Significance
Kisspeptin, a hypothalamic neuropeptide, plays a key role in vertebrate reproduction. In the zebrafish, kisspeptin and its receptor are predominantly expressed in the habenula, a highly evolutionarily conserved brain region mediating behavioral responses to stressful conditions. However, the physiological significance of kisspeptin expressed in the habenula remains unknown. Here we demonstrate a unique role for the kisspeptin system in inhibiting fear response in the zebrafish that extends beyond the control of reproduction.
Keywords: 5-HT, interpeduncular, neuroendocrine, anxiolytic
Abstract
Kisspeptin, a neuropeptide encoded by the KISS1/Kiss1, and its cognate G protein-coupled receptor, GPR54 (kisspeptin receptor, Kiss-R), are critical for the control of reproduction in vertebrates. We have previously identified two kisspeptin genes (kiss1 and kiss2) in the zebrafish, of which kiss1 neurons are located in the habenula, which project to the median raphe. kiss2 neurons are located in the hypothalamic nucleus and send axonal projections to gonadotropin-releasing hormone neurons and regulate reproductive functions. However, the physiological significance of the Kiss1 expressed in the habenula remains unknown. Here we demonstrate the role of habenular Kiss1 in alarm substance (AS)-induced fear response in the zebrafish. We found that AS-evoked fear experience significantly reduces kiss1 and serotonin-related genes (plasmacytoma expressed transcript 1 and solute carrier family 6, member 4) in the zebrafish. Furthermore, Kiss1 administration suppressed the AS-evoked fear response. To further evaluate the role of Kiss1 in fear response, zebrafish Kiss1 peptide was conjugated to saporin (SAP) to selectively inactivate Kiss-R1-expressing neurons. The Kiss1-SAP injection significantly reduced Kiss1 immunoreactivity and c-fos mRNA in the habenula and the raphe compared with control. Furthermore, 3 d after Kiss1-SAP injection, the fish had a significantly reduced AS-evoked fear response. These findings provide an insight into the role of the habenular kisspeptin system in inhibiting fear.
Kisspeptin, a hypothalamic neuropeptide derived from the KISS1/Kiss1, with the ability to activate the kisspeptin receptor (Kiss-R), has proven to play a key role in vertebrate reproduction (1). Kisspeptin neurons are present in the hypothalamic region, but their neural targets are not restricted to the hypothalamic region (2, 3). Furthermore, recent studies in mammals have revealed the expression of Kiss1 in several brain regions, including the medial amygdala (4). However, the knowledge of the potential role of kisspeptin-Kiss-R in nonhypothalamic regions remains limited. Using the teleost fish, we have previously identified two homologous genes (kiss1 and kiss2) encoding kisspeptin (5), of which kiss1 is a conserved ortholog of mammalian KISS1/Kiss1, whereas kiss2 has been found in hypothalamic nuclei of only nonmammalian vertebrates, which include amphibians and teleosts (6). In the zebrafish, kiss1 and kissr1 mRNAs are predominantly expressed in the ventral habenula (vHb) (5, 7). In nonmammalian vertebrates, the dorsal habenula (dHb) and the vHb are homologous to the medial (mHb) and lateral (lHb) habenula in mammals (8, 9). The lHb in primates regulates punishment avoidance behavior (10) and in rodents, it controls anxiety and fear (11), which suggests that nonmammalian vHb, homologous to the mammalian lHb, could modulate fear response. Furthermore, the vHb projects Kiss1 neuronal fibers to the median raphe (MR) (7, 12), a site adjacent to serotonergic (5-hydroxytryptamine, 5-HT) neurons in the zebrafish (13). These results indicate the potential role of habenular Kiss1-Kiss-R1 in the modulation of 5-HT-dependent functions such as anxiety and fear. However, the role of Kiss1 in the control of anxiety and fear has never been tested and remains unknown. In this study, to systematically examine anxiety and fear, we used two different experimental procedures. The novel tank diving test was used to analyze anxiety (bottom dwelling and top-to-bottom transitions in the tank) (14), and alarm substance (also known as alarm pheromone) was introduced to create fear response (erratic movements and freezing) (15). Further, to substantiate the role of endogenous Kiss1 in the absence of fish-specific Kiss-R1 antagonist and zebrafish mutants (or knockout) of kiss1/kissr1, we conjugated zebrafish Kiss1 peptide to saporin (SAP), a ribosome-inactivating protein (16), to selectively inactivate Kiss-R1-expressing neurons, and we examined AS-evoked fear response.
Results
Effect of Kisspeptin on Anxiety.
