Abstract
A hyperplastic polyp (HP) >10 mm is described as a large hyperplastic polyp (LHP). Previous studies have considered LHP and sessile serrated adenoma/polyp (SSA/P) as synonymous. Although HP and SSA/P have previously been morphologically distinguished, differences between LHP and SSA/P have not yet been reported. The present study aimed to define the differences between SSA/P and non-SSA/P in LHP using immunohistochemistry for Ki67. Colorectal serrated lesions (>10 mm) that were completely resected by endoscope and derived from 11 institutions in Japan [Dokkyo Medical University School of Medicine (Mibu), Takahiro Fujii Clinic (Tokyo), Sano Hospital (Kobe), Oda GI Clinic, Hattori GI Endoscopy and Oncology Clinic (Kumamoto), Ohta Clinic (Nagoya), Hiroshima University (Hiroshima), Iwate Medical University (Morioka), Juntendo and Kyorin Universities (Tokyo) as well as Toyama University (Toyama)] affiliated with the Japanese Society for Cancer of the Colon and Rectum (JSCCR) between January 2003 and December 2010 were selected. The histological criteria of the Japanese Society for Cancer of the Colon and Rectum (JSCCR, project meeting; editor-in chief, Takashi Yao) were used to distinguish SSA/P and non-SSA/P from LHP. Non-SSA/P comprises both incomplete SSA/P and HP. A total of 154 samples diagnosed as SSA/P or non-SSA/P from 148 patients were used. This study comprised 107 SSA/P and 47 non-SSA/P cases, whereby lesions were located on the right side of the colon (73.2 and 26.8%, respectively). Ki67-positivity in SSA/Ps was significantly higher compared to non-SSA/Ps. A greater number of SSA/Ps in LHP were located on the right side of the colon compared to the left side. SSA/Ps occurring on the right side of the colon may be precursor lesions of colorectal carcinoma in serrated neoplasia pathways. In conclusion, LHPs and SSA/Ps limited to the right side of the colon are suggested to be clinically treated as the same type of lesions.
Keywords: large hyperplastic polyp, sessile serrated adenoma/polyp, Ki67, tumor location
Introduction
Colorectal serrated lesions were previously classified into hyperplastic polyp (HP) and serrated adenoma (SA). In 1996, Torlakovic and Snover (1) and Torlakovic et al(2) reported that certain HPs may be of neoplastic nature, suggesting sessile serrated adenoma/polyp (SSA/P) as a new concept for serrated polyps. Currently, colorectal serrated lesions are classified as HP, SSA/P and traditional serrated adenoma (TSA), as defined by the World Health Organization (WHO) (3), irrespective of the conventional adenoma-carcinoma sequence.
However, in 1994, Warner et al(4) introduced the term large hyperplastic polyp (LHP) to describe HP >10 mm. LHP and SSA/P were used synonymously by Jass (5), although not supported by the present definition of WHO.
The aim of this study was to clarify the differences between SSA/P and non-SSA/P in LHP by comparing the proliferation rate using immunohistochemical staining for Ki67.
Materials and methods
Study cases and tissue samples
This study comprised 231 colorectal serrated lesions (>10 mm), obtained from 223 patients, which were completely resected by an endoscopist, and derived from 11 institutions in Japan [Dokkyo Medical University School of Medicine (Mibu), Takahiro Fujii Clinic (Tokyo), Sano Hospital (Kobe), Oda GI Clinic, Hattori GI Endoscopy and Oncology Clinic (Kumamoto), Ohta Clinic (Tokyo), Hiroshima University (Hiroshima), Iwate Medical University (Morioka), Juntendo and Kyorin Universities (Tokyo) as well as Toyama University (Toyama)] affiliated with the Japanese Society for Cancer of the Colon and Rectum (JSCCR) between January 2003 and 2010. The study was performed with the approval of the research meeting members of the Dokkyo Medical University Surgical Pathology. Informed consent was obtained from all patients. JSCCR histologic criteria (Japanese Society for Cancer of the Colon and Rectum, project meeting; editor-in-chief, Takashi Yao) of SSA/P were used to distinguish SSA/P and non-SSA/P from LHP. SSA/P was distinguished from conventional HP on the basis of the following criteria: i) crypt dilation, ii) irregularly branching crypts and iii) horizontally arranged basal area crypts at the basal (inverted T and/or L-shaped crypts). The serrated lesions meeting >2 of these criteria were diagnosed as SSA/P, while those meeting 1 or none were diagnosed as non-SSA/P (3,6,7). The selected cases were evaluated by intra- and inter-observation by Y.S., T.F., K.I., S.T., H.T, Y.F., T.F., Y.O., T.S., T.Y., Y.O. and J.I., as part of a project of JSCCR. Only the cases where a consensus regarding the diagnosis had been reached were examined. The locations of the tumors were classified into three groups: the right (cecum, ascending and transverse colon) and left side of the colon (descending and sigmoid colon), as well as the rectum. A total of 154 cases diagnosed as SSA/P or non-SSA/P, obtained from 148 patients were used for this study. Hematoxylin and eosin (H&E) staining was performed as the standard protocol for the pathological examination.
