Significance
Thousands of people each day receive general anesthesia. Recovery from the anesthetic state is a passive process that can be variable and unpredictable. Identifying neural systems involved in recovery of consciousness after general anesthesia is fundamental to optimizing anesthetic safety and our knowledge of anesthetic action. We examined how norepinephrine from the nucleus locus coeruleus (LC) affects general anesthesia, and found that blocking the action of norepinephrine, particularly in the CNS, delayed recovery from general anesthesia. Using genetic tools to selectively activate only LC neurons, we found that LC activation was sufficient to alter EEG measurements of anesthetic depth and accelerate recovery of consciousness. Our data show that LC activity can alter the anesthetic state, and that noradrenergic medications may affect clinical responses to anesthetic agents.
Keywords: DREADD, RASSL
Abstract
Mechanisms driving emergence from general anesthesia are not well understood. The noradrenergic brain nucleus locus coeruleus (LC) modulates arousal and may have effects on general anesthetic state. Using virally delivered designer receptors to specifically control LC norepinephrine (NE) neurons, we investigated the causal relationship between LC-NE activity and general anesthetic state under isoflurane. Selective activation of LC-NE neurons produced cortical electroencephalography (EEG) activation under continuous deep isoflurane anesthesia. Specifically, LC-NE activation reduced burst suppression in EEG and drove a rightward shift in peak EEG frequency with reduced δ EEG power and increased θ EEG power, measures of cortical arousal. LC-NE activation also accelerated behavioral emergence from deep isoflurane anesthesia; this was prevented with β or α1 noradrenergic antagonists. Moreover, these adrenoreceptor antagonists alone were sufficient to markedly potentiate anesthetic duration when delivered centrally or peripherally. Induction of anesthesia also was retarded by LC-NE activation. Our results demonstrate that the LC-NE system strongly modulates the anesthetic state, and that changes in LC-NE neurotransmission alone can affect the emergence from isoflurane general anesthesia. Taken together, these findings extend our understanding of mechanisms underlying general anesthesia and cortical arousal, and have significant implications for optimizing the clinical safety and management of general anesthesia.
Anesthetic agents are critical tools in modern medicine. General anesthesia is akin to a drug-induced transient coma-like state (1). Emergence from general anesthesia is currently a passive process in which the anesthetic agent is discontinued and the patient monitored for spontaneous recovery of physiological and behavioral signs of consciousness, which is a variable and unpredictable process in patient populations (1). There are no drugs available for actively reversing general anesthesia should procedural or safety considerations necessitate rapid emergence. Understanding the neural substrates involved in emergence can identify targets for reversing general anesthesia and reveal mechanisms involved in cortical arousal.
Histaminergic, cholinergic, dopaminergic, and orexinergic/hypocertinergic pathways have been implicated in the emergence from general anesthesia (2–4). These pathways are important in arousal networks that regulate sleep and waking, among other actions. The locus coeruleus (LC)-norepinephrine (NE) brain system is a key arousal node (5); however, the function of LC-NE neurons in anesthetic emergence has been relatively understudied (6–8).
The pontine nucleus LC projects NE fibers throughout the CNS and is the primary source of NE to the cerebral cortex, among other areas (9). The LC receives arousal influences via inputs from orexinergic and histaminergic neurons, as well as an indirect circadian input from suprachiasmatic nucleus via dorsomedial hypothalamus (5), and in turn regulates arousal via connections to multiple forebrain areas (10–12). LC-NE activity can drive waking and is a critical downstream effector site for orexin-mediated sleep–wake transitions (13, 14). Although inhibition of LC-NE neurons is not required for hypnosis with general anesthetic agents (15), dopamine-β-hydroxylase (DBH) knockout (KO) mice (lacking NE) show hypersensitivity to several forms of general anesthesia (6). Considered together, these results indicate a role for NE systems in anesthesia mechanisms that may be critically regulated by the LC.
Here we directly tested the role of LC-NE neurons in the emergence from isoflurane general anesthesia using specific activation of the LC via genetically encoded designer receptors. We generated a vector that uses the synthetic dopamine β hydroxylase (DBH) promoter PRSx8 (16) to selectively express the hM3D Gq-coupled Designer Receptor Exclusively Activated by Designer Drug (DREADD) (17) in LC-NE neurons. We used the selective DREADD ligand clozapine-N-oxide (CNO) to activate hM3Dq-expressing LC-NE neurons in vivo under continuous isoflurane exposure and during isoflurane emergence.
Results
Transgene Expression Is Highly Restricted to LC-NE Neurons Using PRSx8 Promoter.
We targeted expression of the Gq-coupled hM3Dq DREADD (17, 18) to LC-NE neurons using the synthetic promoter PRSx8. PRSx8 is based on an upstream regulatory site in the human DBH promoter and drives high levels of expression in adrenergic neurons (16, 19). Delivery of an adeno-associated virus (AAV) vector containing this construct to the LC region led to expression of the hM3Dq DREADD in NE neurons of the LC and subcoerulear region for at least 3 mo. hM3Dq expression in these neurons was restricted to the plasma membrane of NE cell bodies and their axons in all animals (Fig. 1). In a subset of behavioral subjects (n = 6), we quantified hM3Dq expression in a series of coronal sections (160 µm apart) throughout the LC region. Transgenes expressed well in LC, with expression highly colocalized to tyrosine hydroxylase (a marker of NE cells in this region)-expressing neurons in animals that received HA-hM3Dq vectors (97 ± 1.0% colocalized cells) or mCherry control vectors (97 ± 0.6% colocalized cells). This finding confirmed our ability to selectively target the hM3Dq construct to LC-NE neurons.
