Abstract
Sclerostin is a small protein expressed by the SOST gene in osteocytes, bone cells that respond to mechanical stress applied to the skeleton and appear to play an important role in the regulation of bone remodeling. When sclerostin binds to its receptors on the cell surface of osteoblasts, a downstream cascade of intracellular signaling is initiated, with the ultimate effect of inhibiting osteoblastic bone formation. Recent studies have shown that the SOST gene is also expressed by articular chondrocytes and that modulation of its activity may have effects on articular cartilage and subchondral bone. The role of sclerostin in the pathogenesis of osteoarthritis in humans has not yet been defined, and the potential utility of treating osteoarthritis with interventions that alter sclerostin is not known. Rare genetic skeletal disorders in humans with low sclerostin levels, such as sclerosteosis and van Buchem disease, have been associated with a high bone mineral density (BMD) phenotype and low risk of fractures. This has led to the concept that antisclerostin interventions might be useful in the treatment of patients with osteoporosis and skeletal disorders associated with low bone mass. Compounds that inhibit sclerostin have been shown to stimulate bone formation and reduce bone resorption, with a robust increase in BMD. Investigational monoclonal antibodies to sclerostin, including romosozumab, blosozumab, and BPS804, have advanced to phase II clinical trials or beyond. If antisclerostin therapy is found to have beneficial effects on clinical endpoints, such as reduction of fracture risk or improvement in quality of life in patients with osteoarthritis, with a favorable balance of benefit and risk, then this class of compounds may become a prominent addition to the options for therapy of osteoporosis and other skeletal disorders.
Keywords: anabolic, blosozumab, BPS804, osteoporosis, romosozumab, sclerostin
Introduction
The Wingless-type mouse mammary virus integration site (Wnt) pathway was first identified in mouse breast tumors that were induced by mouse mammary tumor virus [Nusse and Varmus, 1982]. It has subsequently been recognized that Wnt signaling plays a role in many biological processes, including embryogenesis, postnatal development, and tissue homeostasis in adults [Kim et al. 2013]. In recent years, much attention has been focused on Wnt signaling as a regulator of bone formation and regeneration [Ke et al. 2012], raising the possibility that modulation of Wnt signaling might be beneficial in the treatment of skeletal disorders such as osteoporosis [Lewiecki, 2011]. Additionally, Wnt signaling may be involved in cartilage and bone changes in animal models of osteoarthritis [Goldring, 2012], suggesting possible new molecular targets for the treatment of osteoarthritis in humans. Sclerostin, a SOST gene product expressed by osteocytes and articular chondrocytes, is an endogenous inhibitor of Wnt signaling. Investigational agents that inhibit sclerostin are currently being studied for the treatment of skeletal disorders.
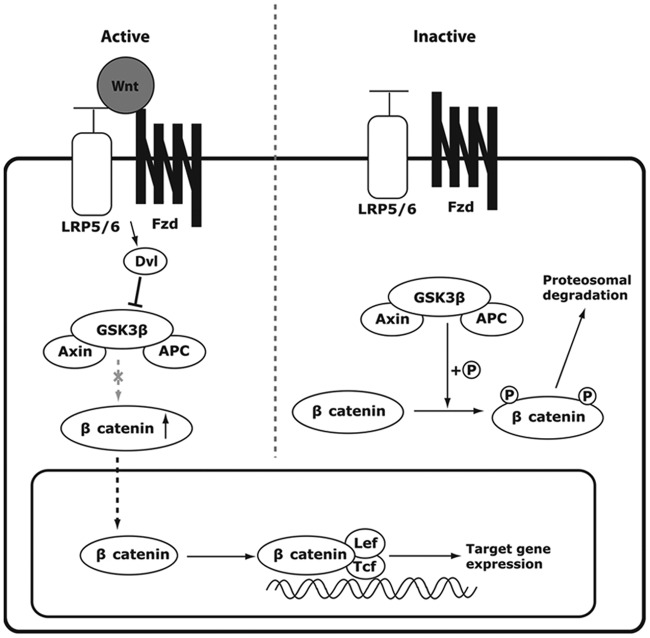
Wnt signaling pathways have been classified into two categories, canonical and noncanonical, with the canonical pathway mediating signaling through stabilization of intracellular β catenin, while noncanonical signaling is independent of β catenin. The canonical (Wnt/β-catenin) pathway is better characterized than the noncanonical pathway and is the one that appears to be most involved in bone disorders. Wnt ligands (Wnts) are glycoproteins that bind to the osteoblast cell surface coreceptors consisting of low-density lipoprotein receptor protein 5 and 6 (LRP5/6) and Frizzled. This is followed by a cascade of intracellular events (Figure 1) that result in intracellular activation of β catenin, translocation of β catenin into the cell nucleus, binding with transcriptional factors, and upregulation of target gene expression.
Figure 1.
