Abstract
Forkhead box protein 3 (FOXP3) regulatory T cells (Tregs) are important in the maintenance of tumor immunity tolerance. Myeloid dendritic cells (mDCs) are antigen-presenting cells (APCs) specialized to initiate and regulate immunity. Tregs and mDCs are suspected of influencing the interaction between the tumor and immune system, and thus the course of tumors. However, the implication and interaction of their concurrent infitration in colorectal cancer (CRC) remain unknown. The aim of this study was to determine FOXP3+ Tregs and CD11c+ mDCs infiltration in CRC and tumor-draining lymph node (TDLN) and to explore the clinical and pathological implication of suppressor and effector immune cell subsets. Immunohistochemical assay was conducted to assess FOXP3+ Tregs and CD11c+ mDCs infiltration in tumor tissue and in metastasis-free TDLN (mfTDLN) and metastatic TDLN (mTDLN). The results showed that FOXP3+ Tregs and CD11c+ mDCs infiltration was higher in tumor tissue compared to adjacent normal mucosa (P<0.001). FOXP3+ Tregs infiltration was associated with advanced tumor-node-metastasis (TNM) stage and lymph node metastasis (P<0.001 and P<0.001, for TNM stage and lymph node metastasis, respectively), whereas less CD11c+ mDCs infiltration of tumor in situ was associated with deeper tumor invasion, advanced TNM stages and lymph node metastasis (P<0.05, P<0.001 and P<0.001, for tumor invasion depth, TNM stages and lymph node metastasis, respectively). Compared to mfTDLN, mTDLN was significantly enriched in FOXP3+ Tregs (P<0.001) and reduced in CD11c+ mDCs (P<0.001). The statistical analysis demonstrated no significant correlations in Tregs and mDCs infiltration. These results suggest that more FOXP3+ Tregs and less CD11c+ mDCs infiltration have stronger prognostic significance in CRC. The presence of tumor cells in mTDLN may contribute to a tolerogenic milieu and facilitate the survival of metastatic tumor cells.
Keywords: colorectal cancer, tumor-draining lymph nodes, tumor metastasis, immune state, regulatory T cells, myeloid dendritic cells
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal tumors and ranks third regarding cancer-associated morbidity. Clinically, due to preoperative medical imaging examination, surgical treatment technology and use of powerful chemotherapeutics, the CRC prognosis has evidently improved. Nevertheless, 40–50% of CRC patients suffer from recurrence and metastasis. In the various factors that affect the prognosis of CRC, a variety of immune cells in CRC microenvironment and draining lymph nodes play a crucial role.
Regulatory T cells (Tregs) are a unique CD4+ helper T cell subset and are thought to modulate the autoimmune response. Tregs are characterized by the CD4+CD25+ phenotype. Forkhead box protein 3 (FOXP3) belongs to the forkhead/winged-helix family of transcriptional regulators and acts as a master regulator of the development and function of Tregs. The most specific and reliable surface marker of Tregs has been identified (1–3). Limited anti-tumor activity of antigen-specific T cells at a clinical level may be limited in CRC patients for various reasons. One major reason for the clinical failure of tumor-directed immune responses might be the generation of the tolerogenic milieu in tumor tissue and tumor-draining lymph nodes (TDLN). Tregs are partly responsible for impeding immune escape against autologous tumor cells (4).
Dendritic cells (DCs) are powerful antigen-presenting cells (APCs) and the interaction of T cells with DCs is fundamental for a favorable immune response to tumors. DCs comprise two phenotypically and functionally distinct subpopulation, conventional myeloid dendritic cells (mDCs) and plasmacytoid DCs (pDCs). pDCs are type-I IFN-producing cells known for their ability to promote anti-viral innate and adaptive immune response. When compared with conventional DCs, pDCs are implicated in protective immunity as well as in tolerance induction (5–6). mDCs function as specific antigen-presenting cells, it can play a critical role in inducing and regulating T cell-mediated immune response, including TH1, TH2 and TH17 response (7). As the strongest APCs, mDCs are able to induce specific cytotoxic T-lymphocyte generation. For this reason, mDCs are closely correlated with tumor occurrence and development (8). Furthermore, mDCs have an important impact on selection of Tregs in thymus and differentiation, proliferation and function regulation in peripheral lymphoid tissues (9–12). In vitro studies also showed that mDCs have the ability to activate Tregs under certain circumstances (13). At present, whether or not the CRC intratumoral presence of mDCs affects the antigen-specific Tregs, clinical course or outcome of the patients has yet to be elucidated.
