Abstract
Vasculitis is characterized by a circumferential vessel-wall thickening (‘halo’), which can be visualized by modern imaging techniques. In particular, the resolution of ultrasound has increased to 0.1 mm. Ultrasound detects abnormalities that are pathognomonic even in arteries with a diameter below 1 mm. It is particularly helpful in the diagnosis of large-vessel vasculitides, such as classic temporal arteritis, large-vessel giant-cell arteritis (GCA), Takayasu arteritis and idiopathic aortitis. Echocardiography is important for determining cardiac involvement in Takayasu arteritis and also for examining the coronary arteries of children with suspected Kawasaki disease, which is a medium-vessel vasculitis. In small vessel vasculitides ultrasound has only a role for determining the distribution or organ involvement.
Fast-track clinics for the diagnosis of GCA help to initiate treatment before complications such as blindness occur; patients receive appointments within 24 h in these clinics. Clinical examination and ultrasound of temporal and axillary arteries are performed by an experienced rheumatologist. In most cases this is able to determine if GCA is present. Temporal artery biopsy can be still carried out in ambivalent cases. The wall swelling of temporal arteries disappears after 2–3 weeks of glucocorticoid treatment. After 3 days of treatment, diagnosis becomes more difficult with ultrasound in some cases. In larger arteries, such as the axillary arteries, wall thickening disappears within months. It tends to be darker (more hypoechoic) in acute disease because of oedema.
Keywords: aortitis, giant-cell arteritis, Kawasaki disease, Takayasu arteritis, temporal arteritis, ultrasound, vasculitis
Introduction
In vasculitides inflammatory leucocytes are present in the vessel walls. This leads to swelling and damage of mural structures. The lumen is narrowed or occluded leading to ischaemia and necrosis [Watts and Scott, 2009].
Vasculitides may primarily affect large, medium-sized or small vessels. They may occur as a primary process or secondary to another underlying disease. An updated nomenclature categorizes vasculitides as large-vessel vasculitis, medium-vessel vasculitis, small-vessel vasculitis (SVV), vasculitides of variable size, single-organ vasculitides (SOV), vasculitides secondary to a systemic disease and vasculitis with suspected aetiology [Jennette et al. 2013].
Small-vessel vasculitides
For SVV like granulomatosis with polyangiitis (formerly, Wegener’s granulomatosis), eosinophilic granulomatosis with polyangiitis (formerly Churg–Strauss syndrome) and microscopic polyangiitis, ultrasound is important for determining disease extension and disease activity. Ultrasound can, for example, evaluate renal, cardiac and pleural involvement [Schmidt, 2013]. The diagnosis of SVV needs to be confirmed histologically. In rare cases, SVV can also affect larger arteries such as the digital arteries [Schmidt et al. 2006, 2008a].
Medium-vessel vasculitides
In the medium-vessel vasculitides, Kawasaki disease and polyarteritis nodosa, aneurysms frequently occur. These may be detected by ultrasound [Wang et al. 2013]. Kawasaki disease is an acute, self-limited vasculitis of childhood. It is characterized by fever, polymorphous exanthema, conjunctivitis, mucositis and unilateral cervical lymphadenopathy. Coronary aneurysms may complicate the course of the disease. In a study of 100 patients with Kawasaki disease 44% of patients had a coronary artery lesion (31% with ectasia, 13% with aneurysm) on the initial echocardiogram [Baer et al. 2006]. Aneurysms may lead to coronary artery occlusion with cardiac infarction and impaired left ventricular function. Echocardiography is the first choice for imaging examination in Kawasaki disease.
Large-vessel vasculitides
This article focuses on the diagnosis of large-vessel vasculitides with ultrasound as this allows the visualization of the characteristic wall thickening in affected arteries. It is increasingly used for diagnosing and monitoring large-vessel vasculitis. In clear cases it may replace histology.
Large-vessel vasculitides include classic temporal arteritis, large-vessel giant-cell arteritis (GCA), Takayasu arteritis and idiopathic aortitis.
Temporal arteritis
Temporal arteritis is characterized by localized headache often accompanied by hard, swollen and tender temporal arteries with reduced pulse. Patients feel very ill and often lose weight. About 50% of patients have concomitant polymyalgia rheumatica (PMR). Night sweats are common. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are usually elevated. ESR is higher than 50 mm/h in 85% of cases. Histology typically shows vasculitis with a predominance of mononuclear-cell infiltration or granulomatous inflammation with or without giant cells. Headaches occur in 74% of cases, tenderness and/or reduced temporal artery pulse in 64%, jaw claudication in 37%, and ophthalmological complications such as anterior ischaemic optic neuropathy or double vision occur in 32% of cases [Schmidt, 2006].