Canonical Wnt β catenin signaling pathway. When a Wnt ligand binds its low-density lipoprotein receptor related protein (LRP5/6) Frizzled (Fzd) coreceptors on the cell surface of osteoblasts, Disheveled (Dvl) is activated, which inhibits glycogen synthase kinase 3β (GSK3β) from phosphorylating β catenin. The cytoplasmic level of β catenin consequently rises, and β catenin translocates into the nucleus to bind with transcriptional factors T-cell factor (Tcf)/lymphoid enhancer-binding factor (Lef-1), upregulating the target gene expression. (Reproduced from Kim et al. [2013])
Evidence from human disorders of sclerostin, such as sclerosteosis and van Buchem disease, and in animal studies investigating the role of sclerostin in Wnt/β-catenin signaling, have led to consideration of sclerostin as a potential target for the treatment of osteoporosis and other skeletal diseases associated with low bone mineral density (BMD) and increased fracture risk. This is based on the concept that an antisclerostin compound would inhibit an inhibitor of Wnt signaling, thereby acting to enhance Wnt signaling and stimulate osteoblastic bone formation. Investigational humanized sclerostin monoclonal antibodies of particular interest include romosozumab (AMG 785, CDP-7851; codeveloped by Amgen, Thousand Oaks, CA, USA, and UCB, Belgium) and blosozumab (Eli Lilly and Company, Indianapolis, IN, USA). BPS804 (Novartis, Basel, Switzerland) is another antisclerostin compound that is in the early stages of development.
Sclerostin
Sclerostin is a monomeric glycoprotein with a cysteine knot-like domain that has homology to the Cerebrus/DAN family of bone morphogenetic protein antagonists [Balemans et al. 2001; Brunkow et al. 2001; Veverka et al. 2009]. It is a SOST gene product expressed by osteocytes [van Bezooijen et al. 2004] and articular chondrocytes [Roudier et al. 2013]. Because of its high conservation across vertebrate species (the amino acid sequences in the vervet, rat, and mouse are 98%, 89%, and 88% identical respectively to the human sequence) [Brunkow et al. 2001], animal models are potentially useful for studying sclerostin. When sclerostin binds to the LRP5/6 and Frizzled coreceptors on the cell surface of osteoblasts, Wnt/β-catenin signaling is inhibited [Li et al. 2005], thereby inhibiting osteoblast differentiation, proliferation, and activity, resulting in reduced osteoblastic bone formation [Baron and Rawadi, 2007; Li et al. 2008].
Sclerostin mutations in humans
Sclerosteosis is a very rare autosomal recessive disorder that principally affects Afrikaners in South Africa [Beighton et al. 1977], with a few sporadic cases reported in other world regions [Hamersma et al. 2003]. It is characterized by progressive bone thickening, tall stature, syndactyly, enlargement of the skull, and foraminal stenosis resulting in entrapment of cranial nerves with facial palsy, with increases in intracranial pressure that may result in sudden death from impaction of the brainstem into the foramen magnum [Gardner et al. 2005; Hamersma et al. 2003]. Affected individuals usually appear normal at birth, with the possible exception of syndactyly; other manifestations begin early in life. It is caused by loss of function mutations of SOST, a gene located on the chromosomal region 17q12-21, resulting in decreased production of sclerostin by osteocytes. As a consequence, osteoblastic bone formation is increased due to enhancement of Wnt/β-catenin signaling. This is reflected in the finding of high levels of bone formation markers, such as procollagen type 1 N-terminal propeptide (P1NP) with normal levels of bone resorption markers, such as C-telopeptide (CTX) [van Lierop et al. 2011] The hyperostotic skeleton of these patients is very strong and highly resistant to fractures [Hamersma et al. 2003]. Heterozygous sclerosteosis carriers are clinically normal but have elevated BMD and P1NP levels that are higher than noncarrier controls [Gardner et al. 2005; van Lierop et al. 2011].
Van Buchem disease is a rare related autosomal recessive genetic disorder, principally reported in a fishing village in The Netherlands. It is caused by a 52 kb deletion in the same chromosomal region as SOST, resulting in downstream impairment of SOST function and defective sclerostin production [Staehling-Hampton et al. 2002]. The skeletal phenotype of individuals with van Buchem disease is similar but less severe than those with sclerosteosis, with normal stature and no syndactyly [Wergedal et al. 2003].
Animal studies of sclerostin
In an animal model of human sclerosteosis, mice lacking the SOST gene (SOST knockout mice) had a high bone mass phenotype that was measurable by dual-energy X-ray absorptiometry (DXA) as early as 1 month of age and peaked at about age 18 months, with histomorphometric data showing high levels of bone formation on trabecular surfaces [Lin et al. 2009]. In contrast, transgenic mice with overexpression of human SOST have been reported to have a low bone mass phenotype [Kramer et al. 2010].
