Abstract
Hypertension is highly prevalent in hemodialysis patients but its management remains a matter of debate. In this review, we discuss the observational studies on the association of blood pressure with outcomes, measurement of blood pressure in hemodialysis patients and present an opinion-based approach to treating hypertension.
Hypertension is a leading cause of end-stage kidney disease (ESRD) and prevalence estimates of hypertension in dialysis patients range from 70% to 90% worldwide (1). Management of hypertension in dialysis patients continues to be a topic of debate in nephrology. Blood pressure (BP) is routinely measured, in a non-standardized manner before, during, and after each hemodialysis treatment. In addition, some nephrologists obtain standardized BP measurements in the dialysis unit and home and perform ambulatory blood pressure monitoring in select patients. Therefore, dialysis providers collect a plethora of BP data. Unfortunately, however, there are few data from randomized controlled trials (RCT) to guide providers on data interpretation, treatment methods and therapeutic goals. This article reviews the salient features of available observational studies and discusses strategies, based on expert opinion, for evaluating and managing hypertension in hemodialysis patients. (2)
Clinical Context
In clinical decision making it is important to recognize the impact of the environment in which BP is measured. In the vast majority of dialysis units, BP is not measured in accordance with the American Heart Association guidelines. Pre-dialysis BP is typically measured when the patient is seated in the dialysis chair. BP is usually measured at a convenient body site with an automated device and then rechecked at approximately thirty minute intervals during dialysis and once at the end of dialysis. The reason for the frequent BP measurements is to ensure safety during the hemodialysis procedure, avoid recurrent symptomatic intradialytic hypotension and marked post-dialysis hypotension, which may pose immediate safety concerns regardless of the long-term consequences of poorly controlled hypertension (3).
Reminder: What is Hypertension?
In 2005 the American Society of Hypertension proposed a revised definition of hypertension: “Hypertension is a progressive cardiovascular syndrome arising from complex and interrelated etiologies. Early markers of the syndrome are often present before blood pressure elevation is observed; therefore, hypertension cannot be classified solely by discrete blood pressure thresholds. Progression is strongly associated with functional and structural cardiac and vascular abnormalities that damage the heart, kidneys, brain, vasculature, and other organs and lead to premature morbidity and death.”(4) In this framework, BP is a “biomarker” for this cardiovascular syndrome and “it is helpful to consider [individual] BP patterns rather than discrete BP thresholds” (4, 5). Therefore, to adequately assess the cardiovascular syndrome in hemodialysis patients, we need to establish the validity of different BP measurements and if we are to develop protocols for optimal management of hypertension we need to understand the implications of different BP patterns.
BP Measurement in Dialysis Patients
The issues surrounding the challenges of BP measurement in dialysis patients have been recently reviewed by Roberts et al(6). The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines recommend standardized measurement of BP in dialysis patients by trained personnel using the auscultatory method in accordance with the AHA guidelines(7). However, the guidelines are generally not followed and the BP is routinely measured using oscillometric devices. Very few studies have validated the use of these devices in dialysis patients (5). Routine' BP measurements may differ significantly from standardized measurements made by trained personnel in accordance with AHA guidelines. Rahman et al. reported that routine BP readings were, on average, higher than standardized readings both pre- (14.3/7 mm Hg) and post-dialysis (13.6/4 mm Hg). In 55% of patients, the routine postdialysis BP readings were ≥10 mm Hg higher than the standardized readings.
If dialysis unit BP assessments are “safety measures” to avoid complications during dialysis then BP measurements between dialysis sessions may be more indicative of the overall impact of hypertension on an individual. The American Heart Association and the European Society of Hypertension recommend home BP monitoring for all patients with hypertension (8, 9). In dialysis patients, home BP monitoring has greater prognostic significance in detecting left ventricular hypertrophy and cardiovascular events than routine dialysis unit BP measurements (10, 11). Ambulatory BP monitoring remains the gold standard for assessing BP control (12) but is unlikely to be routinely available for use in clinical practice due to cost and logistical considerations. Dialysis patients have substantially more healthcare provider contact and opportunity for learning proper use of home BP monitors then non-dialysis patients. However, KDIGO guidelines provide conflicting comments on the use of home BP monitoring stating on one hand that “At present, the evidence for the superiority of self-measured BP at home over pre-hemodialysis BP is impressive.” but recommending that “Although a worthy goal, neither measurement of APBM nor self-measured home BP may be feasible for most patients throughout the world, leaving pre-hemodialysis and post-hemodialysis BP measurements to be used, but with caution and with the knowledge that these are inferior.” (13) Effective management of hypertension in dialysis patients may be difficult to achieve without the use of home BP monitoring (14).