Immunohistochemical staining for Ki67
Immunohisto-chemical staining for Ki67 was performed with a LSAB-2 kit (LSAB®2 System-HRP; Dako, Carpinteria, CA, USA) as previously described (7–9). The 4-μm sections were placed on slides, deparaffinized and dehydrated. The sections were then placed in 0.01 mol/l citrate buffer (pH 6.0) and treated by microwave heating (400 W, 95°C; MI-77; Azumaya, Tokyo, Japan) for 40 min to facilitate antigen retrieval for Ki67. Sections were pretreated with 0.3% H2O2 in methanol at room temperature to quench endogenous peroxidase activity, followed by blocking with Protein Block Serum-Free (Dako) for 30 min, and incubation with anti-Ki67 antibody (1:50 clone MIB-1; Dako Japan, Co., Ltd., Kyoto, Japan) for 1 h. Thereafter, the sections were incubated with biotinylated secondary antibody for 15 min, washed with phosphate-buffered saline (PBS) and treated with peroxidase-conjugated streptavidin for 20 min. The sections were then incubated in 3,3′-diaminobenzidine tetrahydrochloride with 0.05% H2O2 (Liquid DAB+Substrate Chromogen System; Dako) for 3 min and counterstained with Carazzi’s hematoxylin.
Evaluation of immunohistochemical Ki67 expression
To evaluate the target cells, 2 contiguous crypts detected from the bottom to the surface of the colorectal epithelium were selected.
Immunostained sections were evaluated as previously described (7,10,11). In the first evaluation, the proportion of positive cells stained for Ki67 was calculated as the percentage of total cells in 2 crypts. In the second step, to evaluate the asymmetrical staining pattern of Ki67-positive cells in each individual crypt, the length between th e bottom of the crypt to the topmost Ki67-positive cells located at the surface was measured. Computer-assisted cytometrical analysis of Ki67 immunoreactivity was performed using the WinRooF (Fig. 1) image processing software (Mitani Co., Tokyo, Japan) (12,13). Ki67-positivity was evaluated by the surgeons Y.S. and H.U. and the physicians R.K. and Y.F. in our laboratory. Twenty cases were randomly selected and evaluated and remeasured by Y.S. and R.K. to ascertain agreement among the four evaluators.
Figure 1.
