberts N. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain: A Journal of Neurology. 2008;131(8):2181–2191. doi: 10.1093/brain/awn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, Spiers HJ. London Taxi Drivers and Bus Drivers: A Structural MRI and Neuropsychological Analysis. Hippocampus. 2006;16(12):1091–1101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Malenka R. The long-term potential of LTP. Nature Reviews Neuroscience. 2003;4(11):923–926. doi: 10.1038/nrn1258. [DOI] [PubMed] [Google Scholar]
- Markakis E, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA 3 and are surrounded by synaptic vesicles. The Journal of Comparative Neurology. 1999;406(4):449–460. [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies G, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M. Epigenetics and the biological definition of gene-environment interactions. Child Development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Medina AE, Krahe TE. Neocortical plasticity deficits in fetal alcohol spectrum disorders: lessons from barrel and visual cortex. Journal of neuroscience research. 2008;86(2):256–263. doi: 10.1002/jnr.21447. [DOI] [PubMed] [Google Scholar]
- Meltzoff A, Borton R. Intermodal matching by human neonates. Nature. 1979;282(2):403–404. doi: 10.1038/282403a0. [DOI] [PubMed] [Google Scholar]
- Merabet L, Hamilton R, Schlaug G, Swisher J, Kiriakopoulos E, Pitskel N, Pascual-Leone A. Rapid and reversible recruitment of early visual cortex for touch. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich M, Kaas J, Wall J, Nelson R, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19170–19175. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzert J, Lorey B, Zentgraf K. Cognitive motor processes: The role of motor imagery in the study of motor representations. Brain Research Reviews. 2009;60(2):306–326. doi: 10.1016/j.brainresrev.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nature Reviews Neuroscience. 2009;10(12):861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Naegele J, Lombroso P. Genetics of central nervous system developmental disorders. Child and adolescent psychiatric clinics of North America. 2001;10(2):225–239. [PubMed] [Google Scholar]
- Nakamori T, Sato K, Atoji Y, Kanamatsu T, Tanaka K, Ohki-Hamazaki H. Demonstration of a neural circuit critical for imprinting behavior in chicks. The Journal of Neuroscience. 2010;30(12):4467. doi: 10.1523/JNEUROSCI.3532-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. Neural plasticity and human development. Current Directions in Psychological Science. 1999;8(2):42–45. [Google Scholar]
- Nelson C. Cognitive recovery in socially deprived young children: The Bucharest early intervention project. Science. 2007;(318):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nelson C, de Haan M, Thomas KM. Neuroscience of cognitive development: The role of experience and the developing brain. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- Neville H, Bavelier D. Neural organization and plasticity of language. Current Opinion in Neurobiology. 1998;8(2):254–258. doi: 10.1016/s0959-4388(98)80148-7. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annual Review of Physiology. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado J, Ostroff L, Helton T, Robinson C, Harris K, Ehlers M. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52(5):817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annual Reviews Neuroscience. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Dang N, Cohen LG, Brasil-Neto JP. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. Journal of Neurophysiology. 1995;74(3):1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Penn A, Shatz C. Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatric research. 1999;45(4, Part 1 of 2):447–458. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- Perani D, Dehaene S, Grassi F, Cohen L, Cappa S, Dupoux E, Mehler J. Brain processing of native and foreign languages. Neuroreport. 1996;7(15–17):2439. doi: 10.1097/00001756-199611040-00007. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual Learning Directs Auditory Cortical Map Reorganization through Top-Down Influences. Journal of Neuroscience. 2006;26(18):4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons T, Garraghty P, Ommaya A, Kaas J, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252(5014):1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Ptito M, Moesgaard SM, Gjedde A, Kupers R. Cross-modal plasticity revealed by electrotactile stumulation of the tongue in the congenitally blind. Brain and Cognition. 2005;128:606–614. doi: 10.1093/brain/awh380. [DOI] [PubMed] [Google Scholar]
- Rakic P. No more cortical neurons for you. Science. 2006;313(5789):928–929. doi: 10.1126/science.1131713. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS. Behavioral and magnetocephalographic correlates of plasticity in the adult human brain. Proceedings of the National Academy of Science. 1993;1993(90):10413–10420. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Blakeslee S. Phantoms in the brain. London: Fourth Estate; 1998. [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs: The D. O. Hebb lecture. Brain: A Journal of Neurology. 1998;121(9):1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Murre JMJ. Rehabilitation of brain damage: Brain plasticity and principles of guided recovery. Psychological Bulletin. 1999;125(5):544–575. doi: 10.1037/0033-2909.125.5.544. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent J, Lledo P. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. Journal of Neuroscience. 2002;22(7):2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder B, Stock O, Bien S, Neville H, Risler F. Speech processing activates visual cortex in congenitally blind humans. European Journal of Neuroscience. 2002;16(5):930–936. doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR. Modification of brain circuits through experience. In: Bermudez-Rattoni F, editor. Neural plasticity and memory. Boca Raton, FL: Taylor & Francis; 2007. pp. 67–94. [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Diamond MC. Brain changes in response to experience. Scientific American. 1972;226(2):22–29. [Google Scholar]