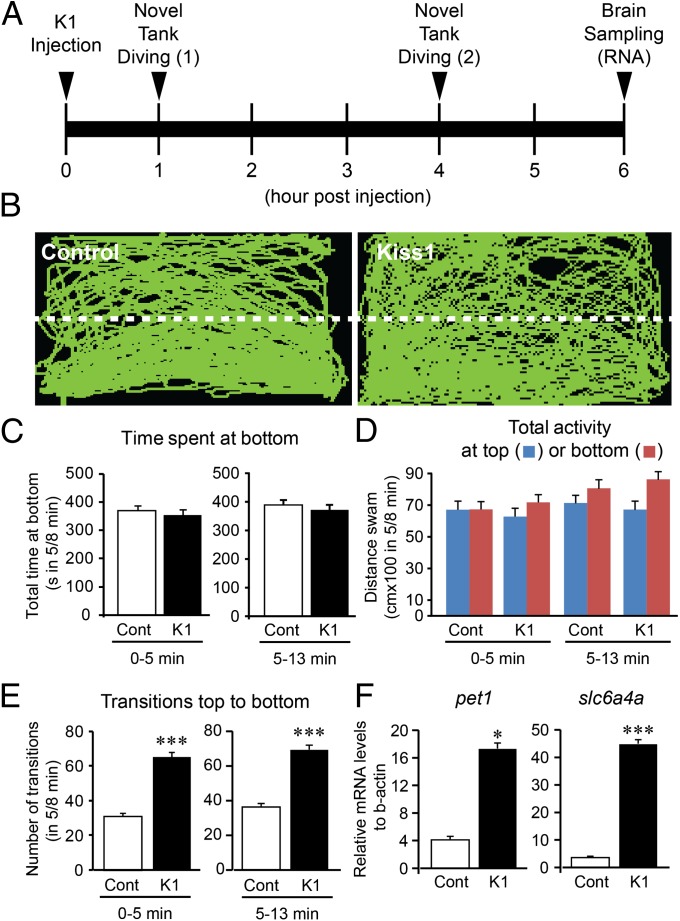
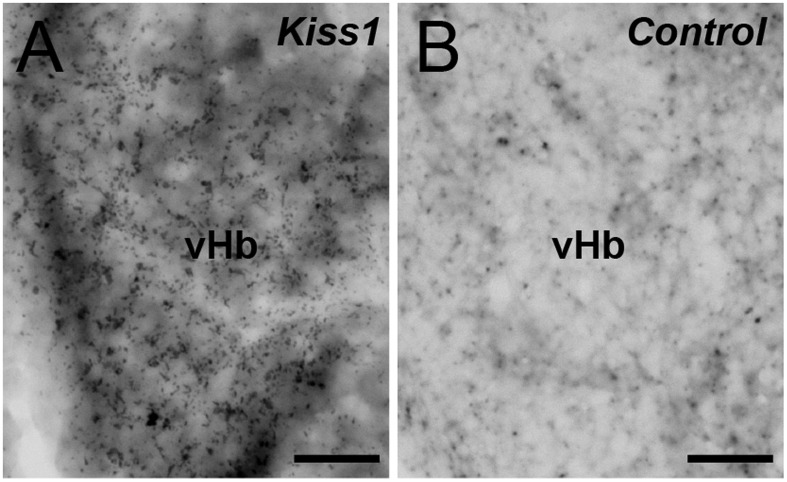
One and 4 h after intracranial administration of Kiss1 (10−11 mol per fish) had no effect on the behavioral parameters of anxiety, such as time spent at the bottom of the tank and total distance traveled during the first 5-min and 8-min observations (Fig. 1 A–D; Fig. S1). However, 4 h after Kiss1 administration saw an increased number of top-to-bottom transitions (Fig. 1E) and mRNA levels of serotonin-related genes [plasmacytoma expressed transcript 1 (pet1) and solute carrier family 6, member 4 (slc6a4a); Fig. 1F]. In contrast, administration of Kiss2 (10−11 mol per fish) had no effect on the observed behavioral parameters of anxiety in the novel tank diving test (Fig. S2 A–D). One hour after intracranial administration of Kiss1 saw significantly increased expression of c-fos mRNA, mainly in the vHb (Fig. 2), as reported previously (7).
Fig. 1.
Effect of Kiss1 administration on anxiety-like behavior and serotonergic genes. (A) Schematic of treatment timeline. Fish were intracranially injected with either distilled water (control) or Kiss1 (10−11 mol per fish) and subjected to a novel tank diving test (13 min observation) at 1 and 4 h after administration. After behavior observation, the fish were returned to their original tank. At 6 h after administration, the fish were killed, and the brain was dissected for gene expression analysis. (B) Side-view video-tracking of swimming behavior in the novel tank diving test at 4 h after administration. A test tank was divided into two equal virtual horizontal portions, marked by a white dividing line. (C–E) Graphs of the novel tank diving test during the first 5 min (0–5 min, Left) and the next 8 min (5–13 min, Right) observation in the fish injected with either distilled water control (Cont, open bars) or Kiss1 (K1, closed bars). There was no difference in behavioral parameters of anxiety: the time spent at the bottom half of the tank (C) and the total distance traveled at the top (blue column) or bottom (red column) half of the tank (D), except for number of transitions from top to bottom (E). (F) Graphs showing the effect of Kiss1 on mRNA levels of pet1 (Left) and slc6a4a (Right) at 6 h after administration. Data are presented as mean ± SEM. *P < 0.05; ***P < 0.001, independent t-test comparisons between control and Kiss1-injected fish.
Fig. 2.
Effect of administration of Kiss1 on c-fos mRNA expression in the habenula. Effect of Kiss1 decapeptide (10−11 mol per fish) (A) or water (B) on the expression of c-fos mRNA in the vHb. Expression of c-fos mRNA in the vHb is seen as black grains with the DAB substrate. (Scale bars, 20 µm.)
Effect of AS on Fear Response.
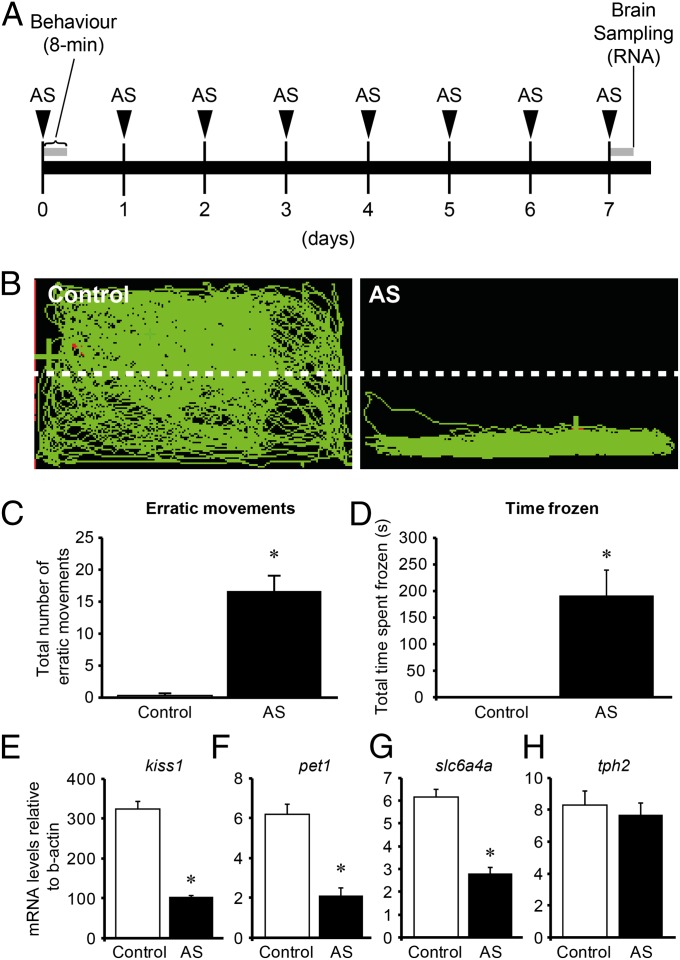
On day 1, AS exposure induced fear response in the zebrafish (Fig. 3 A and B). In the AS-treated zebrafish, the number of erratic movements (Fig. 3C) and the freezing time (Fig. 3D) were significantly (P < 0.01) increased compared with in the control fish, which was exposed to distilled water.