Large-vessel giant-cell arteritis
Increased use and quality of imaging shows that extracranial arterial involvement is much more common than previously assumed. The proximal arm arteries in particular are commonly involved, but also the aorta and femoral and popliteal arteries. When comparing patients with classic cranial temporal arteritis with patients with proximal arm artery involvement (large-vessel GCA), patients with large-vessel GCA tended to be younger (66 years versus 72 years), and there were more females (83% versus 66%). Time between onset of symptoms and diagnosis was longer in large-vessel GCA (7 months versus 2 months). Temporal artery involvement in terms of positive histology or abnormal ultrasound occurred in about 60% of these patients [Schmidt et al. 2008c]. Duration of treatment and prednisolone doses were similar. Patients with large-vessel GCA less commonly developed anterior ischaemic optic neuropathy [Schmidt et al. 2008b, 2009]. Critical limb ischaemia usually does not occur in the course of the disease [Schmidt et al. 2009; Czihal et al. 2013]. The axillary arteries are more commonly involved than the subclavian and brachial arteries [Schmidt et al. 2008c; Czihal et al. 2012b]. Bilateral vessel involvement is present in most patients [Schmidt et al. 2008c; Grayson et al. 2012; Czihal et al. 2012b]. Patients with large-vessel GCA may either present with classic cranial temporal arteritis, with pure PMR, arm claudication or pyrexia of unknown origin.
Takayasu arteritis
Patients with Takayasu arteritis are notably younger than patients with GCA. The disease onset is usually before the age of 40 years. GCA and Takayasu arteritis are similar with regard to histology and response to treatment. Disease duration is longer in Takayasu arteritis. Both entities may involve the aorta. The temporal arteries are never affected in Takayasu arteritis. Patients with Takayasu arteritis experience more severe flares with vascular complications than GCA patients. The pattern of artery involvement may be similar for large-vessel GCA and Takayasu arteritis. However, Takayasu arteritis may occur less symmetrically, most commonly affecting the left subclavian and common carotid arteries [Grayson et al. 2012].
Idiopathic aortitis
Idiopathic aortitis is listed among SOV in the new Chapel Hill nomenclature [Jennette et al. 2013]. However, there is a significant overlap with GCA with regard to symptoms and treatment. Many patients present with pyrexia of unknown origin.
Ultrasound in temporal arteritis
The temporal arteries are localized superficially, about 4 mm below the skin surface. Although they are small with lumen and wall diameters of about 0.7 mm, respectively, they are easily accessible with ultrasound. Ultrasound allows the assessment of the whole length of the superficial temporal arteries.
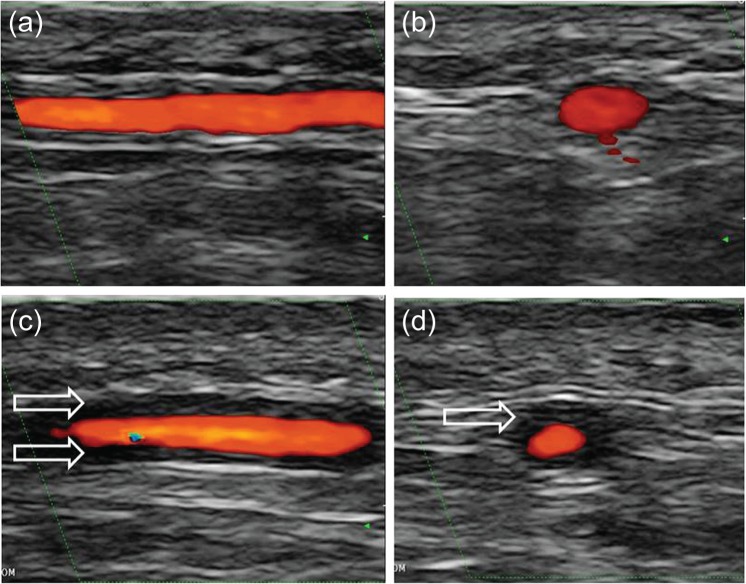
Colour Doppler ultrasound was the first imaging method to show valuable results for the diagnosis of temporal arteritis [Schmidt et al. 1997]. Since the mid-1990s, technology has allowed higher resolution. In fact, ultrasound has the highest resolution of all the imaging techniques used for the diagnosis of vasculitis. Today, a high-frequency probe of more than 10 MHz provides both an axial and lateral resolution of 0.1 mm (100 μm). Ultrasound depicts the artery wall and provides information about blood-flow characteristics within the artery. Colour Doppler ultrasound of temporal arteries shows hypoechoic (dark) oedematous wall swelling in acute temporal arteritis (Figure 1). This tissue is not compressible [Aschwanden et al. 2013]. It disappears with glucocorticoid treatment after 2–3 weeks in most patients. In some patients it becomes difficult to detect a characteristic wall swelling after only 3 days of treatment [Hauenstein et al. 2012].
