Significance
Worker ants are responsible for various tasks for their colony. In their chemical communication, odorant-binding proteins and chemosensory proteins, which accumulate in the sensillum lymph in the antennae, play roles in transporting semiochemicals to chemosensory receptors. However, the number of these proteins is not sufficient to interact with a large number of semiochemicals. Niemann–Pick type C2 protein was identified from the antenna of the worker Japanese carpenter ant, Camponotus japonicus (CjapNPC2). CjapNPC2 accumulated in the sensillum cavity in the basiconic sensillum. The ligand-binding pocket was composed of a flexible β-structure, which allowed binding to various potential semiochemicals, some of which elicited antennal electrophysiological responses. CjapNPC2 might play crucial roles in chemical communication required to perform worker ant tasks.
Keywords: subtraction PCR, fluorescent competitive binding assay, gas chromatography–electroantennogram detection, X-ray crystallography, molecular evolution
Abstract
Ants are eusocial insects that are found in most regions of the world. Within its caste, worker ants are responsible for various tasks that are required for colony maintenance. In their chemical communication, α-helical carrier proteins, odorant-binding proteins, and chemosensory proteins, which accumulate in the sensillum lymph in the antennae, play essential roles in transferring hydrophobic semiochemicals to chemosensory receptors. It has been hypothesized that semiochemicals are recognized by α-helical carrier proteins. The number of these proteins, however, is not sufficient to interact with a large number of semiochemicals estimated from chemosensory receptor genes. Here we shed light on this conundrum by identifying a Niemann–Pick type C2 (NPC2) protein from the antenna of the worker Japanese carpenter ant, Camponotus japonicus (CjapNPC2). CjapNPC2 accumulated in the sensillum cavity in the basiconic sensillum. The ligand-binding pocket of CjapNPC2 was composed of a flexible β-structure that allowed it to bind to a wide range of potential semiochemicals. Some of the semiochemicals elicited electrophysiolgical responses in the worker antenna. In vertebrates, NPC2 acts as an essential carrier protein for cholesterol from late endosomes and lysosomes to other cellular organelles. However, the ants have evolved an NPC2 with a malleable ligand-binding pocket as a moderately selective carrier protein in the sensillum cavity of the basiconic sensillum. CjapNPC2 might be able to deliver various hydrophobic semiochemicals to chemosensory receptor neurons and plays crucial roles in chemical communication required to perform the worker ant tasks.
Ants are eusocial insects comprising 11 subfamilies, 297 genera, and 8,800 species, and they are found in most regions from the Arctic Circle to the southernmost reaches. The insects form 15–20% of the terrestrial animal biomass (1, 2). The colony contains mated queen(s), alate males, virgin queens, and nonreproductive workers. Worker ants are responsible for nurturing the brood, excavating soil for nest construction, procuring food, and protecting their territory against nonnestmates and/or predators. To maintain the colony, worker ants have established a highly sophisticated chemical communication system to detect semiochemicals, molecules that convey information in a task-specific manner (1, 3, 4).
The antenna is the major chemosensory organ in insects. In the worker Japanese carpenter ant, Camponotus japonicus, it is presumed that coeloconic, basiconic, trichoid-I, trichoid-II, and chaetic sensilla play roles in contact chemosensory or olfactory reception (5). Ants have an exceptionally large number of chemosensory receptor genes. For example, genome and transcriptome analyses of the Florida carpenter ant, Camponotus floridanus, identified 407 odorant receptors (Ors), 63 gustatory receptors, and 31 ionotropic glutamate receptors (6). In the peripheral sensory system, the hydrophobic semiochemicals enter the sensillum cavity through pore tubules. The sensillum cavity is completely segregated from the hemocoel by the membranes of tormogen, thecogen, trichogen cells, and receptor dendrite, and it is filled with aqueous sensillum lymph (7). Odorant-binding proteins (OBPs) and chemosensory proteins (CSPs) that accumulate in the sensillum cavity play essential roles in transferring semiochemicals to the Or/odorant receptor coreceptor (Orco) complex, a ligand-gated ion channel on the receptor membrane (8–12). OBPs and CSPs are composed of α-helices that are knitted with disulfide bridges. The result is the formation of a relatively rigid binding cavity. This rigidity results in binding proteins that bind a narrow range of hydrophobic ligands (13–15). Although it has been hypothesized that semiochemicals are recognized by the α-helical carrier protein (16), interestingly, analysis of sequences in an EST library from the red imported fire ant, Solenopsis invicta, has identified only 18 OBPs and 14 CSPs (17, 18). Because ants detect a large variety of chemicals derived from their habitats by chemosensory receptors, they require unidentified carrier protein(s) with a flexible structure that can bind various semiochemicals with moderate selectivity.
To explore the carrier protein(s) from the Japanese carpenter ant, C. japonicus, which widely transfers various hydrophobic semiochemicals, we applied subtraction PCR between worker and male antennae. Here we describe the identification and characterization of a Niemann–Pick type C2 protein of C. japonicus (CjapNPC2). In vertebrates, Niemann–Pick disease is a genetically inherited lipid processing disorder. Mutant Niemann–Pick type C2 (NPC2) inappropriately binds ligands such as cholesterol, causing a fatal neurodegenerative disorder resulting from the endolysosomal accumulation of cholesterol and lipids. Thus, it is known that β-structured NPC2 plays a role in transferring cholesterol (19). However, the ant NPC2 specifically accumulates in the extracellular sensillum cavity in the basiconic sensillum of worker antenna and binds hydrophobic long-chain fatty acids, alcohols, and acetates, but not cholesterol, at neutral pH and dissociates at low pH. In addition, some of these ligands can evoke electrophysiological response from worker antenna. Furthermore, molecular recognition of ligand binding and release is demonstrated on the basis of the crystal structure of apo and fatty acid-bound CjapNPC2. We propose that CjapNPC2 transfers semiochemicals in the sensillum lymph in the basiconic sensillum and plays crucial roles in regulating a plethora of worker ant tasks.