Fig. 3.
Effect of AS exposure on fear response and kiss1 and serotonergic genes. (A) Schematic of treatment timeline. Fish were exposed with AS for 8 min for 7 d. Behavior was observed on the 1 d of AS exposure. Brain samples were collected from the fish treated with 1 d (Fig. S4) and 7 d AS exposure. (B) Side-view video-tracking of swimming behavior of a fish treated with AS for an 8-min recording. A test tank was divided into two equal virtual horizontal portions, marked by a white dividing line. (C and D) Graphs of behavioral parameters of fear response, including the number of erratic movements (C) and the freezing time (D) (n =20 per group). (E–H) Graphs showing the effect of 7 d exposure of AS (8 min/d) on mRNA levels of kiss1 (E), pet1 (F), slc6a4a (G), and tph2 (H) (n =20 per group). Data are presented as mean ± SEM. *P < 0.001, independent t-test comparisons between control and AS-exposed fish.
Effect of Kiss1 on AS-Evoked Fear Response.
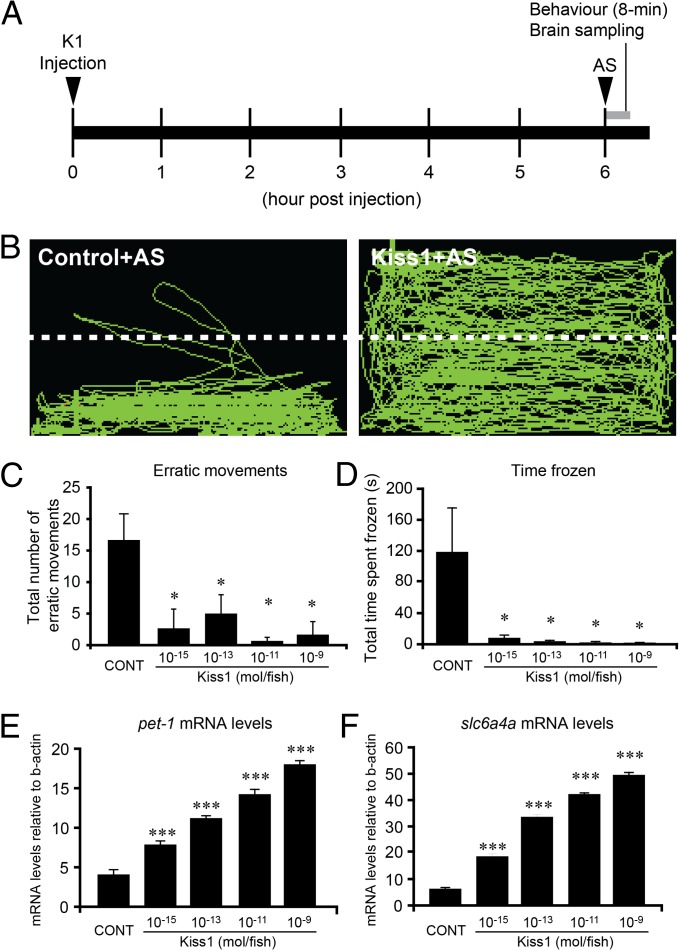
Six hours after Kiss1 administration, AS-evoked fear response was examined (Fig. 4 A and B). In the Kiss1-injected fish, behavioral parameters of fear response such as the number of erratic movements (Fig. 4C) and freezing behavior (Fig. 4D) were significantly decreased at doses of 10−15 to 10−9 mol per fish compared with in the control fish injected with distilled water.
Fig. 4.
Kisspeptin administration interrupted AS-evoked fear response. (A) Schematic of treatment timeline. Fish were injected with either distilled water (control) or Kiss1 (10−11 mol per fish), and AS-evoked fear response (8 min, gray bar) was observed at 6 h after administration. After the behavior observation, the brain sample was collected for gene expression analysis. (B) Side-view video-tracking of swimming behavior of AS-evoked fear response in a fish injected with either distilled water (control, Left) or Kiss1 (Right). A test tank was divided into two equal virtual horizontal portions, marked by a white dividing line. (C and D) Graphs of the effect of different doses of Kiss1 (10−15∼−9mol per fish; n = 20 per group) on fear response, including the number of erratic movements (C) and the freezing time (D). (E and F) Graphs of the effect of different doses of Kiss1 (10−15∼−9mol per fish; n = 20 per group) on mRNA levels of pet1 (E) and slc6a4a (F). Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 vs. control group; one-way ANOVA followed by post hoc Tukey’s test.
Binding Specificity of Kiss1-SAP to Kiss-Rs.
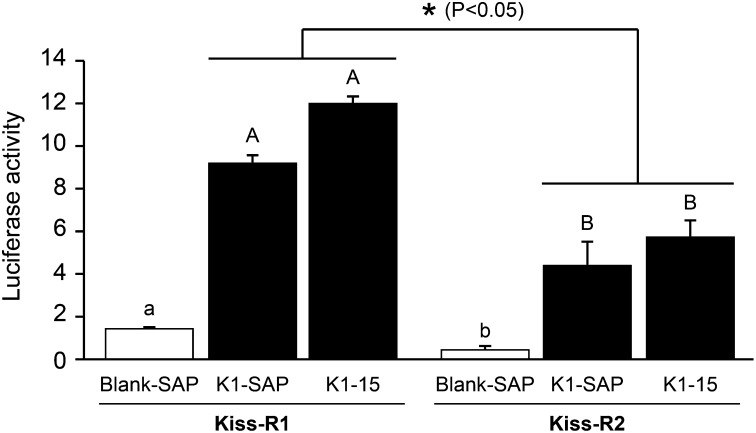
Kiss1-SAP exhibited binding affinity for the zebrafish Kiss-R1 comparable to that of kisspeptin1-15 (Fig. 5). There was no binding affinity of Blank-SAP for both Kiss-R1 and Kiss-R2. Kiss1-SAP and kisspeptin1-15 showed higher ligand selectivity for Kiss-R1 compared with Kiss-R2 (∼twofold; P < 0.05). These data show that conjugation of SAP to kisspeptin1-15 did not significantly alter its affinity for Kiss-R1, which indicates selective inactivation of Kiss-R1 expressing cells via ligand-mediated uptake of Kiss1-SAP.