Figure 1.
Colour Doppler ultrasound showing longitudinal (a) and transverse (b) views of a normal temporal artery and in acute temporal arteritis (c, d). The arrows indicate the vasculitic wall swelling.
The patient lies supine with the head towards the ultrasound monitor. The sonographer may sit in front of the patient. Some sonographers prefer sitting behind the head of the patient. The sonographer places the probe longitudinally in front of the left ear. After detecting the common superficial temporal artery the probe is moved continuously forward to the parietal branch. Then the probe is placed transversely to examine the parietal branch and the common superficial temporal artery in short axis. From the bifurcation the frontal branch is followed longitudinally and transversely [Bruyn and Schmidt, 2011; Schmidt, 2013]. The same procedure is repeated on the right side. The sonographer uses colour Doppler mode and angles the colour box so that the blood flow is not completely parallel to the probe.
Often stenoses of short segments occur. Then colour Doppler shows a blurring mixture of colours (aliasing) together with persistent blood flow in the diastole. Pulsed-wave Doppler then displays at least a two-fold increase in maximum systolic blood-flow velocity in the stenosis compared with the area before or behind the stenosis [Schmidt et al. 1997]. In addition, occlusions may occur, showing an artery that is not filled with colour Doppler signals.
High-end equipment should be used for examining the temporal arteries, although they may also be seen with lesser quality equipment. The frequency of the linear probe should be as high as possible, at least 10 MHz, preferably 15 MHz or higher. There are potential difficulties of which the sonographer should be aware. The sonographer needs to avoid too much pressure as the superficial temporal arteries may be easily compressed. More ultrasound gel is needed to acquire good image quality in areas with hair. If the colour gain (colour sensitivity) is too low, only the centre of the lumen will show colour signals leaving an anechoic (black) rim between the lumen and wall. This phenomenon must not be confused with wall thickening, which is hypoechoic showing tissue. If the colour gain is too high, the inflamed area may be covered and disease may be missed. The sonographer should have examined temporal arteries of about 30–50 healthy subjects and should have seen at least 3–5 patients with definite temporal arteritis before using this method in routine practice.
Many studies have been performed comparing temporal artery ultrasound with histology and with the clinical diagnosis of temporal arteritis. Three meta-analyses have been published [Karassa et al. 2005; Arida et al. 2010; Ball et al. 2010]. The sensitivity of temporal artery duplex ultrasound was 87% with regard to clinical diagnosis, and specificity was 96% in one of the meta-analyses [Karassa et al. 2005]. The presence of a bilateral halo seems to increase the specificity [Arida et al. 2010]. With increasing experience and quality of ultrasound equipment more centres achieve reliable results with ultrasound examination and replace temporal artery biopsy in cases with definitive clinical and ultrasound findings [Schirmer et al. 2011; Porta et al. 2012; Alberts, 2013]. After training for temporal artery ultrasound, inter- and intra-reader agreements have been shown to be excellent with kappa values over 0.8 [De Miguel et al. 2009].
It is also possible to examine the occipital arteries, which may be exclusively involved in some patients, particularly if they are presenting with retroauricular pain [Pfadenhauer and Weber, 2003]. The facial arteries are particularly involved in patients with jaw claudication [Schmidt et al. 2002a].
As the wall swelling of the temporal arteries disappears quickly and recurs only in severe flares, routine follow-up ultrasound examinations are not necessary. Patients’ history and the monitoring of CRP and ESR usually provide enough information to determine whether or not the disease is active.
Ultrasound in large-vessel GCA
About 50% of newly diagnosed GCA patients have axillary artery involvement [Schmidt et al. 2008c; Czihal et al. 2012b]. Including ultrasound examination of the axillary arteries in a protocol increases the diagnostic yield for GCA in routine practice, particularly as only 60% of patients with large-vessel GCA have temporal artery involvement. An experienced sonographer examining both the temporal and axillary arteries should find more GCA patients than biopsy with histology of 1–2 cm-long segments of one of the branches of the common superficial temporal arteries.