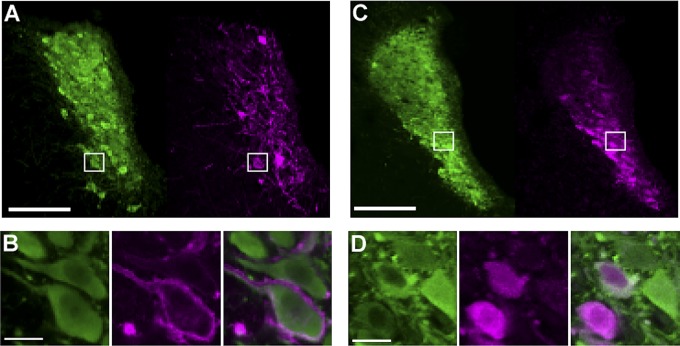
Fig. 1.
PRSx8-driven viral vectors delivered to the LC express transgenes selectively in NE neurons in vivo. (A) TH (green) expression denotes NE neurons and HA tag (magenta) identifies hM3Dq expression in the LC. (B) Boxed region in A showing colocalization of HA-hM3Dq restricted to the plasma membrane of TH-positive neurons. (Left) TH. (Center) hM3Dq (HA tag). (Right) Merge. (C) TH (green) and mCherry (magenta) reporter gene expression in LC of control-virus subject. (D) Boxed region in C confirming colocalization and cytoplasmic deposition of mCherry protein in TH-positive neurons. (Left) TH. (Center) mCherrry. (Right) Merge. (Scale bars: 200 µm in A and C; 20 µm in B and D.)
CNO Stimulation of hM3Dq Receptors Activates LC-NE Neurons.
We used double-barreled recording-microinjection micropipettes in isoflurane-anesthetized rats to validate the functionality of hM3Dq receptors expressed in LC-NE neurons. We identified LC-NE neurons based on standard criteria (SI Materials and Methods) and, after recording >3 min of stable baseline activity, microinjected 5 µM CNO (30–60 nL) from the injection pipette proximal to the recorded neuron. There was no significant difference in pre-CNO mean basal LC firing rates between rats transduced with the PRSx8-hM3Dq vector (n = 6 rats, 16 cells; 2.01 ± 0.54 spikes/s) and those with the control PRSx8-mCherry vector (n = 2 rats, 5 cells; 1.52 ± 0.46 spikes/s; P = 0.67, unpaired t test).
Most LC-NE neurons were activated by local CNO in LC-hM3Dq animals. Overall, the LC-hM3Dq animals demonstrated a significant increase in LC discharge that was not seen in the LC-mCherry animals (n = 21 cells; P = 0.022, unpaired Welch’s t test). In LC-hM3Dq animals, 63% of recorded units were activated by CNO (>10% increase in firing; Fig. 2). Activated units showed an average 152± 50% increase in firing rate above baseline activity (2.01–3.36 Hz; n = 10 cells; P < 0.001, paired t test). In three LC-hM3Dq subjects, some neurons showed a small decrease in discharge rates (−17 ± 9%; n = 6 cells; P = 0.33, paired t test). We hypothesize that these cells might not have expressed hM3Dq sufficiently strongly and were inhibited by NE from neighboring hM3Dq+ LC neurons that were stimulated; additional studies are needed to test this idea. Microinjection of CNO onto LC-NE neurons in LC-mCherry animals did not alter discharge rates (n = 5 cells; P = 0.66, paired t test).
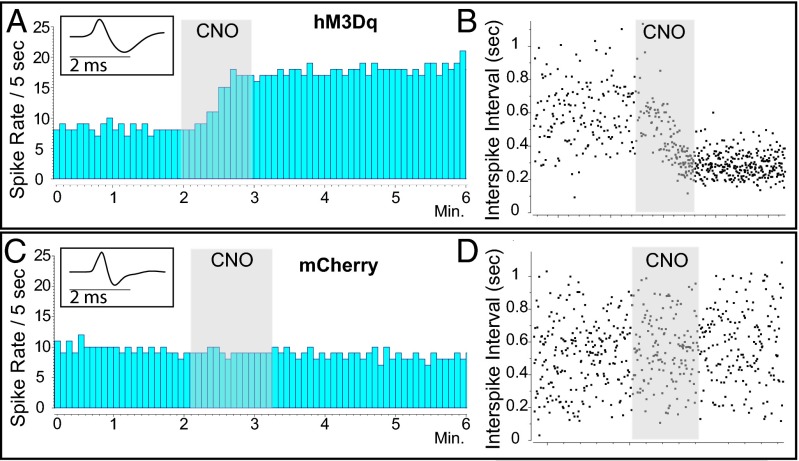
Fig. 2.
CNO delivery activates LC-NE neurons expressing hM3Dq designer receptors. (A) Rate histogram of a representative LC-NE unit (Inset) in an LC-hM3Dq rat excited by a 60-nL CNO microinjection from a double-barrel pipette. Gray shading indicates CNO microinjection. (B) Interspike intervals surrounding CNO microinjection in an LC-NE unit. (C and D) Rate histogram (C) and interspike intervals (D) for a representative LC-NE unit (Inset) in an mCherry rat before, during, and after CNO microinjection in the LC.
We also tested LC-NE responses to systemic CNO in LC-hM3Dq rats (n = 6) by recording multiple units before and after CNO injection (0.1 or 10 mg/kg; n = 3 rats per dose, total 48 neurons). CNO administration significantly increased LC firing rates (F1,44 = 27.88, P < 0.0001; two-way ANOVA); however, there was no main effect of CNO dose, or any interaction (F = 3.3 and 2.7, respectively). Bonferroni posttests confirmed that both 0.1 mg/kg and 10 mg/kg CNO doses significantly increased LC-NE discharge (P < 0.05). Twenty-three of 25 LC-NE neurons (92%) recorded after systemic CNO administration were activated. In those 23 neurons, firing rates were increased an average of 225 ± 29% above baseline. These results confirm that the stimulation of LC-hM3Dq by local or ip CNO activates LC-NE neurons in vivo under isoflurane anesthesia.
hM3Dq-Mediated Activation of LC-NE Neurons Drives Cortical Arousal Under Continuous Isoflurane.