Fig. 5.
Binding selectivity of Kiss1-SAP to Kiss-R types by luciferase reporter assay. Graphs showing induction firefly luciferase activity divided by Renilla luciferase activity in the HEK 293T cells expressing either Kiss-R1 or Kiss-R2 by Blank-SAP, Kiss1-SAP, and zebrafish kisspeptin1-15 (10−5 M). Kiss1-SAP and kisspeptin1-15 induced similar luciferase activities in both Kiss-R1- and Kiss-R2-expressing cells. Luciferase activity induced by Kiss1-SAP and kisspeptin1-15 was significantly higher in Kiss-R1-expressing cells compared with Kiss-R2-expressing cells. Data are presented as mean ± SEM. ANOVA followed by Fisher’s protected least significant difference test among Blank-SAP, Kiss1-SAP, and kisspeptin1-15 (P < 0.05, significant differences between groups are indicated by capitalized letters), and independent t-test comparisons for Kiss1-SAP and kisspeptin1-15 between Kiss-R1 and Kiss-R2 (*P < 0.05).
Effect of Kiss1-SAP Injections on AS-Evoked Fear Response.
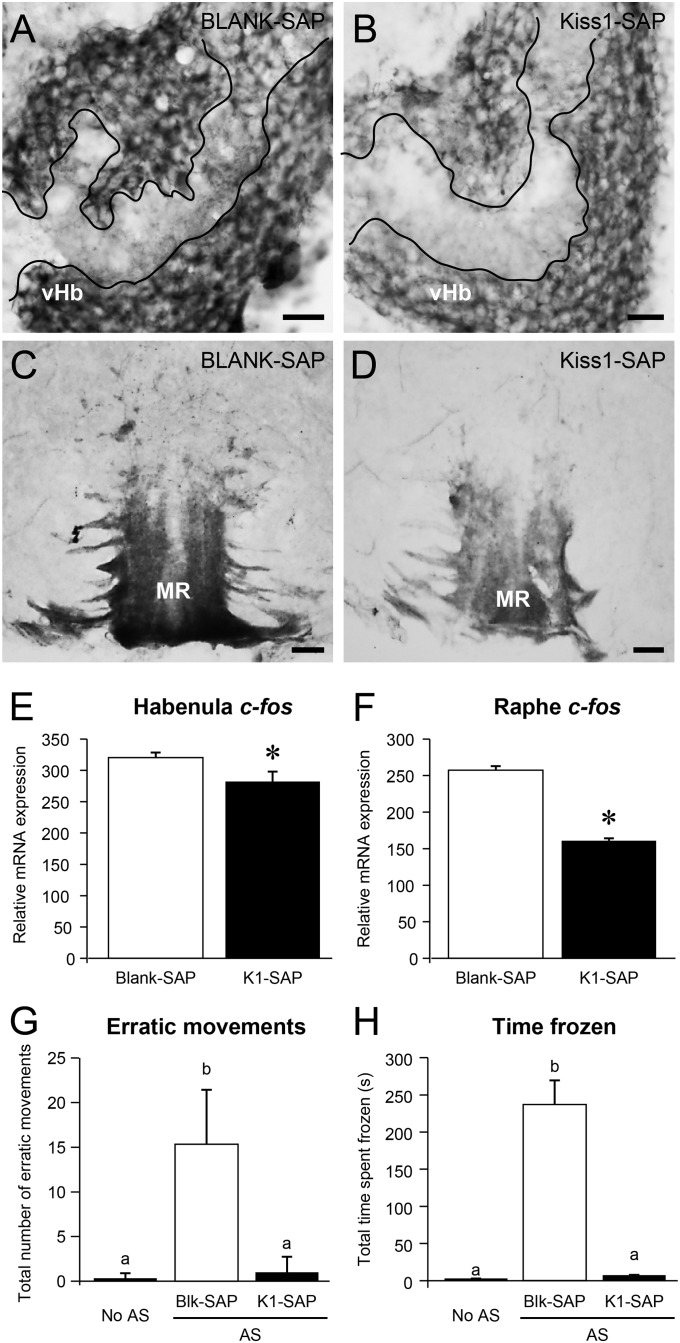
Twelve days after Kiss1-SAP injection, Kiss1 immunorectivity was reduced in the neuropil of the vHb (Fig. 6 A and B), and Kiss1 immunoreactive axons were reduced in the MR (Fig. 6 C and D) compared with fish injected with Blank-SAP. Three days after Kiss1-SAP injection, there was no difference in Kiss1 immunoreactivities compared with the Blank-SAP group (Fig. S3), whereas c-fos mRNA levels were significantly (P < 0.05) decreased in the habenula and raphe regions (Fig. 6 E and F) compared with in fish injected with Blank-SAP. Three days of Kiss1-SAP treatment significantly reduced the AS-evoked fear response (the number of erratic movements and total freezing time compared with fish injected with Blank-SAP), but there was no difference in fear response compared with non-AS-treated (treated with distilled water) fish (Fig. 6 G and H).
Fig. 6.
Effect of Kiss1-SAP on Kiss1 immunoreactivity, on c-fos mRNA expression levels in the habenula and the median raphe, and on AS-evoked fear response. (A–D) Transverse sections of Kiss1 immunoreactivity in the ventral habenula (vHb; A and B) and the median raphe (MR; C and D) in the brain of zebrafish injected with Blank-SAP (A and C) or Kiss1-SAP (B and D) at 12 d after injection (10−3 mol per fish; intracranial administration, n = 3 per group). Kiss1 immunoreactive fibers were relatively reduced in the area of neuropil of the vHb (encircled by white lines) in Kiss1-SAP-treated fish compared with the Blank-SAP group (A and B). Kiss1 immunoreactive axons entering the MR were relatively weak, and fewer fibers were seen in the superior raphe regions in Kiss1-SAP-treated fish compared with in the Blank-SAP group (C and D). (Scale bars, 20 µm.) (E and F) Graphs showing the effect of Kiss-SAP on mRNA levels of c-fos in the habenula (E) and raphe (F) regions at 3 d after injection (n = 10 per group). (G and H) Graphs of behavioral parameters of fear response: the number of erratic movements (G) and the freezing time (H) in the zebrafish injected with Blank-SAP (open column) or Kiss1-SAP (closed column) (10−5 mol per fish; intracranial administration, n =10 per group) at 3 d after injection. Behavior data of control (no AS, fish treated with distilled water) are provided for comparison. Data are presented as mean ± SEM. Significant differences (P < 0.05) between groups are indicated by different letter codes; one-way ANOVA followed by post hoc Tukey’s test.
