Abstract
Campylobacter jejuni is a significant cause of human gastroenteritis worldwide. In an attempt to further define bacterial factors that influence infectivity, Cj0596 was identified as playing a role in C. jejuni virulence. Cj0596 is a periplasmic chaperone that is similar to proteins involved in outer membrane protein (OMP) folding in other bacteria. Mutation of cj0596 caused an alteration in the levels of eight OMPs, compared to wild-type bacteria. Replacement of the cj0596 mutation with the wild-type cj0596 gene restored a wild-type OMP profile. The altered OMP profile in the cj0596 mutant was accompanied by significant changes in several virulence properties, including an increase in the ability to autoagglutinate, increased susceptibility to several antimicrobial agents, and increased biofilm formation. In summary, mutation of cj0596 alters the C. jejuni OMP profile and leads to changes in OMP-related phenotypes involved in C. jejuni pathogenesis.
Keywords: Campylobacter jejuni, PPIase, Cj0596
Introduction
Campylobacter jejuni is a microaerophilic Gram-negative bacterium that is a major cause of human gastroenteritis (Miller & Mandrell, 2005). C. jejuni gastroenteritis is usually associated with consumption of undercooked poultry that has been contaminated with the bacterium, as a natural reservoir for the bacterium is the cecum of poultry (Miller & Mandrell, 2005). C. jejuni colonizes the small and large intestines in humans, causing severe abdominal pain, fever, and watery or bloody diarrhea, in addition to possible bacteremia, other extraintestinal symptoms, and sequelae of infection including Guillain-Barré Syndrome and reactive arthritis (Nachamkin, et al., 2000).
C. jejuni surface structures including outer membrane proteins (OMPs) affect virulence in several ways. Flagella are important for C. jejuni invasion of host cells in two ways; by motility, and by secretion of proteins including Campylobacter invasion antigens (Cia proteins), FlaC, and FspA (Larson, et al., 2008). In addition to flagella, several OMPs (CadF, PEB1, JlpA, CapA and OMPs of 32 kDa and 36 kDa) are known to play a role in adherence to host cells (Larson, et al., 2008). C. jejuni CfrA, ChuA, Cj0178 and Cj0444 are OMPs that allow C. jejuni to acquire iron and hemin from the host (Stintzi, et al., 2008). Two membrane efflux pumps, CmeABC and CmeDEF, and MOMP are involved in transport of a variety of antibiotics, detergents and dyes in Campylobacter (Zhang & Plummer, 2008).
The role of peptidyl prolyl cis-trans isomerases (PPIases) in virulence may be related to their ability to fold outer membrane proteins as well as some secreted proteins (Sklar, et al., 2007). Cj0596 (the strain 81-176 designation is CJJ81176_0624) is a PPIase that is located in the periplasm, is similar to SurA in E. coli and other orthologs in numerous bacteria, and was previously shown to play a role in C. jejuni virulence (Asakuray, et al., 2007, Rathbun, et al., 2009). Other bacteria in which PPIases have been characterized as virulence factors include Shigella flexneri, Legionella pneumophila, Chlamydia trachomatis, and Neisseria gonorrhoeae (Cianciotto, et al., 1990, Lundemose, et al., 1993, Leuzzi, et al., 2005, Purdy, et al., 2007).
In E. coli, SurA is the primary periplasmic chaperone and plays a role in folding the outer membrane proteins LamB, OmpA, OmpF, and OmpC. A surA mutant showed reduced piliation resulting from decreased levels of PapC and FimD (Sklar, et al., 2007). SurA mutation also results in an increased sensitivity to hydrophobic dyes, detergents, novobiocin, bacitracin, and vancomycin (Sklar, et al., 2007).
Previously, we showed that mutation of cj0596 increased C. jejuni motility and ability to invade human intestinal epithelial cells in vitro, but decreased growth rate and colonization of the intestinal tracts of mice (Rathbun, et al., 2009). Here we investigate the effect of cj0596 mutation on the C. jejuni outer membrane.
Materials and Methods
Bacterial strains and culture conditions
Campylobacter jejuni was routinely maintained with minimal passage on blood agar plates (Remel; Lenexa, KS) at 37°C in sealed culture boxes (Mitsubishi Gas Chemical [MGC], New York, NY) containing a microaerobic atmosphere generated by Pack-Micro Aero (MGC). For the growth of 81-176 and 81-176cj0596+, the complemented mutant derived from 81-176 cj0596 (Rathbun, et al., 2009), streptomycin was added to 30 μg/ml. For the growth of 81-176cj0596, an isogenic mutant derived from 81-176, chloramphenicol (30 μg/ml) was added to select for the mutation. Liquid cultures of all three strains were grown in Mueller-Hinton (MH) broth and cultured in the same environments described above.