Preclinical studies are consistent with a skeletal mechanosensory function for the osteocyte, with bone loading and unloading being associated with modulation of sclerostin levels followed by changes in bone mass. Ulnar loading (mechanical stimulation of bone) in rodents was followed by a dramatic decrease in SOST transcription and sclerostin expression by osteocytes, with an increase in bone formation on histomorphometry consistent with enhanced Wnt signaling [Robling et al. 2008]. This suggests that an osteoanabolic response to weight-bearing physical activity or other types of mechanical stress may be mediated, at least in part, through osteocyte expression of sclerostin. In the same study, hindlimb unloading by tail suspension of healthy mice (a surrogate for disuse osteoporosis in humans) was associated with a transient increase in SOST transcription, although sclerostin levels did not appear to change. In other studies in mice, mechanical unloading was followed by increased sclerostin levels and decreased Wnt signaling, with SOST knockout mice being resistant to mechanical unloading-induced bone loss [Lin et al. 2009]. Taken as a whole, the data in animals suggest that sclerostin may play a role in the skeletal response to loading (e.g. exercise) and unloading (e.g. bed rest) in humans.
The Wnt signaling pathway is known to have effects on chondrocytes as well as osteoblasts and may play a role in the development of cartilage and bone changes with osteoarthritis [Lories and Luyten, 2011; Luyten et al. 2009]. However, the relationship between Wnt/β-catenin signaling and osteoarthritis is complex and not fully defined, with apparently inconsistent findings in some experiments. Studies in adult transgenic mice have demonstrated that activation of Wnt/β-catenin signaling leads to premature chondrocyte differentiation and an osteoarthritis-like phenotype [Zhu et al. 2009], while other studies have shown that inhibition of Wnt/β-catenin signaling is associated with an increase in apoptosis of articular chondrocytes and destruction of articular cartilage [Zhu et al. 2008]. It has recently been demonstrated that a biologically active SOST gene is expressed by articular chondrocytes, with the finding of increased sclerostin in focal areas of damaged cartilage in sheep and mice with surgically induced osteoarthritis, while decreased sclerostin levels were reported in subchondral bone of sheep in association with bone sclerosis [Chan et al. 2011].
Preclinical studies of sclerostin inhibition
The effects of antisclerostin monoclonal antibodies have been studied in animals [Babcook et al. 1996; Veverka et al. 2009]. A significant increase in BMD was reported in mice treated with an antisclerostin monoclonal antibody (Scl-AbI) [Veverka et al. 2009]. Osteoanabolic effects of a different antisclerostin monoclonal antibody (Scl-AbII) were observed in ovariectomized rats, with reversal of bone loss due to estrogen deficiency at some skeletal sites. Bone histomorphometry demonstrated an increase in osteoblast surface and mineralizing surface, with a decrease in osteoclast surface, suggesting uncoupling of bone formation and bone resorption.
The effects of antisclerostin therapy have been further characterized in nonhuman primates, whose bone remodeling is similar to humans. Humanized sclerostin monoclonal antibody (Scl-AbIV) was administered to healthy adolescent gonad-intact female cynomolgus monkeys in variable doses given once monthly for two consecutive months [Ominsky et al. 2010]. BMD was assessed by DXA and peripheral quantitative computed tomography. Bone remodeling was evaluated with osteocalcin and P1NP, both markers of bone formation, and CTX, a marker of bone resorption. Bone histomorphometry was performed and biomechanical tests of bone strength were conducted. There was a significant increase followed by a decline of osteocalcin and P1NP after each dose of Scl-AbIV, with no consistent alteration of CTX. This pattern of bone marker response suggests reversibility of bone-forming effects within 1 month of dosing and possibly a disassociation of bone formation and bone resorption during the time of observation. Increases in bone mineral content (BMC) and BMD were reported at the femoral neck, radial metaphysic, and tibial metaphysis, with dose-dependent increases in bone formation on trabecular, periosteal, endocortical, and intracortical surfaces.
Antisclerostin therapy has also been shown to have beneficial effects on fracture healing and bone repair in rodents, with acceleration of bone repair, increased bone strength and increased callus density [Agholme et al. 2010; Li et al. 2010]. In a fracture-healing study in male cynomolgus monkeys, it was found that treatment with a sclerostin monoclonal antibody for 10 weeks improved fracture healing, with an increase in callus area, callus BMC, and torsional stiffness compared with vehicle [Ominsky et al. 2009]. Importantly, antisclerostin therapy increased bone formation, mass, and strength.
A recent study found that absence of sclerostin in mice with genetic knockout of sclerostin did not alter development of age-dependent osteoarthritis, and that antisclerostin therapy with a monoclonal antibody in rats with post-traumatic osteoarthritis had no effect on articular cartilage remodeling [Roudier et al. 2013].