Expression of kiss1 and 5-HT-Related Genes.
AS exposure had no effect on kiss1 and 5-HT-related genes [pet1, slc6a4a, and tryptophan hydroxylase 2 (tph2)] mRNA levels at 1 d treatment (Fig. S4); however, 7 d of repeated AS treatment significantly reduced kiss1 (P < 0.01), pet1, and slc6a4a mRNA levels (P < 0.001) compared with in the controls (Fig. 3 E–G). There was no effect of AS exposure on tph2 mRNA levels (Fig. 3H). Kiss1 administration significantly (P < 0.001) increased pet1 and slc6a4a mRNAs levels in AS-treated and control fish at doses of 10−15 to 10−9 mol per fish (Fig. 4 E and F). There was a dose-dependent effect of Kiss1 on serotonin-related genes (ANOVA, followed by Tukey’s test). There was no effect of Kiss2 (10–11 mol per fish) on mRNA levels of 5-HT-related genes (Fig. S2 E–G).
Discussion
Kiss1 administration specifically induced c-fos expression in the vHb, a symmetric aggregation of cells in the habenula identified by cytoarchitectural observations (17), as well as expression of marker genes such as dao and pcdh10a (9), but not in the dHb, which suggests successful delivery of Kiss1 to its target site.
Fish with anxiety spent more time at the bottom of the tank (bottom dwelling) when exposed to a novel environment (18). In the novel tank diving test, we found that neither Kiss1 nor Kiss2 administration had any effect on behavioral parameters for anxiety during the first 5-min and the following 8-min observations. However, Kiss1 increased the number of transitions from top to bottom, indicating that administration of Kiss1 stimulated exploratory behavior (19). In the AS exposure experiment, zebrafish showed erratic movements followed by freezing, a typical feature of fear response in the zebrafish (15). Interestingly, Kiss1 administration significantly reduced AS-evoked freezing and erratic behaviors, which indicates the role of habenular Kiss1 in fear response. Dose-dependent effects of Kiss1 on AS-evoked fear response and 5-HT-related gene expression suggests its neuromodulatory effect.
Although the administration of exogenous Kiss1 inhibited AS-evoked fear response, the role of endogenous habenular Kiss1 remains undetermined. Therefore, to address this, we used Kiss1-SAP to selectively inactivate Kiss-R1-expressing neurons and examined AS-evoked fear response. Luciferase assays confirmed that Kiss1-SAP retains higher affinity for Kiss-R1 compared with Kiss-R2. Three days after Kiss1-SAP administration, c-fos mRNA levels significantly decreased in the vHb and the MR, whereas Kiss1 immunoreactivity decreased only after 12 d. This indicates successful suppression of Kiss1, and in addition, it shows that the efficacy of Kiss1-SAP is different at the level of neural activity and death of Kiss1 neurons. That the incomplete ablation of Kiss1 immunoreactivity is a time course effect is also seen in hypocretin 2-SAP-treated rats (20). In our real-time PCR assay, we noted a high basal level of c-fos mRNA in the Blank-SAP and Kiss1-SAP injected fish, which could be partly a result of the inclusion of the dHb, as the whole habenula was used for mRNA quantification. In our behavioral assay, Blank-SAP treated fish (control) showed fear response to AS exposure, whereas Kiss1-SAP-treated fish showed no response to AS, as did non-AS (water)-exposed fish. This suggests that inactivation of the habenular Kiss-R1 neurons by Kiss1-SAP prohibits AS triggered signals from being transmitted downstream to the raphe nuclei. In addition, the Kiss1-SAP treatment significantly suppressed AS-evoked erratic movements and freezing, but there was no anxiolytic effect (increase in top-to-bottom transitions) compared with non-AS-treated fish. Taken together, the inactivation of Kiss-R1 neurons by the Kiss1-SAP supports our hypothesis that the Kiss1-Kiss-R1 system is crucial in the signaling pathway of AS-evoked fear response.
On the basis of these results, we speculate on the potential role of Kiss1 in the modulation of fear response in the zebrafish. Fear is considered the most primitive of emotions and is expressed from early stages of life, throughout the phyla within the vertebrates, as a normal reaction to threatening situations (21). Therefore, the neural mechanisms underlying fear are highly conserved across species. A recent biochemical study identified the glycosaminoglycan chondroitins as the major components of AS in the zebrafish, which activate the olfactory bulbular region (22). Therefore, the habenular Kiss1 neurons could receive AS signals from the olfactory neurons. However, these olfactory bulb neurons innervate the medial subnucleus of the dHb (23), but not the vHb, where Kiss1-Kiss-R1 are expressed in the zebrafish. In addition, a very recent study in the zebrafish has shown the failure of neural activation of the dHb neurons after exposure to the AS and chondroitin sulfate (24). Therefore, the effect of AS on Kiss1 neurons could be modulated via signaling inside the habenula subnuclei (from dHb to vHb), similar to the rat habenula (25), or by other afferent pathways in the forebrain.
In the zebrafish, the lateral dHb (dHbL) innervates the dorsal interpeduncular nucleus, and the nucleus further innervates the griseum centrale (26), a homolog of the mammalian dorsal periaqueductal gray, a core structure of the brain aversion system (27). The dHbL-silenced zebrafish show increased freezing in the course of a fear-conditioned assay (26, 28), suggesting that this subnucleus is crucial for the modifications of behavior responses in an experience-dependent manner. A very recent study demonstrated that the dHb-interpeduncular nucleus silenced zebrafish exhibits intense innate fear expression, indicating a state of elevated baseline anxiety (29). We also found that Kiss1 administration increased mRNA levels of 5-HT-related genes (pet1 and slc6a4a) in AS-exposed fish, suggesting the potential role of Kiss1 in serotonin modulation, as we reported previously (7). 5-HT has been widely implicated in the development and expression of fear and depression-like behavior (30, 31). The mammalian lHb plays a pivotal role in escape behavior by influencing the activity of 5-HT neurons (32). These results suggest the potential role of Kiss1 in the modulation of 5-HT-dependent behaviors. Our morphological analysis showed no innervation of Kiss1 immunoreactive axons in the serotonergic superior raphe, but they terminated at the median raphe. This supports our hypothesis that serotonergic neurons are indirectly modulated by the habenular Kiss1 neurons via nonserotonergic interneurons in the MR (7). In the novel tank diving test, we also found some anxiolytic effects of Kiss1 (increase in top-to-bottom transitions) and an increase in 5-HT-related genes by Kiss1 administration. This is in good agreement with the antidepressant-like effects and stimulation of locomotor activities by kisspeptin-13 reported in rodents (33, 34). In addition, cross-referencing between treatments (Figs. 3 and 4) also reveals that expression of 5-HT-related genes elevated by Kiss1 injection was much above those levels suppressed by AS treatments. This indicates that Kiss1 does not return various parameters to controls but far exceeds them. This raises the possibility that the kisspeptin “reversal” of AS effects, as well as anxiolytic effect, could be partly occurring through an interaction of other neurotransmitters, such as adrenergic and cholinergic neurons (33, 35).