In each subject, we recorded cortical EEG from a bipolar electrode over the frontal lobe ipsilateral to the LC recording site during local microinjection of CNO. We examined EEG activity in premicroinjection and postmicroinjection epochs as with the single unit recordings described above. We found that cortical EEG in rats deeply anesthetized under 2% isoflurane was acutely activated after local unilateral LC-hM3Dq stimulation by microinjection of 5 µM CNO into the LC (Fig. 3). Changes in cortical EEG occurred with local unilateral LC CNO delivery in all LC-hM3Dq subjects (n = 6). On local delivery of 5 µM CNO to the LC, we saw a rightward shift in peak EEG frequency in LC-hM3Dq rats, but not in LC-mCherry rats (n = 2, n = 20 microinjections; P = 0.028, unpaired Welch’s t test).
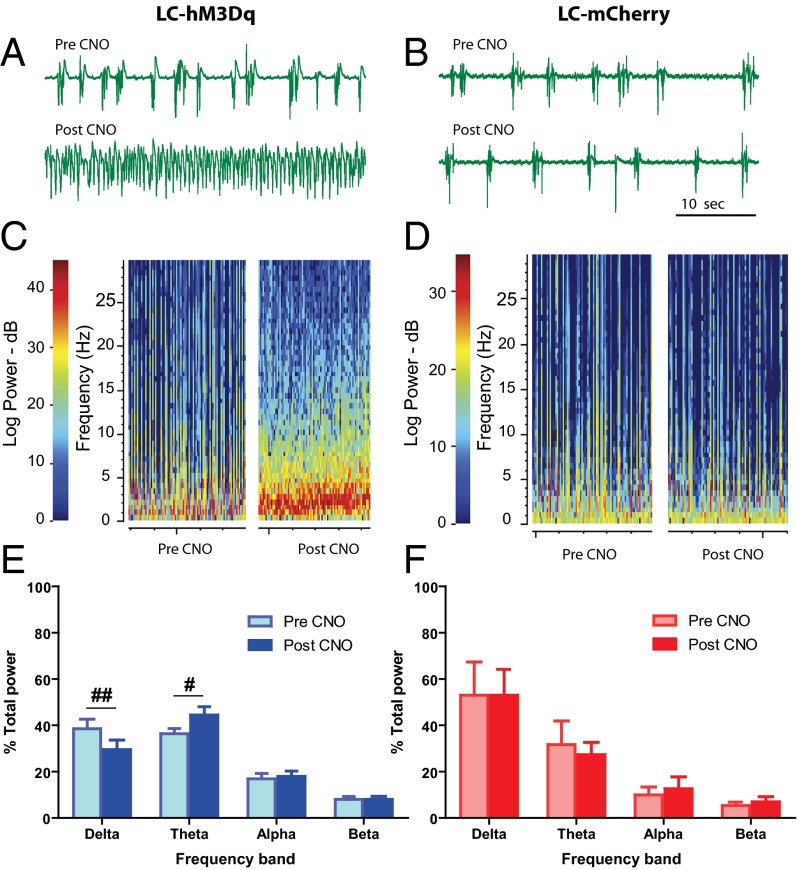
Fig. 3.
(A–D) CNO microinjection into LC-hM3Dq drives cortical EEG changes under deep anesthesia. Examples of raw EEG traces (50 s duration; A and B) from spectrograms (100 s duration each; C and D) of cortical EEG immediately before and after CNO microinjection. LC-hM3Dq stimulation yields reduced burst suppression (A) and increased total EEG power, as well as a rightward shift in peak EEG frequency (red band in C). No such changes occurred in LC-mCherry rats (B and D). (E and F) CNO microinjection decreases the relative EEG δ power and increases θ power (#P < 0.05, ##P < 0.01, Bonferroni posttest) in LC-hM3Dq rats (blue; E), but not in LC-mCherry rats (red; F) (n = 20).
We saw increases in total EEG power of 141 ± 12% in LC-hM3Dq subjects after CNO microinjection (n = 16; P = 0.004, one sample t test), with no overall change in total EEG power from LC-mCherry subjects after CNO microinjection (100 ± 9%; n = 4; P = 0.96). The increase in total power was consistent with less burst suppression in cortical EEG (a measure of anesthetic depth), indicating a transition away from deep anesthesia (20). In LC-hM3Dq rats, CNO produced a significant decrease in the absolute burst suppression ratio from 69% to 54% burst suppression (−15 ± 5%; P = 0.006, Wilcoxon signed-rank test). This effect was not seen in LC-mCherry animals (−4 ± 3%; P = 0.25, paired t test).
In LC-hM3Dq animals, we found a significant interaction between CNO and EEG spectral frequency (F3,60 = 6.26; P = 0.0009, two-way repeated-measures ANOVA). Specifically, CNO activation of LC led to a significant decrease in δ band power and a significant increase in θ band power, with no changes in α or low-β frequencies (Bonferroni posttests; Fig. 3 E and F). No such interaction was seen in LC-mCherry animals (F3,12 = 0.55; P = 0.66, two-way repeated-measures ANOVA). When directly comparing relative power in the δ and θ bands between LC-hM3Dq and LC-mCherry animals, no significant differences in either frequency band were seen before CNO exposure (n = 20 microinjections; P = 0.19 and P = 0.47, respectively, unpaired t test). After CNO application, hM3Dq animals showed decreased δ power (P = 0.029, unpaired t test;) and increased θ power (P = 0.040, unpaired t test) compared with LC-mCherry subjects. These findings demonstrate that selective activation of LC-NE neurons is sufficient to elicit cortical arousal, as defined by a reduction in burst suppression, a rightward shift in peak EEG frequencies, decreased δ power, and increased θ power during continuous deep isoflurane anesthesia. Thus, we next tested whether LC-NE activation could alter the anesthetic state, measured as time to emergence from isoflurane anesthesia.
