Abstract
Objective
Tenofovir is used commonly in HIV treatment and prevention settings, but factors that correlate with tenofovir exposure in real-world setting are unknown.
Design
Intensive pharmacokinetic (PK) studies of tenofovir in a large, diverse cohort of HIV-infected women over 24-hours at steady-state were performed and factors that influenced exposure (assessed by areas-under-the-time-concentration curves, AUCs) identified
Methods
HIV-infected women (n=101) on tenofovir-based therapy underwent intensive 24-hour PK sampling. Data on race/ethnicity, age, exogenous steroid use, menstrual cycle phase, concomitant medications, recreational drugs and/or tobacco, hepatic and renal function, weight and body mass index (BMI) were collected. Multivariable models using forward stepwise selection identified factors associated with effects on AUC. Glomerular filtration rates (GFR) prior to starting tenofovir were estimated by the CKD-EPI equation using both creatinine and cystatin-C measures
Results
The median (range) of tenofovir AUCs was 3350 (1031–13,911) ng x h/mL. Higher AUCs were associated with concomitant ritonavir use (1.33-fold increase, p 0.002), increasing age (1.21-fold increase per decade, p=0.0007) and decreasing BMI (1.04-fold increase per 10% decrease in BMI). When GFR was calculated using cystatin-C measures, mild renal insufficiency prior to tenofovir initiation was associated with higher subsequent exposure (1.35-fold increase when pre-tenofovir GFR <70mL/min, p=0.0075).
Conclusions
Concomitant ritonavir use, increasing age, decreasing BMI and lower GFR prior to tenofovir initiation as estimated by cystatin C were all associated with elevated tenofovir exposure in a diverse cohort of HIV-infected women. Clinicians treating HIV-infected women should be aware of common clinical conditions that affect tenofovir exposure when prescribing this medication.
Keywords: Tenofovir, pharmacokinetics, HIV-infected women, diverse populations, GFR, cystatin C
INTRODUCTION
Since approval by the Federal Drug Administration in 2001, tenofovir disoproxil fumarate (TDF) has become one of the most frequently prescribed antiretrovirals in the management of HIV infection[1]. Moreover, TDF is co-formulated into several fixed-dose combinations, which can help promote adherence to combination antiretroviral therapy (cART) [2], and the co-formulation of TDF and emtricitabine is the only agent approved for pre-exposure prophylaxis in the United States [3, 4]. While mostly effective and safe, TDF has been associated with several major adverse effects in terms of renal function [5] and bone mineral density loss [6, 7], both of which can trigger discontinuation of the drug. Adverse effects of medications are generally correlated with systemic levels of the drug and, as is common with many antiretrovirals, TDF demonstrates significant inter-individual variability in plasma drug levels [8, 9]. The factors that contribute to inter-patient variability in TDF pharmacokinetics in diverse and real-world populations, however, are largely unknown.
As with most medications, the dose of TDF that was ultimately marketed for adults (300 milligrams (mg) once daily) with normal renal function was determined during Phase I and II studies of the drug [10]. Dose-finding studies usually entail intensive pharmacokinetic (PK) evaluations after short-term use in a limited number of volunteers (either HIV-infected or non-infected) who are often homogeneous in regards to race/ethnicity, gender and/or comorbidities. The generalizability of these PK studies to patients with different characteristics is thereby limited by their inclusion criteria and small sample sizes [11–13]. Broad recognition of important factors that modify a drug’s PK parameters once it is used in more diverse populations can be delayed due to limitations in post-marketing tracking procedures or publication bias [14]. To address some of these limitations, we conducted intensive PK studies of TDF in a sample of HIV-infected women in the setting of routine clinical use in order to identify factors associated with drug exposure.