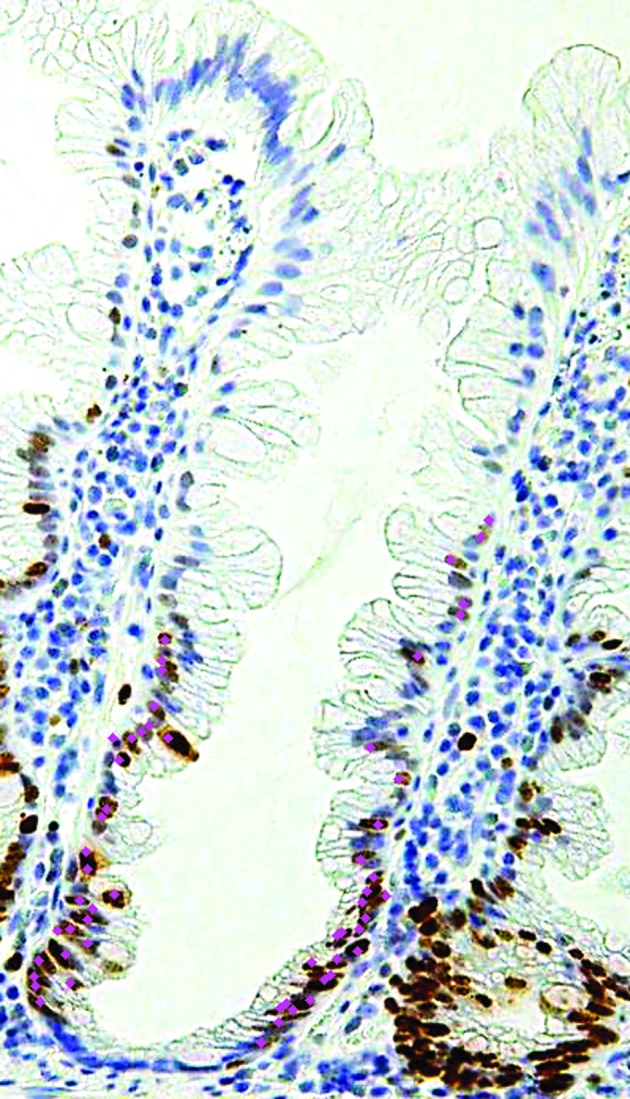
Computer-assisted cytometrical analysis of Ki67 immunoreactivity was performed using the WinRooF image processing software (Mitani Co., Tokyo, Japan). Count of the touched nucleus.
Statistical analysis
Statistical analysis was performed using the Stateflex program package (version 6). For the statistical analysis of the association of SSA/P and non-SSA/P with gender, age, location of tumor and endoscopic morphology, Fisher’s exact test or χ2 test were used. The proportion of positive cells stained for Ki67 and the asymmetrical location of proliferated cells in the crypts were analyzed using the Mann-Whitney U test. The agreement between evaluators was analyzed using the Wilcoxon signed-rank test. Continuous variables were expressed as the mean ± SD, or, where indicated, the median and interquartile range (IQR). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics
The selected cases were classified into two groups based on the evaluation by H&E staining: SSA/P and non-SSA/P. This study comprised 107 SSA/P cases in 101 patients and 47 non-SSA/P cases in 47 patients. The number of males and females diagnosed with SSA/P was almost identical, yet there were more males compared to females with non-SSA/P. Thus, there tended to be a larger number of females with SSA/P compared to non-SSA/P. The most common location of tumors was the right side of the colon in both SSA/P and non-SSA/P (SSA/P, 84.1%; non-SSA/P, 70.2%) cases, showing no statistically significant difference. The most common endoscopic morphology was type IIa lesion in both SSA/P and non-SSA/P cases. No statistically significant difference was identified between SSA/P and non-SSA/P with regard to endoscopic morphology. The patient characteristics are outlined in Table I.
Table I.
Clinicopathological characteristics of SSA/P and non-SSA/P.
| Characteristics | SSA/P n=107 | non-SSA/P n=47 | P-value |
|---|---|---|---|
| Gender | |||
| Male | 55 | 34 | |
| Female | 52 | 13 | 0.015 |
| Age (years) | 57.1±9.9 | 57.9±10.0 | NS |
| Location | |||
| Right side | 90 (84.1%) | 33 (70.2%) | |
| Left side | 17 (15.9%) | 13 (27.7%) | |
| Rectum | 0 | 1 (2.1%) | NS |
| Endoscopic morphology | |||
| Is | 19 (17.8%) | 5 (10.6%) | |
| Isp | 1 (0.9%) | 1 (2.1%) | |
| Ip | 5 (4.7%) | 0 | |
| IIa | 78 (72.9%) | 39 (83.0%) | |
| Is + IIa | 4 (3.7%) | 1 (2.1%) | |
| IIa + IIc | 0 | 1 (2.1%) | NS |
SSA/P, sessile serrated adenoma/polyp; non-SSA/P, sessile serrated adenoma/polyp; NS, not significant.