Results and Discussion
Identification and Structural Characterization of CjapNPC2.
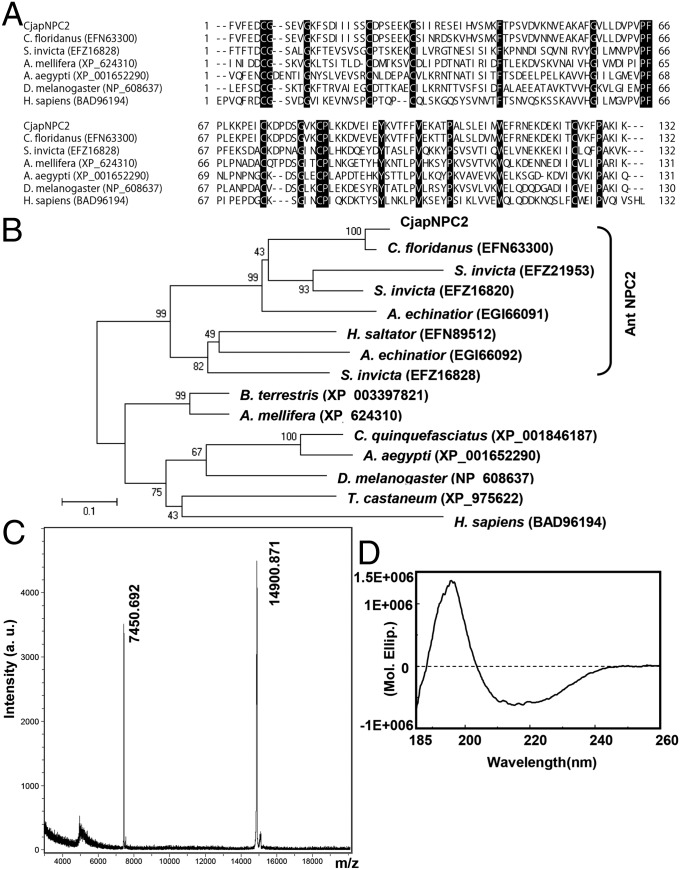
To identify worker-antenna-specific genes that are responsible for chemical communication in C. japonicus, reciprocal subtraction PCR between worker and male antennae was carried out. We determined eight full-length cDNA sequences from worker-antennal flagellum cDNA and two full-length cDNA sequences from male-antennal flagellum cDNA [DNA Data Bank of Japan (DDBJ) accession nos. AB734099–AB734108; Tables S1 and S2 and Fig. S1). One of the predicted proteins identified from the worker antenna was composed of 154 amino acid residues, including a 22-amino-acid-long signal peptide (DDBJ accession no. AB734104). This secreted protein had six cysteine residues that are observed in those of the Classic OBP family (8, 20). However, Blastp search, ClustalW alignment, and phylogenetic tree analyses indicated that this protein had 92% amino acid sequence identity with a NPC2-like protein from C. floridanus (GenBank accession no. EFN63300) (21) and belonged to a family of NPC2, which is widely identified not only from vertebrate genome but also from genome of hymenopteran, dipteran, and coleopteran insects and yeast. The ant NPC2s form a specific clade in the phylogenetic tree (Fig. 1 A and B). On the basis of these results, we named this protein CjapNPC2. Mature CjapNPC2 had a calculated molecular mass of 14,906.39 Da and an isoelectric point of 5.16 and was predicted as a β-sheet protein (Fig. S2). A recombinant mature CjapNPC2 that was expressed by using a pET-22b(+) vector had a molecular mass of 14,900.871 Da by MALDI-TOF mass spectrometry analysis (Fig. 1C). Far-UV circular-dichroism analysis of the CjapNPC2 showed a spectrum with a positive peak at 193 nm and a negative peak at 211 nm (Fig. 1D). These results suggest that the CjapNPC2 contains three disulfide bridges and β-sheet structures. These properties were similar to those observed with vertebrate NPC2s (22, 23).
Fig. 1.
Alignment, phylogenetic tree, and structural analysis of CjapNPC2. (A and B) ClustalW alignment (A) and phylogenetic tree (B) of NPC2 from various insects and human. Identical amino acid residues are shaded in black. GenBank accession numbers are shown in parentheses. The bracket to the right of the tree indicates the clade encompassing NPC2 from ants. Bootstrap values were determined from 1,000 replications. The bar indicates 10% divergence. (C) MALDI-TOF mass spectrometry of recombinant CjapNPC2. (D) Circular dichroism analysis of purified recombinant CjapNPC2. A positive peak of the far-UV circular dichroism spectrum at 193 nm and a negative peak at 211 nm were observed at pH 7, indicating that CjapNPC2 is a β-structure-rich protein.
CjapNPC2 Accumulates in the Sensillum Cavity in Worker Basiconic Sensillum.