Clinical studies of sclerostin inhibition
Romosozumab
The first in-human study of romosozumab was a phase I randomized, double-blind, placebo-controlled, ascending single-dose study in healthy men and postmenopausal women [ClinicalTrials.gov identifier NCT01059435] [Padhi et al. 2011]. The primary objectives of this study were to evaluate its safety and tolerability, with secondary objectives to assess the pharmacodynamics (PD), pharmacokinetics (PK), and the effects on BMD and bone turnover markers. The study subjects were randomized to receive either subcutaneous romosozumab (0.1, 0.3, 1.0, 3.0, 5.0, or 10.0 mg/kg) or intravenous romosozumab (1.0 or 10.0 mg/kg) or placebo in a 3:1 ratio of romosozumab to placebo. After receiving romosozumab, a greater than dose-proportional increase in serum concentrations was observed, with clearance or apparent clearance decreasing as dose increased. PK was nonlinear as has been reported with other therapeutic monoclonal antibodies [Wang et al. 2008]. Peak romosozumab serum concentrations occurred within the first week after subcutaneous administration. Administration of romosozumab was followed by a dose-dependent increase of serum P1NP, bone-specific alkaline phosphatase (BSAP), and osteocalcin compared with baseline. The maximum increases from baseline for P1NP, BSAP, and osteocalcin were 184%, 126%, and 176% for the 10.0 mg/kg subcutaneous dose and 167%, 125%, and 143% for the 5.0 mg/kg intravenous dose respectively (p < 0.01 compared with placebo). In contrast, serum CTX levels decreased in an approximately dose-dependent manner after a dose of romosozumab, with maximum significant decreases from baseline of 54% with the 10.0 mg/kg subcutaneous dose and 49% for the 5.0 mg/kg intravenous dose (p < 0.01 compared with placebo). Compared with placebo, a single subcutaneous dose of romosozumab increased BMD at the lumbar spine and total hip in all cohorts at days 29, 57, and 85, with the exception of total hip BMD for the 5.0 mg/kg cohort at day 29, generally in a dose-dependent manner. The largest significant increase in lumbar spine BMD was 5.3% on day 85 with a subcutaneous dose of 10.0 mg/kg (p < 0.01 compared with placebo). Romosozumab was generally well tolerated with all administered doses. At least one adverse event (AE) was reported by 64% or 60% of subjects receiving subcutaneous placebo or romosozumab respectively, and 50% or 25% of subjects receiving intravenous placebo or romosozumab respectively. Most AEs were considered mild by the investigator, with none resulting in discontinuation from the study. There were no deaths. The AEs most commonly reported for subcutaneous administration of placebo or romosozumab were injection site erythema, back pain, headache, constipation, injection site hemorrhage, arthralgia, and dizziness, all of which were considered mild. One subject who received 10 mg/kg subcutaneous romosozumab was reported to have a serious AE (SAE) of nonspecific hepatitis, with an elevated liver function test beginning 1 day after dosing and liver enzymes that peaked at 6–13 times the upper limit of normal. Six to eight days after romosozumab was administered, abdominal ultrasound and hepatitis panels were normal, with resolution of the SAE by day 26. In subjects receiving intravenous placebo or romosozumab, none reported more than one mild AE, and there were no SAEs. Mild, transient asymptomatic decreases in mean serum ionized calcium levels (about 4% below baseline) were reported after dosing of romosozumab, with values returning to baseline during the study or follow-up period. This was associated with transient increases in serum intact parathyroid hormone levels that returned to baseline levels by the end of the study. Of the 54 subjects receiving romosozumab, 6 (11%) tested positive for binding antiromosozumab antibodies and one of these, who received a 10.0 mg/kg subcutaneous dose, tested positive for romosozumab-neutralizing antibodies at study end and up to day 283; and one subject receiving 5.0 mg/kg intravenously tested positive for neutralizing antibodies during follow up on day 132 and up to day 252. There were no symptoms or abnormalities of other laboratory tests, vital signs, or electrocardiogram associated with the development of neutralizing antibodies.
In a phase II randomized, placebo-controlled, multidose, multinational study [ClinicalTrials.gov identifier: NCT01059435], the efficacy, safety, and tolerability of romosozumab were evaluated in postmenopausal women aged 55–85 years (N = 419) with lumbar spine, total hip, or femoral neck T score up to −2.0 and at least −3.5 [McClung et al. 2012]. The subjects were randomized to one of nine groups, receiving once monthly subcutaneous dosing of romosozumab (70 mg, 140 mg, 210 mg) or once monthly subcutaneous placebo, three-monthly subcutaneous dosing of romosozumab (140 mg, 210 mg) or three-monthly subcutaneous placebo, or an open-label active comparator of either subcutaneous teriparatide 20 μg daily or oral alendronate 70 mg once weekly. The primary endpoint was the percentage change in lumbar spine BMD with romosozumab compared with placebo at month 12. The mean age of subjects was 67 years, with the mean baseline T score at the lumbar spine, total hip, and femoral neck of −2.3, −1.5, and −1.9, respectively. All doses of romosozumab increased BMD at the lumbar spine, total hip, and femoral neck at month 12 compared with placebo (p < 0.005), and all doses significantly increased serum P1NP and reduced serum CTX from baseline as early as week 1. The greatest BMD increase was with once monthly subcutaneous romosozumab 210 mg, with a reported increase of 11.3% at the lumbar spine and 4.1% at the total hip. The BMD increases with romosozumab were significantly greater than those achieved with alendronate and teriparatide (p < 0.0001). All doses of romosozumab increased serum P1NP and reduced serum CTX by week 1 compared with baseline, while bone turnover marker changes with alendronate and teriparatide were as expected (i.e. a decrease of both markers with alendronate and an increase in both markers with teriparatide). Although both romosozumab and teriparatide may be classified as osteoanabolic agents, the differences in bone marker response are consistent with different mechanisms of action that could potentially lead to differences in clinical effects. Romosozumab was generally well tolerated, with overall AEs similar between groups, although mild injection site reactions were more common with romosozumab (12%) compared with placebo (4%).