The aim of our study was to analyze the infiltration of CRC by FOXP3+ Tregs and CD11c+ mDCs in tumor in situ and TDLN, and to investigate whether or not there is a correlation with the clinicopathological characteristics of the disease. We applied an immunohistochemical method with FOXP3-Ab and CD11c-Ab to examine Tregs and mDCs infiltration and to identify the potential roles of Tregs and mDCs in CRC immunity.
Patients and methods
Patients and tissue specimens
Fifty two formalin-fixed paraffin-embedded (FFPE) CRC samples and 20 FFPE normal colorectal mucosa samples obtained from >5 cm from the tumor margin were used in this study from patients who underwent treatment at the General Hospital of CNPC in Jilin between 2008 and 2011. The investigation was approved by the Ethics Committee of CNPC. The patients (29 males, 23 females; mean age, 64 years, ranging from 47 to 82 years) were diagnosed as CRC without preoperative radiotherapy or chemotherapy. A number of clinicopathological characteristics, such as gender, age, location, histological type, tumor-node-metastasis (TNM), depth of tumor invasion, lymph node metastasis, and the differentiation degree were included in clinicopathological studies. According to the classification of CRC (WHO 2000), 47 samples were tubular adenocarcinoma and 5 were mucoid carcinoma. Regarding tumor differentiation, 6 samples were well-differentiated and 41 samples were moderately-poorly differentiated. Twenty of 52 patients had lymph node metastasis, while 32 of 52 patients had no lymph node metastasis. According to the Union for International Cancer Control (UICC 2002) criteria, 14 patients were in the grade T1+T2 group and 38 patients were in the grade T3+T4 group. Thirty patients were in the I+II tumor group, 22 were in the III+IV tumor group according to TNM staging criteria of the 2002 UICC staging system.
Immunohistochemical analysis
FFPE tissue sections (4 μm) were prepared for immunohistochemistry using the Ready-to-Use Immunohistochemistry Hypersensitivity UltraSensitive™ S-P kit (Fuzhou Maxim, Fuzhou, China) according to the manufacturer’s instructions. Tissue sections were first deparaffinized in xylene and rehydrated in a series of graded alcohols. After hydration, endogenous peroxidase activity was blocked using 3% H2O2 (v/v) for 10 min at room temperature. Standard antigen retrieval was then performed using heat-induced epitope retrieval by heating the slides immersed in the boiling retrieval solution (EDTA, pH 8.0) in pressure boiler for 2 min. Gradually cooling at room temperature, the sections were washed three times with phosphate-buffered saline (PBS), then non-immune animal serum was dropped onto the sections for 10 min at room temperature and the serum was wiped off. The sections were subsequently incubated with monoclonal antibody against either FOXP3 (Wuhan Boster Biological Technology, Ltd., Wuhan, China), or monoclonal antibody against CD11c (Beijing Fir Jinqiao Biotechnology Company, Beijing, China), followed by a biotin-labeled secondary antibody and streptomycin antibiotin peroxidase. Diaminobenzidine staining reaction was then performed, followed by hematoxylin counterstaining. The slides were then dehydrated, cleared and mounted as normal. Negative controls were performed by omitting the primary antibody instead of using PBS. Internal positive control was used for quality assurance. Ten randomly selected high-power fields (HPF; 1 HPF=0.237 mm2) were analyzed for Tregs and mDCs infiltration in the two tumors in situ and draining lymph nodes, and 10 HPF were averaged in each case.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software, version 13.0. Correlation of Tregs and mDCs infiltration with the clinicopathological characteristics of the tumors was evaluated using the Student’s t-test. Tregs and mDCs infiltration in metastatic TDLN (mTDLN) and in metastasis-free TDLN (mfTDLN) was also assessed using the Student’s t-test. Correlation of Tregs infiltration with mDCs infiltration was analyzed using the Spearman’s rank-order correlation analysis. For the comparisons, P<0.05 was considered to indicate a statistically significant difference.