Outer Membrane Profile Comparison
To determine whether the C. jejuni OMP composition was altered by mutation of cj0596, OMPs were isolated from 81-176, 81-176cj0596, and 81-176cj0596+ by a modified Sarkosyl extraction method (Hobb, et al., 2009) and 15 μg of protein was run on a single dimension 12% SDS-PAGE gel, which was stained with Coomassie Blue (Hobb, et al., 2009). Densitometry measurements were performed on digital images of the gel using ImageJ software (Abramoff, et al., 2004), by defining small, identical rectangular boundaries around protein bands to be compared and determining the relative number of pixels in each as described (http://rsb.info.nih.gov/ij/index.html). Bands of interest were cut from the gel and the proteins identified using MALDI-ToF-ToF tandem mass spectrometry (MCG Mass Spectrometry Core Facility), using a combination of MS spectra (tryptic fingerprint) and collision-mediated fragmentation MS/MS of peaks isolated from first-dimension MS. Identifications were made using GPS Explorer software (Applied Biosystems) and MASCOT searches of an NCBI database of all known proteobacterial proteins. Two biological replicates were performed, with essentially identical results.
Autoagglutination Assay
The autoagglutination abilities of 81-176, 81-176cj0596, and 81-176cj0596+ were assessed by growing cells overnight in MH broth, then diluting the following morning in MH broth to OD600=1.0. The suspensions were aliquoted into borosilicate glass tubes (2 ml per tube) and allowed to sit undisturbed in room air at 25°C. The OD600 of each cell suspension was measured every 30 min for 2 h, with a decrease in OD600 reflecting autoagglutination (Misawa & Blaser, 2000). Three biological replicates were performed, each in triplicate, with essentially identical results.
Biofilm Formation Assay
The abilities of 81-176, 81-176cj0596, and 81-176cj0596+ to form biofilms were assessed as previously described (Candon, et al., 2007). Briefly, cells were grown overnight in MH broth, then diluted the following morning in MH broth to OD600=0.05. Sterile 96-well polystyrene plates were inoculated with 100 μl C. jejuni cells per well (6 wells per strain) and incubated at 37°C under microaerobic conditions without agitation. After 72 h, biofilm formation was assessed by staining the plates with crystal violet (CV) solution (1% CV in 95% ethanol) and incubating at room temperature for 15 min, washing twice with distilled water to remove unbound CV, and dissolving the bound CV by adding 1.5 ml DMSO and incubating at room temperature for 48 h. The OD570 was then measured, with OD570 reflecting biofilm formation.
Antimicrobial agent sensitivity assays
The sensitivities of C. jejuni 81-176, 81-176cj0596, and 81-176cj0596+ to a variety of antimicrobial compounds were determined using disk diffusion assays. Bacterial suspensions (OD600=1.0) were streaked on MH plates and 7 mm filter paper disks impregnated with an antimicrobial agent were placed on the plate. Plates were incubated at 37°C under microaerobic conditions for 48 h and the zones of inhibition were measured. The antimicrobial agents tested were ampicillin (6.3 ng/disk), polymyxin B (25 ng/disk), vancomycin (500 ng/disk), gentamicin (3.1 ng/disk), ethidium bromide (3.1 ng/disk), and CV (7.8 ng/disk).