METHODS
Study population
The Women’s Interagency HIV Study (WIHS) is a large, multicenter, prospective cohort study of HIV-infected women and at-risk HIV uninfected women in the United States[15]. The WIHS is highly representative of U.S. women living with HIV in terms of age, race/ethnicity, socioeconomic status, concomitant medications, comorbid medical conditions, etc. We previously described the “WIHS Intensive PK Study,” [16, 17] which enrolled HIV-infected women on cART for 24 hour sampling of antiretroviral plasma levels after administration of a dose witnessed by study team members under conditions of routine participant use. For this analysis, our study sample consisted of WIHS participants who used TDF for at least six months prior to PK evaluation. Institutional review boards at all participating institutions approved the consent and protocol materials for this study.
Intensive PK protocol methods
Pharmacokinetic protocols were conducted in clinical research centers or other facilities associated with collaborating WIHS sites. Plasma samples were drawn over 24 hours for drug levels under conditions of actual use (including simulation of the participants’ customary diet and administration of other medications). Participants were seen for the PK visit within six weeks of their core WIHS visit and data were collected at both visits on weight, comorbidities, HIV RNA level, CD4 cell counts, self-reported adherence, hepatic and renal function and illicit substance use. All participants received standard dosing of TDF (300 mg orally once daily) and drug levels were measured in specimens collected at 0, 4, 8, 15, 18 and 24 hours after a witnessed dose.
Laboratory procedures
Plasma levels of TDF were determined by liquid chromatography/tandem mass spectrometry (LC-MS/MS) with TDF-d6 as the internal standard [18]. The plasma sample was pretreated with trifluoroacetic acid for protein precipitation before injecting into the Micromass Quattro Ultima LC-MS/MS system. The assay was validated from 10 to 1000 nanogram (ng)/milliliter (mL) of TDF with a coefficient of variation < 15% for quality control samples at low, medium and high concentrations.
Cystatin C was measured in 67 of the 101 women who contributed data to these analyses. Cystatin C in plasma samples was quantified as described previously [19] at the University of California, Los Angeles (UCLA) Clinical Immunology Research Laboratory by a particle-enhanced immunoturbidimetric assay (Gentian, Moss, Norway), which has been calibrated against the new World Standard Reference material ERM-DA471/IFCC [20]. Intra-assay coefficients of variation, based on 10 replicates, were <2% at serum concentrations of 0.7 and 1.1 mg/Liter (L). Inter-assay coefficients of variation were 4.4% and 3.9% at serum concentrations of 0.8 and 2.2 mg/L, respectively.
Predictor variables
We analyzed the following variables in relationship to exposure: race (self-reported), age, exogenous sex steroid use, phase in menstrual cycle by self-report, concomitant medications (including other antiretrovirals), use of recreational drugs and/or tobacco, hepatic and renal function parameters, weight and estimated lean body mass. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using serum creatinine from the recent WIHS visit was employed to estimate glomerular filtration rate measures (eGFRcr) [21] in one set of models. GFR was dichotomized as being more or less than 70 mL/minute (min)/1.73 meters (m)2 in our models. The models were then repeated using the CKD-EPI equation for cystatin C (eGFRcys) [20–22]. Renal function prior to the initiation of TDF for each participant was also assessed using creatinine measures from prior visits (going back up to 4 visits before starting TDF if there was missing data). To obtain pre-TDF cystatin C for as many women as possible, we used values more than 4 visits back when necessary (n=16 of 67) from archived plasma specimens. All demographic data was collected at the core WIHS visit.
Outcome variables
Areas under the concentration-time curves (AUCs) were used to estimate TDF exposure over the dosing interval; these were calculated for each individual using the trapezoidal rule [23].
Statistical analysis
All analyses were conducted using Stata (version 11.2, College Station, TX) and SAS (version 9.2, SAS Institute, Cary, NC). For multivariate modeling, AUC was logarithmically transformed, and predictors’ coefficients were back-transformed to produce estimated multiplicative effects on geometric mean AUCs. The multivariable model was constructed by forward stepwise selection, with p<0.05 required for entry and retention, but with race (African American versus others) included because of high a priori interest. Since 33% of the participants did not have available cystatin C measures from visits that preceded the start of TDF, we used multiple imputation [24] to reduce the likelihood of possible bias from excluding so many observations from analysis. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing eGFR estimates using cystatin C, with 10 imputations performed to yield ~95% relative efficiency [25].