Systemic CNO Activation of LC-NE Accelerates Emergence from Isoflurane General Anesthesia.
To investigate behavioral consequences of LC-hM3Dq activation, we examined anesthetic emergence by measuring the return of righting reflex (RORR) after discontinuation of 2% isoflurane. Animals were allowed to stabilize under isoflurane anesthesia, and were then tested with systemic delivery of vehicle or CNO (0.1, 1.0, and 10 mg/kg i.p.) for 20 min before discontinuation of isoflurane in a within-subjects manner. We found no difference in baseline (i.e., vehicle treatment) emergence time between LC-hM3Dq and LC-mCherry rats (n = 16; P = 0.43, unpaired t test). However, as shown in Fig. 4, LC-hM3Dq rats (n = 11), but not LC-mCherry rats (n = 5), exhibited a significant reduction in time to emergence after CNO treatment (F1,53 = 31.23; P < 0.0001, two-way ANOVA) (Movie S1). An interaction between hM3Dq expression and CNO dose (F3,53 = 3.74; P = 0.0162, two-way ANOVA) confirmed that effects of CNO were restricted to LC-hM3Dq rats.
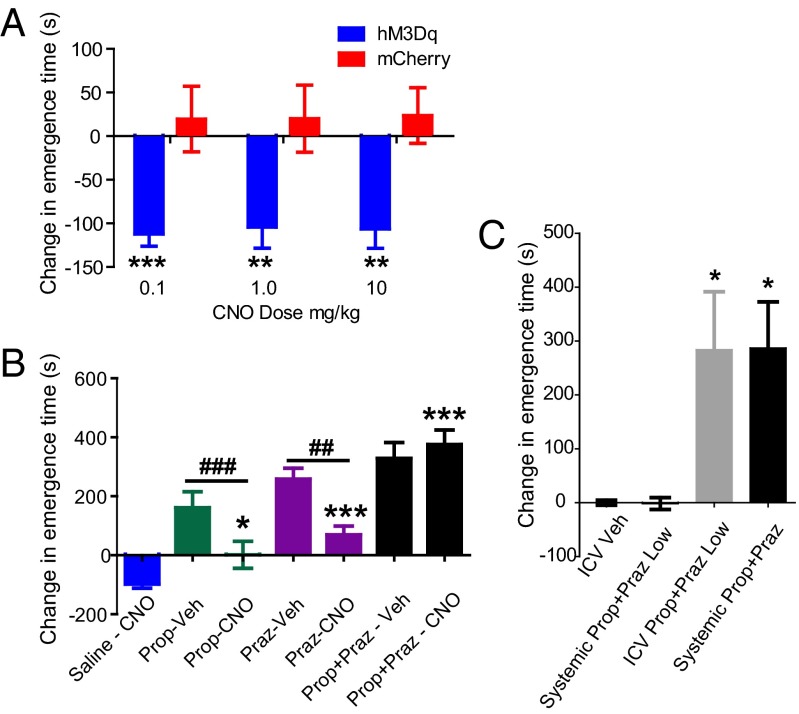
Fig. 4.
CNO facilitates emergence from general anesthesia in LC-hM3Dq rats. (A) CNO speeds emergence in LC-hM3Dq rats (blue), but not LC-mCherry rats (red), across all doses tested (**P < 0.01, ***P < 0.001, Bonferroni posttest; n = 16) (Movie S1). (B) Blocking adrenergic signaling delays emergence time and prevents CNO-mediated acceleration of emergence. Propranolol (β antagonist, 10 mg/kg; green bars) or prazosin (α1 antagonist, 0.42 mg/kg; purple) alone or in combination (propranolol-prazosin, 10/0.42 mg/kg; black) delayed emergence from isoflurane anesthesia. Antagonists also attenuated the effect of CNO (1 mg/kg) on emergence in LC-hM3Dq rats, and α plus β antagonism completely abolished the effect of CNO (*P < 0.05, ***P < 0.001, Bonferroni posttest vs. saline-CNO; n = 20). Note the change in graph scale from A. CNO decreased the emergence time relative to single antagonist treatment alone (##P < 0.01, ###P < 0.001, paired t test). (C) A low-dose adrenoreceptor antagonist mixture (propranolol 0.20 mg/kg + prazosin 0.0084 mg/kg) had no systemic effect, but significantly delayed emergence when given centrally (P = 0.0026, one-way repeated-measures ANOVA; n = 5). Bonferroni multiple comparison posttests confirmed the emergence times for the i.c.v. low-dose propranolol-prazosin combination (gray bar) and the 50-fold–higher systemic propranolol propranolol-prazosin mixture (10 mg/kg + 0.42 mg/kg; black) were not significantly different (*P < 0.05. Bonferroni posttest vs. i.c.v. vehicle and systemic low-dose mixture).
This finding demonstrates that specifically enhancing LC-NE transmission accelerates the emergence from isoflurane general anesthesia. Emergence latency was highly sensitive to LC-NE activation; Bonferroni post hoc tests confirmed differences in emergence latency between LC-hM3Dq and LC-mCherry rats at 0.1, 1.0, and 10 mg/kg doses of CNO (t = 3.28–4.26; P < 0.01). No overall effect of dose was seen across the range tested (F3,53 = 0.98; P = 0.41, two-way ANOVA). Collapsed across all doses, LC-hM3Dq activation decreased the emergence time from 387 ± 25 s to 284 ± 10 s (−103s) (Fig. 4).