Results
Mutation of cj0596 alters the outer membrane composition of C. jejuni
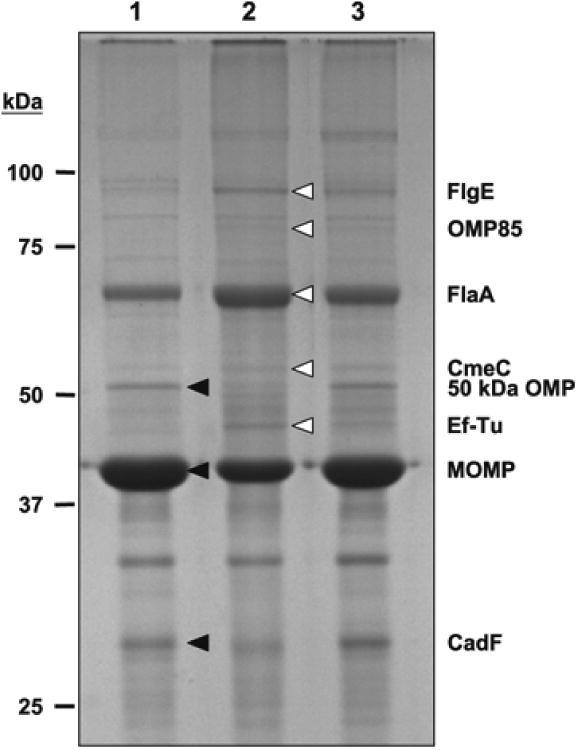
The effect of cj0596 mutation on the C. jejuni outer membrane was studied by purifying the outer membrane fractions from 81-176, 81-176cj0596, and 81-176cj0596+, and comparing the OMP profiles using SDS-PAGE. Several proteins showed altered abundance in the cj0596 mutant compared to the wild-type (Fig. 1); those bands were excised and the proteins identified by using MALDI-ToF/ToF mass spectrometry (MS/MS data shown in Fig. S1). The proteins that were more abundant in the mutant were FlgE (Cj0043, flagellar hook protein; 2.5-fold), OMP85 (Cj0129, a predicted component of the OMP insertion machinery; 3.0-fold), FlaA (Cj1339, flagellar filament protein; 1.8-fold), CmeC (Cj0365, the outer membrane component of the CmeABC efflux pump; 1.9-fold), and Ef-Tu (Cj0470, a translation elongation factor; 2.4-fold). The proteins that were less abundant in the mutant were 50 kDa OMP (Cj1170, minor porin; 1.5-fold), major outer membrane protein (Cj1259, MOMP; 1.5-fold), and CadF (Cj1478, fibronectin binding protein; 1.3-fold). All proteins that showed altered abundance in the mutant returned to wild-type or near wild-type levels in the complemented mutant.
Figure 1.
SDS-PAGE gel (12%) showing differences in OMP profiles of 81-176 (lane 1), 81-176cj0596 (lane 2), 81-176cj0596+ (lane 3). Proteins with altered expression are indicated with arrowheads. Proteins with greater expression in the cj0596 mutant: FlgE, OMP85, FlaA, CmeC, Ef-Tu. Proteins with lesser expression in the cj0596 mutant: 50 kDa OMP, MOMP, CadF.
Autoagglutination ability of C. jejuni is enhanced by mutation of cj0596
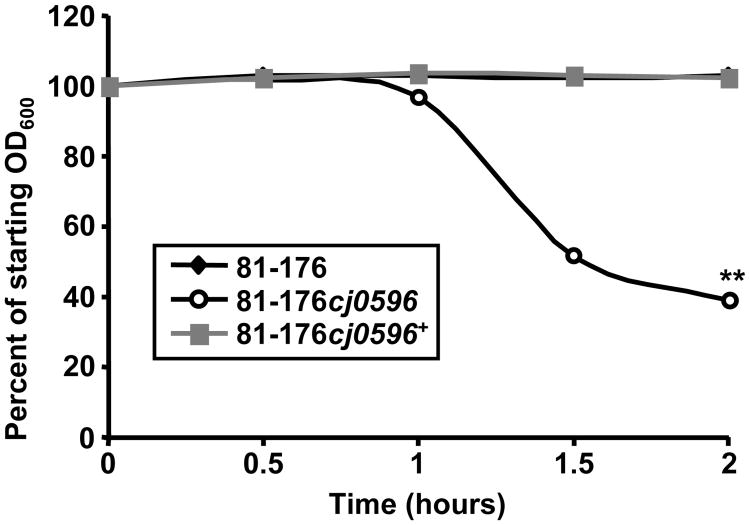
To examine the role of Cj0596 in autoagglutination, the abilities of C. jejuni 81-176, 81-176cj0596, and 81-176cj0596+ to autoagglutinate were compared. The 81-176cj0596 mutant strain autoagglutinated to a greater extent than the wild-type and complemented mutant (Fig 2). Under these conditions, the wild-type and complemented mutant showed no decrease in OD600 at 2 h, whereas the OD600 of the mutant was decreased to 38.7% of the initial OD600 (the experiment was performed in triplicate, p<0.001).
Figure 2.