Results
Tregs and mDCs infiltration in CRC
FOXP3 protein expression was found in the nucleus of Tregs. FOXP3+ Tregs were expressed in CRC tumor tissue and adjacent normal mucosa. However, the frequency of cells expressing FOXP3 protein in CRC tissue was much higher compared to adjacent normal mucosa (8.07/HPF vs. 0.96/HPF, P<0.01, Fig. 1A).
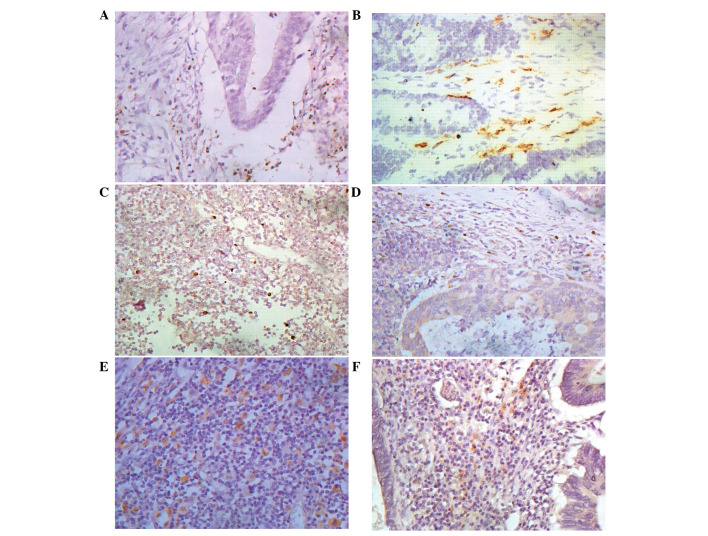
Figure 1.
Immunostaining in colorectal cancer (CRC) tissues and tumor-draining lymph node (TDLN). Nuclear staining for (A) FOXP3 in CRC and membranous staining for (B) CD11c in CRC. Nuclear staining for FOXP3 in (C) metastasis-free TDLN (mfTDLN) and (D) metastatic TDLN (mTDLN) and membranous staining for (E) CD11c in mfTDLN and (F) mTDLN. Original magnification, ×400.
The expression of CD11c protein was also analyzed in CRC tumor tissue and adjacent normal mucosa, and was primarily expressed in the cell membrane of mDCs. CD11c+ mDC exhibited a higher infiltration in CRC, which was in variable shapes, irregular and varying in size, and certain cells had extended cell processes. CD11c+ mDCs mainly scattered in the tumor stroma, or in the surrounding edge of tumor cell clusters, and were often accompanied by infiltration with lymphocytes. CD11c+ DCs infiltration was significantly increased in CRC tumor tissue compared to adjacent normal mucosa (6.72/HPF vs. 2.32/HPF, P<0.01, Fig. 1B).
Clinical and pathological implication of Tregs and mDCs infiltration
The correlations between the clinicopathological characteristics of the tumor samples and Tregs or mDCs infiltration are summarized in Table I. Significant increase in Tregs infiltration was associated with tumor samples that had advanced TNM stages (9.56/HPF vs. 6.97/HPF, P<0.01) and lymph node metastasis (9.72/HPF vs. 7.04, P<0.01). However, Tregs infiltration showed no statistically significant difference in patient age, tumor size, histology, invasion depth and tumor differentiation (P>0.05). By contrast, tumors with the deeper tumor invasion were found to have lower infiltration of CD11c+ mDCs (5.81/HPF vs. 8.51/HPF, P<0.05). The CD11c+ mDCs were significantly lower in advanced TNM stages showing signs of decreasing immunity against tumor (3.75/HPF vs. 9.69/HPF, P<0.01). As expected, CD11c+ mDCs infiltration decreased in tumors with the presence of lymph node metastasis compared to the absence of lymph node metastasis (3.73/HPF vs. 8.99/HPF, P<0.01). No statistically significant difference was found in various age, size, histology and differentiation groups with regard to CD11c expression (P>0.05).
Table I.