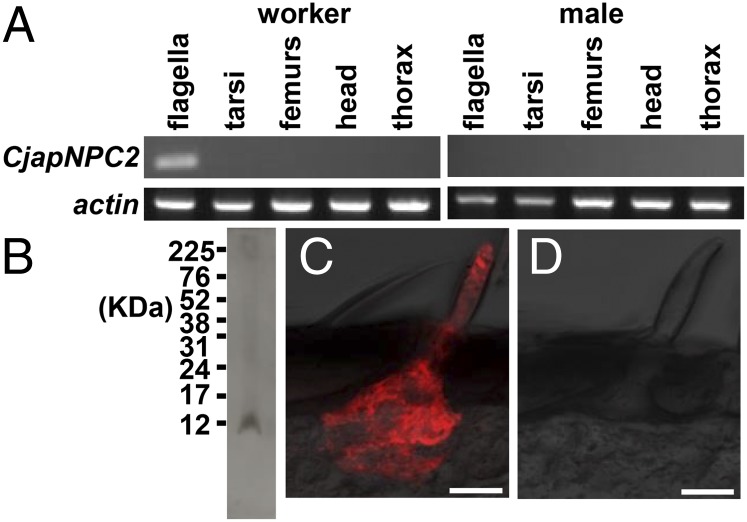
In vertebrates, NPC2 is a water-soluble protein that plays a role in transporting cholesterol from late endosomes and lysosomes to other cellular organelles (22). In the fruit fly, NPC2 is thought to regulate sterol homeostasis and the biosynthesis of 20-hydroxyecdysone, a steroidal insect molting hormone (24). To evaluate the function of CjapNPC2 in various tissues, RT-PCR was carried out. Contrary to our expectation, CjapNPC2 was expressed only in the flagella of the worker antenna (Fig. 2A). To localize the ant NPC2 at the cellular level, immunohistochemical analysis was performed. By Western blot analysis, anti-CjapNPC2 antiserum detected a single immunoreactive band from the worker antennal extract (Fig. 2B). The immunoreactive material was specifically accumulated in the sensillum cavity in the basiconic sensillum (Fig. 2C). The sensillum houses ∼130 sensory neurons that presumably express a large number of chemosensory receptor genes (5, 6). Preimmune serum as a negative control only showed background staining (Fig. 2D). The localization of CjapNPC2 was similar to that of OBP (25), suggesting that CjapNPC2 is likely to carry hydrophobic compounds in the sensillum cavity in the basiconic sensillum.
Fig. 2.
Expression and localization of CjapNPC2. (A) RT-PCR analysis of CjapNPC2 expression. Actin was expressed in all experimental tissues, whereas CjapNPC2 was expressed only in worker flagella. (B) Western blot analysis of CjapNPC2. An immunoreactive material having a molecular mass of 12 KDa was detected from worker antennal extract by using rabbit anti-CjapNPC2 serum. (C) Immunohistochemical localization of CjapNPC2. The immunoreactive material to rabbit anti-CjapNPC2 antibody was specifically localized in the sensillum cavity of the basiconic sensillum on the worker antenna. (Scale bar: 20 μm.) (D) Basiconic sensillum in control experiment using preimmune serum as a primary antibody. (Scale bar: 20 μm.)
CjapNPC2 Carries Hydrophobic Semiochemicals Evoking Antennal Electrophysiological Responses.
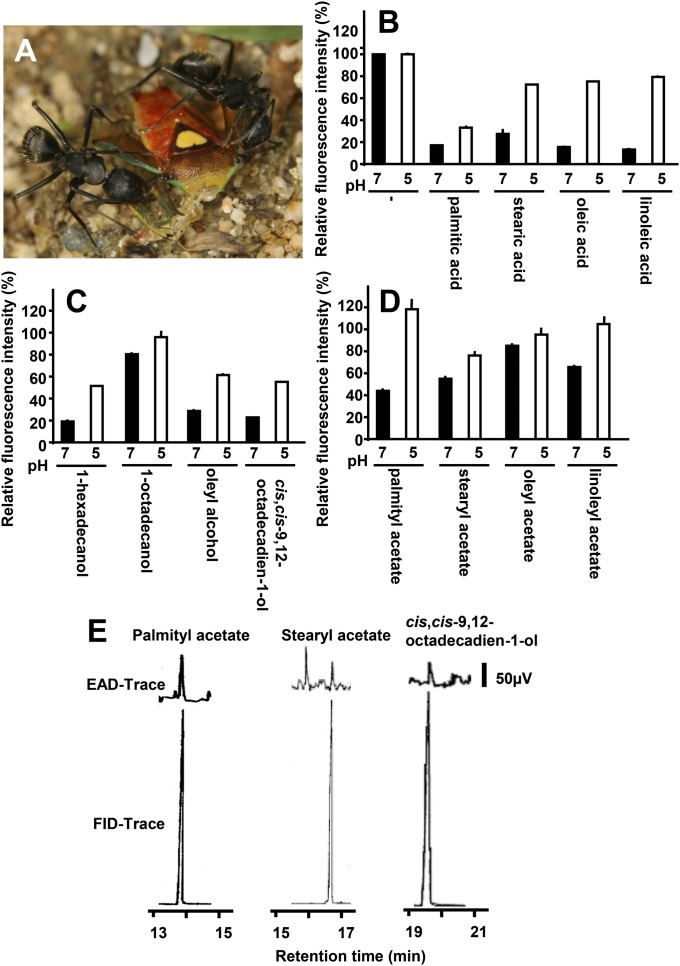
The Japanese carpenter ant is an omnivore. In the field, the worker ants are occasionally observed in groups hunting other insects and subsequently harvesting the soft tissues of the prey and bringing the food back to the nest. During hunting the worker ants repeatedly use their antennae to check the body surface of the prey and harvest muscle tissues (Fig. 3A). The internal organs are rich in long-chain fatty acids derived from triglycerides and cholesterol. Fatty acids that are derived from seed elaiosomes are also used as rewards and/or semiochemicals that help trigger seed-dispersal behavior of ants (26, 27). These behaviors indicate that ant antennae are able to detect fatty acids. Thus, we hypothesized that CjapNPC2 binds to long-chain fatty acids. In fluorescence competitive binding assays, CjapNPC2 bound to palmitic, stearic, oleic, and linoleic acids in a pH-dependent manner (Fig. 3B). Specifically, CjapNPC2 bound these fatty acids at neutral pH (i.e., pH 7), but not at an acidic pH (i.e., pH 5). In contrast, CjapNPC2 did not bind cholesterol at pH 7 and showed poor binding at pH 5 (Fig. S3). Next, we examined the binding of CjapNPC2 to the related long-chain alcohols and acetates. CjapNPC2 showed pH-dependent binding and dissociation to 1-hexadecanol, oleyl alcohol, cis,cis-9,12-octadecadien-1-ol, palmityl acetate, and linoleyl acetate (Fig. 3 C and D). We also investigated binding of CjapNPC2 to other candidate ligands, n-tricosane (a cuticular hydrocarbon that is presumably related to nestmate recognition) and linalool (a plant volatile molecule that is found in the Japanese carpenter ant habitat). CjapNPC2, however, did not bind to either compound (Fig. S3). These results suggest that CjapNPC2 not only binds long-chain fatty acids but also partly binds their related alcohols and acetates.