Immunohistochemical Ki67 expression in SSA/P and non-SSA/P
The median Ki67-positivity was 40.5% (range, 33.6–47.3%) and 32.2% (range, 8.8–36.5%) in SSA/P and non-SSA/P lesions, respectively, with a statistically significant difference (P<0.01). The median asymmetry of the location was 15.0% (range, 8.3–20.7%) and 11.3% (range, 5.8–15.9%) in SSA/P and non-SSA/P lesions, respectively, with a statistically significant difference (P<0.02). Based on these results, SSA/P was demonstrated to be higher in immunopositivity for Ki67 and asymmetic in the location compared to non-SSA/P (Table II).
Table II.
Immunohistochemical Ki67 expression in SSA/P and non-SSA/P.
| SSA/P, % | non-SSA/P, % | P-value | |
|---|---|---|---|
| Ki67-positivity (IQR) | 40.5 (33.6–47.3) | 32.2 (8.8–36.6) | <0.0 |
| Asymmetry of the location (IQR) | 15.0 (8.3–20.6) | 11.3 (5.8–15.10) | 10.02 |
SSA/P, sessile serrated adenoma/polyp; non-SSA/P, sessile serrated adenoma/polyp; IQR, median and interquartile range; NS, not significant.
Moreover, no statistically significant differences were found in the assessments of the evaluators (Table III), thus, the evaluations were deemed to be sound and accurate.
Table III.
Correlation of immunohistochemical Ki67 expression between the two observers.
| Observer
|
|||
|---|---|---|---|
| Y.S., % | R.K., % | P-value | |
| Ki67-positivity (IQR) | 37.5 (23.9–46.3) | 36.1 (22.8–43.1) | NS |
| Asymmetry of the location (IQR) | 10.2 (7.0–17.9) | 11.2 (5.9–20.3) | NS |
IQR, median and interquartile range; NS, not significant.
The images showing H&E staining and immunohisto-chemistry for Ki67 in SSA/P and non-SSA/P are shown in Figs. 2 and 3.
Figure 2.
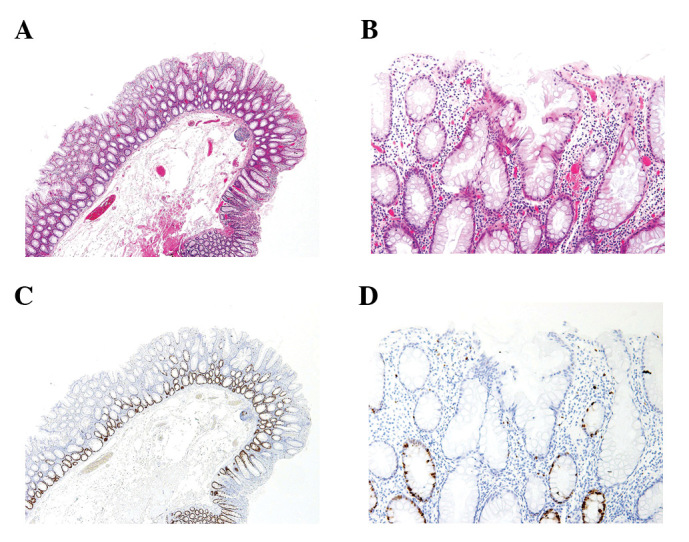
Morphology of the non-SSA/P. (A) Non-SSA/P showing hyper-plastic glands without neoplastic atypia. (B) Magnified view of (A). (C) Ki67-positive cell is observed in the lower portion of the tubules. (D) Magnified view of (C). SSA/P, sessile serrated adenoma/polyp; non-SSA/P, sessile serrated adenoma/polyp.
Figure 3.