Correlation between Tregs and mDCs infiltration and clinicopathological characteristics in CRC.
| Clinicopathological characteristics | No. | Treg/HPF ± SD | P-value | mDC/HPF ± SD | P-value |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≥60 | 33 | 7.77±2.47 | 0.165 | 6.81±4.30 | 0.430 |
| <60 | 19 | 8.59±3.49 | 6.58±4.31 | ||
| Histology | |||||
| Tubular adenocarcinoma | 47 | 8.11±3.51 | 0.369 | 6.97±4.25 | 0.135 |
| Mucious carcinoma | 5 | 7.52±5.53 | 4.72±4.07 | ||
| Invasion depth | |||||
| T1+T2 | 14 | 7.25±2.82 | 0.109 | 8.51±4.39 | <0.05 |
| T3+T4 | 38 | 8.37±2.88 | 5.81±3.88 | ||
| Differentiation | |||||
| Well | 6 | 8.12±3.96 | 0.485 | 8.26±4.61 | 0.198 |
| Moderately-poorly | 41 | 8.07±2.87 | 6.52±4.23 | ||
| Lymph node metastasis | |||||
| Yes | 20 | 9.72±3.16 | <0.001 | 3.73±1.55 | <0.001 |
| No | 32 | 7.04±2.17 | 8.99±4.26 | ||
| TNM stage | |||||
| I+II | 30 | 6.97±2.18 | <0.001 | 9.69±4.01 | <0.001 |
| III+IV | 22 | 9.56±3.10 | 3.75±1.56 |
Values in bold indicate statistical significance. Tregs, regulatory T cells; HPF, high-power fields; SD, standard deviation; mDCs, myeloid dendritic cells; CRC, colorectal cancer.
Tregs and mDCs infiltration in mTDLN and mfTDLN
We aimed to assess whether or not metastatic cancer cells had any impact on Tregs and mDCs infiltration in TDLN. Therefore, 52 CRC cases, including 20 cases with mTDLN and 32 cases with mfTDLN were detected. FOXP3+ Tregs were diffusely distributed in the paracortical areas of lymph node and metastatic carcinoma stroma. Tregs infiltration was significantly higher in mTDLN compared to mfTDLN (8.2/HPF vs. 5.5/HPF, P<0.01, Fig. 1C and D). The mDCs status was observed, and results showed that CD11c+ mDCs mainly were distributed in the T cell-rich zones of lymph nodes. They also accumulated in the vicinity of tumor nests, and CD11c+ mDCs infiltration decreased significantly in mTDLN compared to mfTDLN (3.55/HPF vs. 9.03/HPF, P<0.01, Fig. 1 E and F).
Correlation of Tregs and mDCs infiltration
The correlation between FOXP3 and CD11c expression was also analyzed in CRC tissue and TDLN (Table II). The statistical analysis suggested that no significant correlation was observed in the Tregs and mDCs infiltrations.
Table II.
Correlation between FOXP3+ Tregs and mDCs in CRC tissue and TDLN.
| R | P-value | |
|---|---|---|
| CRC tissue | −0.29 | 0.063 |
| TDLN | −0.30 | 0.137 |
R, Spearman’s coefficient correlation; signifcant correlations (P<0.05). FOXP3; forkhead box protein 3; Tregs, regulatory T cells; CRC, colorectal cancer; TDLN, tumor-draining lymph node.
Discussion
FOXP3, a member of the forkhead/winged-helix transcriptional factor family, has been demonstrated to be the master regulator of Tregs development in the thymus, as well as Tregs suppressive function (1–3,14). A series of studies demonstrated that Tregs was closely correlated with the tumor immune escape mechanism. In previous studies, increased proportions of CD4+CD25+ Tregs in the total CD4+ T-cell populations were detected in various types of cancer, including lung, breast and ovarian tumors, and metastatic melanoma in lymph nodes (15–18). Tregs infiltrating human tumors were shown to be tumor antigen-specific as indicated by experiments showing that Tregs suppressed the proliferation of naïve CD4+ T cells and inhibited IL-2 secretion by CD4+ effector cells upon activation by tumor-specific antigens (19–20). Sato and colleagues found that ovarian cancer patients with a higher CD8+/Treg-cell ratio in tumor tissue had an increased survival chance (21). Their study suggested that Tregs negatively affects the antitumor immune response mediated by effector CD8+ T cells. This finding suggests that strategies aiming to enhance tumor-specific CD8+ T cells while decreasing Tregs may be effective in improving the outcome in cancer patients. Consistent with those studies, we found significantly higher numbers of Tregs in CRC compared to the adjacent normal mucosa. Although numbers varied from patient to patient, there was an ∼8-fold increase of Tregs infiltration in CRC compared to healthy mucosa.