BP in Dialysis Patients and Outcomes – Observational Studies
Landmark observational studies were instrumental in highlighting the risk of cardiovascular disease and kidney failure with hypertension(15, 16). These studies were followed by pivotal RCTs that demonstrated the benefit of BP lowering (17-19). Together these studies form the basis for recommendations for BP targets in the general population (20-22). However, recent reviews of observational studies in hemodialysis patients have suggested that pre-dialysis systolic BP <140 mmHg may be associated with increased mortality versus a systolic BP of 140-150 mm Hg. Even more surprising is that higher systolic BP values were not associated with increased risk and might even be associated with increased short-term survival (23, 24). These findings have been recently described in detail (25-27) and have led some to surmise that BP per se should not be the focus of treatment in dialysis patients(25, 28).
There appears to be a potential dilemma for the casual reader. If the findings of observational studies of BP and outcomes in the general population are valid then why should we question the results of similar studies in hemodialysis patients? To begin to answer this question it is important to recognize how the observational studies in the general population and hemodialysis patients differ. First, most studies in hemodialysis patients use routine BP measurements while those in the general population used BP measurements made by trained personnel using standardized protocols. Errors in the measurement of BP may lead to differential misclassification, which can bias apparent associations between BP and outcomes. (29) Second, in most well designed general population observational studies, there is careful assessment of baseline comorbidities and outcomes are adjudicated. In contrast, many hemodialysis studies using registry data rely only on claims data for comorbidities and as a result underestimate the comorbidities(30). Third, antihypertensive medication data are incomplete in the dialysis registries and most analyses do not adequately account for the potential impact of medications on outcomes. Finally, it is often inferred that the higher risk of death with lower BP in dialysis patients means that lowering BP in dialysis patients is harmful. However, the correct interpretation of these findings is that people with lower BP are at increased risk of adverse outcomes compared to people with higher BP. The effect of lowering BP on outcomes while accounting for comorbidities, volume removal on dialysis and antihypertensive medications use has not been evaluated in studies of dialysis patients. In summary, observational data on the association between BP and outcomes in dialysis patients cannot be used to infer BP goals in dialysis patients. An RCT of different blood pressure targets in hemodialysis patients is underway but results are not yet available (31).
Goal BP in Hemodialysis Patients
Since BP is a biomarker of the “cardiovascular syndrome”, management of hypertension should address prevention and treatment of the full range of cardiovascular complications of hypertension. In the absence of data from RCT in dialysis patients, the goals have to be extrapolated from general population studies. Based on these guidelines, it is reasonable to aim for a predialysis BP ≤ 140/90 mm Hg or a home BP ≤ 135/85 mm Hg (13, 32, 33). Although the recent KDIGO BP guidelines did not recommend BP goals in dialysis patients due to lack of evidence to support any guidelines in dialysis patients, the general strategies to “Individualize BP targets and agents according to age, co-existent cardiovascular disease and other co-morbidities … and tolerance of treatment” and “Inquire about postural dizziness and check for postural hypotension” are relevant to the management of hypertension in dialysis patients.
BP Patterns in Hemodialysis Patients
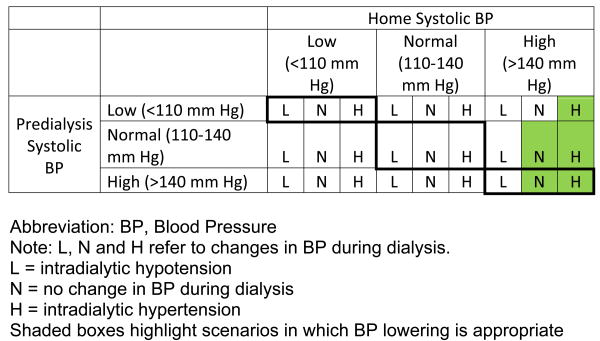
There are a large number of potential BP patterns in hemodialysis patients. Home and pre-dialysis systolic BP can be low (<110 mm Hg), normal (110 to 140 mm Hg) or high (>140 mm Hg). During dialysis the BP may be stable, decrease or increase. Combining all these scenarios may result in 27 different patterns of systolic BP in hemodialysis patients (Figure 1). Individual patients may also move from one pattern to the other over time with treatment of hypertension. These large numbers of patterns contribute to the difficulty in managing hypertension in hemodialysis patients. The current KDOQI recommendations are applicable to only two patterns; those with high predialysis and home BP without intradialytic hypotension. In the absence of home BP data, persistent hypertension (≥160 mm Hg), before, during and at the end of hemodialysis sessions suggests the presence of sustained hypertension and warrants investigation of possible contributing factors and initiation or modification of treatment.
Figure 1.