Autoagglutination abilities of C. jejuni strains. Culture suspensions (OD600=1.0) of strains 81-176 (black line), 81-176cj0596 (dashed line) and 81-176cj0596+ (gray line) were allowed to sit undisturbed at 25°C and OD600 was measured every 30 min for 2 h. Statistical significance (p <0.001) is represented by two asterisks. Error bars are present but are smaller than the symbols designating data points.
cj0596 mutation increases Campylobacter biofilm formation
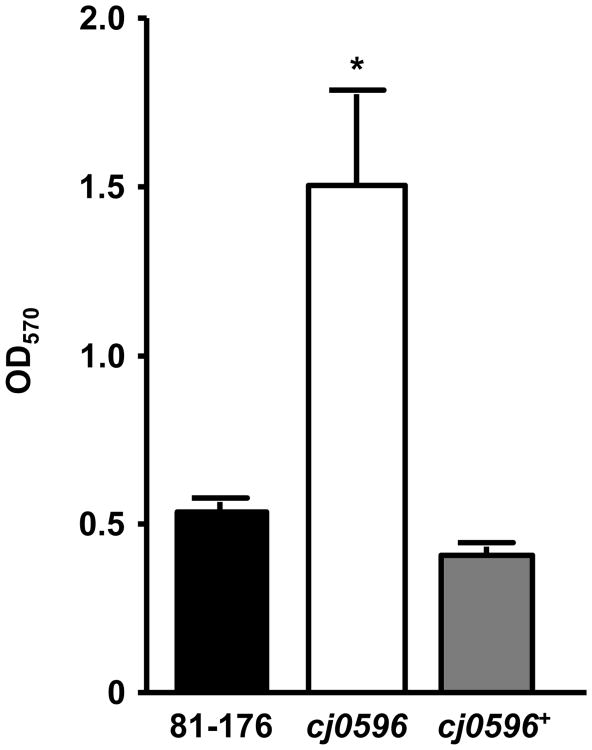
The role of Cj0596 in biofilm formation was assessed by comparing the biofilm forming capacities of C. jejuni 81-176, 81-176cj0596, and 81-176cj0596+. The OD570 measurements for the wild-type and complemented mutant (0.53 and 0.41, respectively) differed significantly (p<0.01) from the OD570 measured for the mutant (1.50), indicating that the cj0596 mutant showed a much greater ability to form biofilms than either the wild-type or complemented mutant (Fig. 3).
Figure 3.
Biofilm formation of C. jejuni strains. Culture suspensions (OD600=0.05) of strains 81-176 (black), 81-176cj0596 (white) and 81-176cj0596+ (gray) were incubated statically in 96-well polystyrene plates for 72 h at 37°C, then stained with CV. Biofilm formation was quantified by measuring OD570. Statistical significance (p <0.05) is represented by an asterisk.
Mutation of cj0596 increases the susceptibility of C. jejuni to antimicrobial agents
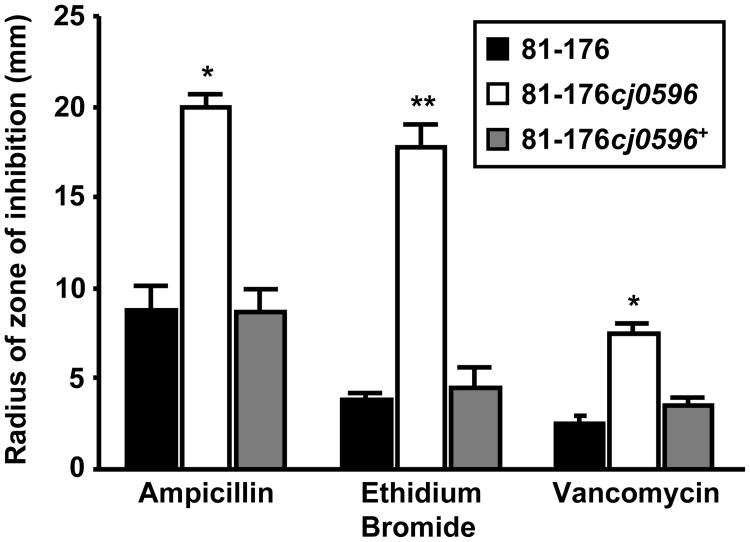
A disk diffusion method was used to determine whether deletion of cj0596 affected the susceptibilities of C. jejuni 81-176, 81-176cj0596, and 81-176cj0596+ to a variety of antimicrobial agents. The cj0596 mutant was significantly more susceptible (the experiment was performed in triplicate, p<0.001) to ampicillin (6.3 ng/disk). ethidium bromide (3.1 ng/disk), and vancomycin (500 ng/disk) (Fig 4); whereas no significant increase in susceptibility to CV (7.8 ng/disk), polymyxin B (25 ng/disk), or gentamicin (3.1 ng/disk) was observed (data not shown).