Fig. 3.
Binding properties of CjapNPC2 to various potential ligands and gas chromatography–electroantennogram detection (GC-EAD) of CjapNPC2-interacting semiochemicals. (A) Two workers cooperatively hunt a stink bug, Sastragala esakii, and harvest the internal organs. (B–D) Binding of CjapNPC2 to long-chain fatty acids (B), long-chain alcohols (C), and acetates (D). Binding activity of CjapNPC2 under conditions at pH 7 and 5 was evaluated by fluorescence competitive binding assay using N-phenyl-1-naphthylamine (1-NPN) as a fluorescence reporter. Values are means ± SD; n = 3. (E) Typical traces of GC-EAD run to volatile organic compounds. The antenna responded to palmityl acetate (retention time, 13.81 min), stearyl acetate (16.69 min), and cis,cis-9,12-octadecadien-1-ol (19.62 min).
OBP is able to bind its ligand at neutral pH and to eject it in a very fast process (t1/2 = 9 ms) in low pH condition localized on adjacent negatively charged olfactory receptor neuron (28, 29). Our binding assay showed that CjapNPC2 bound to a wide variety of long-chain fatty acids, alcohols, and acetates at neutral pH and dissociated with these ligands at low pH. Furthermore, human NPC2 can deliver ligand to acceptor membranes in a flash by a collision transfer mechanism (30). By using a pH-dependent dissociation and/or collision transfer mechanism, CjapNPC2 might transfer semiochemicals to the chemosensory receptors in the sensillum cavity at the same speed as OBPs. To better understand the physiological function of CjapNPC2, further biophysical analysis is required.
In the lepidopteran insects, the porous sensillum is classified as the olfactory sensillum (31). The basiconic sensillum, where CjapNPC2 expression is observed, has many pits on the cuticular surface (5). On the basis of the morphological evidence, we assumed that the basiconic sensillum acts not only as the contact chemosensory sensillum but also as the olfactory sensillum. Thus, we sought to record the electrophysiological response to these CjapNPC2-bound compounds using a gas chromatography–electroantennogram detector (GC-EAD). The electroantennogram response to n-undecane, a major component of the alarm pheromone (32), was repetitively observed, although the response was much smaller than that found in lepidopteran insects (33, 34) (Fig. S4). The worker antenna also showed the electrophysiological response to palmityl acetate, stearyl acetate, and cis,cis-9,12-octadecadien-1-ol (Fig. 3E) compounds, which have not been reported semiochemicals in C. japonicus. The weak antennal response from ants simply indicates the small number of firing neurons, which is significantly smaller than those of pheromone-responding neurons of lepidopteran insects (180 sensilla in C. japonicus vs. 17,000 sensilla in Bombyx mori) (5, 35). Conversely, responses to the fatty acids showed low reproducibility in our preliminary experiments. We assume that this low reproducibility resulted from the low volatility of fatty acids and/or detection limit by the GC-EAD. The basiconic sensillum with many olfactory receptor neurons inside expressing CjapNPC2 seems to have been evolved as a generalist for detection of a wide range of semiochemicals rather than as a specialist for detection of a single compound. To confirm the functions of the basiconic sensillum in the worker antenna, further electrophysiological evidence at the single unit level is required. Although the antenna did not respond all CjapNPC2-interacting compounds, these results suggest that CjapNPC2 plays roles in solubilization of hydrophobic semiochemicals in the sensillum cavity in the basiconic sensillum and delivery to the Or/Orco complex on the chemosensory receptor neuron.
Crystal Structures of the Apo and Fatty-Acid-Bound Forms of CjapNPC2.