The axillary arteries can be easily and quickly examined with ultrasound. The probe is placed longitudinally in the axilla along the humeral head and neck [Bruyn and Schmidt, 2011; Schmidt, 2013]. This scan is identical with the axillary shoulder scan for detecting glenohumeral joint effusions. The axillary artery localizes either at the level of the humerus or 1–2 cm medially to it. The axillary artery is localized proximally to the circumflexa humeri artery. The area distal to the circumflexa humeri artery is the proximal brachial artery, which may also show vasculitis. In order to obtain a good colour Doppler image the colour box needs to be steered as with the temporal arteries to avoid a blood flow perpendicular to the sound waves. In evaluating the artery wall the grey-scale image probe and vessel should be as parallel as possible. A normal vessel shows an intima-media complex of less than 1 mm. A bright line represents the interface between artery lumen and vessel wall followed by a dark line representing wall tissue and another bright line representing the interface between media and adventitia.
In large-vessel vasculitis the artery wall is thickened, usually to more than 1 mm (Figure 2) [Schmidt et al. 2008c; Czihal et al. 2012b]. A homogeneous wall swelling, preferably circumferential, of 1.5 mm or more is pathognomonic for the diagnosis of axillary vasculitis [Schmidt et al. 2008c]. Vasculitis may lead to stenosis showing aliasing as explained above and characteristic PW-Doppler curves with increased systolic and diastolic flow velocities. Sometimes axillary or proximal brachial arteries are occluded due to vasculitis.
Figure 2.
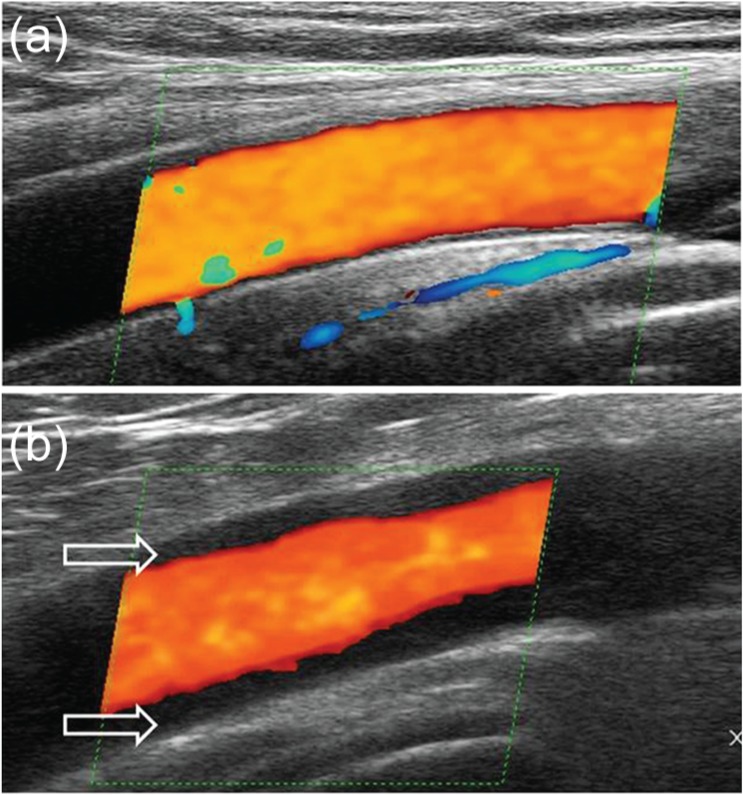
A longitudinal ultrasound image of a normal axillary artery (a) and an axillary artery in large-vessel giant-cell arteritis. The arrows indicate the vasculitic wall swelling with a diameter of 1.8 mm.
Arteriosclerosis is uncommon both in temporal or axillary arteries. It is hyperechoic, heterogeneous and often asymmetric. The carotid, femoral and popliteal arteries are much more often affected by arteriosclerosis. In a few cases it may be difficult to differentiate hypoechoic plaques from vasculitic lesions. However, usually it is easy to differentiate arteriosclerosis from vasculitis with ultrasound.