Figure 4.
Susceptibilities of C. jejuni strains to antimicrobial agents. The disk diffusion method was used to compare the susceptibilities of strains 81-176 (black), 81-176cj0596 (white) and 81-176cj0596+ (gray) to ampicillin (6.3 ng/disk), ethidium bromide (3.1 ng/disk), and vancomycin (500 ng/disk). Susceptibilities are expressed as the radius of the zone of inhibition by each compound, and statistical significance is represented by one asterisk (p <0.05) or two asterisks (p <0.001).
Discussion
C. jejuni is an important human pathogen, but many details of the mechanisms involved in infection are unknown. Previously, we and others found that mutation of cj0596 affects several virulence-related phenotypes (Asakura, et al., 2007, Rathbun, et al., 2009). In our previous study, we found that a cj0596 mutant showed an increase in motility and invasion of INT407 intestinal epithelial cells, but also showed a modestly lower growth rate and decreased ability to colonize mice (Rathbun, et al., 2009). Furthermore, proteomics experiments comparing the whole-cell protein profiles of C. jejuni strains 81-176, 81-176cj0596, and 81-176cj0596+ also indicated that the changes in virulence properties were accompanied by changes in the C. jejuni whole-cell proteome. While our prior studies may have identified some proteins involved in the observed mutant phenotypes, we considered that analysis of the OMPs expressed by these strains may be informative given that Cj0596 is a periplasmic PPIase involved in folding integral OMPs.
OMPs play a large role in C. jejuni pathogenesis. It should be noted that the analysis of OMP profiles can be inherently challenging if the expression of an abundant protein (including a porin such as MOMP, see below) is altered, due to the relatively small total number of proteins in the outer membrane. While this can complicate the analysis somewhat, careful loading of the samples such that the abundance of the majority of the proteins is unchanged still allows important comparisons to be made. Consequently, in the present study, the 81-176 cj0596 mutant showed altered levels of eight OMPs, five of which were more abundant in the cj0596 mutant and three proteins which were less abundant in the mutant. The five proteins that were more abundant in the cj0596 mutant were FlaA (Cj1339), FlgE (Cj0043), OMP85 family protein (Cj0129), CmeC (Cj0365), and Ef-Tu (Cj0470). Upregulation of FlaA, the major subunit of the C. jejuni flagellum, was seen previously in a proteomic study done on whole cells (Rathbun, et al., 2009). FlgE is the flagellar hook protein, which is required for flagellar structure, flagellin secretion, and motility. The increased levels of both FlgE and FlaA is consistent with the increase in motility and invasion of INT407 cells seen in the cj0596 mutant (Rathbun, et al., 2009), possibly by increasing the functionality and/or efficiency of the flagella. Alternatively, the greater abundance of these flagellar proteins may reflect that a larger proportion of the C. jejuni population is expressing the phase-variable motile phenotype (Hendrixson, 2006).
Cj0129 is a member of the highly conserved Omp85 protein family that is involved in insertion of OMPs into the outer membrane of bacteria, as well as of mitochondria and chloroplasts (Schleiff & Soll, 2005). The upregulation of Cj0129 in the cj0596 mutant may be a response to the altered levels of other OMPs and/or an increase in incorrectly folded proteins in the periplasm. CmeC is the outer membrane component of the CmeABC efflux pump, which is responsible for the intrinsic resistance of Campylobacter to a variety of antimicrobial agents (see also below)(Lin, et al., 2002). Although Ef-Tu is primarily cytoplasmic and involved in protein translation, it has been identified in the outer membrane of several bacterial species, including E. coli and Mycoplasma pneumoniae (Berrier, et al., 2000, Pancholi & Chhatwal, 2003, Prokhorova, et al., 2006, Kolberg, et al., 2008). In our previous C. jejuni whole-cell proteomic study, Ef-Tu was less abundant overall in the cj0596 mutant (Rathbun, et al., 2009). It is possible that this reflects increased localization of EF-Tu to the outer membrane fraction, despite an overall decrease in cellular EF-Tu.