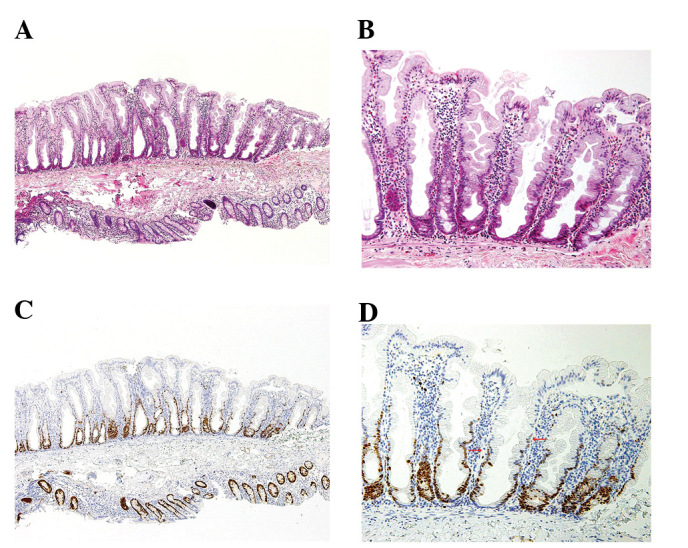
Morphology of the SSA/P. (A) SSA/P showing crypt dilation. (B) Magnified view of (A). (C) Ki67-positive cell is observed in the lower to higher portion of the tubules and asymmetry of the location. (D) Magnified view of (C).
Discussion
There is a variety of benign epithelial tumors and tumor-like lesions (non-neoplastic polyps, including inflammatory and hamartomatous polyps) in the colorectum. Sporadic adenoma presents as a benign epithelial tumor excluding polyposis possibly hereditary and a cancer/dysplasia associated with inflammatory bowel disease (neoplasia associated with ulcerative colitis). SA is a specific type of sporadic adenoma, while HP is an SA-like lesion (14,15). Both are morphologically serrated lesions. According to the Japanese Classification of Colorectal Carcinoma (second English edition) (16), most adenomas in the large intestine are circumscribed, elevated lesions (protruded or superficial type) and their surface is usually granular, lobulated or gyrus-like, although papillary or villous structure is sometimes observed. Based on their microscopic architecture, adenomas are classified as tubular, villous, tubulovillous or serrated. Papillary adenoma is not the term to describe any adenomas, and serrated adenomas resemble HPs due to the serrated morphology of the epithelium (saw-toothed epithelial infoldings) in the upper half of the duct. By contrast, in the superficial part nuclear swelling, pseudostratification, mitotic figures and hypereosinophilic cytoplasms are clearly visible. These lesions are correlated with mixed adenoma-serrated polyp (MSAP), mixed adenoma-hyperplastic polyp (MAHP) and SSA/P. SSA/P is a synonym for atypical HP, giant HP and serrated polyp with abnormal proliferation. In general, SSA/P is different from adenoma in structure and pathogenesis, the structure of SSA/P containing saw-toothed epithelial infoldings with no overt cytologic dysplasia, and its pathogenesis being correlated with the serrated neoplasia pathway (17–19), which differs from the adenoma-carcinoma sequence (20–22) and the de novo carcinogenesis of adenoma (22–24). Furthermore, Chung et al(25) suggested that large (>10 mm) SSA/Ps occurring on the right side of the colon are precursor lesions of colorectal carcinoma with microsatellite instability (MSI) mutation in the genetic background. Clinically, the incidence rate of SSA/P is 2–20% of colon polyps and 8–18% of serrated polyps, its morphology is flat or sessile, possibly exceeding 10 mm in diameter, having a predilection for the right side of the colon (2). The diagnosis of SSA/P lesions differs between institutions due to the lack of clear criteria. Although a study by Jass (5) had used the two terms LHP (>1 cm in diameter) and SSA/P interchangeably, however, another study by Fujii and Fujimori (26) had used the two terms differentially.