RESULTS
Characteristics of patient population
TDF levels were measured over a 24 hour period for 101 WIHS participants. Table 1 shows the patient characteristics of the study sample (n=101) and of the covariates included in the final multivariate models. The mean age (range) of the participants was 43.1 (21.7–64.9) years. Sixty four women (63%) reported their race as African American, 24 (24%) Hispanic and 10 (10%) Caucasian.
Table 1.
Participant characteristics of entire study population (n = 101).
| Parameter | All HIV-positive participants (n=101) |
|---|---|
| Age (years) | 43.1 (21.7 64.9) |
| Race (self-report) | |
| Black | 64 (63%) |
| Hispanic | 24 (24%) |
| White | 10 (10%) |
| Other | 3 (3%) |
| BMI (kg/m2) | 28.6 (15.0 62.0) |
| Concomitant ritonavir use | 62 (61%) |
| eGFRcr less than 70ml/min per 1.73m2 | 15 (14.9%) |
| Hepatitis C antibody positive | 37 (37%) |
| Concomitant diabetes | 26 (26%) |
| Concomitant hypertension | 36 (36%) |
| Current smoking | 54 (53%) |
| Current alcohol use (versus abstainer) | 36 (36%) |
| Current crack or powdered cocaine use | 16 (16%) |
| Detectable HIV RNA at time of sampling | 36 (36%) |
| Current CD4+ cell count (cells/μl) | 409 (4–1461) |
Data above are presented as mean (range) or numbers (percentage). Covariates in italics were retained in final multivariate model due to statistically significant association with tenofovir disoproxil fumarate (TDF) exposure. eGFRcr, creatinine-based estimated glomerular filtration rate.
Summary of pharmacokinetic parameters for tenofovir
The TDF PK parameters for the study population demonstrated significant inter-individual variation. The median (range) for TDF AUC was 3350 (1031 – 13,911) ng x hours (h)/mL, for minimum plasma drug concentration (Cmin) was 69.7 (0–363) ng/mL, for maximum plasma drug concentration (Cmax) was 251 (81.1–1020) ng/mL, for the time after administration at which Cmax was reached (tmax) was 4.1 (0–24) hours and for TDF clearance from plasma (CL/F) was 322 (77–1047) mL/h. This data is summarized in Table 2 and Figure 1 shows the time-concentration curves for the 101 participants who underwent intensive PK sampling for TDF levels.
Table 2.
Summary exposure metrics for tenofovir in the Women’s Interagency HIV intensive pharmacokinetic study.
| Tenofovir (n = 101) | AUC (ng h/ml) | Cmin (ng/ml) | Cmax (ng/ml) | tmax (h) | CL/F (ml/h) |
|---|---|---|---|---|---|
| Median | 3350 | 69.7 | 251 | 4.1 | 322 |
| Range | 1031–13911 | 0–363 | 81.1–1020 | 0–24 | 77–1047 |
AUC, area under the curve; CL/F, clearance/bioavailability; Cmax, maximum plasma concentration; Cmin, trough plasma concentration (all values below the LLOQ of 10 ng/ml were set to 0 ng/ml); tmax, time of maximum plasma concentration.
Fig. 1.
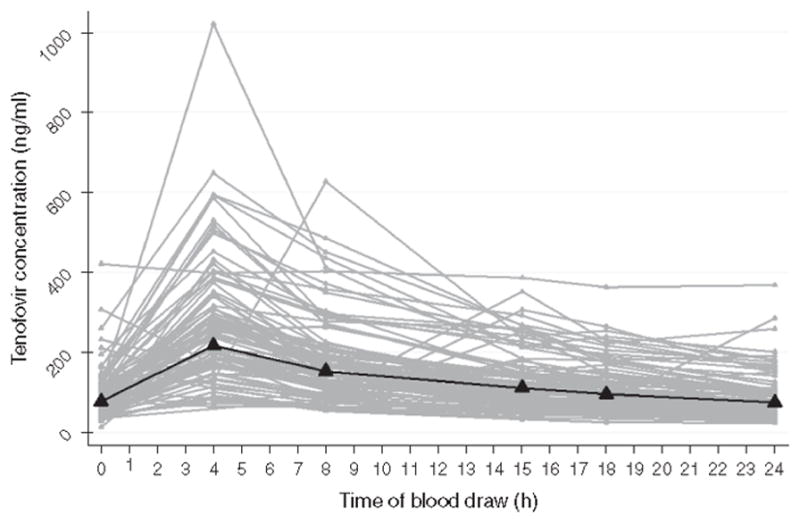
Time–concentration curves for 101 women in intensive pharmacokinetic studies for tenofovir
Bold line represents the median.