Factors Contributing to Uncontrolled Hypertension in Hemodialysis Patients
Sodium and volume excess are perhaps the most important factors contributing to uncontrolled hypertension in dialysis patients. Sodium and volume excess may result from patient level factors such as non-adherence to dietary salt and fluid restriction, treatment level factors such as use of high dialysate sodium concentrations and sodium modeling (34) and limitations imposed by thrice weekly dialysis. In the Frequent Hemodialysis Network Trial, dialysis six versus three times per week was associated with an 18% increase in fluid removal per week and a 10 mm Hg decrease in systolic BP and lower use of antihypertensive medications (34) implying improved dry weight. In the Dry-Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) trial, dry weight probing reduced postdialysis weight by 1 kg at 8 weeks and resulted in 6.6/3.3 mm Hg lower BP in the intervention arm (35).
Non adherence with prescribed medications is common and may contribute to uncontrolled hypertension. The prescribed pill burden for hemodialysis patients may exceed 25 pills per day (36). Non-adherence to beta blockers and clonidine may cause rebound hypertension. Sleep apnea is common in hemodialysis patients and may be associated with hypertension (37). Treatment of obstructive sleep apnea with continuous positive airway pressure (CPAP) therapy can reduce BP and may also have beneficial effects on left ventricular ejection fraction (38, 39). Other secondary causes of hypertension such as primary hyperaldosteronism, Cushing's syndrome and pheochromocytoma should also be considered in patients with uncontrolled BP (40, 41).
Special Considerations
Blood Pressure Variability
BP variability is a risk factor for adverse outcomes in patients with hypertension (42, 43). Dialysis patients are particularly prone to higher BP variability due to vascular stiffness and dialysis related volume changes. We recently reported that in a cohort of incident hemodialysis patients a one standard deviation increase in pre-dialysis systolic BP variability was associated with an 18% higher risk for all-cause and CVD mortality. Greater fluid removal during dialysis was associated with lower BP variability(44). Increased pre-dialysis systolic BP variability was also associated with increased all-cause mortality in the HEMO trial (45). In a retrospective analysis of the Fosinopril in Dialysis (FOSIDIAL) study, increased pre-dialysis BP variability was associated with a higher risk of CV events (46). Flythe et al. recently reported that greater fluid removal during dialysis was associated with higher intradialytic systolic BP variability and the higher variability was associated with an increased risk of mortality (47, 48). Therefore, in addition to absolute BP levels, we should also pay attention to pre-dialysis and intradialytic BP variability.
Intradialytic Hypertension
Intradialytic hypertension can occur frequently and is often under-recognized, since unlike intradialytic hypotension it is not associated with immediate complications. However, even over a short-term follow-up of six months, an intradialytic rise in systolic BP ≥10 mm Hg was associated with a two-fold greater risk of hospitalization or death (49). Lower ultrafiltration during dialysis leading to volume overload is a major contributing factor to intradialytic hypotension. Novel mechanisms for intradialytic hypotension include endothelial cell dysfunction and sympathetic hyperactivity during dialysis are putative mechanisms for intradialytic hypertension (50-52). In one small prospective open label study, carvedilol reduced the frequency of intradialytic hypertension episodes by approximately 50% (p<0.001). (53)
Management of Hypertension
Standardization of BP measurements
Reliable data to guide management is the first step towards management of hypertension in dialysis patients. BP measurement in the dialysis unit should be standardized. AHA guidelines for BP measurements should be used to identify the correct cuff size and position (54). Devices used to measure BP should be validated and calibrated. Site of BP measurement should be kept constant and should be clearly identified in medical records. NKF KDOQI guidelines recommend measurement of BP in thighs or legs of patients that have undergone multiple surgical vascularization procedures in both arms (55). However, the goal BP recommendations are valid only for brachial BP measurement and the reference values for lower limb BP are unknown (55).
Home BP Monitoring
Home BP monitoring is recommended for all patients with hypertension by the AHA and the European Society of Hypertension (8, 9). In a single unit open label randomized controlled trial, decision-making based on home BP monitoring compared with predialysis BP measurements led to better BP control as assessed by ABPM (56). Importantly, symptomatic intradialytic hypotension did not increase. It is possible that home BP monitoring may increase patients' self-efficacy and involvement in care although this has not been scientifically investigated.
Patient-Centered Management
Although dialysis patients have much greater access to trained healthcare professionals than non-dialysis patients, much of the emphasis on self-care is focused on dietary phosphate management. Compounding the problem is low health literacy, which is common in dialysis patients and is associated with higher BP (57, 58). Educating and empowering dialysis patients to take responsibility for their own care may improve self-efficacy and improve BP control and quality of life (58). In a recent study, patient education and counseling regarding BP, salt and volume intake reduced BP levels (59). Moattari et al. conducted a randomized controlled trial of six-week intervention that included a combination of individual and group empowerment counseling sessions (60). The intervention improved self-efficacy, decreased interdialytic weight gain by 0.4 kg and reduced predialysis BP by 14/5 mm Hg.