The subclavian, common carotid and vertebral arteries can also be easily examined by ultrasound. However, these arteries are rarely affected in GCA without the involvement of either the temporal or axillary arteries. Therefore, routinely examining these arteries will not greatly increase the sensitivity of ultrasound. Carotid artery stenoses are rarely due to GCA. Most stenoses in the age group around 70–75 years are due to arteriosclerosis. However, vasculitic stenoses and occlusions may occur in the vertebral arteries causing cerebral ischaemia and stroke [Garcia-Garcia et al. 2011].
The clinical examination of patients with GCA should include the palpation of the pedal pulses. If these pulses are not palpable and particularly if patients complain about lower limb claudication, the arteries of the lower leg should be examined by ultrasound. The superficial femoral arteries and popliteal arteries in particular are commonly involved in GCA [Czihal et al. 2012a].
With medical treatment alone, without any interventions such as stenting or bypass surgery, the prognosis of GCA of the arm arteries is benign in nearly all cases [Schmidt et al. 2008b; Czihal et al. 2013]. Ultrasound may be carried out every 6 months for follow up in order to measure the maximum wall thickness. If the diameter decreases or remains the same, then it can be presumed that disease activity has been under control during this period.
Polymyalgia rheumatica
Shoulder and hip ultrasound increases the specificity for diagnosing PMR, therefore musculoskeletal ultrasound has been incorporated into the new European League against Rheumatism/American College of Rheumatology (EULAR/ACR) collaborative initiative classification criteria [Dasgupta et al. 2012]. Patients in whom ultrasound revealed inflammation in the shoulder and hip region responded better to glucocorticoid treatment [Matteson et al. 2012]. A subgroup of PMR patients revealed large-vessel vasculitis. In 7% of patients with ‘pure PMR’, for example, PMR without symptoms of temporal arteritis, temporal artery ultrasound was positive [Schmidt and Gromnica-Ihle, 2002b]. When ultrasound of the axillary arteries was added about 15% of patients with ‘pure PMR’ were diagnosed with large-vessel vasculitis [Schmidt et al. 2008c].
Ultrasound in Takayasu arteritis
The ultrasound findings in Takayasu arteritis are similar to those in GCA. In acute flares the wall is hypoechoic because of wall oedema, whereas in most cases it tends to be hyperechoic [Schmidt et al. 2007; Keo et al. 2013]. In most cases the diagnosis of Takayasu arteritis is not established before stenoses occur. However, early prestenotic disease can be detected by ultrasound because of its high resolution and excellent depiction of the artery walls [Schmidt et al. 2002b]. In suspected disease the carotid, subclavian and vertebral arteries should be examined together with the abdominal aorta. The renal arteries should be examined in case of arterial hypertension.
Echocardiography may reveal the following abnormalities: (a) left ventricular hypertrophy as arterial hypertension caused by renal artery stenosis may be missed because of subclavian artery stenoses or occlusions; (b) vasculitic wall swelling of the ascending aorta; the first 4 cm of the ascending aorta are visible with transthoracic echocardiography; (c) aortic valve insufficiency due to vasculitis of the ascending aorta; (d) pericardial effusion; (e) pulmonary hypertension due to vasculitic stenosis of the pulmonary arteries [Fateh-Moghadam et al. 2010].
Follow-up examinations are carried out as with large-vessel GCA to evaluate increase or decrease of wall swelling and stenoses [Schmidt, 2013].
Idiopathic aortitis
Aortitis is common in GCA and Takayasu arteritis; it rarely occurs as isolated aortitis. The abdominal aorta is fairly well visible with ultrasound if the patient is not obese. The thoracic aorta is more commonly affected by vasculitis than the abdominal aorta. However, most parts of the thoracic aorta are not accessible by transthoracic ultrasound because the air in the lungs inhibits visibility. Transoesophageal ultrasound provides excellent information on whether aortitis is present, but this is an invasive imaging technique.
Two similar entities affect the wall and/or the tissue around the abdominal aorta: aortitis/periaortitis and retroperitoneal fibrosis. CRP and ESR are increased in both entities. However, the increase is most commonly greater in aortitis. Usually, only retroperitoneal fibrosis leads to hydronephrosis. Treatment of both diseases includes immunosuppressive therapy, notably glucocorticoids. Both magnetic resonance imaging (MRI) and computed tomography (CT) are the imaging procedures of choice for the diagnosis of retroperitoneal fibrosis [Pipitone et al. 2012]. Ultrasound is disappointing in detecting retroperitoneal fibrosis as it fails to differentiate clearly the affected tissue from the surrounding tissue. However, ultrasound clearly delineates an inflamed aortic wall in abdominal aortitis [Schmidt, 2013].