Three proteins with decreased abundance in the cj0596 mutant were 50 kDa OMP (Cj1170), MOMP (Cj1259), and CadF (Cj1478). The 50kDa OMP belongs to the OmpA family and forms a cation selective pore (Bolla, et al., 2000). It is upregulated in C. jejuni during in vivo growth (Stintzi, et al., 2005). Because it is upregulated during in vitro growth, the decreased abundance of the 50 kDa OMP in the cj0596 mutant may play a role in the decrease in maximum culture density described previously (Rathbun, et al., 2009). In addition to its porin activity, MOMP is involved in the structural organization of the outer membrane and in adherence to cultured cells (Schroder & Moser, 1997). The change in MOMP abundance in the outer membrane therefore could be responsible for, or reflective of, overall changes in outer membrane architecture. CadF promotes the binding of C. jejuni to fibronectin on host cells and is required for maximal adherence and invasion of INT407 cells and colonization of the chicken cecum (Larson, et al., 2008). As fibronectin is found in the basement membrane of the mouse intestine, it is possible that the decreased amount of CadF found in the cj0596 mutant may play a part in the mouse colonization defect previously seen in the mutant (Rathbun, et al., 2009). It is also possible that the changes in the abundance of each these OMPs is related to the modest growth defect of the cj0596 mutant, although clearly both the altered OMP profile and growth defect result from mutation of cj0596 as WT characteristics for both are restored in the complemented mutant.
While the levels of eight OMPs were altered in the cj0596 mutant, the mutation may affect other OMPs whose abundances are not altered. Such proteins may be improperly folded and non-functional, as was shown to occur with the aberrant presentation of Shigella flexneri IcsA in a surA mutant (Purdy, et al., 2007). Any poorly folded, non-functional proteins may affect the various phenotypes seen in the cj0596 mutant, but would not have been detected by SDS-PAGE.
Accompanying the altered cj0596 mutant OMP profile were changes in surface-related characteristics. Autoagglutination is a known virulence factor associated with pili or other OMPs in bacteria including enteropathogenic E. coli and Vibrio cholerae (Chiang, et al., 1995, Knutton, et al., 1999). In C. jejuni, autoagglutination requires glycosylated flagella (Guerry, 2007). The cj0596 mutant autoagglutinated more quickly than the wild-type and complemented mutant. Because deletion of cj0596 alters the C. jejuni OMP profile, including the expression of flagellar proteins, the increased ability of the cj0596 mutant to autoagglutinate is consistent with increased expression of flagella.
Biofilms are groups of cells enclosed in a matrix of extracellular polymeric substances. Biofilm formation is important in bacterial pathogenesis as the ability of bacteria to form biofilms may allow them to better survive both in the environment and during infection. C. jejuni forms biofilms (Svensson, et al., 2009), and a recent proteomic analysis identified Cj0596 as being more highly expressed in biofilm-forming cells compared to planktonic cells (Kalmokoff, et al., 2006). Previously, a C. jejuni NCTC 11168 cj0596 mutant was found to show a decreased ability to form biofilms when compared to the wild-type (Asakura, et al., 2007). However, our assays showed that an 81-176 cj0596 mutant formed significantly more biofilms. It is possible that the difference between the two studies lies in interstrain differences (81-176 vs. NCTC 11168). However, it is also possible that the biofilm phenotype in the previous study was due to an unlinked mutation, as the cj0596 mutation was not complemented in that work. The increased ability of the cj0596 mutant to form biofilms may be due to altered outer membrane architecture, or may be partially due to the increased motility of this mutant.
Alteration of the outer membrane can render a bacterium more susceptible to antimicrobial agents, particularly those that are hydrophobic, through increased permeability or alteration of drug efflux pumps. Efflux pumps in C. jejuni include CmeABC (predominant) and CmeDEF (secondary), which confer resistance to fluoroquinolones, other antibiotics, dyes, detergents, disinfectants, and bile (Lin, et al., 2002, Akiba, et al., 2006). An E. coli surA mutant was more sensitive to bacitracin, vancomycin, and bile salts due to an alteration in the outer membrane (Lazar & Kolter, 1996). Similarly, the cj0596 mutant was more susceptible to ethidium bromide, vancomycin, and ampicillin (Fig. 4) whereas no effect was seen with crystal violet, polymyxin B, or gentamicin (data not shown). Of the compounds tested in this study, ampicillin, ethidium bromide, gentamicin, and polymyxin B were previously tested in an 81-176 cmeB mutant (Lin, et al., 2002). The cmeB mutant was more susceptible to ampicillin (32-fold), ethidium bromide (8-fold), and gentamicin (2-fold); polymyxin B showed no change in susceptibility. The change in susceptibilities may be due to an alteration of antimicrobial targets in the membrane, an overall increase in membrane permeability, or a change in efflux pump structure due to changes in the stoichiometry of its subunits.