In mammals, the MR projects to the hippocampus and the amygdala and regulates contextual fear conditioning (36, 37), which could be similar in the zebrafish because fish also possess the ability to contextualize fear (38). However, the mammalian hippocampus and the amygdala-homologous brain regions have not been defined in fish. Therefore, in the zebrafish, the dHbL and vHb pathways may play different roles in processing fear responses, which could be similar to the two independent amygdale circuits for predator odor-induced unconditioned and conditioned fear in rodents (39). The existence of Kiss-R in the habenula of fish and mammals (2, 3) suggests that the role of kisspeptin signaling in odor-dependent fear response could be evolutionarily conserved in vertebrates. In rats, the medial nucleus of the amygdala processes chemosensory stimuli and projects to the ventromedial nucleus of the hypothalamus and dorsal periaqueductal gray, which modulates predator odor-induced unconditioned fear (39), possibly through the habenula (40). In animals, fear is a signal of an external danger that can be triggered without any learning (41), and it is biologically inherent in humans to learn to fear objects and situations that threaten the survival of the species (42). As the mammalian brain functions advanced, along with the development of additional brain structures such as the cortex and the hippocampus, during evolution, the classical fear pathways have also evolved to prepare and respond to aversive stimuli that are potentially life threatening. Interestingly, a very recent report demonstrated the effects of kisspeptin-13 on passive avoidance learning in mice (35). Because the hypothalamus and the amygdala express kisspeptin and Kiss-R (2, 4, 43), it is possible that kisspeptin-Kiss-R signaling could be involved in the contextualization of fear in mammals (44).
To date, the neural mechanisms and pathways that subdue or remove fear have not been elucidated to comprehend psychiatric disorders such as phobia, which is characterized by marked and persistent fear. Importantly, the present study shows that kisspeptin in the habenula may facilitate removing fear. As fear is a strong stressor and impairs reproductive performance (45), recovery from threat is important to reboot impaired reproductive functions. Therefore, kisspeptin, an evolutionarily conserved neuropeptide in reproduction, may subserve an additional role for fear modulation to maintain emotional aspects of reproductive capability such as sexual motivation and arousal. The present study in the zebrafish provides a unique role for the habenular kisspeptin system in the brain that extends beyond the control of reproduction.
Materials and Methods
Animals and Housing.
Sexually mature male zebrafish (Danio rerio, ∼35 mm in body length and ∼350 mg in body weight) were maintained in groups of 10 fish per 20 L freshwater aquaria at 28 ± 0.5 °C with a controlled natural photoregimen (14/10 h, light/dark). RIKEN Wako wild-type strain was obtained from the Zebrafish National BioResource Center of Japan (www.shigen.nig.ac.jp/zebra/). The zebrafish were fed with adult zebrafish food (Zeigler). All experiments were carried out only after 2 wk of fish acclimatization. The fish were anesthetized by immersion in water containing benzocaine (0.1g benzocaine/200 mL water; Sigma) before the injections and the dissection of tissues. This research was conducted under the ethical approval of Monash University Animal Ethics Committee (MARP/2011/41 and MARP/2012/093).
Intracranial Administration of Kiss1.
Administration of peptide was carried out using the method described previously (7). Briefly, anesthetized fish (n = 20) were placed on a sponge soaked with water, and the skulls were punctured with a 25 G × 1” needle (Terumo) in the midline at the telencephalon–diencephalon border. The fish were intracranially injected with 1 µL of either distilled water or kisspeptins into the cranial cavity by a heat-pulled glass capillary micropipette attached with a microinjector (IM-9B; Narishige).
Source of Peptides.
Commercially synthesized peptides, zebrafish kisspeptin1-15 (pyroglut-NVAYYNLNSFGLRY-NH2; Open Biosystems), and zebrafish kisspeptin2-10 (FNYNPFGLRF-NH2; BioGenes) were used for injecting in this study. For selective inactivation of the habenular Kiss1 neurons, zebrafish kisspeptin1-15 conjugated with saporin (Kiss1-SAP; Advanced Targeting Systems) was used. Nontargeted SAP control (Blank-SAP; Advanced Targeting Systems), which is an 11-amino acid peptide conjugated to SAP, was used as a control. The amino acid sequence of the peptide in Blank-SAP (scrambled human melanocyte-stimulating hormone) has no significant homology to known G protein-coupled receptor ligands.
DIG-in Situ Hybridization of c-fos.
The digoxigenin (DIG)-in situ hybridization was performed as described previously (7) (see SI Materials and Methods for details).
Novel Tank Diving Test.
To evaluate the effect of Kiss1 on anxiety, a novel tank diving test was performed (n = 20 per group) according to the protocol implemented by Cachat and coworkers (14). In brief, the following behavioral parameters were recorded: total time spent at the bottom-half of the tank, number of top-to-bottom transitions, and total distance traveled in either the top or bottom half of the tank. As a control, the effect of Kiss2 on anxiety behavior was also examined (n = 10–20 per group). One and 4 h after administration of either kisspeptins (Kiss1 or Kiss2) or distilled water (DW), the treated fish was placed individually in a glass tank (361 mm length, 218 mm width, 256 mm height) containing 11 L dechlorinated water at the same temperature as the home tank. The lighting condition used was 802.4 lx. After the fish was relocated to a novel tank, a side view of swimming behavior was recorded by a video camera (Sony Handycam DCR-SX83E, positioned approximately 1 m away from the tank) for a period of 13 min per fish, was conducted between 2.00:00 PM and 4.00:00 PM The data were divided into the first 5 min and the second 8 min because anxiety in the zebrafish is most apparent within the first 5 min of exposure to a novel environment (46). Video data were analyzed using automated tracking software, LoliTrack 2.0 (Loligo Systems). A visible line was drawn on the surface of the glass tank for an imaginary separation of the water level to top and bottom halves. Six hours after administration of kisspeptin, the fish were killed by immersing them in water containing benzocaine, and the raphe region of the brain was dissected and stored at −80 °C until RNA extraction.