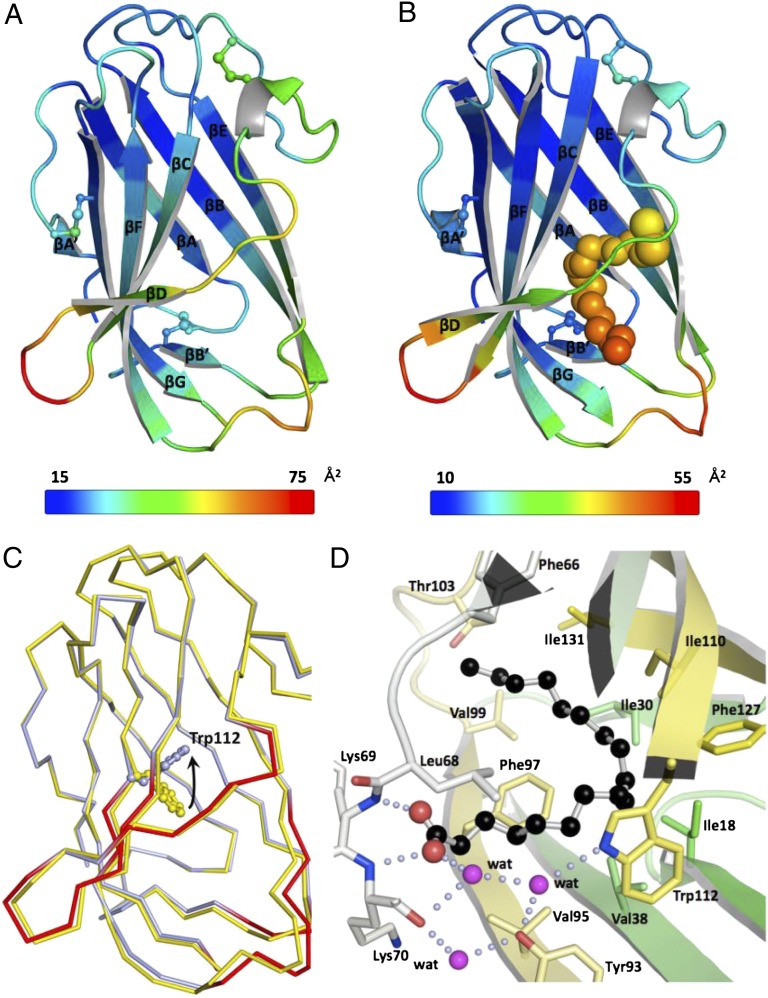
The crystal structures of the apo form of CjapNPC2 and CjapNPC2 in complex with oleic acid as a typical ligand were determined to better understand the molecular mechanism of ligand binding and release (Table 1 and Fig. 4). CjapNPC2 adopts an Ig-like β-sandwich fold conformation with a large cavity in the interior of the protein that is stabilized by three disulfide bonds. This structure is similar to that found with bovine NPC2 (22, 23). The apo and ligand-bound CjapNPC2 structures are well aligned with root-mean-square difference (rmsd) of 0.44 Å across all Cα atoms (Table 1 and Fig. 4C). Although a detailed comparison revealed substantial displacements (rmsd > 0.7 Å) at the entrance of the cavity, these displacements may not result from the ligand binding because the corresponding regions have extremely higher B factors above the average in both forms (Fig. 4 A and B). These results suggest that the ligand-binding cavity, particularly the entrance regions, of CjapNPC2 is relatively flexible.
Table 1.
Data collection and refinement statistics
| Data collection | Apo | Oleic acid complex |
| PDB ID code | 3WEA | 3WEB |
| Space group | P21 | P21212 |
| Cell parameters, Å | a = 40.4, b = 35.7, c = 104.9 | a = 34.8, b = 103.2, c = 39.3 |
| Cell parameters, ° | β = 92.3 | |
| X-ray source | PF-AR BL-NW12 | PF BL-5 |
| Wavelength, Å | 1.00000 | 1.00000 |
| Resolution, Å | 100–1.8 (1.86–1.80) | 100–1.7 (1.76–1.70) |
| No. of reflections | 167,212 | 499,517 |
| No. of unique reflections | 28,146 (2,747) | 66,810 (6,533) |
| Completeness, % | 99.0 (98.1) | 99.8 (97.9) |
| Multiplicity | 5.9 (4.8) | 7.5 (6.6) |
| R merge | 0.101 (0.495) | 0.106 (0.443) |
| Average I/σ | 7.1 (3.0) | 16.3 (8.2) |
| Refinement | ||
| Resolution, Å | 22.4–1.8 (1.85–1.80) | 31.3–1.7 (1.74–1.70) |
| R factor | 0.258 (0.352) | 0.215 (0.259) |
| R free | 0.317 (0.421) | 0.267 (0.256) |
| No. of waters | 121 | 140 |
| Average B value, Å2 | 38.4 | 22.3 |
| rmsd from ideals | ||
| Lengths, Å | 0.009 | 0.006 |
| Angles, ° | 1.34 | 1.13 |
| Ramachandran plot, favored/allowed/disallowed, % | 97.7/2.3/0.0 | 99.2/0.8/0.0 |
Values in parentheses refer to the highest resolution shell.
Fig. 4.
Crystal structures of CjapNPC2. (A and B) Ribbon representations of the apo form (A) and the oleic acid-bound form (B) colored according to the crystallographic B factors. The color bars below the structures show the B-factor scales for the corresponding forms. Average B factors are 31 and 20 Å3 for the apo and ligand-bound structures, respectively. The three disulfide bonds are shown in the ball-and-stick model. The oleic acid is shown as a space-filling model. β-strands (βA′–βG) are labeled from the N to the C terminus, and η represents a 310-helix. (C) Cα traces of superimposed models of CjapNPC2 in the apo form (yellow) and complexed with oleic acid (light blue) are shown with regions of the largest displacements (rmsd > 0.7 Å) highlighted in red in the model of the ligand-bound CjapNPC2. Trp-112 localized at the bottom of the hydrophobic cavity is shown as a ball-and-stick model. The arrow indicates rotation of the side-chain conformation of the Trp-112 from the apo form to the ligand-bound form. (D) Molecular basis for recognition of oleic acid by CjapNPC2. Oleic acid is shown as a ball-and-stick model. Residues involved in the recognition are shown as stick models and water molecules as pink spheres. Hydrogen bonds are indicated by light blue dotted lines.