A phase III randomized, double-blind, placebo-controlled, parallel-group, 2-year registration trial of romosozumab in postmenopausal women aged 55–90 years with osteoporosis (estimated enrollment = 6000) is currently underway [ClinicalTrials.gov identifier: NCT01575834]. The primary endpoints are the incidence of new vertebral fractures at 12 months and 24 months. There are two study arms, with the active treatment group receiving subcutaneous romosozumab injections for 12 months, followed by open-label subcutaneous denosumab injections for another 12 months, and the placebo comparator group receiving subcutaneous placebo injections for 12 months followed by subcutaneous open-label denosumab injections for another 12 months. The study start date was March 2012, with an estimated primary study completion date of October 2015 (final data collection date for primary outcome measure) and an estimated study completion date of January 2016.
Blosozumab
In two phase I, randomized, double-blind, placebo-controlled, single and multiple dose-escalating studies of blosozumab administered subcutaneously and intravenously to healthy postmenopausal women [ClinicalTrials.gov identifiers: NCT01742078, NCT01742091], statistically significant changes in levels of sclerostin, BSAP, osteocalcin, and CTX were observed [McColm et al. 2012]. Lumbar spine BMD increased up to 3.41% following a single dose and up to 7.71% following multiple doses at day 85, compared with baseline. The effects were generally dose dependent over the dose range tested. Antibodies to blosozumab were detected, with no evidence of a neutralizing effect on PK or PD parameters.
The efficacy, safety, and tolerability of blosozumab were evaluated in a phase II randomized, parallel-design, double-blind, placebo-controlled study in postmenopausal women with low BMD [ClinicalTrials.gov identifier: NCT01144377] [Benson et al. 2013]. The study enrolled 154 women with a mean baseline age of 65 years and a mean baseline lumbar spine T score of −2.76. The subjects were randomized to receive subcutaneous blosozumab 180 mg every 2 weeks, subcutaneous blosozumab 270 mg every 2 weeks, subcutaneous blosozumab 180 mg every 4 weeks, or subcutaneous placebo every 2 weeks. In an addendum to the study, additional participants received subcutaneous blosozumab 270 mg every 12 weeks with subcutaneous placebo every 2 weeks in the weeks when blosozumab was not given. The primary endpoint was lumbar spine BMD change at 52 weeks compared with baseline. There were dose-related increases in lumbar spine BMD in each of the groups receiving blosozumab, with each superior to placebo. The lumbar spine BMD increase at 52 weeks was 6.7% with the dose of 270 mg every 12 weeks, 8.4% with 180 mg every 4 weeks, 13.9% with 180 mg every 2 weeks, and 17.8% with 270 mg every 2 weeks. AEs were similar across all groups, with the exception of increased injection site reactions with blosozumab.
Plans for a phase III study to evaluate fracture risk with blosozumab in postmenopausal women have not been announced at the time of writing.
BPS804
A randomized, double-blind, placebo-controlled phase II study is evaluating the safety and efficacy of multiple dosing regimens of BPS804 in postmenopausal women with low BMD [ClinicalTrials.gov identifier: NCT01406548]. The primary efficacy endpoint is change in lumbar spine BMD at month 9 compared with baseline. Subjects for this study are postmenopausal women aged 45–85 years with a baseline lumbar spine T score from −2.0 to −3.5. The estimated study completion date is September 2013.
Discussion
Osteoporosis is a major public health concern that affects over 75 million people in the USA, Europe, and Japan, with more than 8.9 million fractures occurring each year [Kanis, 2007]. Osteoporosis is defined as a skeletal disease characterized by low BMD and poor bone quality, resulting in reduced bone strength and increased risk of fractures [Klibanski et al. 2001]. Fractures of the hip and spine are associated with chronic pain, deformity, depression, disability, and death. As the world population ages, there will be an increased number of older people at risk of fractures and their consequences. Despite the availability of medications proven to reduce fracture risk, osteoporosis is underdiagnosed [Delmas et al. 2005] and undertreated [Foley et al. 2007]. With a better understanding of the regulators of bone remodeling, new targets for therapeutic intervention have emerged [Lewiecki, 2011]. Sclerostin is expressed by osteocytes, which appear to be important mechanosensors that detect skeletal loading and unloading, acting to initiate the bone remodeling cycle. It is particularly intriguing that antisclerostin compounds have been reported, at least in short-term studies, to increase bone formation markers while decreasing bone resorption markers; this is in contrast to teriparatide, the only osteoanabolic agent currently approved in the USA for treating osteoporosis, with which an initial increase in bone formation markers is followed by an increase in bone resorption markers. If uncoupling of bone resorption and formation with antisclerostin therapy leads to improved clinical outcomes with a favorable balance of benefits and risks, then this approach to osteoporosis treatment will be a welcome addition to current options.