Activation of LC-NE Neurons Drives Cortical EEG Changes During Isoflurane General Anesthesia and Emergence.
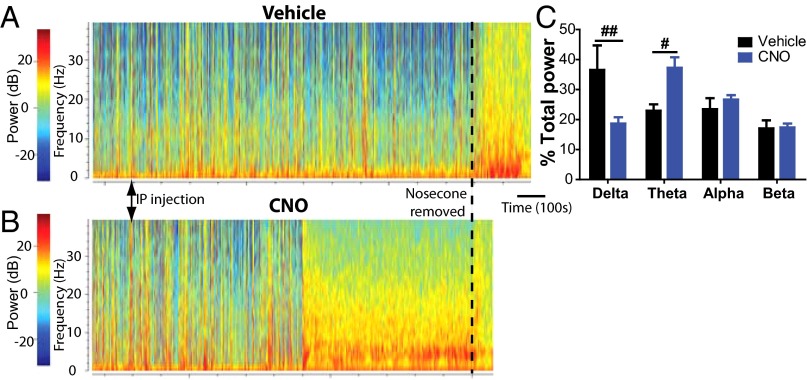
During continuous 2% isoflurane exposure, we compared cortical EEG activity at 15 min after vehicle or CNO injection (1.0 mg/kg i.p., 5-min epoch). We saw clear cortical EEG changes after CNO injection despite a lack of purposeful movements from the subjects (Fig. 5 and Fig. S1). As with local microinjections to the LC, systemic CNO administration caused a significant reduction in burst suppression, from 74.4% to 44.7% (−30 ± 8%; P = 0.0036, paired t test). There was also a significant rightward shift in peak frequency compared with vehicle injections (P = 0.046, paired t test). We found a strong interaction between CNO and EEG frequency band (F3,36 = 7.29; P = 0.0006, two-way repeated-measures ANOVA). This was driven primarily by a reduction in the proportion of total EEG in δ (−18%; P < 0.01) and a simultaneous increase in θ (+14%; < 0.05; Bonferroni post hoc test) (Fig. 5). This finding confirms that systemic CNO also drove measures of arousal in cortical EEG during continuous deep (2%) isoflurane anesthesia.
Fig. 5.
LC-hM3Dq stimulation modulates cortical arousal during continuous isoflurane administration. (A and B) Spectrograms of EEG power during continuous isoflurane after delivery of vehicle (A) or CNO i.p. (B) in the same LC-hM3Dq rat (injections at arrowheads). Sustained cortical EEG changes occur abruptly at ∼10 min after i.p. CNO. Isoflurane was removed (dashed line) at 20 min after CNO, and the rat was allowed to emerge from anesthesia in room air. The spectrogram terminated at the point of first purposeful movement in both cases. (C) Across all subjects (n = 10), CNO i.p. drove significant decreases in relative δ power and increases in θ power at 15 min after i.p. injection during continuous 2% isoflurane (last 5 min before discontinuation of isoflurane). #P < 0.05; ##P < 0.01, Bonferroni posttest, vehicle vs. CNO.
After removal from isoflurane, further EEG transitions were seen before purposeful movement in both vehicle- and CNO-treated subjects. The onset of the final EEG pattern before purposeful movement was significantly faster in CNO-treated animals compared with vehicle-treated animals (P = 0.0046, paired t test). At the time when this final EEG pattern was observed after CNO (Fig. S2A), we compared EEG power spectral densities in vehicle- and CNO-treatment animals, and observed significant differences in EEG power (paired t test; P < 0.0001) (Fig. S2B), demonstrating that LC-NE activation accelerates cortical as well as behavioral emergence from general anesthesia.
LC-NE–Facilitated Emergence Is Mediated Through Both β and α Adrenergic Receptors.
Because there was no difference in emergence effect across CNO doses, we used the 1-mg/kg dose for additional studies with noradrenergic antagonists to help identify the signaling mechanisms underlying accelerated emergence. LC-hM3Dq animals (n = 20) received saline, propranolol (β antagonist; 10 mg/kg), prazosin (α1 antagonist; 0.42 mg/kg), or propranolol-prazosin combination (10 mg/kg + 0.42 mg/kg) pretreatment 30 min before isoflurane induction. They were then given either vehicle or CNO 20 min before discontinuation of isoflurane and measurement of RORR.
Pretreatment with either the β or α antagonist or the β + α combination significantly extended emergence time when vehicle was delivered before removal from isoflurane, compared with CNO (F3,46 = 41.64; P < 0.0001, one-way ANOVA). This finding confirms that noradrenergic transmission is involved in the normal physiological process of emergence from isoflurane anesthesia. As shown in Fig. 4B, when CNO was delivered 20 min before removal from isoflurane, the β adrenergic antagonist propranolol blocked the CNO-mediated reduction in time to emergence in LC-hM3Dq rats (t = 2.69; P < 0.05, Bonferroni post hoc test); however, the emergence time after CNO was still shorter in propranolol-pretreated animals compared with animals given vehicle after propranolol pretreatment (P = 0.0009, paired t test). This demonstrates partial efficacy of LC activation despite β adrenergic blockade, and indicates that non-β adrenergic mechanisms are also involved in facilitated emergence driven by stimulation of LC-hM3Dq receptors in LC-NE neurons.
Pretreatment with the α1 antagonist prazosin prevented hM3Dq-mediated accelerated emergence (t = 4.42; P < 0.001, Bonferroni post hoc test) (Fig. 4).
The emergence time was also reduced with CNO compared with vehicle after prazosin pretreatment (P = 0.0022, paired t test). However, pretreatment with the propranolol-prazosin combination prevented hM3Dq-mediated acceleration of emergence (t = 11.06; P < 0.001, Bonferroni post hoc test), as well as the partial effects of CNO compared with vehicle (P = 0.52, paired t test). The partial efficacy of CNO in the presence of either noradrenergic antagonist alone, but not in combination, demonstrates that LC-NE transmission can drive emergence from general anesthesia through transmission at either β or α1 adrenoreceptors, and that the facilitation of emergence by hM3Dq LC stimulation is driven by noradrenergic signaling.