Acknowledgments
This study was supported by National Institutes of Health grants AI055715 and AI058284 to SAT.
We thank Rhonda I. Hobb for sharing her expertise regarding OMP purification experiments, and members of the Thompson Lab for helpful comments and discussions. We also thank John Nechtman and Eric Miller (MCG Proteomics Core Laboratory) for the mass spectrometry work.
Contributor Information
Kimberly M Rathbun, Email: krathbunmd@students.mcg.edu.
Stuart A Thompson, Email: stthomps@mcg.edu.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Akiba M, Lin J, Barton YW, Zhang Q. Interaction of CmeABC and CmeDEF in conferring antimicrobial resistance and maintaining cell viability in Campylobacter jejuni. J Antimicrob Chemother. 2006;57:52–60. doi: 10.1093/jac/dki419. [DOI] [PubMed] [Google Scholar]
- Asakura H, Yamasaki M, Yamamoto S, Igimi S. Deletion of peb4 gene impairs cell adhesion and biofilm formation in Campylobacter jejuni. FEMS Microbiol Lett. 2007;275:278–285. doi: 10.1111/j.1574-6968.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- Berrier C, Garrigues A, Richarme G, Ghazi A. Elongation factor Tu and DnaK are transferred from the cytoplasm to the periplasm of Escherichia coli during osmotic downshock presumably via the mechanosensitive channel mscL. J Bacteriol. 2000;182:248–251. doi: 10.1128/jb.182.1.248-251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla JM, De E, Dorez A, Pages JM. Purification, characterization and sequence analysis of Omp50, a new porin isolated from Campylobacter jejuni. Biochem J. 2000;352:637–643. [PMC free article] [PubMed] [Google Scholar]
- Candon HL, Allan BJ, Fraley CD, Gaynor EC. Polyphosphate kinase 1 (PPK1) is a pathogenesis determinant in Campylobacter jejuni. J Bacteriol. 2007;189:8099–8108. doi: 10.1128/JB.01037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SL, Taylor RK, Koomey M, Mekalanos JJ. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- Cianciotto NP, Eisenstein BI, Mody CH, Engleberg NC. A mutation in the mip gene results in an attenuation of Legionella pneumophila virulence. J Infect Dis. 1990;162:121–126. doi: 10.1093/infdis/162.1.121. [DOI] [PubMed] [Google Scholar]
- Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007;15:456–461. doi: 10.1016/j.tim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Hendrixson DR. A phase-variable mechanism controlling the Campylobacter jejuni FlgR response regulator influences commensalism. Mol Microbiol. 2006;61:1646–1659. doi: 10.1111/j.1365-2958.2006.05336.x. [DOI] [PubMed] [Google Scholar]
- Hobb RI, Fields JA, Burns CM, Thompson SA. Evaluation of procedures for outer membrane isolation from Campylobacter jejuni. Microbiology. 2009;155:979–988. doi: 10.1099/mic.0.024539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmokoff M, Lanthier P, Tremblay TL, et al. Proteomic analysis of Campylobacter jejuni 11168 biofilms reveals a role for the motility complex in biofilm formation. J Bacteriol. 2006;188:4312–4320. doi: 10.1128/JB.01975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S, Shaw RK, Anantha RP, Donnenberg MS, Zorgani AA. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol Microbiol. 1999;33:499–509. doi: 10.1046/j.1365-2958.1999.01495.x. [DOI] [PubMed] [Google Scholar]
- Kolberg J, Hammerschmidt S, Frank R, Jonak J, Sanderova H, Aase A. The surface-associated elongation factor Tu is concealed for antibody binding on viable pneumococci and meningococci. FEMS Immunol Med Microbiol. 2008;53:222–230. doi: 10.1111/j.1574-695X.2008.00419.x. [DOI] [PubMed] [Google Scholar]
- Larson CL, Christensen JE, Pacheco SA, Minnich SA, Konkel ME. Campylobacter jejuni secretes proteins via the flagellar type III secretion system that contribute to host cell invasion and gastroenteritis. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. ASM press; Washington, DC: 2008. pp. 315–332. [Google Scholar]