Management of Volume Overload
Volume overload is common among dialysis patients and may precede initiation of dialysis (61). Dry weight attainment is the most important factor for BP control in dialysis patients (62). Greater fluid removal with dialysis and lowering of dry-weight is associated with BP lowering, decreased predialysis systolic BP variability, lower left ventricular mass, and less use of antihypertensive medications (34, 35, 44). In the DRIP trial, the following technique was used to probe and decrease dry-weight successfully (35): at each dialysis treatment, an additional ultrafiltration of 0.1-kg/10 kg-body weight was prescribed. If the ultrafiltration was not tolerated, the additional removal was reduced by 50% and another 50% if still not tolerated. Dry weight was established when a 0.2-kg incremental ultrafiltration was not tolerated.
Hur et al. recently reported the results of a randomized controlled trial designed to evaluate the impact of bioimpedance-guided assessment of dry weight in dialysis patients(63). Bioimpedance spectroscopy was performed pre-dialysis at the mid-week dialysis treatment twice a month. In the intervention group, bioimpedance data was provided to the treating physician and used to adjust ultrafiltration targets. In the intervention group, volume overload gradually decreased by 0.5 L at year one and predialysis systolic BP decreased by 4.5/2.6 mm Hg, antihypertensive medication use decreased by 9% and left ventricular mass index decreased by 10.2 g/m2. The advantage of this technique over dry-weight probing as performed in the DRIP trial appears to be the reduction of fluid overload without an increase in intradialytic hypotension or vascular access complications. However, bioimpedance remains a research tool whereas dry-weight probing can be performed routinely without requiring purchase of additional equipment.
Relative plasma volume (RPV) monitoring during hemodialysis can be used to infer the presence of volume overload. During ultrafiltration, patients with volume overload continue to fill the intravascular space from interstitial space and maintain plasma volume resulting in flat RPV slopes whereas patients at dry weight start developing hemoconcentration and have steep RPV slopes as there is no refill from interstitial to intravascular space (64). However, inference of volume overload can only be made retrospectively after the treatment has been completed. Moreover, a RCT of RPV versus conventional monitoring showed increased risk of access and non-access related hospitalizations in the RPV group (65). While the results of the trial have been questioned, the role of RPV for determining volume overload in dialysis patients remains unclear.
Dietary and Dialysate Sodium
The KDIGO 5D guidelines emphasize the importance of restriction of dietary salt intake for control of BP (13). In a recent secondary analysis of the HEMO trial, higher dietary salt intake was associated with higher predialysis systolic BP and a linear increase in the hazard for death (66). A positive dialysate-serum sodium gradient is another potential cause of net positive sodium balance during dialysis (67). Lowering dialysate sodium may reduce thirst, lower interdialytic weight gain; reduce BP and antihypertensive medication use (68). It may also be associated with lower carotid intimal medial thickness and flow-mediated dilatation (69). Individualized dialysate sodium prescription can also reduce interdialytic weight gain and lower BP (70). In the absence of RCTs, it is difficult to recommend whether dialysate sodium should be lowered for all dialysis patients or individualized based on serum sodium concentrations. A randomized control trial to assess the effectiveness of lower dialysate sodium concentrations is underway in Australia and New Zealand(71).
Antihypertensive Medications
Many dialysis patients will require antihypertensive medications to control BP. The KDIGO 5D report stated “Beyond preferences based on the literature, guidelines in the nondialysis population, and the use of common wisdom, there is no compelling evidence to recommend one class of antihypertensive agents over another” (13). Most patients on dialysis have a longstanding history of hypertension prior to the start of dialysis and have been receiving antihypertensive medications. Antihypertensive regimens are often modified in the pre-ESRD period due to volume overload and hyperkalemia. Most patients with ESRD take multiple antihypertensive medications, and the optimal regimen to control BP and reduce morbidity and mortality is unknown.
We recently assessed the relationships of antihypertensive medications to mortality in two retrospective cohorts of incident hemodialysis patients; a cohort of 11,291 patients treated in facilities operated by Dialysis Clinic Inc. (DCI), a not-for-profit provider and a cohort of all US incident dialysis patients with Medicare parts A, B and D eligibility (72, 73). In both cohorts, a RAS-based regimen without a β-blocker was associated with significant decrease in mortality risk compared to a β-blocker-based regimen that did not contain a RAS agent. A regimen that combined a RAS agent and a β-blocker was associated with a further decrease in mortality risk(73). These findings provide strong evidence of the beneficial effects of RAS agents in dialysis patients. However, a randomized controlled trial is needed to confirm the results of these observational studies.
Renal Denervation
Sympathetic hyperactivity is common in ESRD and is contributing factor to hypertension and CVD (74-76). Bilateral nephrectomy may help control resistant hypertension in part by reducing renal sympathetic activity (77). However, there is significant morbidity associated with nephrectomy and it is not routinely performed. Renal denervation alone may also lower BP by decreasing renal sympathetic activity (78, 79). In a recent study, renal denervation was attempted in 12 hemodialysis patients with uncontrolled hypertension. Three patients could not undergo denervation due to atrophic renal arteries. In the remaining nine patients, systolic BP was reduced by 28 mm Hg at 12 months (80). These potential benefits will need to be weighed against the risk of significant complications and at present this approach should be considered experimental.