Normal Emergence from Isoflurane General Anesthesia Is Highly Sensitive to Central Noradrenergic Function.
The antagonism of CNO-mediated facilitation of emergence demonstrates that noradrenergic antagonists potently extend unconsciousness and retard anesthetic emergence (Fig. 4B). However, noradrenergic antagonists have peripheral effects that could alter anesthetic state through pharmacokinetic modulation (21), along with their central effects on noradrenergic arousal circuits. To differentiate central from peripheral sites of action, we delivered a low-dose propranolol-prazosin combination (0.2 mg/kg + 0.0084 mg/kg) systemically and also centrally through i.c.v. cannulae (n = 5 rats). As shown in Fig. 4C, neither systemic low-dose propranolol-prazosin nor i.c.v. vehicle had an effect on emergence times; however, centrally administered low-dose propranolol-prazosin significantly extended baseline emergence times (F3,12 = 8.60; P = 0.0026, one-way repeated-measures ANOVA). Application of Bonferroni’s multiple-comparisons test confirmed that central low-dose propranolol-prazosin was equipotent on behavioral emergence (t = 0.038; P > 0.05) with the 50-fold-higher propranolol-prazosin dose (10 mg/kg + 0.42 mg/kg) used systemically to antagonize emergence. The relative potency of central vs. peripheral noradrenergic antagonism demonstrates that central noradrenergic activity plays a prominent role in regulating normal anesthetic emergence.
LC-NE Activation Alters Anesthetic Induction.
Given the effects of LC-NE activation on emergence, we sought to determine whether the LC-NE system is also capable of modulating induction of anesthesia. Hysteresis in transitions of consciousness has been previously demonstrated with many anesthetics, including isoflurane (22, 23); specifically, the concentrations of isoflurane required for loss of righting reflex (LORR; anesthetic induction) are significantly higher than those needed to maintain anesthesia and prevent RORR. The biological mechanisms underlying this pharmacologic disconnect are not understood, but previous results indicate involvement of NE signaling (22). Here we found that in LC-hM3Dq subjects (n = 9), systemic CNO (1 mg/kg) given 20 min before induction significantly delayed the time to LORR by 128 ± 8% (P = 0.0071, paired t test). Thus, in both directions (induction and emergence), LC-NE activation can affect behavioral state transitions with isoflurane anesthesia, providing evidence that LC activity may affect anesthetic hysteresis.
LC-NE hM3Dq Effects Do Not Extend to Locomotion.
We sought to determine whether the effects of stimulation of LC-NE neurons on anesthetic state resulted from generalized locomotor activation. After completion of the emergence experiments, rats (n = 14) underwent locomotor assessment after administration of CNO 1 mg/kg or vehicle. Locomotor testing was performed during the rats’ active phase (as were emergence experiments) in an unfamiliar testing chamber. We saw no effect of CNO on locomotor activity during a 1-h locomotion test that encompassed the time of emergence experiments (F1,30 = 0.23; P = 0.65, two-way ANOVA) (Fig. S3). Thus, our behavioral results on emergence are not related to enhanced motor output, but likely are related to NE action in specific networks that are responsible for the anesthetic state.
Discussion
In this study, we expressed hM3Dq DREADDs selectively in NE neurons of the LC using microinjections of a viral vector driven by the PRSx8 promoter. Stimulation of hM3Dq-expressing LC-NE neurons with CNO accelerated behavioral emergence (RORR) from deep isoflurane anesthesia. Moreover, pharmacologic antagonism of NE neurotransmission at β or α1 adrenoreceptors delayed spontaneous or LC-NE–stimulated RORR. We confirmed that hM3Dq stimulation activates LC-NE neurons and concurrently decreases δ power and increases θ EEG activity, indicating cortical arousal. Our results indicate a role for central noradrenergic signaling in endogenous anesthetic emergence, and demonstrate that increased noradrenergic transmission from the LC alone is sufficient to facilitate anesthetic emergence.
We were able to reversibly drive LC-NE neurons through synthetic Gq signaling using DREADD receptors. Inducible hM3Dq control of neural signaling (and lack of constitutive hM3Dq activity) has been validated in vitro and in several other neural systems (17, 18, 24). In concordance with previous findings, we found no basal differences in LC-NE activity or emergence times for subjects expressing hM3Dq receptors when not exposed to CNO. Moreover, neither we nor others found behavioral or physiological effects in rats or mice from CNO alone (25). In contrast, CNO delivered locally or systemically to LC-hM3Dq–expressing rats potently activated NE neurons in the LC, drove cortical arousal and accelerated behavioral emergence from isoflurane anesthesia. All previous manipulations of LC function in WT subjects used electrical stimulation, lesions, or pharmacologic interventions that do not allow both selective and reversible manipulations of LC-NE neurons. Our use of virally delivered hM3Dq with the PRSx8 promoter allowed us to specifically target NE neurons in the LC and subcoeruleus, avoiding potentially confounding effects from other noradrenergic (or non-NE) systems. In addition, this approach avoided adaptive developmental actions that can alter endogenous circuits in transgenic models.
The preparation for our acute single-unit electrophysiology required a surgical plane of anesthesia (2% isoflurane). During our recordings, as seen previously with deep anesthesia, cortical EEG was in a burst suppression pattern that was significantly decreased after stimulation of LC-NE neurons via hM3Dq receptors (3, 26). Additional measures of increased cortical arousal, including increased peak frequency and decreased δ and increased θ power, were seen after local hM3Dq-mediated activation of LC-NE neurons. Cortical arousal after LC-NE activation under deep anesthesia validates the potency of LC-mediated action on the cortical state and is consistent with the concept of the LC as an initiator node for anesthetic emergence.