It has been estimated that the failure rate for fracture fixation in patients with osteoporosis is 10–25% [Cornell, 2003]. An agent that improves bone repair, fracture healing, and fixation of surgical hardware would be of great benefit in the management of fractures. A consensus statement released by the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis addressed fracture healing with current and emerging osteoporosis therapy [Goldhahn et al. 2012]. It was concluded that bisphosphonates and raloxifene did not impair fracture healing. Preclinical studies and several case reports suggested that strontium ranelate might enhance fracture healing [Goldhahn et al. 2012]. Studies with teriparatide had mixed results, but overall were consistent with a beneficial effect on fracture healing [Goldhahn et al. 2012]. Clinical trials with denosumab have shown no adverse effects on fracture healing [Adami et al. 2012; Cummings et al. 2009]. Sclerostin monoclonal antibody therapy has been shown to enhance fracture healing in rats and cynomolgus monkeys [Ominsky et al. 2011]. Although several phase II studies to investigate the effects of romosozumab on fracture healing in humans have been conducted [ClinicalTrials.gov identifiers: NCT00907296, NCT01081678], there is currently no ongoing effort to pursue regulatory approval of this agent for acceleration of fracture healing.
Osteoarthritis is the most common type of arthritis and one of the most common disorders encountered in general practice [Neogi and Zhang, 2013]. It has a predilection for affecting the lower extremity joints, especially the hips and knees, and is a leading cause of disability in aging adults. Osteoarthritis affects about 14% of adults aged 25 and older, and about 34% of those aged 65 and older [Centers for Disease Control and Prevention, 2011]. It accounts for 55% of all arthritis-related hospitalizations, with hip and knee replacement procedures accounting for 35% of all arthritis-related procedures during hospitalization [Centers for Disease Control and Prevention, 2011]. Although osteoarthritis was once felt to be due to degeneration of cartilage as a normal consequence of aging, more recent models consider it to be a complex joint disease that affects cartilage, subchondral bone, and other adjacent structures, including synovium, bone marrow, menisci, ligaments, and muscles [Lories and Luyten, 2011]. Articular chondrocytes may be the most important cells involved in the pathogenesis of osteoarthritis. Improved understanding of the role of chondrocytes in chondrogenesis and repair of damaged cartilage may lead to the development of more effective treatment strategies for osteoarthritis. The importance of Wnt/β-catenin signaling in the pathogenesis of osteoarthritis in humans is not well understood. The findings in preclinical studies using antisclerostin therapy in animal models of osteoarthritis have been disappointing, with no reported benefit on cartilage remodeling during aging or mechanical injury [Roudier et al. 2013].
The potential risks of antisclerostin therapy should be considered. These include the possibility of oncogenic effects and arthritis. Teriparatide, the only osteoanabolic agent that is currently approved for the treatment of osteoporosis, is associated with an increased risk of osteosarcoma in rats that is dependent on dose and duration of treatment [Vahle et al. 2004], although there is no evidence for increased risk in humans [Cipriani et al. 2012], who have important differences in skeletal physiology, dose, and duration of treatment. Activating Wnt pathway mutations have been linked to colon neoplasms and hepatocellular carcinoma [Clevers, 2006]. Activation of Wnt signaling has been observed in studies of human osteosarcoma cell lines [Kansara et al. 2009]. The relevance of these findings to antisclerostin therapy in humans is uncertain, with no reports of increased cancer risk at this time. It is reassuring to note that no increase in malignancies has been reported in patients with sclerosteosis and van Buchem disease. The finding that activation of Wnt signaling in articular chondrocytes results in an osteoarthritis-like phenotype in mice [Zhu et al. 2009] raises concerns of antisclerostin therapy causing arthritis in humans. However, this has not been reported to date in any reports of clinical trials. Finally, there is the theoretical concern that prolonged extreme stimulation of bone formation with antisclerostin therapy might cause bony overgrowth that results in nerve impingement syndromes, as may occur in patients with sclerosteosis and van Buchem disease.