Management of Hypertension – A Suggested Approach
Effective management of hypertension in dialysis patients will require system-level changes in addition to individual physician- and patient-level interventions. For many nephrologists, the patterns of BP change during the dialysis procedure may take precedence over hypertension control. Current clinical guidelines do not offer any recommendations on treatment based on these BP patterns. In a recent nonrandomized controlled trial, a computer-based clinical decision support reminded physicians of the pre- and postdialysis BPs and the current BP guidelines but did not improve hypertension control (81). These findings reflect the complexity of hypertension management in dialysis patients.
An opinion-based approach to management of hypertension in dialysis patients is described in Figure 2. The first step in management is to obtain standardized BP measurements in accordance with AHA guidelines. Next, patients and caregivers must be educated regarding salt restriction and use of home BP devices. The data from home devices and dialysis unit BP measurements should be presented to the nephrologist in a monthly composite report that also includes the frequency and severity of intradialytic hypotension and hypertension. RAS agents should be considered for all dialysis patients that require antihypertensive medications. Dry-weight probing protocols should be adapted for use in clinical practice in a manner similar to the use of anemia management protocols in dialysis units. Serum and dialysate sodium concentrations should be included in the BP section of the monthly physician reports and dialysate sodium should be individualized to avoid positive sodium balance. Organized delivery of dialysis care in the US offers a great opportunity to implement such a change and improve outcomes in dialysis patients.
Figure 2.
Acknowledgments
We are grateful to Dr. Bruce L. Horowitz for a careful review and thoughtful comments on the manuscript.
Funding Sources: Dr. Shafi was supported by K23-DK-083514 from the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health, Bethesda MD.
Dr. Zager is supported by R01-DK0-83424 083514 from the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health, Bethesda MD.
Research reported in this publication was supported by the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number K23DK083514. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115(4):291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Arbor Research Collaborative for Health; Ann Arbor, MI: [Accessed on 8/24/2013]. 2010 Annual Report of the Dialysis Outcomes and Practice Patterns Study Hemodialysis Data 1999-2008. at http://www.dopps.org/annualreport/ [Google Scholar]
- 3.Henderson LW. Symptomatic intradialytic hypotension and mortality: an opinionated review. Semin Dial. 2012;25(3):320–325. doi: 10.1111/j.1525-139X.2012.01068.x. [DOI] [PubMed] [Google Scholar]
- 4.Giles TD, Berk BC, Black HR, Cohn JN, Kostis JB, Izzo JL, Jr, Weber MA. Expanding the definition and classification of hypertension. J Clin Hypertens (Greenwich) 2005;7(9):505–512. doi: 10.1111/j.1524-6175.2005.04769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czarkowski M, Staszkow M, Kostyra K, Shebani Z, Niemczyk S, Matuszkiewicz-Rowinska J. Determining the accuracy of blood pressure measurement by the Omron HEM-907 before and after hemodialysis. Blood Press Monit. 2009;14(5):232–238. doi: 10.1097/mbp.0b013e328331d5b5. [DOI] [PubMed] [Google Scholar]
- 6.Roberts MA, Pilmore HL, Tonkin AM, Garg AX, Pascoe EM, Badve SV, Cass A, Ierino FL, Hawley CM Beta-Blocker to Lower Cardiovascular Dialysis Events (BLOCADE) Feasibility Study Trial Management Committee. Challenges in blood pressure measurement in patients treated with maintenance hemodialysis. Am J Kidney Dis. 2012;60(3):463–472. doi: 10.1053/j.ajkd.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 7.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 8.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D American Heart Association, American Society of Hypertension, Preventive Cardiovascular Nurses Association. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):1–9. doi: 10.1161/HYPERTENSIONAHA.107.189011. [DOI] [PubMed] [Google Scholar]
- 9.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T, O'Brien E, Ohkubo T, Padfield P, Palatini P, Pickering TG, Redon J, Revera M, Ruilope LM, Shennan A, Staessen JA, Tisler A, Waeber B, Zanchetti A, Mancia G ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24(12):779–785. doi: 10.1038/jhh.2010.54. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47(1):62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 11.Moriya H, Oka M, Maesato K, Mano T, Ikee R, Ohtake T, Kobayashi S. Weekly averaged blood pressure is more important than a single-point blood pressure measurement in the risk stratification of dialysis patients. Clin J Am Soc Nephrol. 2008;3(2):416–422. doi: 10.2215/CJN.03490807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson AM, Pickering TG. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006;70(6):1000–1007. doi: 10.1038/sj.ki.5001695. [DOI] [PubMed] [Google Scholar]