Ultrasound in comparison with other imaging techniques
Ultrasound has many advantages: It is widely available and it can be performed by the rheumatologist himself. The suspected diagnosis can be confirmed or excluded immediately. Ultrasound is not invasive and has no relevant side effects. Findings can be explained to the patient during the examination. The sonographer needs to be well trained in vascular ultrasound in order to provide reliable results. Good reliability is possible as mentioned above. An experienced sonographer can perform a standardized ultrasound examination of temporal and axillary arteries in 10 min. An extensive ultrasound examination of all arteries that may be potentially affected by vasculitis would be very time consuming.
High-quality MRI with dedicated coils can be used for examining the temporal arteries. The diagnostic power for diagnosing temporal arteritis is similar in both MRI and ultrasound [Bley et al. 2008]. MRI can also depict the artery walls and it is superior to ultrasound for examining the thoracic aorta. MR-angiography (MRA) provides a good overview of affected arteries. CT and CT-angiography (CTA) are alternatives to MRI and MRA [Prieto-Gonzalez et al. 2012]. However, CT has not yet been used for examining the temporal arteries. Positron emission tomography (PET) or PET-CT also provides a good overview, but is limited by high radiation exposition, high costs and the inability to examine the temporal arteries [Blockmans et al. 2009]. Ultrasound and PET correlate well in diagnosing GCA [Forster et al. 2011]. Conventional angiography is invasive and does not depict the artery wall. It has still its place in interventional treatment, particularly in Takayasu arteritis. Table 1 provides an overview of imaging techniques used for the diagnosis of large-vessel vasculitis.
Table 1.
Comparison of imaging techniques in the diagnosis of large-vessel vasculitides [Schmidt, 2013].
| Diagnostic test | Economical | Noninvasive | Depicting artery wall | Depicting aorta | Intervention | Temporal arteries |
|---|---|---|---|---|---|---|
| Angiography | + | − | − | ++ | ++ | − |
| Ultrasound | ++ | ++ | ++ | (+) | − | ++ |
| Computed tomography | + | + | ++ | ++ | − | − |
| Computed tomography-angiography | + | + | − | ++ | − | − |
| Magnetic resonance imaging | + | + | ++ | ++ | − | ++ |
| Magnetic resonance-angiography | + | + | − | ++ | − | + |
| Positron emission tomography | − | + | − | ++ | − | − |
Practical application of ultrasound
Fast-track clinics for the diagnosis of GCA help initiate treatment before complications such as blindness occur. In such clinics, patients receive appointments within 24 h. Clinical examination and ultrasound of temporal and axillary arteries are performed by an experienced rheumatologist. Other arteries may be examined depending on clinical findings. In most cases this clearly determines if GCA is present. Temporal artery biopsy is performed in ambivalent cases. Other imaging techniques such as MRI, MRA, CT, CTA or PET can be carried out, particularly if aortitis is suspected and ultrasound findings are not clear. MRA may provide an overview of involved vessels particularly in patients with Takayasu arteritis in order to obtain information about the vascular pattern before treatment. Conventional angiography is only used for interventional treatment. Ultrasound in particular is a valuable tool for follow up, especially for large-vessel GCA and Takayasu arteritis.
In conclusion, ultrasound has become a valuable diagnostic tool for large-vessel vasculitis. It helps establish the diagnosis early and thus may prevent complications such as blindness.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Receipt of grants/research supports: Abbvie, Actelion, Esaote, GE, Merck, Mundipharma, Novartis, Roche, Savient, Siemens. Receipt of honoraria or consultation fees: Abbvie, Berlin-Chemie, Novartis, Roche. Participation in a company sponsored speaker’s bureau: Abbvie, Berlin-Chemie, Medac, Merck, Mundipharma, Pfizer, Roche, UCB.