AS Exposure.
To examine the effect of Kiss1 on fear response, fish were exposed to AS. In brief, the following behavioral parameters were recorded: number (frequency) of erratic movements and duration of freezing. Erratic behavior was defined as a characteristic fast zig-zagging response associated with rapid direction changes (47). Freezing was defined as the total absence of movement for 1 s or longer (19).
The procedure of AS extraction was carried out according to the protocol implemented by Speedie and Gerlai (15). Male fish (n = 11) were killed by submerging them in cold water. Fifteen shallow cuts were made on the right trunk of the zebrafish with a razor blade, and the cuts were washed with 5 mL cold DW. This was then repeated on the left trunk of the fish to obtain a total of 10 mL AS solution per fish. The AS solution was then aliquoted into a 1.5-mL Eppendorf tube and stored at −20 °C until use.
The influence of fear stimuli on the habenular kiss1 neurons was evaluated by exposure to AS. Fish were individually placed in glass tanks (361 mm length, 218 mm width, 256 mm height) containing 11 L water at the same temperature as the home tank and allowed to acclimatize for 5 min. The 1 mL of DW or AS was then administered through a glass capillary positioned at the corner of the tank about 0.5 cm below the water level. The mean time for introduction through the capillary was 5 s. Swimming behaviors of the fish exposed to AS or DW (n = 10 per group) were recorded for 8 min, as described earlier, and then returned to their original housing tanks. The same protocol was repeated for another 6 d (no behavioral observation). On the first (day 0) and the last day (day 7) of the experiment, the fish were anesthetized and the habenula and raphe region were dissected and placed into a 1.5-mL Eppendorf tube containing TRIzol (Invitrogen) and stored at −80 °C until RNA extraction for gene expression analysis.
To examine the neuromodulatory effect of Kiss1 on AS-evoked fear response, fish were administered with varying concentrations (10−15, 10−13, 10−11, and 10−9 mol per fish) of Kiss1, as described earlier. Six hours after administration of Kiss1, the fish were individually placed in the behavioral tank and exposed to either AS or DW (n = 10 per group) for 8 min of monitoring fear response.
Real-time PCR for kiss1 and Serotonin-Related Genes.
The brain cDNA samples of the fish obtained from the novel tank diving test, alarm substance exposure, and kisspeptin injection studies were subjected to real-time PCR. Gene expression levels of kiss1 and serotonin-related genes (pet1, slc6a4a, and tph2) were examined by real-time PCR with specific primers, as described previously (7) (see SI Materials and Methods for details).
Luciferase Assay for Binding Affinity of Kiss1-SAP.
To validate the binding affinity of Kiss1-SAP (Advanced Targeting Systems) to zebrafish Kiss-R1, luciferase assays were performed (see SI Materials and Methods for details).
Effect of Kiss1-SAP on Fear Response.
The fish were intracranially injected with 1 µL of either Kiss1-SAP or Blank-SAP (10−5 mol per fish; Advanced Targeting Systems). Three days after the injection, the fish were used for behavior tests. To confirm the effect of Kiss1-SAP on the habenula kisspeptin neurons, Kiss1 immunoreactivity was examined in the brain of zebrafish injected with either Kiss1-SAP or Blank-SAP (n = 3 each) 3 d after the injection, using our newly generated specific antibody to prepro-zebrafish Kiss1 (antibody code: PAS 15133/15134; Fig. S5). Furthermore, neural activity of kisspeptin neurons in the Kiss1-SAP-injected fish was evaluated by c-fos mRNA levels, using real-time PCR. Immunohistochemistry and real-time PCR for c-fos mRNA were performed as described previously (7).
Statistical Analysis.
All experimental data were analyzed using the Statistical Package for the Social Sciences 18 (SPSS Inc). All data are expressed as mean ± SEM, and statistical analyses were performed using independent t-test to observe statistical significance between controls and experimental groups for the AS exposure test. To analyze the dose-dependent effect of Kiss1 injection, data were analyzed using one-way ANOVA and post hoc Tukey’s test for statistical significance; P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank T. Soga and Y. M. Khor for advice on fish behavior analysis, S. Moriya and T. Kitahashi for technical support for luciferase assays, A. Thanabalan and K. W. Ng for their technical assistance with gene expression analysis, and Professor D. W. Pfaff for his constructive comments on the manuscript. This work was supported by grants from the Malaysian Ministry of Higher Education (FRGS/2/2010/ST/MUSM/03/2; to S.O. and I.S.P.); the Malaysian Ministry of Science, Technology, and Innovation (02-02-10-SF0044; to I.S.P. and S.O.); and Monash University Malaysia (SO-10-01; to S.O.), IP-09-01 (to I.S.P.), and Neuroscience Research Strength grant (to I.S.P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314184111/-/DCSupplemental.