- 13.Levin NW, Kotanko P, Eckardt KU, Kasiske BL, Chazot C, Cheung AK, Redon J, Wheeler DC, Zoccali C, London GM. Blood pressure in chronic kidney disease stage 5D-report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2010;77(4):273–284. doi: 10.1038/ki.2009.469. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal R. Managing hypertension using home blood pressure monitoring among haemodialysis patients--a call to action. Nephrol Dial Transplant. 2010;25(6):1766–1771. doi: 10.1093/ndt/gfq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 16.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358(9294):1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 17.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288(23):2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 18.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 19.Anonymous. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265(24):3255–3264. [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.Authors/Task Force Members. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F ESH Scientific Council; Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P ESC Committee for Practice Guidelines (CPG); Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S Document Reviewers. Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 22.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, Lefevre ML, Mackenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014 2013 Dec 18; doi: 10.1001/jama.2013.284427. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45(4):811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 24.Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, Kerr PG, Morgenstern H, Li Y, Pisoni RL, Saran R, Tentori F, Akizawa T, Fukuhara S, Port FK. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2012;82(5):570–580. doi: 10.1038/ki.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacson E, Jr, Lazarus JM. The association between blood pressure and mortality in ESRD-not different from the general population? Semin Dial. 2007;20(6):510–517. doi: 10.1111/j.1525-139X.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R. The controversies of diagnosing and treating hypertension among hemodialysis patients. Semin Dial. 2012;25(4):370–376. doi: 10.1111/j.1525-139X.2012.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrotra R, Agarwal R, Townsend R, Goldfarb S. End-Stage Renal Disease and Dialysis. NephSAP. 2012 [Google Scholar]
- 28.Weir MR. Debate from the 2012 ASH Annual Scientific Sessions: should blood pressure be reduced in hemodialysis patients? Con position. J Am Soc Hypertens. 2012;6(6):443–447. doi: 10.1016/j.jash.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H. Bias due to non-differential misclassification of polytomous confounders. J Clin Epidemiol. 1993;46(1):57–63. doi: 10.1016/0895-4356(93)90009-p. [DOI] [PubMed] [Google Scholar]
- 30.Longenecker JC, Coresh J, Klag MJ, Levey AS, Martin AA, Fink NE, Powe NR. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11(3):520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 31.Gul A, Miskulin D, Gassman J, Harford A, Horowitz B, Chen J, Paine S, Bedrick E, Kusek JW, Unruh M, Zager P BID Pilot Study Investigators. Design of the Blood Pressure in Dialysis Pilot Study. Am J Med Sci. 2013 doi: 10.1097/MAJ.0b013e31827daee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taler SJ, Agarwal R, Bakris GL, Flynn JT, Nilsson PM, Rahman M, Sanders PW, Textor SC, Weir MR, Townsend RR. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for Management of Blood Pressure in CKD. Am J Kidney Dis. 2013;62(2):201–213. doi: 10.1053/j.ajkd.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.KDIGO: KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. 2012;2(Suppl) [Google Scholar]
- 34.FHN Trial Group. Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53(3):500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dharia SM, Brown LK, Unruh ML. Recognition and treatment of obstructive sleep apnea. Semin Dial. 2013;26(3):273–277. doi: 10.1111/sdi.12067. [DOI] [PubMed] [Google Scholar]
- 38.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50(2):417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 39.Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, Ando S, Bradley TD. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348(13):1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda K, Shimamoto K, Ura N, Ogata H, Shizukuda Y, Iwakura M, Nozawa A, Kikuchi K, Iimura O. A case of primary aldosteronism with chronic renal failure undergoing hemodialysis treatment. Endocrinol Jpn. 1989;36(5):681–686. doi: 10.1507/endocrj1954.36.681. [DOI] [PubMed] [Google Scholar]