According to Higuchi et al(10) the histologic features of SSA/P are: i) exaggerated serrations in the lower crypt, increased surface callosity or papillarity; ii) increased crypt branching and/or horizontally arranged crypts at the base (inverted T and L-shaped crypts); iii) crypt dilation; iv) increased epithelial:stromal ratio (>50%); v) mitoses in the upper crypt; vi) cytological atypia in the upper crypt, particularly enlarged vesicular nuclei with prominent nucleoli and vii) increased mucin production (intracellular and/or luminal). SSA/P is diagnosed when the suspected lesion has four or more of these features, however, their histology assessment is complex during routine work. Recently, the characteristics of SSA/P were defined as follows: crypt dilation, horizontally arranged basal area crypt at the basal (inverted T and L-shaped crypts), prominent serration of crypts and although some areas of SSA/P may have straight crypts similar to those of microvesicular hyperplastic polyps (MVHP), in the latter, the straight crypts usually account for less than half of the lesion and if more than two or three contiguous crypts demonstrate features of SSA/P, the lesion should be classified as SSA/P, according to the WHO classification (3). For the facilitation of diagnosis, the research project ‘Potential of Cancerization of Colorectal Serrated Lesions’ supported by the Japanese Society for Cancer of the Colon and Rectum (6), further refined the histo-logic criteria of SSA/P, as defined by the WHO classification. In brief, SSA/P is composed of serrated cryptal epithelium with aberrant compartmentalization, essentially characterized by architectural abnormalities, including i) crypt dilation, ii) irregularly branching crypts and iii) horizontally arranged basal crypts (inverted T and/or L-shaped crypts). The lesion is diagnosed as SSA/P if at least two of these findings are present in >10% of the lesion (the criteria of SSA/P by JSCCR).
SSA/P and HP were diagnosed according to these criteria (JSCCR criteria) and the differences in proliferation were examined by evaluating the Ki67 index (using image analyzer and criteria as the current manuscript) and the reg1α protein expression within the framework of the research project ‘Potential of Cancerization of Colorectal Serrated Lesions’ (13). Consequently, SSA/Ps was found to have a higher proliferation rate compared to the HPs diagnosed in this study. Thus, SSA/Ps are not conventional cytologic dysplasias, rather neoplastic lesions (27).
The microscopic pathology of LHP (>10 mm) was also evaluated according to these criteria and classified into two categories: SSA/P and non-SSA/P (incomplete SSA/P + HP). The differences between SSA/P and non-SSA/P were defined by comparing the proliferation rates in this study. There were statistically significant differences in the proliferation between SSA/Ps and non-SSA/Ps, and with the latter comprising almost 40% of LHPs. Thus, we concluded that LHP and SSA/P are not the same type of lesion in the intestine. More than 70% of LHPs restricted to the right side of the colon were SSA/P, and in general, these lesions predominantly occur at this location. Thus, the same clinical management should be administered to LHP and SSA/P occurring on the right side of the colon. In this study, no typical SSA/Ps were found in the rectum.
Subsequently, the usefulness of endoscopic diagnosis of SSA/P (26,28,29) was evaluated. The crypt dilation in the lower portion of mucosa was found to reflect tubular structure in the surface mucosa, regardless of the structural variant of the tubules, whether IIIH- or expanded II-type pit pattern. However, the differentiation of the diagnosis of SSA/P and HP remains difficult by the sole evaluation of magnified endoscopic images of pit patterns at the present stage. Therefore, the diagnosis of SSA/P is suggested to be performed using a combination of endoscopic diagnosis, as well as the assessment of tumor location and endoscopic morphology as described above, since SSA/P has a preference for the right side of the colon and the IIIH- or expanded II-type pit pattern (Fig. 4). The canceration cases in these SSA/Ps according to the criteria are to be investigated in further studies.
Figure 4.
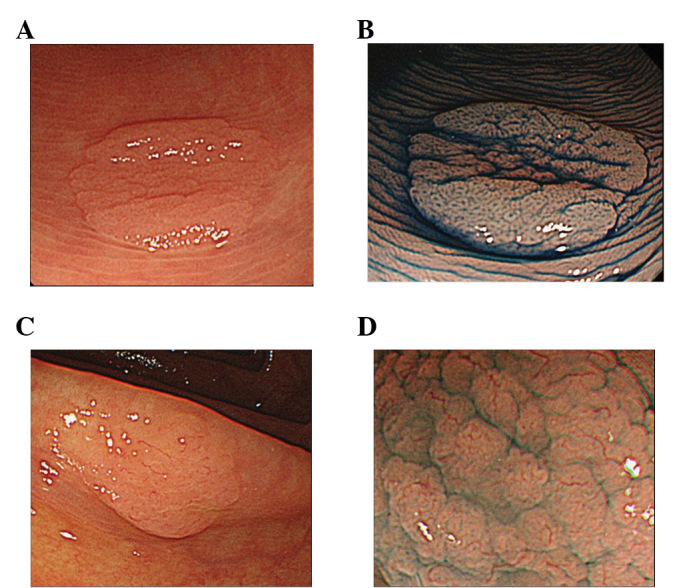
Endoscopy and magnified endoscopy images of non-SSA/P and SSA/P is shown. (A) and (B) are non-SSA/P, while (C) and (D) are SSA/P. Upper-side images are conventional endoscopic, while lower-side images are magnifying endoscopic images with indigo carmine solution. (A) Small flat elevated lesion located in the sigmoid colon. (B) Magnified view of the star-like pit pattern. (C) Small flat elevated lesion located in the ascending colon. (D) Magnified view of the expanded/fernlike pit pattern is shown (IIIH by T. Fujii, type 2: brown sicker vessels by Y. Sano). SSA/P, sessile serrated adenoma/polyp; non-SSA/P, sessile serrated adenoma/polyp.