The structure of the CjapNPC2–oleic acid complex reveals that the binding pocket of CjapNPC2 accommodates one molecule of the ligand in a U-shaped conformation. The binding pocket is lined exclusively by hydrophobic amino acid residues. Most of these hydrophobic residues make contacts with the aliphatic chain of the bound oleic acid and stabilize its U-shaped conformation. The U-shaped conformation of the ligand is dictated by the shape of the binding cavity rather than the existence of the cis double bond between C9 and C10 of the oleic acid. The ability to conform the ligand into a U shape may be a characteristic for CjapNPC2-bound fatty acids because palmitic acid and stearic acid show comparable affinities as oleic acid despite the absence of a double bond (Fig. 3B). Among the amino acid residues that make hydrophobic contacts with oleic acid, Trp-112 located deep inside the ligand-binding pocket flips its side chain by ∼120° between the apo and ligand-bound forms (Fig. 4C). This flipping of the Trp-112 side chain is required to avoid a steric clash with oleic acid and suggests that the Trp-112 side-chain reorientation seems critical for lacing the hydrophobic cavity where the fatty-acid aliphatic chain can fit.
A specific set of hydrogen bonds for the deprotonated carboxyl group of oleic acid completes anchoring of the ligand (Fig. 4D). The O1 and O2 atoms of oleic acid form direct hydrogen bonds with the backbone amides of Lys-70 and -69, respectively. The O1 atom is linked further to Lys-70 CO, Trp-112 Nε1, and Tyr-93 Oη by means of three water molecules. Such a hydrogen-bond network, in particular the two direct intermolecular hydrogen bonds, may be critical for high-affinity binding of fatty acids to CjapNPC2 because no binding was observed for n-tricosane (Fig. S3). It is noteworthy that the Lys-69–Lys-70 sequence of CjapNPC2 is substituted by a Glu–Lys sequence in NPC2s from other ant species (Fig. 1A). This replacement suggests that ant NPC2s could form two direct hydrogen bonds with the carboxyl group of fatty acids. In contrast, the same sequence is replaced by Pro–Asn/Glu sequences in other insect and mammalian NPC2-like proteins (Fig. 1A). Because proline does not have an amide proton, NPC2s with the Pro–Asn/Glu sequences cannot make two hydrogen bonds with the fatty acid carboxylate in the same manner as CjapNPC2. In fact, it has been reported that no fatty acid binding was detected for the bovine NPC2 (19). Together, these findings suggest that the fatty acid binding is a characteristic feature for the ant NPC2s.
Conclusion
Here we demonstrate that CjapNPC2 specifically accumulates in the sensillum cavity of the basiconic sensillum of the antenna of worker C. japonicus (Figs. 1 A–C and 2). The protein is composed of a flexible β-structure and binds several types of potential hydrophobic semiochemicals, and by binding it evokes antennal electrophysiological responses (Figs. 1D and 3). In addition, we solve the crystallographic structures of the apo and ligand-bound forms of CjapNPC2 and unveil the potential mechanism of molecular recognition of a fatty acid by CjapNPC2 (Fig. 4 and Table 1). Together, the data suggest that CjapNPC2 delivers various hydrophobic semiochemicals to Or/Orco complex on the 130 receptor neurons housed in the basiconic sensillum and plays crucial roles in chemical communication required to perform the worker ant tasks. In vertebrates including human, NPC2 acts as an essential carrier protein for cholesterol from late endosomes and lysosomes to other cellular organelles. Ants appear to exploit the NPC2 with a malleable binding pocket as a moderately selective carrier protein for various hydrophobic semiochemicals in the sensillum lymph. Synthesis of CjapNPC2 may also save energy for the production of >100 OBPs and CSPs required for the transport of various ligands to an inordinately large number of chemosensory receptors. Our results provide insight into protein function and structure of NPC2 between vertebrates and ants in molecular evolution.
Invasive ants such as the red imported fire ant, S. invicta, and the Argentine ant, Linepithema humile, cause environmental and economic impacts throughout the world (36, 37). In Culex mosquitoes, a reverse chemical ecological approach using a carrier protein as a selective filter has been used to identify the minor components of oviposition attractant (38). A combination of a binding assay using the ant NPC2 and an electrophysiological assay using Ors/Orco complex expressed in Xenopus oocyte (6) could pave the way for the development of an assay to identify the specific chemical components to repel and/or to regulate the behavior of these invasive ants.