References
- 1.Messager S, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102(5):1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbison AE, de Tassigny Xd, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(1):312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- 3.Lee DK, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446(1):103–107. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, et al. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020–2030. doi: 10.1210/en.2010-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitahashi T, Ogawa S, Parhar IS. Cloning and expression of kiss2 in the zebrafish and medaka. Endocrinology. 2009;150(2):821–831. doi: 10.1210/en.2008-0940. [DOI] [PubMed] [Google Scholar]
- 6.Gopurappilly R, Ogawa S, Parhar IS. Functional significance of GnRH and kisspeptin, and their cognate receptors in teleost reproduction. Front Endocrinol (Lausanne) 2013;4:24. doi: 10.3389/fendo.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa S, Ng KW, Ramadasan PN, Nathan FM, Parhar IS. Habenular Kiss1 neurons modulate the serotonergic system in the brain of zebrafish. Endocrinology. 2012;153(5):2398–2407. doi: 10.1210/en.2012-1062. [DOI] [PubMed] [Google Scholar]
- 8.Aizawa H, et al. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr Biol. 2005;15(3):238–243. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amo R, et al. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010;30(4):1566–1574. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 11.Pobbe RLH, Zangrossi H., Jr The lateral habenula regulates defensive behaviors through changes in 5-HT-mediated neurotransmission in the dorsal periaqueductal gray matter. Neurosci Lett. 2010;479(2):87–91. doi: 10.1016/j.neulet.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Servili A, et al. Organization of two independent kisspeptin systems derived from evolutionary-ancient kiss genes in the brain of zebrafish. Endocrinology. 2011;152(4):1527–1540. doi: 10.1210/en.2010-0948. [DOI] [PubMed] [Google Scholar]
- 13.Lillesaar C, Tannhäuser B, Stigloher C, Kremmer E, Bally-Cuif L. The serotonergic phenotype is acquired by converging genetic mechanisms within the zebrafish central nervous system. Dev Dyn. 2007;236(4):1072–1084. doi: 10.1002/dvdy.21095. [DOI] [PubMed] [Google Scholar]
- 14.Cachat J, et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc. 2010;5(11):1786–1799. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- 15.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188(1):168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stirpe F, Barbieri L, Battelli MG, Soria M, Lappi DA. Ribosome-inactivating proteins from plants: Present status and future prospects. Biotechnology (N Y) 1992;10(4):405–412. doi: 10.1038/nbt0492-405. [DOI] [PubMed] [Google Scholar]
- 17.Aizawa H. Habenula and the asymmetric development of the vertebrate brain. Anat Sci Int. 2013;88(1):1–9. doi: 10.1007/s12565-012-0158-6. [DOI] [PubMed] [Google Scholar]
- 18.Blaser RE, Chadwick L, McGinnis GC. Behavioral measures of anxiety in zebrafish (Danio rerio) Behav Brain Res. 2010;208(1):56–62. doi: 10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Egan RJ, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205(1):38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerashchenko D, et al. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21(18):7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeDoux JE. Evolution of human emotion: A view through fear. Prog Brain Res. 2012;195:431–442. doi: 10.1016/B978-0-444-53860-4.00021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathuru AS, et al. Chondroitin fragments are odorants that trigger fear behavior in fish. Curr Biol. 2012;22(6):538–544. doi: 10.1016/j.cub.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Miyasaka N, et al. From the olfactory bulb to higher brain centers: Genetic visualization of secondary olfactory pathways in zebrafish. J Neurosci. 2009;29(15):4756–4767. doi: 10.1523/JNEUROSCI.0118-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.deCarvalho TN, Akitake CM, Thisse C, Thisse B, Halpern ME. Aversive cues fail to activate fos expression in the asymmetric olfactory-habenula pathway of zebrafish. Front Neural Circuits. 2013;7:98. doi: 10.3389/fncir.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim U, Chang SY. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J Comp Neurol. 2005;483(2):236–250. doi: 10.1002/cne.20410. [DOI] [PubMed] [Google Scholar]
- 26.Agetsuma M, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010;13(11):1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 27.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 28.Lee A, et al. The habenula prevents helpless behavior in larval zebrafish. Curr Biol. 2010;20(24):2211–2216. doi: 10.1016/j.cub.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Mathuru AS, Jesuthasan S. The medial habenula as a regulator of anxiety in adult zebrafish. Front Neural Circuits. 2013;7:99. doi: 10.3389/fncir.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDevitt RA, et al. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol Psychiatry. 2011;69(8):780–787. doi: 10.1016/j.biopsych.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Amat J, et al. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917(1):118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka M, Csabafi K, Telegdy G. Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul Pept. 2013;180:1–4. doi: 10.1016/j.regpep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Csabafi K, Jászberényi M, Bagosi Z, Lipták N, Telegdy G. Effects of kisspeptin-13 on the hypothalamic-pituitary-adrenal axis, thermoregulation, anxiety and locomotor activity in rats. Behav Brain Res. 2013;241:56–61. doi: 10.1016/j.bbr.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Telegdy G, Adamik Á. The action of kisspeptin-13 on passive avoidance learning in mice. Involvement of transmitters. Behav Brain Res. 2013;243:300–305. doi: 10.1016/j.bbr.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Silva RC, Gárgaro AC, Brandão ML. Differential regulation of the expression of contextual freezing and fear-potentiated startle by 5-HT mechanisms of the median raphe nucleus. Behav Brain Res. 2004;151(1-2):93–101. doi: 10.1016/j.bbr.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Avanzi V, Castilho VM, de Andrade TG, Brandão ML. Regulation of contextual conditioning by the median raphe nucleus. Brain Res. 1998;790(1-2):178–184. doi: 10.1016/s0006-8993(97)01538-2. [DOI] [PubMed] [Google Scholar]
- 38.Eisenberg M, Dudai Y. Reconsolidation of fresh, remote, and extinguished fear memory in Medaka: Old fears don’t die. Eur J Neurosci. 2004;20(12):3397–3403. doi: 10.1111/j.1460-9568.2004.03818.x. [DOI] [PubMed] [Google Scholar]
- 39.Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118(2):324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- 40.Felton TM, Linton L, Rosenblatt JS, Morell JI. First and second order maternal behavior related afferents of the lateral habenula. Neuroreport. 1999;10(4):883–887. doi: 10.1097/00001756-199903170-00039. [DOI] [PubMed] [Google Scholar]
- 41.Cyrulnik B. Ethology of anxiety in phylogeny and ontogeny. Acta Psychiatr Scand. 1998;98(s393):44–49. doi: 10.1111/j.1600-0447.1998.tb05966.x. [DOI] [PubMed] [Google Scholar]
- 42.McNally RJ. Preparedness and phobias: A review. Psychol Bull. 1987;101(2):283–303. [PubMed] [Google Scholar]
- 43.Arai AC. The role of kisspeptin and GPR54 in the hippocampus. Peptides. 2009;30(1):16–25. doi: 10.1016/j.peptides.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 44.LeDoux J. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry. 1998;44(12):1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 45.Kongsted AG. Relation between reproduction performance and indicators of feed intake, fear and social stress in commercial herds with group-housed non-lactating sows. Livest Sci. 2006;101(1-3):46–56. [Google Scholar]
- 46.Wong K, et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio) Behav Brain Res. 2010;208(2):450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: Comparison of three behavioral quantification methods. Behav Res Methods. 2006;38(3):456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.