SSA/P has a generally acknowledged methylation background, associated with the serrated neoplasia pathway (18). Epigenetic changes including DNA methylation are important mechanisms of carcinogenesis, particularly in the elderly (30). The right-side shift of colorectal carcinomas in Japan has been increasing along with the aging population. Therefore, findings of the present study highlight the importance of a complete resection of LHPs of the right-side colon, which are synonymous with SSA/P, and if not applicable, the careful observation and biopsy at short intervals are recommended.
Further studies, comprising pathological as well as endoscopic analyses of SSA/Ps, need to be conducted to confirm results regarding SSA/P and carcinogenesis, based on neoplasia cases associated with SSA/Ps, according to the same criteria.
Acknowledgments
This study was financed by the Japanese Society for Cancer of the Colon and Rectum, the Dokkyo Medical University, the Young Investigator award to Y.S. (no. 2011-03-5) and a Grant-in-Aid for Scientific Research to T. Fujimori (C).
The authors are grateful to A. Iwashita (Fukuoka University Chikushi Hospital), K. Sugihara (Surgical Oncology, Tokyo Medical and Dental University), Y. Katou (Department of Pathology, Cancer Institute), T. Shimoda (National Cancer Center), H. Watanabe, S. Ishiguro (PCL Japan) and T. Mutou (The Cancer Institute Hospital of JFCR) for their help in organizing our project research. The authors would also like to thank C. Matsuyama, A. Shimizu, T. Ono, M. Katayama, N. Nagashima, S. Kidate and A. Kikuchi (Department of Surgical and Molecular Pathology, Dokkyo Medical University School of Medicine, Mibu, Japan) for their excellent technical assistance.
References
- 1.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 2.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. International Agency for Research on Cancer; Lyon: 2010. pp. 160–165. [Google Scholar]
- 4.Warner AS, Glick ME, Fogt F. Multiple large hyperplastic polyps of the colon coincident with adenocarcinoma. Am J Gastroenterol. 1994;89:123–125. [PubMed] [Google Scholar]
- 5.Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol. 2005;2:398–405. doi: 10.1038/ncponc0248. [DOI] [PubMed] [Google Scholar]
- 6.Yao T, Sugai T, Iwashita A, et al. Histopathological characteristics and diagnostic criteria of SSA/P, project research ‘potential of cancerization of colorectal serrated lesions’ of Japanese Society for Cancer of the Colon and Rectum. Stomach and Intestine (Tokyo) 2011;46:442–448. (In Japanese) [Google Scholar]
- 7.Fujimori Y, Fujimori T, Imura J, et al. An assessment of the diagnostic criteria for SSA/Ps using image processing software analysis for Ki67 immunohistochemistry. Diagn Pathol. 2012;7:59. doi: 10.1186/1746-1596-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura T, Fukui H, Sekikawa A, et al. Involvement of REG Ialpha protein in the regeneration of ductal epithelial cells in the minor salivary glands of patients with Sjögren’s syndrome. Clin Exp Immunol. 2009;155:16–20. doi: 10.1111/j.1365-2249.2008.03806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torlakovic E, Gomez JD, Driman DK, Parfitt JR, Wang C, Benerjee T, Snover DC. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA) Am J Surg Pathol. 2008;32:21–29. doi: 10.1097/PAS.0b013e318157f002. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- 11.Kusaka T, Fukui H, Sano Y, Ueda Y, Chiba T, Fujimori T. Analysis of K-ras codon 12 mutations and p53 overexpression in colorectal nodule-aggregating tumors. J Gastroenterol Hepatol. 2000;15:1151–1157. doi: 10.1046/j.1440-1746.2000.02280.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Fujimori Y, Ogata H, et al. Immunohistochemical analysis of Ki-67 expression in so-called SSA/P; In comparison with hyperplastic polyps. Prog Dig Endosc. 2011;78:50–52. (In Japanese) [Google Scholar]