Noradrenergic transmission, particularly from the LC, is known to be a key modulator of arousal from sleep (13, 27), and LC-NE is one of the systems posited to also regulate arousal in anesthetic contexts (28, 29). Delivery of NE directly to the nucleus basalis, another node in sleep–wake circuits and one of many regions that receive NE innervation from the LC, produces transient microarousals in desflurane-anesthetized rats, supporting a role for NE in emergence functions (7). Although elimination of LC-NE discharge is not necessary for volatile anesthetic action (15, 30), increases in LC activity and central NE levels after cessation of halothane anesthesia have been documented (31, 32). In addition, during halothane anesthesia, LC discharge rate has been positively correlated with arousal-related changes in cortical and hippocampal EEG (8, 33).
In the present study, antagonism of NE signaling at α1 or β receptors blocked LC-hM3Dq–facilitated emergence and also significantly extended baseline emergence times. This confirms a role for NE in endogenous anesthetic emergence in WT subjects, as reported recently in DBH KO mice that do not produce NE. DBH KO mice have hypersensitivity to, and extended emergence times from, multiple volatile anesthetics and dexmedetomidine (6, 22); however, those KO studies could not specify whether the adrenergic component involved in emergence was the LC or other brain or peripheral NE systems. Our findings identify the LC as a likely major contributor to the results in these KO studies. Moreover, by administering low doses of noradrenergic antagonists i.c.v., we provide strong evidence that central NE plays a substantial role in regulation of the anesthetic state and transitions of consciousness. Finally, LC and other noradrenergic neurons release compounds in addition to NE; our findings with NE antagonists support those of DBH KOs and exclude those cofactors as possible substrates of facilitated emergence.
Methylphenidate, a potent inhibitor of dopamine (DA) and NE transporters, has been shown to induce active emergence during isoflurane or propofol anesthesia, indicating potential synergy for dopaminergic and noradrenergic emergence mechanisms and efficacy across anesthetic modalities (4, 34). However, because DBH KO mice show heightened DA levels owing to release of DA from noradrenergic terminals (35), the methylphenidate studies in conjunction with the KO model discussed above support our findings that an NE mechanism is a primary contributor to anesthetic emergence. Our findings extend the previous studies by directly identifying LC-NE activation as sufficient to accelerate anesthetic emergence.
Critical targets of LC-NE neurons to drive emergence from anesthesia remain to be defined. LC has widespread anatomic projections, and provides a direct excitatory tone to the cortex and many wake-promoting regions, including other brainstem nuclei, basal forebrain cholinergic regions, the dorsal raphe serotonergic system, and the intralaminar region of the thalamus (36). Moreover, NE exerts its excitatory influence in these regions through postsynaptic α1 and β receptors (37). Blockade of either of these receptors inhibited CNO-facilitated emergence here, reciprocally validating that enhanced NE from LC-hM3Dq activation mediates accelerated emergence. Hypnotic doses of isoflurane have been shown to activate putative sleep-promoting ventrolateral preoptic (VLPO) area neurons; complementary to its excitatory actions in other regions, NE specifically hyperpolarizes the isoflurane-sensitive population of VLPO neurons (38). In the present study, we have shown that LC-NE activation also antagonizes induction of isoflurane anesthesia. Actions of the LC on the induction and emergence of anesthesia may reflect its widespread efferent circuits and ability to act concurrently at multiple sites.
We found no effects of LC-hM3Dq activation on locomotor activity, indicating that the effects reported here are not simply a reflection of altered locomotor activity. Intraventricular NE or optogenetic stimulation of the LC has mixed effects on locomotor activity in previous studies, with increasing locomotion in some cases and no effect in others (13, 39).
Our present results extend and clarify previous investigations into the circuitry regulating emergence from general anesthesia. Our findings strengthen the existing evidence suggesting a possibly important role for homeostatic sleep-wake mechanisms (e.g., LC-NE neurons) in broader realms of consciousness, such as general anesthesia. Our findings also have implications for clinical practice, indicating that LC-NE has a clear role in emergence from general anesthesia, and that adrenoreceptor antagonists may prolong anesthetic effects even after isoflurane discontinuation. Currently, many patients who undergo general anesthesia receive adrenoreceptor drugs to, for example, regulate autonomic function either chronically or acutely during anesthetic procedures (e.g., to control blood pressure). Our findings suggest that such treatments may interact with anesthetic induction and emergence mechanisms, potentially compromising optimal anesthetic management (29).
In view of previous findings (6, 22), our data indicate that diurnal and stress-induced variations in LC-NE activity may affect clinical responses to anesthetic agents. They also suggest that the use of noradrenergic antagonists in patients undergoing surgical procedures should be strongly considered to optimize the safety and regulation of general anesthesia. By causally demonstrating that LC activation alone is sufficient to accelerate behavioral emergence from isoflurane general anesthesia, this study identifies the LC as an important effector node in anesthetic emergence networks.