To exhibit potential functions of Tregs in CRC, we analyzed the correlation between Tregs and clinicopathological characteristics. Results showed that Tregs infiltration was closely correlated with lymph node metastasis and TNM staging. Tregs in patients with lymph node metastasis were significantly more expressed compared to those without lymph node metastasis and Tregs infiltration showed an increase in TNM staging. Lymph node metastasis and TNM staging are important indications for judging the prognosis and survival time of cancer patients. Therefore, the results suggest that Tregs infiltration correlates with CRC metastasis and poor prognosis. Tumor cells have potentially more opportunity to be exposed to immune cells directly or indirectly with the progression of disease. Furthermore, tumor cells secrete factors such as transforming growth factor β (TGF-β), COX-2 and IL-10, thus recruiting and expanding Tregs in tumor tissue (22–23). In addition, tumor cells secrete chemokine CCL22, which can be combined with a high expression of CCR4 receptors on the surface of FOXP3+ Tregs. It also contributes to the recruitment of Tregs to tumor tissue (17).
mDCs are specialized APCs, characterized by the efficient induction of primary T-cell responses and are thus able to initiate body immune responses, and produce anti-tumor immunity (24). Of note, we found an ∼3-fold increase of mDCs at the tumor site in CRC patients compared to normal mucosa patients. The mechanism of this phenomenon is unclear. However, it underlines the importance of anti-tumor immunity responses in in situ analyses. We hypothesize that on CRC tumor-antigen stimulation, the body part of the mDCs migrates to tumor tissue. Previous studies have suggested that mDCs density ≥20/HPF in tumor tissue was identified as high infiltration. According to this standard, mDCs infiltrated in CRC tumor tissue were 6.72/HPF and did not reach the level of anti-tumor immune response, which might be a reason for CRC local tissue immunodeficiency (25). Since the decrease in the frequency and function of mDCs was considered a negative factor in anti-tumor immunity (26), we performed an analysis of mDCs infiltration in correlation with the clinicopathological characteristics. The study showed that mDCs infiltration negatively correlated with lymph node metastasis and invasion depth. The mDCs infiltration in patients with lymph node metastasis was significantly lower than that in patients without lymph node metastasis, and a decrease in mDCs infiltration occurred with an increase in TNM staging. Therefore, the extent of infiltrated mDCs in CRC tumor tissue generally reflects the tumor immune state.
In tumor immunity, the presentation of tumor antigen to naive CD4+ T cells occurs in regional lymph nodes, known as TDLN, in principle the optimal environment for the generation of an effective anti-tumor immune response. However, TDLN are under the effect of tolerogenic factors produced by tumor cells (17,27,28). Tumor immune tolerance rather than activation is induced to hinder the immune attack and promote tumor progress. In other words, mfTDLN and mTDLN are under the effect of tolerogenic factors imported from the tumor site. It was thought that immune tolerance should be more evident in mTDLN, which are mostly exposed to tolerogenic factors locally produced by tumor cells. In the present study, we aimed to confirm whether or not mTDLN exposed to mostly tolerogenic factors were more immunosuppressed compared to mfTDLN. We analyzed Tregs and mDCs infiltration and found a significant excess of Tregs in mTDLN. By contrast, mDCs infiltration in mTDLN was much lower compared to mfTDLN. This observation suggests that in mTDLN the presence of tumor cells could break the in situ anti-tumor immune response down, while facilitating tumor progression.