- Rutter M, O'Connor TG. Are There Biological Programming Effects for Psychological Development? Findings From a Study of Romanian Adoptees. Developmental Psychology. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage. 2009;44(4):1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J, Donoghue J. Plasticity and primary motor cortex. Annual Review of Neuroscience. 2000;23(1):393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Arancio O. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sato M, Cattaneo L, Rizzolatti G, Gallese V. Numbers within our hands: Modulation of corticospinal excitability of hands muscles during numerical judgment. Journal of Cognitive Neuroscience. 2007;19(4):684–693. doi: 10.1162/jocn.2007.19.4.684. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive Functions Correlate With White Matter Architecture in a Normal Pediatric Population: A Diffusion Tensor MRI Study. Human Brain Mapping. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein M, Behrens T, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JM, Begley S. The mind and the brain: Neuroplasticity and the power of mental force. New York, NY: HarperCollins; 2002. [Google Scholar]
- Senghas A, Kita S, Ozyurek A. Children Creating Core Properties of Language: Evidence from an Emerging Sign Language in Nicaragua. Science. 2004;305(5691):1779–1782. doi: 10.1126/science.1100199. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Simmel ML. Phantom experiences following amputation in childhood. Journal of Neurology, Neurosurgery & Psychiatry. 1962;25:69–78. doi: 10.1136/jnnp.25.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270(5237):758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- Smith G, Heynen A, Bear M. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1515):357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorra K, Harris K. Stability in synapse number and size at 2 hr after long-term potentiation in hippocampal area CA1. Journal of Neuroscience. 1998;18(2):658–671. doi: 10.1523/JNEUROSCI.18-02-00658.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield B, Trice J. Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Experimental Brain Research. 1988;72(2):399–406. doi: 10.1007/BF00250261. [DOI] [PubMed] [Google Scholar]
- Stettler D, Yamahachi H, Li W, Denk W, Gilbert C. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49(6):877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene◊ environment interactions in rhesus monkeys. Annals of the New York Academy of Sciences. 2006;1094(1):52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Sur M, Learney CA. Development and plasticity of cortical areas and networks. Nature Reviews Neuroscience. 2001;2:261. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea L, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. International Journal of Developmental Neuroscience. 1998;16(3–4):235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings N, Reeves A, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. Journal of Neuroscience. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences. 2010;107(35):15649. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. Journal of Neuroscience. 2007;27(12):3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R, DeTeresa R, Hansen L. Neocortical cell counts in normal human adult aging. Annals of Neurology. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Thomas A, Marrett S, Saad Z, Ruff D, Martin A, Bandettini P. Functional but not structural changes associated with learning: An exploration of longitudinal Voxel-Based Morphometry (VBM) Neuroimage. 2009;48(1):117–125. doi: 10.1016/j.neuroimage.2009.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Johnson M. New advances in understanding sensitive periods in brain development. Current directions in psychological science. 2008;17(1):1–5. [Google Scholar]
- Trachtenberg J, Chen B, Knott G, Feng G, Sanes J, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Tsao F-M, Liu H-M, Kuhl P, Tsao F-Mtmct. Speech Perception in Infancy Predicts Language Development in the Second Year of Life: A Longitudinal Study. Child Development. 2004;75(4):1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: Past and future directions. Neuromolecular medicine. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker J, Lalonde C. Cross-language speech perception: Initial capabilities and developmental change. Developmental psychology. 1988;24(5):672–683. [Google Scholar]
- Werker J, Tees R. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development. 2002;25(1):121–133. [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM. Associations between Regional Cortical Thickness and Attentional Networks as Measured by the Attention Network Test. Cerebral Cortex. 2011;21(2):345. doi: 10.1093/cercor/bhq101. [DOI] [PubMed] [Google Scholar]
- Whitlock J, Heynen A, Shuler M, Bear M. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of Neurophysiology. 1965a;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. Journal of Neurophysiology. 1965b;28(6):1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Holtmaat A, Wright N, Fox K, Svoboda K. Structural Plasticity Underlies Experience-Dependent Functional Plasticity of Cortical Circuits. Journal of Neuroscience. 2010;30(14):4927–4932. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett K, Maguire E. Navigational expertise may compromise anterograde associative memory. Neuropsychologia. 2009;47(4):1088–1095. doi: 10.1016/j.neuropsychologia.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett K, Spiers HJ, Maguire EA. Talkent in the taxi: A model system for exploring expertise. Tansactions of the Royal Society. 2009;364:1407–1416. doi: 10.1098/rstb.2008.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nature Neuroscience. 2001;4(11):1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]