References
- Alberts M. (2013) Temporal arteritis: improving patient evaluation with a new protocol. Perm J 17: 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arida A., Kyprianou M., Kanakis M., Sfikakis P. (2010) The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord 11: 44–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschwanden M., Daikeler T., Kesten F., Baldi T., Benz D., Tyndall A., et al. (2013) Temporal artery compression sign – a novel ultrasound finding for the diagnosis of giant cell arteritis. Ultraschall Med 34: 47–50 [DOI] [PubMed] [Google Scholar]
- Baer A., Rubin L., Shapiro C., Sood S., Rajan S., Shapir Y., et al. (2006) Prevalence of coronary artery lesions on the initial echocardiogram in Kawasaki syndrome. Arch Pediatr Adolesc Med 160: 686–690 [DOI] [PubMed] [Google Scholar]
- Ball E., Walsh S., Tang T., Gohil R., Clarke J. (2010) Role of ultrasonography in the diagnosis of temporal arteritis. Br J Surg 97: 1765–1771 [DOI] [PubMed] [Google Scholar]
- Bley T., Reinhard M., Hauenstein C., Markl M., Warnatz K., Hetzel A., et al. (2008) Comparison of duplex sonography and high-resolution magnetic resonance imaging in the diagnosis of giant cell (temporal) arteritis. Arthritis Rheum 58: 2574–2578 [DOI] [PubMed] [Google Scholar]
- Blockmans D., Bley T., Schmidt W. (2009) Imaging for large-vessel vasculitis. Curr Opin Rheumatol 21: 19–28 [DOI] [PubMed] [Google Scholar]
- Bruyn G., Schmidt W. (2011) Introductory Guide to Musculoskeletal Ultrasound for the Rheumatologist. Houten: Bohn Stafleu van Loghum/Springer [Google Scholar]
- Czihal M., Piller A., Schroettle A., Kuhlencordt P., Schulze-Koops H., Hoffmann U. (2013) Outcome of giant cell arteritis of the arm arteries managed with medical treatment alone: cross-sectional follow-up study. Rheumatology (Oxford) 52: 282–286 [DOI] [PubMed] [Google Scholar]
- Czihal M., Tato F., Rademacher A., Kuhlencordt P., Schulze-Koops H., Hoffmann U. (2012a) Involvement of the femoropopliteal arteries in giant cell arteritis: clinical and color duplex sonography. J Rheumatol 39: 314–321 [DOI] [PubMed] [Google Scholar]
- Czihal M., Zanker S., Rademacher A., Tato F., Kuhlencordt P., Schulze-Koops H., et al. (2012b) Sonographic and clinical pattern of extracranial and cranial giant cell arteritis. Scand J Rheumatol 41: 231–236 [DOI] [PubMed] [Google Scholar]
- Dasgupta B., Cimmino M., Maradit-Kremers H., Schmidt W., Schirmer M., Salvarani C., et al. (2012) 2012 provisional classification criteria for polymyalgia rheumatica: a European League against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 71: 484–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel E., Castillo C., Rodriguez A., De Agustin J. (2009) Learning and reliability of colour Doppler ultrasound in giant cell arteritis. Clin Exp Rheumatol 27: S53–S58 [PubMed] [Google Scholar]
- Fateh-Moghadam S., Huehns S., Schmidt W., Dietz R., Bocksch W. (2010) Pericardial effusion as primary manifestation of Takayasu arteritis. Int J Cardiol 145: 33–35 [DOI] [PubMed] [Google Scholar]
- Forster S., Tato F., Weiss M., Czihal M., Rominger A., Bartenstein P., et al. (2011) Patterns of extracranial involvement in newly diagnosed giant cell arteritis assessed by physical examination, colour coded duplex sonography and FDG-PET. Vasa 40: 219–227 [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia J., Ayo-Martin Ã., Argandona-Palacios L., Segura T. (2011) Vertebral artery halo sign in patients with stroke: a key clue for the prompt diagnosis of giant cell arteritis. Stroke 42: 3287–3290 [DOI] [PubMed] [Google Scholar]
- Grayson P., Maksimowicz-Mckinnon K., Clark T., Tomasson G., Cuthbertson D., Carette S., et al. (2012) Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis 71: 1329–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauenstein C., Reinhard M., Geiger J., Markl M., Hetzel A., Treszl A., et al. (2012) Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford) 51: 1999–2003 [DOI] [PubMed] [Google Scholar]
- Jennette J., Falk R., Bacon P., Basu N., Cid M., Ferrario F., et al. (2013) 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1–11 [DOI] [PubMed] [Google Scholar]
- Karassa F., Matsagas M., Schmidt W., Ioannidis J. (2005) Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 142: 359–369 [DOI] [PubMed] [Google Scholar]