- 41.Mise K, Ubara Y, Sumida K, Hiramatsu R, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, Hoshino J, Sawa N, Hashimoto M, Fujii T, Sasano H, Takaichi K. Cushing's syndrome after hemodialysis for 21 years. J Clin Endocrinol Metab. 2013;98(1):13–19. doi: 10.1210/jc.2012-2766. [DOI] [PubMed] [Google Scholar]
- 42.Rossignol P, Kessler M, Zannad F. Visit-to-visit blood pressure variability and risk for progression of cardiovascular and renal diseases. Curr Opin Nephrol Hypertens. 2013;22(1):59–64. doi: 10.1097/MNH.0b013e32835b489f. [DOI] [PubMed] [Google Scholar]
- 43.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 44.Shafi T, Sozio S, Bandeen-Roche K, Ephraim P, Luly J, McDermott A, Scialla J, Crews D, Tangri N, Miskulin D, Michels W, Peter W, St, Wu A, Jaar B, Zager P, Boulware L. Predialysis Systolic Blood Pressure Variability and Outcomes in Incident Hemodialysis Patients. The DEcIDE Network Patient Outcomes in ESRD Study. JASN-in review [Google Scholar]
- 45.Chang TI, Flythe JE, Brunelli SM, Muntner P, Greene T, Cheung AK, Chertow GM. Visit-to-visit systolic blood pressure variability and outcomes in hemodialysis. J Hum Hypertens. 2013 doi: 10.1038/jhh.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossignol P, Cridlig J, Lehert P, Kessler M, Zannad F. Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension. 2012;60(2):339–346. doi: 10.1161/HYPERTENSIONAHA.111.190397. [DOI] [PubMed] [Google Scholar]
- 47.Flythe JE, Kunaparaju S, Dinesh K, Cape K, Feldman HI, Brunelli SM. Factors associated with intradialytic systolic blood pressure variability. Am J Kidney Dis. 2012;59(3):409–418. doi: 10.1053/j.ajkd.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 48.Flythe JE, Inrig JK, Shafi T, Chang TI, Cape K, Dinesh K, Kunaparaju S, Brunelli SM. Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am J Kidney Dis. 2013;61(6):966–974. doi: 10.1053/j.ajkd.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inrig JK, Oddone EZ, Hasselblad V, Gillespie B, Patel UD, Reddan D, Toto R, Himmelfarb J, Winchester JF, Stivelman J, Lindsay RM, Szczech LA. Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int. 2007;71(5):454–461. doi: 10.1038/sj.ki.5002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Buren PN, Kim C, Toto RD, Inrig JK. The prevalence of persistent intradialytic hypertension in a hemodialysis population with extended follow-up. Int J Artif Organs. 2012;35(12):1031–1038. doi: 10.5301/ijao.5000126. [DOI] [PubMed] [Google Scholar]
- 51.Rubinger D, Backenroth R, Sapoznikov D. Sympathetic activation and baroreflex function during intradialytic hypertensive episodes. PLoS One. 2012;7(5):e36943. doi: 10.1371/journal.pone.0036943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inrig JK, Van Buren P, Kim C, Vongpatanasin W, Povsic TJ, Toto R. Probing the mechanisms of intradialytic hypertension: a pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol. 2012;7(8):1300–1309. doi: 10.2215/CJN.10010911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inrig JK, Van Buren P, Kim C, Vongpatanasin W, Povsic TJ, Toto R. Probing the mechanisms of intradialytic hypertension: a pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol. 2012 Aug;7(8):1300–9. doi: 10.2215/CJN.10010911. Epub 2012 Jun 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in hum ans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 55.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 Suppl 1):S1–290. [PubMed] [Google Scholar]
- 56.da Silva GV, de Barros S, Abensur H, Ortega KC, Mion D, Jr Cochrane Renal Group Prospective Trial Register: CRG060800146. Home blood pressure monitoring in blood pressure control among haemodialysis patients: an open randomized clinical trial. Nephrol Dial Transplant. 2009;24(12):3805–3811. doi: 10.1093/ndt/gfp332. [DOI] [PubMed] [Google Scholar]
- 57.Adeseun GA, Bonney CC, Rosas SE. Health literacy associated with blood pressure but not other cardiovascular disease risk factors among dialysis patients. Am J Hypertens. 2012;25(3):348–353. doi: 10.1038/ajh.2011.252. [DOI] [PubMed] [Google Scholar]
- 58.Mason J, Khunti K, Stone M, Farooqi A, Carr S. Educational interventions in kidney disease care: a systematic review of randomized trials. Am J Kidney Dis. 2008;51(6):933–951. doi: 10.1053/j.ajkd.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 59.Kauric-Klein Z. Blood pressure knowledge in hypertensive hemodialysis patients. CANNT J. 2012;22(4):18–25. [PubMed] [Google Scholar]