Factors associated with tenofovir AUC using eGFRcr
In the final multivariate model using the creatinine-based estimate of GFR, race did not substantially influence TDF exposure (Table 3), although older age was associated with higher exposure (increase in AUC by 1.21-fold for every decade of age, p=0.0007). Concomitant ritonavir (RTV) use (present in 61% of all participants) was associated with increased TDF AUC by an average of 1.33-fold (p=0.0020). Each 10% increase in body mass index (BMI, kilogram (kg)/m2) was associated with a 0.96-fold reduction in TDF AUC (p=0.019). An eGFRcr of <70 mL/min/1.73m2 prior to initiation of TDF was associated with a 1.31-fold higher AUC (p-value showed a trend towards statistical significance at 0.094).
Table 3.
Multivariate model showing fold-effects on area under the curve by covariate (renal parameter: Chronic Kidney Disease Epidemiology Collaboration equation, using creatinine prior to visit on tenofovir, n=101).
| Parameter | Estimate (95% CI), P value |
|---|---|
| Concomitant RTV use | ↑1.33 (1.11 – 1.59), 0.0020 |
| Per decade of age | ↑1.21 (1.08 – 1.34), 0.0007 |
| Black versus non-black | ↑1.04 (0.86 – 1.25), 0.68 |
| Per 10% increase in BMI | ↓0.96 (0.93 – 0.99), 0.019 |
| eGFRcr <70 ml/min per 1.73 m2 | ↑1.31 (0.95 – 1.81), 0.094 |
CI, confidence interval; eGFRcr, the CKD-EPI estimate for glomerular filtration rate; RTV, ritonavir.
Factors associated with tenofovir AUC using eGFRcys
In an alternative multivariate analysis (Table 4), we estimated GFR using cystatin C measures and dichotomized eGFRcys as being ≥ or < than 70 mL/min/1.73m2. As with the models using eGFRcr, race did not substantially affect exposure in this model (p=0.97). The effect of age on TDF exposure (1.20 fold increase in TDF AUC per decade of age, p=0.0003) was still prominent. Concomitant RTV use similarly increased exposure by an average of 1.33-fold (p=0.0014), and higher BMI was similarly associated with a lower (0.96-fold per 10% increase in BMI) TDF AUC (p=0.025). Mild renal insufficiency (eGFRcys of <70 mL/min/1.73m2) preceding the initiation of the TDF-based cART regimen was significantly associated with a 1.35-fold higher AUC for TDF (p=0.0075).
Table 4.
Multivariate model showing fold-effects on area under the curve by covariate (renal parameter: Chronic Kidney Disease Epidemiology Collaboration equation, using cystatin C prior to visit on tenofovir, n = 101, after multiple imputation of cystatin C).
| Parameter | Estimate (95% CI), P value |
|---|---|
| Concomitant RTV use | ↑1.33 (1.12–1.58), 0.0014 |
| Per decade of age | ↑1.20 (1.09–1.33), 0.0003 |
| Black versus non-Black | ↓0.97 (0.81–1.16), 0.73 |
| Per 10% increase in BMI | ↓0.96 (0.93–1.00), 0.025 |
| eGFRcys <70 ml/min per 1.73 m2 | ↑1.35 (1.08–1.69), 0.0075 |
CI, confidence interval; eGFRcys, the cystatin C estimate for glomerular filtration rate; RTV, ritonavir.