Materials and Methods
C. japonicus pupae and adults were collected from laboratory colonies or on the campus of Kobe University. Total RNA was isolated from each experimental tissue by using a TRIzol Reagent (Life Technologies). cDNAs were identified by using a PCR-Select cDNA Subtraction Kit, a SMART RACE cDNA Amplification Kit (Clontech Laboratories), and gene-specific primers (Table S1). RT-PCR was performed as described (39). Phylogenetic analysis was performed by using MEGA software (Version 5.2.1) (40). Signal peptide, molecular mass, and secondary structure were predicted by using Signal P Server (Version 4.1) (41), PeptideMass (42), and Jpred (43), respectively. MALDI-TOF mass spectrometry and circular dichroism analyses were carried out as described (44). The bacterial expression vector was constructed and transformed to BL21(DE3) (38). After culture of the transformant, IPTG induction, and osmotic shock procedures, the recombinant CjapNPC2 was purified by a combination of hydrophobic interaction, ion exchange, and gel-filtration chromatography. Western blotting and immunohistochemistry were performed as described (45) by using antiserum, which was raised in rabbits against the purified recombinant CjapNPC2 (Operon Biotechnologies). Fluorescence competitive binding assay and GC-EAD analyses were carried out as described, respectively (33, 46). For crystallography, diffraction data were collected at a wavelength of 1.0000 Å with CCD detectors (Area Detector Systems), integrated, and scaled by using the program DENZO and SCALPACK in the HKL2000 program suite (47). The crystal structure of CjapNPC2 was determined by the molecular replacement method using the bovine NPC2 structure (Protein Data Bank ID code 2HKA) as a reference model by the program MOLREP (48). Initial model building was conducted by the ARP/wARP program (49). Manual model building and molecular refinement were performed by using Coot (50) and Refmac5 (51, 52). The stereochemistry of the models was analyzed with the program Rampage (53). Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Shizuo G. Kamita (University of California, Davis) and Dr. Zainulabeuddin Syed (University of Notre Dame) for comments during the preparation of our manuscript. We also thank Dr. Naoaki Saito, Dr. Tetsuro Mimura, Asuka Nakano, Dr. Midori Kidokoro-Kobayashi, and Dr. Mamiko Ozaki (Kobe University) for assistance with immunohistochemistry, supporting the fluorescence competitive binding assay, and supplying cocoons; and the beamline researchers and staff at the Photon Factory for X-ray diffraction data collection. This work was partly supported by the Global Centers of Excellence Program for Global Center for Education and Research in Integrative Membrane Biology and Japan Society for the Promotion of Science KAKENHI Grant 23580070 (to Y. Ishida).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The cDNA sequences reported in this paper have been deposited in the DNA Data Bank of Japan, http://www.ddbj.nig.ac.jp (accession nos. AB734099–AB734108). Atomic coordinates for the crystal structures of CjapNPC2 have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 3WEA (apo form) and 3WEB (in complex with oleic acid)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323928111/-/DCSupplemental.
References
- 1. Holldobler B, Wilson EO (1990) The Ants (Belknap/Harvard Univ Press, Cambridge, MA) p 733.
- 2.Schultz TR. In search of ant ancestors. Proc Natl Acad Sci USA. 2000;97(26):14028–14029. doi: 10.1073/pnas.011513798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Meer R. Ant interactions with soil organisms and associated semiochemicals. J Chem Ecol. 2012;38(6):728–745. doi: 10.1007/s10886-012-0140-8. [DOI] [PubMed] [Google Scholar]
- 4.Holldobler B, Carlin NF. Anonymity and specificity in the chemical communication singals of social insects. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1987;161(4):567–581. [Google Scholar]
- 5.Nakanishi A, Nishino H, Watanabe H, Yokohari F, Nishikawa M. Sex-specific antennal sensory system in the ant Camponotus japonicus: Structure and distribution of sensilla on the flagellum. Cell Tissue Res. 2009;338(1):79–97. doi: 10.1007/s00441-009-0863-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhou X, et al. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 2012;8(8):e1002930. doi: 10.1371/journal.pgen.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keil TA, Steinbrecht RA. Diffusion barriers in silkmoth sensory epithelia: application of lanthanum tracer to olfactory sensilla of Antheraea polyphemus and Bombyx mori. Tissue Cell. 1987;19(1):119–134. doi: 10.1016/0040-8166(87)90063-2. [DOI] [PubMed] [Google Scholar]
- 8.Vogt RG. Molecular basis of pheromone detection in insects. In: Gilbert LI, Iatro K, Gills S, editors. Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology. Vol 3. London: Elsevier; 2005. pp. 753–804. [Google Scholar]
- 9.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 10.Pelosi P, Zhou J-J, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63(14):1658–1676. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallem EA, Dahanukar A, Carlson JR. Insect odor and taste receptors. Annu Rev Entomol. 2006;51:113–135. doi: 10.1146/annurev.ento.51.051705.113646. [DOI] [PubMed] [Google Scholar]
- 12.Leal WS. Proteins that make sense. In: Blomquist GJ, Vogt RG, editors. Insect Pheromone Biochemistry and Molecular Biology: The Biosynthesis and Detection of Pheromones and Plant Volatiles. San Diego: Elsevier Academic; 2003. pp. 447–476. [Google Scholar]
- 13.Sandler BH, Nikonova L, Leal WS, Clardy J. Sexual attraction in the silkworm moth: Structure of the pheromone-binding-protein-bombykol complex. Chem Biol. 2000;7(2):143–151. doi: 10.1016/s1074-5521(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 14.Lartigue A, et al. X-ray structure and ligand binding study of a moth chemosensory protein. J Biol Chem. 2002;277(35):32094–32098. doi: 10.1074/jbc.M204371200. [DOI] [PubMed] [Google Scholar]