- 13.Hatanaka Y, Hashizume K, Nitta K, Kato T, Itoh I, Tani Y. Cytometrical image analysis for immunohistochemical hormone receptor status in breast carcinomas. Pathol Int. 2003;53:693–699. doi: 10.1046/j.1440-1827.2003.01547.x. [DOI] [PubMed] [Google Scholar]
- 14.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862–876. doi: 10.1053/gast.2002.35392. [DOI] [PubMed] [Google Scholar]
- 16.Sugihara K, Kusunoki M, Watanabe T, Sakai Y, Sekimoto M, Ajioka Y. Japanese Classification of Colorectal Carcinoma. Japanese Society for Cancer of the Colon and Rectum. Kanehara & Co; Tokyo: 2009. Guidelines for classification; pp. 1–34. [Google Scholar]
- 17.Hawkins NJ, Bariol C, Ward RL. The serrated neoplasia pathway. Pathology. 2002;34:548–555. [PubMed] [Google Scholar]
- 18.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 19.García-Solano J, Pérez-Guillermo M, Conesa-Zamora P, Acosta-Ortega J, Trujillo-Santos J, Cerezuela-Fuentes P, Mäkinen MJ. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: further insights into the full recognition of a new subset of colorectal carcinoma. Hum Pathol. 2010;41:1359–1368. doi: 10.1016/j.humpath.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. International Agency for Research on Cancer; Lyon: 2010. pp. 134–146. [Google Scholar]
- 22.Tanaka T. Colorectal carcinogenesis: review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimoda T, Ikegami M, Fujisaki J, Matsui T, Aizawa S, Ishikawa E. Early colorectal carcinoma with special reference to its development de novo. Cancer. 1989;64:1138–1146. doi: 10.1002/1097-0142(19890901)64:5<1138::aid-cncr2820640529>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Kudo S, Tamura S, Hirota S, et al. The problem of de novo colorectal carcinoma. Eur J Cancer. 1995;31A:1118–1120. doi: 10.1016/0959-8049(95)00251-d. [DOI] [PubMed] [Google Scholar]
- 25.Chung SM, Chen YT, Panczykowski A, Schamberg N, Klimstra DS, Yantiss RK. Serrated polyps with ‘intermediate features’ of sessile serrated polyp and microvesicular hyperplastic polyp: a practical approach to the classification of nondysplastic serrated polyps. Am J Surg Pathol. 2008;32:407–412. doi: 10.1097/PAS.0b013e318158dde2. [DOI] [PubMed] [Google Scholar]
- 26.Fujii T, Fujimori T. Magnifying endoscopic diagnosis in large serrated lesion-LHP vs. SSA/P. Stomach and Intestine (Tokyo) 2011;46:449–457. (In Japanese) [Google Scholar]
- 27.Shida Y, Fujimori T, Tanaka H, et al. Clinicopathological features of serrated adenocarcinoma defined by Mäkinen in dukes’ B colorectal carcinoma. Pathobiology. 2012;79:169–174. doi: 10.1159/000334837. [DOI] [PubMed] [Google Scholar]
- 28.Tajiri H, Niwa H. Proposal for a consensus terminology in endoscopy: how should different endoscopic imaging techniques be grouped and defined? Endoscopy. 2008;40:775–778. doi: 10.1055/s-2008-1077507. [DOI] [PubMed] [Google Scholar]
- 29.Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An B, Kondo Y, Okamoto Y, et al. Characteristic methylation profile in CpG island methylator phenotype-negative distal colorectal cancers. Int J Cancer. 2010;127:2095–2105. doi: 10.1002/ijc.25225. [DOI] [PubMed] [Google Scholar]