- Lazar SW, Kolter R. SurA assists the folding of Escherichia coli outer membrane proteins. J Bacteriol. 1996;178:1770–1773. doi: 10.1128/jb.178.6.1770-1773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzzi R, Serino L, Scarselli M, et al. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol Microbiol. 2005;58:669–681. doi: 10.1111/j.1365-2958.2005.04859.x. [DOI] [PubMed] [Google Scholar]
- Lin J, Michel LO, Zhang Q. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrob Agents Chemother. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundemose AG, Kay JE, Pearce JH. Chlamydia trachomatis Mip-like protein has peptidyl-prolyl cis/trans isomerase activity that is inhibited by FK506 and rapamycin and is implicated in initiation of chlamydial infection. Mol Microbiol. 1993;7:777–783. doi: 10.1111/j.1365-2958.1993.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Miller WG, Mandrell RE. Prevalence of Campylobacter in the food and water supply: Incidence, outbreaks, isolation and detection. In: Ketley JM, Konkel ME, editors. Campylobacter: Molecular and cellular biology. Horizon Bioscience; Norfolk, UK: 2005. pp. 101–163. [Google Scholar]
- Misawa N, Blaser MJ. Detection and characterization of autoagglutination activity by Campylobacter jejuni. Infect Immun. 2000;68:6168–6175. doi: 10.1128/iai.68.11.6168-6175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I, Allos BM, Ho TW. Campylobacter jejuni infection and the association with Guillain-Barré Syndrome. In: Nachamkin I, Blaser MJ, editors. Campylobacter. ASM Press; Washington, DC: 2000. pp. 155–175. [Google Scholar]
- Pancholi V, Chhatwal GS. Housekeeping enzymes as virulence factors for pathogens. Int J Med Microbiol. 2003;293:391–401. doi: 10.1078/1438-4221-00283. [DOI] [PubMed] [Google Scholar]
- Prokhorova TA, Nielsen PN, Petersen J, et al. Novel surface polypeptides of Campylobacter jejuni as traveler's diarrhoea vaccine candidates discovered by proteomics. Vaccine. 2006;24:6446–6455. doi: 10.1016/j.vaccine.2006.05.085. [DOI] [PubMed] [Google Scholar]
- Purdy GE, Fisher CR, Payne SM. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J Bacteriol. 2007;189:5566–5573. doi: 10.1128/JB.00483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun KM, Hall JE, Thompson SA. Cj0596 is a periplasmic peptidyl-prolyl cis-trans isomerase involved in Campylobacter jejuni motility, invasion, and colonization. BMC Microbiology. 2009 doi: 10.1186/1471-2180-9-160. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff E, Soll J. Membrane protein insertion: mixing eukaryotic and prokaryotic concepts. EMBO Rep. 2005;6:1023–1027. doi: 10.1038/sj.embor.7400563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder W, Moser I. Primary structure analysis and adhesion studies on the major outer membrane protein of Campylobacter jejuni. FEMS Microbiol Lett. 1997;150:141–147. doi: 10.1111/j.1574-6968.1997.tb10362.x. [DOI] [PubMed] [Google Scholar]
- Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 2007;21:2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, van Vliet AHM, Ketley JM. Iron metabolism, transport and regulation. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. ASM press; Washington, DC: 2008. pp. 591–610. [Google Scholar]
- Stintzi A, Marlow D, Palyada K, Naikare H, Panciera R, Whitworth L, Clarke C. Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect Immun. 2005;73:1797–1810. doi: 10.1128/IAI.73.3.1797-1810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson SL, Davis LM, MacKichan JK, Allan BJ, Pajaniappan M, Thompson SA, Gaynor EC. The CprS sensor kinase of the zoonotic pathogen Campylobacter jejuni influences biofilm formation and is required for optimal chick colonization. Mol Microbiol. 2009;71:253–272. doi: 10.1111/j.1365-2958.2008.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Plummer PJ. Mechanisms of antibiotic resistance in Campylobacter. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. ASM press; Washington, DC: 2008. pp. 263–276. [Google Scholar]