- 15.Damberger FF, Ishida Y, Leal WS, Wüthrich K. Structural basis of ligand binding and release in insect pheromone-binding proteins: NMR structure of Antheraea polyphemus PBP1 at pH 4.5. J Mol Biol. 2007;373(4):811–819. doi: 10.1016/j.jmb.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 16.Damberger FF, Michel E, Ishida Y, Leal WS, Wüthrich K. Pheromone discrimination by a pH-tuned polymorphism of the Bombyx mori pheromone-binding protein. Proc Natl Acad Sci USA. 2013;110(46):18680–18685. doi: 10.1073/pnas.1317706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González D, et al. The major antennal chemosensory protein of red imported fire ant workers. Insect Mol Biol. 2009;18(3):395–404. doi: 10.1111/j.1365-2583.2009.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotzek D, Robertson HM, Wurm Y, Shoemaker D. Odorant binding proteins of the red imported fire ant, Solenopsis invicta: An example of the problems facing the analysis of widely divergent proteins. PLoS ONE. 2011;6(1):e16289. doi: 10.1371/journal.pone.0016289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liou H-L, et al. NPC2, the protein deficient in Niemann-Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem. 2006;281(48):36710–36723. doi: 10.1074/jbc.M608743200. [DOI] [PubMed] [Google Scholar]
- 20.Leal WS. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373–391. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- 21.Bonasio R, et al. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. Science. 2010;329(5995):1068–1071. doi: 10.1126/science.1192428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu S, Benoff B, Liou H-L, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282(32):23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedland N, Liou H-L, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci USA. 2003;100(5):2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Warren JT, Buchanan J, Gilbert LI, Scott MP. Drosophila Niemann-Pick type C-2 genes control sterol homeostasis and steroid biosynthesis: A model of human neurodegenerative disease. Development. 2007;134(20):3733–3742. doi: 10.1242/dev.004572. [DOI] [PubMed] [Google Scholar]
- 25.Shanbhag SR, et al. Expression mosaic of odorant-binding proteins in Drosophila olfactory organs. Microsc Res Tech. 2001;55(5):297–306. doi: 10.1002/jemt.1179. [DOI] [PubMed] [Google Scholar]
- 26.Boulay R, Coll-Toledano J, Manzaneda AJ, Cerdá X. Geographic variations in seed dispersal by ants: Are plant and seed traits decisive? Naturwissenschaften. 2007;94(3):242–246. doi: 10.1007/s00114-006-0185-z. [DOI] [PubMed] [Google Scholar]
- 27.Hughes L, Westoby M, Jurado E. Convergence of elaiosomes and insect prey: Evidence from ant foraging behaviour and fatty acid composition. Funct Ecol. 1994;8:358–365. [Google Scholar]
- 28.Leal WS, et al. Kinetics and molecular properties of pheromone binding and release. Proc Natl Acad Sci USA. 2005;102(15):5386–5391. doi: 10.1073/pnas.0501447102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keil TA. Surface coats of pore tubules and olfactory sensory dendrites of a silkmoth revealed by cationic markers. Tissue Cell. 1984;16(5):705–717. doi: 10.1016/0040-8166(84)90004-1. [DOI] [PubMed] [Google Scholar]
- 30.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281(42):31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 31.Steinbrecht RA. Pore structures in insect olfactory sensilla: A review of data and concepts. Int J Insect Morphol Embryol. 1997;26(3–4):229–245. [Google Scholar]
- 32.Hayashi N, Komae H. Components of the ant secretions. Biochem Syst Ecol. 1980;8(3):293–295. [Google Scholar]
- 33.Fujii T, et al. Female sex pheromone of a lichen moth Eilema japonica (Arctiidae, Lithosiinae): Components and control of production. J Insect Physiol. 2010;56(12):1986–1991. doi: 10.1016/j.jinsphys.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Struble DL, Arn H. Combined gas chromatography and electroantennogram recording of insect olfactory responses. In: Hummel HE, Miller TA, editors. Techniques in Pheromone Research. New York: Springer; 1984. [Google Scholar]
- 35.Steinbrecht RA. Zur morphometrie der antenne des seidenspinners, Bombyx mori L.: Zahl und verteilung der riechsensillen (Insecta, Lepidoptera) Z Morphol Tiere. 1970;68(2):93–126. [Google Scholar]
- 36.Ascunce MS, et al. Global invasion history of the fire ant Solenopsis invicta. Science. 2011;331(6020):1066–1068. doi: 10.1126/science.1198734. [DOI] [PubMed] [Google Scholar]
- 37.Suarez AV, Holway DA, Case TJ. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc Natl Acad Sci USA. 2001;98(3):1095–1100. doi: 10.1073/pnas.98.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leal WS, et al. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE. 2008;3(8):e3045. doi: 10.1371/journal.pone.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida Y, Leal WS. Cloning of putative odorant-degrading enzyme and integumental esterase cDNAs from the wild silkmoth, Antheraea polyphemus. Insect Biochem Mol Biol. 2002;32(12):1775–1780. doi: 10.1016/s0965-1748(02)00136-4. [DOI] [PubMed] [Google Scholar]
- 40.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 42.Gasteiger E, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The Proteomics Protocols Handbook. Humana, Clifton, NJ; 2005. pp. 571–607. [DOI] [PubMed] [Google Scholar]
- 43.Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36(Web Server issue):W197–W201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishida Y, Ishibashi J, Leal WS. Fatty acid solubilizer from the oral disk of the blowfly. PLoS ONE. 2013;8(1):e51779. doi: 10.1371/journal.pone.0051779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishida Y, Ozaki M. Aversive odorant causing appetite decrease downregulates tyrosine decarboxylase gene expression in the olfactory receptor neuron of the blowfly, Phormia regina. Naturwissenschaften. 2012;99(1):71–75. doi: 10.1007/s00114-011-0865-1. [DOI] [PubMed] [Google Scholar]
- 46.Xu W, Cornel AJ, Leal WS. Odorant-binding proteins of the malaria mosquito Anopheles funestus sensu stricto. PLoS ONE. 2010;5(10):e15403. doi: 10.1371/journal.pone.0015403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in ocillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 48.Vagin A, Teplyakov A. MOLREP: An automated program for molecular replacement. J Appl Cryst. 1997;30:1022–1025. [Google Scholar]
- 49.Perrakis A, Morris R, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nat Struct Biol. 1999;6(5):458–463. doi: 10.1038/8263. [DOI] [PubMed] [Google Scholar]
- 50.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 51.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53(Pt 3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 53.Lovell SC, et al. Structure validation by Calpha geometry: φ,ψ and Cbeta deviation. Proteins. 2003;50(3):437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.