Summary
Sclerostin is a SOST gene product that reduces osteoblastic bone formation by inhibiting canonical Wnt/β-catenin signaling. Investigational monoclonal antibodies to sclerostin have been to shown to increase bone formation markers and decrease bone resorption markers, with an increase in bone mass. Several of these agents are now in clinical trials, with romosozumab now being evaluated in a phase III trial to determine its efficacy in reducing fracture risk in postmenopausal women with osteoporosis, in the most advanced stage of development. Antisclerostin therapy appears be a promising approach to the treatment of osteoporosis. Wnt/β-catenin signaling has also been implicated in the pathogenesis of osteoarthritis, with the potential for therapeutic intervention yet to be determined.
Footnotes
Funding: The author has received grant/research support from Amgen, Merck, Eli Lilly, and Novartis.
Conflict of interest statement: The author has served as a consultant, advisory board member, speakers’ bureau participant, or given presentations at sponsored speaking events for Amgen, Eli Lilly, Novartis, Radius Health, and AgNovos Healthcare.
References
- Adami S., Libanati C., Adachi J., Boonen S., Cummings S., de Gregorio L., et al. (2012) Denosumab administration is not associated with fracture healing complications in postmenopausal women with osteoporosis: results from the FREEDOM trial. J Bone Miner Res 25(Suppl. 1): S478 [Google Scholar]
- Agholme F., Li X., Isaksson H., Ke H.Z., Aspenberg P. (2010) Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res 25(11): 2412–2418 [DOI] [PubMed] [Google Scholar]
- Babcook J., Leslie K., Olsen O., Salmon R., Schrader J. (1996) A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proc Natl Acad Sci U S A 93(15): 7843–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W., Ebeling M., Patel N., Van H., Olson P., Dioszegi M., et al. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10: 537–543 [DOI] [PubMed] [Google Scholar]
- Baron R., Rawadi G. (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148: 2635–2643 [DOI] [PubMed] [Google Scholar]
- Beighton P., Davidson J., Durr L., Hamersma H. (1977) Sclerosteosis – an autosomal recessive disorder. Clin Genet 11: 1–7 [DOI] [PubMed] [Google Scholar]
- Benson C., Robins D., Recker R., Alam J., Chiang A., Mitlak B., et al. (2013) Effect of blosozumab on bone mineral density: results of a phase 2 study of postmenopausal women with low bone mineral density. Bone Abstracts 1: OC5.3 [Google Scholar]
- Brunkow M., Gardner J., Van N., Paeper B., Kovacevich B., Proll S., et al. (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011) Osteoarthritis. Available at: http://www.cdc.gov/arthritis/basics/osteoarthritis.htm (accessed 15 October 2013).
- Chan B., Fuller E., Russell A., Smith S., Smith M., Jackson M., et al. (2011) Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage 19: 874–885 [DOI] [PubMed] [Google Scholar]
- Cipriani C., Irani D., Bilezikian J. (2012) Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res 27: 2419–2428 [DOI] [PubMed] [Google Scholar]
- Clevers H. (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Cornell C. (2003) Internal fracture fixation in patients with osteoporosis. J Am Acad Orthop Surg 11: 109–119 [DOI] [PubMed] [Google Scholar]
- Cummings S., San Martin J., McClung M., Siris E., Eastell R., Reid I., et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756–765 [DOI] [PubMed] [Google Scholar]
- Delmas P., van de Langerijt L., Watts N., Eastell R., Genant H., Grauer A., et al. (2005) Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res 20: 557–563 [DOI] [PubMed] [Google Scholar]
- Foley K., Foster S., Meadows E., Baser O., Long S. (2007) Assessment of the clinical management of fragility fractures and implications for the new HEDIS osteoporosis measure. Med Care 45: 902–906 [DOI] [PubMed] [Google Scholar]
- Gardner J., van Bezooijen R., Mervis B., Hamdy N., Lowik C., Hamersma H., et al. (2005) Bone mineral density in sclerosteosis; affected individuals and gene carriers. J Clin Endocrinol Metab 90: 6392–6395 [DOI] [PubMed] [Google Scholar]
- Goldhahn J., Feron J., Kanis J., Papapoulos S., Reginster J., Rizzoli R., et al. (2012) Implications for fracture healing of current and new osteoporosis treatments: an ESCEO consensus paper. Calcif Tissue Int 90: 343–353 [DOI] [PubMed] [Google Scholar]
- Goldring M. (2012) Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis 4: 269–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamersma H., Gardner J., Beighton P. (2003) The natural history of sclerosteosis. Clin Genet 63: 192–197 [DOI] [PubMed] [Google Scholar]
- Kanis J. (2007) Assessment of Osteoporosis at the Primary Health-care Level. Technical report. World Health Organization Collaborating Centre for Metabolic Bone Diseases. Sheffield: University of Sheffield [Google Scholar]
- Kansara M., Tsang M., Kodjabachian L., Sims N., Trivett M., Ehrich M., et al. (2009) Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest 119: 837–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H., Richards W., Li X., Ominsky M. (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33: 747–783 [DOI] [PubMed] [Google Scholar]