- Keo H., Caliezi G., Baumgartner I., Diehm N., Willenberg T. (2013) Increasing echogenicity of diffuse circumferential thickening (“macaroni sign”) of the carotid artery wall with decreasing inflammatory activity of Takayasu arteritis. J Clin Ultrasound 41: 59–62 [DOI] [PubMed] [Google Scholar]
- Matteson E., Maradit-Kremers H., Cimmino M., Schmidt W., Schirmer M., Salvarani C., et al. (2012) Patient-reported outcomes in polymyalgia rheumatica. J Rheumatol 39: 795–803 [DOI] [PubMed] [Google Scholar]
- Pfadenhauer K., Weber H. (2003) Duplex sonography of the temporal and occipital artery in the diagnosis of temporal arteritis. A prospective study. J Rheumatol 30: 2177–2181 [PubMed] [Google Scholar]
- Pipitone N., Vaglio A., Salvarani C. (2012) Retroperitoneal fibrosis. Best Pract Res Clin Rheumatol 26: 439–448 [DOI] [PubMed] [Google Scholar]
- Porta F., Gargani L., Kaloudi O., Schmidt W., Picano E., Damjanov N., et al. (2012) The new frontiers of ultrasound in the complex world of vasculitides and scleroderma. Rheumatology (Oxford) 51(Suppl. 7): 26–30 [DOI] [PubMed] [Google Scholar]
- Prieto-Gonzalez S., Arguis P., Garcia-Martinez A., Espigol-Frigole G., Tavera-Bahillo I., Butjosa M., et al. (2012) Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis 71: 1170–1176 [DOI] [PubMed] [Google Scholar]
- Schirmer M., Duftner C., Schmidt W., Dejaco C. (2011) Ultrasonography in inflammatory rheumatic disease: an overview. Nat Rev Rheumatol 7: 479–488 [DOI] [PubMed] [Google Scholar]
- Schmidt W. (2006) Current diagnosis and treatment of temporal arteritis. Curr Treat Options Cardiovasc Med 8: 145–151 [DOI] [PubMed] [Google Scholar]
- Schmidt W. (2013) Imaging in vasculitis. Best Pract Res Clin Rheumatol 27: 107–118 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Gromnica-Ihle E. (2002) Incidence of temporal arteritis in patients with polymyalgia rheumatica: a prospective study using colour Doppler ultrasonography of the temporal arteries. Rheumatology 41: 46–52 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Kraft H., Vorpahl K., Volker L., Gromnica-Ihle E. (1997) Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 337: 1336–1342 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Krause A., Schicke B., Kuchenbecker J., Gromnica-Ihle E. (2009) Do temporal artery duplex ultrasound findings correlate with ophthalmic complications in giant cell arteritis? Rheumatology (Oxford) 48: 383–385 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Krause A., Schicke B., Wernicke D. (2008a) Color Doppler ultrasonography of hand and finger arteries to differentiate primary from secondary forms of Raynaud’s phenomenon. J Rheumatol 35: 1591–1598 [PubMed] [Google Scholar]
- Schmidt W., Moll A., Seifert A., Schicke B., Gromnica-Ihle E., Krause A. (2008b) Prognosis of large-vessel giant cell arteritis. Rheumatology (Oxford) 47: 1406–1408 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Natusch A., Moller D., Vorpahl K., Gromnica-Ihle E. (2002a) Involvement of peripheral arteries in giant cell arteritis: a color Doppler sonography study. Clin Exp Rheumatol 20: 309–318 [PubMed] [Google Scholar]
- Schmidt W., Nerenheim A., Seipelt E., Poehls C., Gromnica-Ihle E. (2002b) Diagnosis of early Takayasu arteritis with sonography. Rheumatology (Oxford) 41: 496–502 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Seifert A., Gromnica-Ihle E., Krause A., Natusch A. (2008c) Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford) 47: 96–101 [DOI] [PubMed] [Google Scholar]
- Schmidt W., Seipelt E., Krause A., Wernicke D. (2007) Carotidynia in Takayasu arteritis. J Rheumatol 34: 231–232 [PubMed] [Google Scholar]
- Schmidt W., Wernicke D., Kiefer E., Gromnica-Ihle E. (2006) Colour duplex sonography of finger arteries in vasculitis and in systemic sclerosis. Ann Rheum Dis 65: 265–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Li J., Jiang Y., Dai Q., Jiang Y., Hou Y., et al. (2013) Polyarteritis nodosa with multiple aneurysms and renal arteriovenous fistula successfully diagnosed by colour Doppler sonography. Clin Rheumatol 32(Suppl. 1): 89–92 [DOI] [PubMed] [Google Scholar]
- Watts R., Scott D. (2009) Recent developments in the classification and assessment of vasculitis. Best Pract Res Clin Rheumatol 23: 429–443 [DOI] [PubMed] [Google Scholar]