Materials and Methods
Subjects (n = 32) received PRSx8-hM3Dq DREADD or mCherry control vectors in the LC. Single-unit electrophysiology, cortical EEG recording, and microinjection via double-barreled pipette in anesthetized subjects were carried out as described previously (8, 40, 41). Rats received a 40-min exposure to 2% isoflurane before undergoing emergence testing in room air. Antagonists were delivered 30 min before anesthesia induction, and CNO was delivered 20 min before emergence testing, during isoflurane exposure (with the exception of LORR experiments, where it was delivered before induction). Bilateral cortical electrophysiology during emergence was recorded differentially from frontal skull screws 3 mm apart. Animals underwent a 60-min locomotor assessment after CNO injection. All subjects were histologically confirmed for vector expression. All single-unit data were normalized to baseline firing before CNO. Cortical arousal was defined as concurrent reductions in burst suppression and rightward changes in peak EEG frequency, with decreases in δ power and increases in θ power. Burst suppression was calculated as EEG ±20 µV for 100 ms. Relative power for EEG frequency bands was used owing to changes in burst suppression and total power after CNO delivery. P < 0.05 was the acceptable α level for all experiments. Details of the experimental procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Bryan Roth, Patrice Guyunet, and Ruth Stornetta for source plasmids and Audrey Postma for histological assistance. Clozapine-n-oxide was supplied by National Institutes of Health-National Cancer Institute under the auspices of NS064882-01, and by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program. This research was supported by Public Health Service Grants R01 MH092868 and C06 RR015455, the Parkinson’s Disease Foundation (E.M.V.), and the Neuroscience Institute of Medical University of South Carolina.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310025111/-/DCSupplemental.
References
- 1.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363(27):2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelz MB, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci USA. 2008;105(4):1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology. 2009;111(4):725–733. doi: 10.1097/ALN.0b013e3181b061a0. [DOI] [PubMed] [Google Scholar]
- 4.Solt K, et al. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115(4):791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4(7):732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 6.Hu FY, et al. Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine β-hydroxylase knockout mice. Anesthesiology. 2012;117(5):1006–1017. doi: 10.1097/ALN.0b013e3182700ab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology. 2011;115(4):733–742. doi: 10.1097/ALN.0b013e31822c5ee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11(10):3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aston-Jones G, Shipley MT, Grazanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Nervous System. 2nd Ed. New York: Academic Press; 1995. pp. 183–214. [Google Scholar]
- 10.Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429(4):638–653. [PubMed] [Google Scholar]
- 11.Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function, part I: Principles of functional organisation. Curr Neuropharmacol. 2008;6(3):235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: New evidence of anatomical and physiological specificity. Physiol Rev. 1983;63(3):844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 13.Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter ME, et al. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci USA. 2012;109(39):E2635–E2644. doi: 10.1073/pnas.1202526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gompf H, et al. Halothane-induced hypnosis is not accompanied by inactivation of orexinergic output in rodents. Anesthesiology. 2009;111(5):1001–1009. doi: 10.1097/ALN.0b013e3181b764b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang DY, Carlezon WA, Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther. 2001;12(14):1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- 17.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander GM, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63(1):27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott SB, et al. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29(18):5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijn PC, Sneyd JR. I.v. anaesthesia and EEG burst suppression in rats: Bolus injections and closed-loop infusions. Br J Anaesth. 1998;81(3):415–421. doi: 10.1093/bja/81.3.415. [DOI] [PubMed] [Google Scholar]
- 21.Foëx P, Francis CM, Cutfield GR. The interactions between beta-blockers and anaesthetics: Experimental observations. Acta Anaesthesiol Scand Suppl. 1982;76:38–46. doi: 10.1111/j.1399-6576.1982.tb01887.x. [DOI] [PubMed] [Google Scholar]
- 22.Friedman EB, et al. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: Evidence for neural inertia. PLoS ONE. 2010;5(7):e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steyn-Ross ML, Steyn-Ross DA, Sleigh JW. Modelling general anaesthesia as a first-order phase transition in the cortex. Prog Biophys Mol Biol. 2004;85(2-3):369–385. doi: 10.1016/j.pbiomolbio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson SM, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14(1):22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Broek PL, van Rijn CM, van Egmond J, Coenen AM, Booij LH. An effective correlation dimension and burst suppression ratio of the EEG in rat: Correlation with sevoflurane-induced anaesthetic depth. Eur J Anaesthesiol. 2006;23(5):391–402. doi: 10.1017/S0265021505001857. [DOI] [PubMed] [Google Scholar]
- 27.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1(8):876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9(5):370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 29.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasui Y, Masaki E, Kato F. Sevoflurane directly excites locus coeruleus neurons of rats. Anesthesiology. 2007;107(6):992–1002. doi: 10.1097/01.anes.0000291453.78823.f4. [DOI] [PubMed] [Google Scholar]
- 31.Saunier CF, et al. Activation of brain noradrenergic neurons during recovery from halothane anesthesia: Persistence of phasic activation after clonidine. Anesthesiology. 1993;79(5):1072–1082. doi: 10.1097/00000542-199311000-00026. [DOI] [PubMed] [Google Scholar]
- 32.Chave S, et al. Effects of two volatile anesthetics (sevoflurane and halothane) on the hypothalamic noradrenaline release in rat brain. Brain Res. 1996;706(2):293–296. doi: 10.1016/0006-8993(95)01080-7. [DOI] [PubMed] [Google Scholar]
- 33.Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55(2):381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- 34.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116(5):998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374(6523):643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 36.Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function, part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol. 2008;6(3):254–285. doi: 10.2174/157015908785777193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholas AP, Hökfelt T, Pieribone VA. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol Sci. 1996;17(7):245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- 38.Moore JT, et al. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr Biol. 2012;22(21):2008–2016. doi: 10.1016/j.cub.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal DS, Mandell AJ. Behavioral activation of rats during intraventricular infusion of norepinephrine. Proc Natl Acad Sci USA. 1970;66(2):289–293. doi: 10.1073/pnas.66.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jodo E, Aston-Jones G. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res. 1997;768(1-2):327–332. doi: 10.1016/s0006-8993(97)00703-8. [DOI] [PubMed] [Google Scholar]
- 41.Shiekhattar R, Aston-Jones G. Local application of bicuculline potentiates NMDA receptor-mediated sensory responses of brain noradrenergic neurons. Synapse. 1992;10(1):54–61. doi: 10.1002/syn.890100108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.