- Kim J., Liu X., Wang J., Chen X., Zhang H., Kim S., et al. (2013) Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis 5: 13–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanski A., Adams-Campbell L., Bassford T., Blair S., Boden S., Dickersin K., et al. (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785–795 [DOI] [PubMed] [Google Scholar]
- Kramer I., Loots G., Studer A., Keller H., Kneissel M. (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25: 178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewiecki E. (2011) New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol 7: 631–638 [DOI] [PubMed] [Google Scholar]
- Li X., Ominsky M., Niu Q., Sun N., Daugherty B., D’Agostin D., et al. (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869 [DOI] [PubMed] [Google Scholar]
- Li X., Warmington K.S., Niu Q.T., Asuncion F.J., Barrero M., Grisanti M., et al. (2010) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass and bone strength in aged male rats. J Bone Miner Res 25(12): 2647–2656 [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., et al. (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280: 19883–19887 [DOI] [PubMed] [Google Scholar]
- Lin C., Jiang X., Dai Z., Guo X., Weng T., Wang J., et al. (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24: 1651–1661 [DOI] [PubMed] [Google Scholar]
- Lories R., Luyten F. (2011) The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 7: 43–49 [DOI] [PubMed] [Google Scholar]
- Luyten F., Tylzanowski P., Lories R. (2009) Wnt signaling and osteoarthritis. Bone 44: 522–527 [DOI] [PubMed] [Google Scholar]
- McClung M., Grauer A., Boonen S., Brown J., Diez-Perez A., Langdahl B., et al. (2012) Inhibition of sclerostin with AMG 785 in postmenopausal women with low bone mineral density: phase 2 trial results. J Bone Miner Res 27(Suppl. 1): S8 [Google Scholar]
- McColm J., Womack T., Hu L., Tang C., Chiang A. (2012) Blosozumab, a humanized monoclonal antibody against sclerostin, demonstrated anabolic effects on bone in postmenopausal women.J Bone Miner Res 27(Suppl. 1): S9. [DOI] [PubMed] [Google Scholar]
- Neogi T., Zhang Y. (2013) Epidemiology of osteoarthritis. Rheum Dis Clin North Am 39: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99–109 [DOI] [PubMed] [Google Scholar]
- Ominsky M., Li C., Li X., Tan H., Lee E., Barrero M., et al. (2011) Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res 26: 1012–1021 [DOI] [PubMed] [Google Scholar]
- Ominsky M., Samadfan R., Jolette J., Vlasseros F., Smith S., Kostenuik P., et al. (2009) Sclerostin monoclonal antibody stimulates bone formation and improves the strength and density of the fracture callus and lumbar spine in a primate fibular osteotomy model. J Bone Miner Res 24(Suppl. 1): S89–S90 [Google Scholar]
- Ominsky M., Vlasseros F., Jolette J., Smith S., Stouch B., Doellgast G., et al. (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25: 948–959 [DOI] [PubMed] [Google Scholar]
- Padhi D., Jang G., Stouch B., Fang L., Posvar E. (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26: 19–26 [DOI] [PubMed] [Google Scholar]
- Robling A., Niziolek P., Baldridge L., Condon K., Allen M., Alam I., et al. (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283: 5866–5875 [DOI] [PubMed] [Google Scholar]
- Roudier M., Li X., Niu Q., Pacheco E., Pretorius J., Graham K., et al. (2013) Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum 65: 721–731 [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K., Proll S., Paeper B., Zhao L., Charmley P., Brown A., et al. (2002) A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet 110: 144–152 [DOI] [PubMed] [Google Scholar]
- Vahle J., Long G., Sandusky G., Westmore M., Ma Y., Sato M. (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol 32: 426–438 [DOI] [PubMed] [Google Scholar]
- van Bezooijen R., Roelen B., Visser A., Van der Wee-Pals L., de Wilt E., Karperien M., et al. (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199: 805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lierop A., Hamdy N., Hamersma H., van Bezooijen R., Power J., Loveridge N., et al. (2011) Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J Bone Miner Res 26: 2804–2811 [DOI] [PubMed] [Google Scholar]
- Veverka V., Henry A., Slocombe P., Ventom A., Mulloy B., Muskett F., et al. (2009) Characterization of the structural features and interactions of sclerostin: molecular insight into a key regulator of Wnt-mediated bone formation. J Biol Chem 284: 10890–10900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang E., Balthasar J. (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84: 548–558 [DOI] [PubMed] [Google Scholar]
- Wergedal J., Veskovic K., Hellan M., Nyght C., Balemans W., Libanati C., et al. (2003) Patients with Van Buchem disease, an osteosclerotic genetic disease, have elevated bone formation markers, higher bone density, and greater derived polar moment of inertia than normal. J Clin Endocrinol Metab 88: 5778–5783 [DOI] [PubMed] [Google Scholar]
- Zhu M., Chen M., Zuscik M., Wu Q., Wang Y., Rosier R., et al. (2008) Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum 58: 2053–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Tang D., Wu Q., Hao S., Chen M., Xie C., et al. (2009) Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res 24: 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]