- 60.Moattari M, Ebrahimi M, Sharifi N, Rouzbeh J. The effect of empowerment on the self-efficacy, quality of life and clinical and laboratory indicators of patients treated with hemodialysis: a randomized controlled trial. Health Qual Life Outcomes. 2012;10:115–7525. 10–115. doi: 10.1186/1477-7525-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai YC, Tsai JC, Chen SC, Chiu YW, Hwang SJ, Hung CC, Chen TH, Kuo MC, Chen HC. Association of Fluid Overload With Kidney Disease Progression in Advanced CKD: A Prospective Cohort Study. Am J Kidney Dis. 2013 doi: 10.1053/j.ajkd.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5(7):1255–1260. doi: 10.2215/CJN.01760210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, Kayikcioglu M, Demirci MS, Ozkahya M, Duman S, Ok E. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013;61(6):957–965. doi: 10.1053/j.ajkd.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Hecking M, Karaboyas A, Antlanger M, Saran R, Wizemann V, Chazot C, Rayner H, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Moissl U, Kotanko P, Levin NW, Säemann MD, Kalantar-Zadeh K, Port FK, Wabel P. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol. 2013;38(1):78–90. doi: 10.1159/000353104. Epub 2013 Jul 6. [DOI] [PubMed] [Google Scholar]
- 65.Reddan DN, Szczech LA, Hasselblad V, Lowrie EG, Lindsay RM, Himmelfarb J, Toto RD, Stivelman J, Winchester JF, Zillman LA, Califf RM, Owen WF., Jr Intradialytic blood volume monitoring in ambulatory hemodialysis patients: a randomized trial. J Am Soc Nephrol. 2005 Jul;16(7):2162–9. doi: 10.1681/ASN.2004121053. Epub 2005 Jun 1. [DOI] [PubMed] [Google Scholar]
- 66.Mc Causland FR, Waikar SS, Brunelli SM. Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients. Kidney Int. 2012;82(2):204–211. doi: 10.1038/ki.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munoz Mendoza J, Sun S, Chertow GM, Moran J, Doss S, Schiller B. Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant. 2011;26(4):1281–1287. doi: 10.1093/ndt/gfq807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thein H, Haloob I, Marshall MR. Associations of a facility level decrease in dialysate sodium concentration with blood pressure and interdialytic weight gain. Nephrol Dial Transplant. 2007;22(9):2630–2639. doi: 10.1093/ndt/gfm220. [DOI] [PubMed] [Google Scholar]
- 69.Gumrukcuoglu HA, Ari E, Akyol A, Akdag S, Simsek H, Sahin M, Gunes Y, Tuncer M. Effects of lowering dialysate sodium on carotid artery atherosclerosis and endothelial dysfunction in maintenance hemodialysis patients. Int Urol Nephrol. 2012;44(6):1833–1839. doi: 10.1007/s11255-011-0117-5. [DOI] [PubMed] [Google Scholar]
- 70.Elshahawy Y, Sany D, Shawky S. Outcome of individualized dialysate sodium concentration for hemodialysis patients. Saudi J Kidney Dis Transpl. 2013;24(3):507–513. doi: 10.4103/1319-2442.111025. [DOI] [PubMed] [Google Scholar]
- 71.Dunlop JL, Vandal AC, de Zoysa JR, Gabriel RS, Haloob IA, Hood CJ, Matheson PJ, McGregor DO, Rabindranath KS, Semple DJ, Marshall MR. Rationale and design of the Sodium Lowering In Dialysate (SoLID) trial: a randomised controlled trial of low versus standard dialysate sodium concentration during hemodialysis for regression of left ventricular mass. BMC Nephrol. 2013;14:149–2369. 14–149. doi: 10.1186/1471-2369-14-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sozio S, Shafi T, Peter W, St, Bandeen-Roche K, Ephraim P, Luly J, Boulware L. Antihypertensive Medications and Mortality in Incident Hemodialysis Patients: A Marginal Structural Analysis of a National Cohort. Abstract, American Society of Nephrology Kidney Week 2013 J Am Soc Nephrol xx. 2013 xx. [Google Scholar]
- 73.Shafi T, Peter W, St, Sozio S, Ephraim P, Bandeen-Roche K, Boulware L. Renin-Angiotensin System (RAS) Blocking Drugs Reduce the Risk of Death in US Incident Hemodialysis (HD) Patients. , (abstract) American Society of Nephrology Kidney Week 2013 J Am Soc Nephrol xx. 2013 xx. [Google Scholar]
- 74.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327(27):1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 75.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106(15):1974–1979. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 76.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105(11):1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 77.Zazgornik J, Biesenbach G, Janko O, Gross C, Mair R, Brucke P, Debska-Slizien A, Rutkowski B. Bilateral nephrectomy: the best, but often overlooked, treatment for refractory hypertension in hemodialysis patients. Am J Hypertens. 1998;11(11 Pt 1):1364–1370. doi: 10.1016/s0895-7061(98)00154-x. [DOI] [PubMed] [Google Scholar]
- 78.Symplicity HTN-2 Investigators. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 79.Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA Symplicity HTN-2 Investigators. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 80.Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F, Bohm M, Lambert EA, Krum H, Sobotka PA, Schmieder RE, Ika-Sari C, Eikelis N, Straznicky N, Lambert GW, Esler MD. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2013.01.218. [DOI] [PubMed] [Google Scholar]
- 81.Thompson S, Hemmelgarn B, Wiebe N, Majumdar S, Klarenbach S, Jindal K, Manns B, Mortis G, Campbell P, Tonelli M Alberta Kidney Disease Network. Clinical decision support to improve blood pressure control in hemodialysis patients: a nonrandomized controlled trial. J Nephrol. 2012;25(6):944–953. doi: 10.5301/jn.5000238. [DOI] [PubMed] [Google Scholar]