The above results have demonstrated that Tregs and mDCs infiltration correlates with a malignant biologic phenotype in CRC. Additionally, studies have shown that mDCs were associated with Tregs selection in thymus and differentiation, proliferation and function regulation in peripheral lymphoid tissues (9–12). Increasing evidence suggests that intratumoral DCs are actively immunosuppressive and induce tolerance to tumor (29). However, understanding of the correlation of DCs and Tregs interaction, modulation of activity and function of various DCs subtypes at the tumor site is incomplete. Thus, we also analyzed the correlation between mDCs Tregs and in CRC tumor tissue and TDLN and did not find any significant correlation in the tumor tissue or in TDLN. As a tolerogenic DCs, pDCs may induce tolerance and promote Tregs development rather than tumor immunity (30–32). The shift of DCs subset in tumor tissue may tip the balance against anti-tumor immune response and facilitate the survival of tumor cells (33). The correlation and significance of pDCs and FOXP3+ Tregs in CRC is to be discussed in a future study.
In summary, FOXP3 are highly expressed in CRC tumor tissue and TDLN. Nevertheless, CD11c expression decreased in TDLN and in CRC tumor stroma with CRC development, and are strongly correlated with malignant tumor phenotypes. Thus, the concurrent increased expression of FOXP3 and decreased expression of CD11c may contribute to an efficient metastasis of CRC to the lymph node and to tumor progress.
Acknowledgments
This study was supported by grant no. 2011151 from the Department of Education of Jilin Province and National Natural Science Foundation of China (grant no. 81170632).
References
- 1.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 3.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 4.Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287–302. doi: 10.1007/3-540-27702-1_13. [DOI] [PubMed] [Google Scholar]
- 5.Gilliet M, Liu Y. Human plasmacytoid-derived dendritic cells and the induction of T-regulatory cells. Hum Immunol. 2002;63:1149–1155. doi: 10.1016/s0198-8859(02)00753-x. [DOI] [PubMed] [Google Scholar]
- 6.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 7.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269:64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarbell KV, Petit L, Zuo X, Toy P, et al. Dendritic cell-expanded, islet-specific CD4+CD25+CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 11.Liu YJ. A unified theory of central tolerance in the thymus. Trends Immunol. 2006;27:215–221. doi: 10.1016/j.it.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki S, Patel M, Harper A, et al. Effective expansion of alloantigen-specific Foxp3+ CD25+ CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc Natl Acad Sci USA. 2006;103:2758–2763. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee DK, Dhodapkar MV, Matayeva E, et al. Expansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 15.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 16.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 17.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 18.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 19.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang HY, Peng G, Guo Z, et al. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174:2661–2670. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 21.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 23.Rolle CE, Sengupta S, Lesniak MS. Mechanisms of immune evasion by gliomas. Adv Exp Med Biol. 2012;746:53–76. doi: 10.1007/978-1-4614-3146-6_5. [DOI] [PubMed] [Google Scholar]
- 24.Vinay DS, Kim CH, Choi BK, Kwon BS. Origins and functional basis of regulatory CD11c+ CD8+ T cells. Eur J Immunol. 2009;39:1552–1563. doi: 10.1002/eji.200839057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsujitani S, Kakeji Y, Watanabe A, et al. Infiltration of dendritic cells in relation to tumor invasion and lymph node metastasis in human gastric cancer. Cancer. 1990;66:2012–2016. doi: 10.1002/1097-0142(19901101)66:9<2012::aid-cncr2820660928>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 26.Schuler PJ, Börger V, Bölke E, et al. Dendritic cell generation and CD4+ CD25 high FOXP3+ regulatory T cells in human head and neck carcinoma during radio-chemotherapy. Eur J Med Res. 2011;16:57–62. doi: 10.1186/2047-783X-16-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cochran AJ, Huang RR, Lee J, et al. Tumor-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6:659–670. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 28.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 29.Shurin MR, Shurin GV, Lokshin A, et al. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: friends or enemies? Cancer Metastasis Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- 30.Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 31.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubert P, Jacobs N, Caberg JH, et al. The cross-talk between dendritic and regulatory T cells: good or evil? J Leukoc Biol. 2007;82:781–794. doi: 10.1189/jlb.1106694. [DOI] [PubMed] [Google Scholar]
- 33.Battaglia A, Buzzonetti A, Baranello C, et al. Metastatic tumour cells favour the generation of a tolerogenic milieu in tumour draining lymph node in patients with early cervical cancer. Cancer Immunol Immunother. 2009;58:1363–1373. doi: 10.1007/s00262-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]