Addition of any one of the remaining unselected candidate predictor variables resulted in less than 6% change in the estimated effects shown in Tables 3 and 4.
DISCUSSION
Although tenofovir is one of the most commonly used antiretroviral agents in both HIV treatment and pre-exposure prophylaxis settings, limited information is available on the factors that influence its pharmacokinetics under conditions of actual use and in diverse populations. Our study examined factors associated with TDF exposure at steady state in a relatively large sample of HIV-infected women who were taking the drug as part of their prescribed cART regimens. The study participants were highly varied in terms of age, race, comorbid conditions, concomitant medications and body habitus, similar to patients in clinical practice. We found significant inter-individual variation in plasma drug levels, and pharmacokinetic parameters (Figure 1) in this sample. Four common factors were independently associated with greater TDF exposure: older age, pre-existing mild renal insufficiency, lower BMI and concomitant RTV use.
Ritonavir use increased TDF levels, a finding that is consistent with previous studies that have noted a 32–50% increase in TDF AUC when TDF is co-administered with ritonavir boosted-lopinavir [26, 27]. This association is particularly relevant as the concomitant use of TDF and ritonavir-boosted protease inhibitors is common [28]. A recent analysis in the Adults Clinical Trials Group (ACTG) A5208 study demonstrated that young African women randomized to tenofovir/emtricitabine/lopinavir/ritonavir had a higher incidence of renal insufficiency compared to women randomized to tenofovir/emtricitabine/nevirapine regimens [29]. Our study also demonstrates that lower body mass index is associated with modest increases in TDF AUC. Prior studies have demonstrated that low body weight is associated with decreased clearance of TDF [30] and that higher body weight is associated with increased clearance of TDF [31]. Lower eGFR as estimated by cystatin C measured prior to starting TDF (< 70 mL/min/1.73m2) was associated with a 35% increase in TDF AUC. Although eGFR <70 mL/min/1.73m2 as estimated by creatinine measures was associated with higher TDF exposure, the link between pre-existing kidney function and TDF-AUC was strengthened by estimating GFR using cystatin C measures. Because cystatin C levels are independent of muscle mass, cystatin C is a particularly useful measure in chronically ill, aging populations, or in patients with HIV infection, because artifacts in creatinine-based GFR estimates can occur in individuals with debility or loss of muscle mass [32, 33].
Adverse effects of TDF on renal function have been described [34–36] and dose reductions are currently recommended for patients with marked renal insufficiency, but no modification is recommended for persons with more modest renal dysfunction [1]. There is relatively little available information on TDF pharmacokinetics in patients with mild renal dysfunction and long term use of the drug. A previous study [37] found that the median AUC was 1.41-fold higher in 10 HIV uninfected subjects with a creatinine clearance from 50 to 79 mL/min/1.73m2 than in 3 subjects with creatinine clearance >80 mL/min/1.73m2 and another recent study demonstrated that higher TDF troughs are associated with renal impairment[38]. In the previously-cited analysis in ACTG5208, women in the tenofovir/emtricitabine/lopinavir/ritonavir arm with lower pre-randomization creatinine clearance were at the highest risk of developing renal insufficiency events [29].
Our findings are consistent with these reports, but the longitudinal nature of our cohort uniquely allowed us to model the effect of renal function prior to TDF initiation. We found that common factors can combine to significantly increase TDF levels and that mild renal impairment prior to TDF use can significantly impact subsequent TDF exposure. This finding suggests that mild renal insufficiency prior to TDF use could result in a spiral of increased TDF exposure and subsequent renal injury. Adjustments of TDF dosing based on exposure measures could enhance the safety profile of this important medication.
The WIHS intensive PK studies demonstrate the feasibility and utility of measuring 12 to 24 hour AUCs in large, unselected and diverse populations under actual-use conditions to determine factors associated with exposure in the clinical setting. One limitation is that this study was performed exclusively in a cohort of women, and the results may not be directly applicable to HIV-infected men on TDF-based therapy. We also have not yet examined inter-patient variability as a function of underlying host genetic characteristics, although such studies are underway. In spite of these limitations, there are several strengths to this study. Notably, there were a large number of individuals included in the analysis with longitudinal data collected over time, including renal function prior to the initiation of TDF-based cART. The study was also conducted under conditions that are representative of how antiretroviral medications are actually taken in routine practice. The study used a robust measure of tenofovir exposure as assessed by AUCs from intensive PK studies performed over 24 hours. Finally, renal function was assessed both by cystatin-C and creatinine measures in our study.
In conclusion, concomitant ritonavir use, lower BMI, older age and lower eGFR prior to starting TDF were all associated with higher TDF exposure in a cohort of HIV positive women under conditions of routine clinical use. Estimates of GFR using cystatin C may enhance the evaluation of pre-existing renal dysfunction on subsequent TDF exposure and were used in our models. Clinicians providing care to individuals with HIV should be aware of the impact of these common clinical conditions when using TDF. More studies are needed to identify clinically relevant factors contributing to elevated TDF exposure, the pharmacodynamic relationship between exposure and adverse effects, as well as the genetic factors that may contribute to inter-patient variability of antiretroviral concentrations in real-world practice.
Acknowledgments
FUNDING: This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) (K23 A1067065 and RO1 AI098472 to M.G. and R01 AI65233 to R.M.G.). Additional funding is also provided by the National Center for Research Resources (NCRR)/NIH via the UCSF-CTSI (UL1 RR024131) and the NIH Roadmap for Medical Research (KL2 RR024130 to B.A.).
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt);; Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by NIAID (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Some study visits were conducted in Clinical Research Centers funded under: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank the WIHS participants who contributed to making this study possible. We also thank Ms. Niloufar Ameli for her excellent statistical programming.
Footnotes
Disclaimers: None.
References
- 1.d’Arminio Monforte A, Rezza LA, Pezzotti G, Antinori P, Phillips A, Angarano AN, Colangeli G, De Luca V, Ippolito A, Caggese G, Soscia L, Filice F, Gritti G, Narciso F, Tirelli P, Moroni UM. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naïve patients. I.C.O.N.A. Study Group. Italian Cohort of Antiretroviral-Naïve Patients. AIDS. 2000:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg DR, Ragland K, Monk A, Deeks SG. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS. 2010;24:2835–2840. doi: 10.1097/QAD.0b013e328340a209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65–68. [PubMed] [Google Scholar]
- 4.Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586–589. [PubMed] [Google Scholar]
- 5.Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagnieu MC, Barkil ME, Livrozet JM, Cotte L, Miailhes P, Boibieux A, et al. Population pharmacokinetics of tenofovir in AIDS patients. J Clin Pharmacol. 2008;48:1282–1288. doi: 10.1177/0091270008322908. [DOI] [PubMed] [Google Scholar]
- 9.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55:5294–5299. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45:2733–2739. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JA, Johnson K, Paulauskis J, Cook J. So many studies, too few subjects: establishing functional relevance of genetic polymorphisms on pharmacokinetics. J Clin Pharmacol. 2006;46:258–264. doi: 10.1177/0091270005283463. [DOI] [PubMed] [Google Scholar]
- 12.Huang SM, Lesko LJ, Williams RL. Assessment of the quality and quantity of drug-drug interaction studies in recent NDA submissions: study design and data analysis issues. J Clin Pharmacol. 1999;39:1006–1014. doi: 10.1177/00912709922011764. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi M, Ameli N, Bacchetti P, Sharp GB, French AL, Young M, et al. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols. AIDS. 2005;19:1885–1896. doi: 10.1097/01.aids.0000189866.67182.f7. [DOI] [PubMed] [Google Scholar]
- 14.Rising KBP, Bero L. Reporting bias in drug trials submitted to the Food and Drug Administration: review of publication and presentation. PLoS Med. 2008;5:e217. doi: 10.1371/journal.pmed.0050217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 16.Gandhi M, Benet LZ, Bacchetti P, Kalinowski A, Anastos K, Wolfe AR, et al. Nonnucleoside reverse transcriptase inhibitor pharmacokinetics in a large unselected cohort of HIV-infected women. Journal of Acquired Immune Deficiency Syndromes. 2009;50:482–491. doi: 10.1097/qai.0b013e31819c3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi M, Greenblatt RM, Bacchetti P, Jin C, Huang Y, Anastos K, et al. A single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. J Infect Dis. 2012;206:1453–1461. doi: 10.1093/infdis/jis508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delahunty T, Bushman L, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1907–1914. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. Journal of Acquired Immune Deficiency Syndromes. 2012;61:565–573. doi: 10.1097/QAI.0b013e3182737706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. American Journal of Kidney Diseases. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England Journal of Medicine. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. American Journal of Kidney Diseases. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 24.Schafer JL. Multiple imputation: a primer. Statistical Methods in Medical Research. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 25.Gilks WRRS, Spiegehalter DJ, editors. Markov chain Monte Carlo in practice. London: Chapman & Hall; 1996. [Google Scholar]
- 26.Pruvost A, Negredo E, Theodoro F, Puig J, Levi M, Ayen R, et al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrobial Agents and Chemotherapy. 2009;53:1937–1943. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. Journal of Acquired Immune d\Deficiency syndromes. 2006;43:278–283. doi: 10.1097/01.qai.0000243103.03265.2b. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Nacher I, Garcia B, Barreiro P, Rodriguez-Novoa S, Morello J, Gonzalez-Lahoz J, et al. Trends in the prescription of antiretroviral drugs and impact on plasma HIV-RNA measurements. The Journal of Antimicrobial Chemotherapy. 2008;62:816–822. doi: 10.1093/jac/dkn252. [DOI] [PubMed] [Google Scholar]
- 29.Mwafongo ANK, Hosseinipour M, Lockman S, Hughes M, Currier J. ACTG 5208. Renal Insufficiency among Women Treated with Tenofovir/Emtricitabine/Lopinavir/Ritonavir or Tenofovir/Emtricitabine/Nevirapine. 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 30.Nishijima T, Komatsu H, Gatanaga H, Aoki T, Watanabe K, Kinai E, et al. Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PloS One. 2011;6:e22661. doi: 10.1371/journal.pone.0022661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiser JJ, Fletcher CV, Flynn PM, Cunningham CK, Wilson CM, Kapogiannis BG, et al. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrobial Agents and Chemotherapy. 2008;52:631–637. doi: 10.1128/AAC.00761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. American Journal of Kidney Diseases. 2012;59:628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odden MC, Scherzer R, Bacchetti P, Szczech LA, Sidney S, Grunfeld C, Shlipak MG. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Archives of Internal Medicine. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szczech LA. Renal dysfunction and tenofovir toxicity in HIV-infected patients. Topics in HIV medicine : a publication of the International AIDS Society, USA. 2008;16:122–126. [PubMed] [Google Scholar]
- 35.Rodriguez-Novoa S, Alvarez E, Labarga P, Soriano V. Renal toxicity associated with tenofovir use. Expert Opinion on Drug Safety. 2010;9:545–559. doi: 10.1517/14740331003627458. [DOI] [PubMed] [Google Scholar]
- 36.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. American Journal of Kidney Diseases. 2011;57:773–780. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Kearney BP, Yale K, Shah J, Zhong L, Flaherty JF. Pharmacokinetics and dosing recommendations of tenofovir disoproxil fumarate in hepatic or renal impairment. Clinical Pharmacokinetics. 2006;45:1115–1124. doi: 10.2165/00003088-200645110-00005. [DOI] [PubMed] [Google Scholar]
- 38.Poizot-Martin I, Solas C, Allemand J, Obry-Roguet V, Pradel V, Bregigeon S, et al. Renal impairment in patients receiving a tenofovir-cART regimen: Impact of tenofovir trough concentration. Journal of Acquired Immune Deficiency syndromes. 2012 doi: 10.1097/QAI.0b013e31827ce4ee. [DOI] [PubMed] [Google Scholar]


