Abstract
Background
Glioblastoma multiforme (GBM) is a rare tumor, which affects 1/100 000 individuals, but it represents 30% of central nervous system malignancies. GBM is a severe tumor responsible for 2% of all cancer-related deaths. Although characterized by genotypic and phenotypic heterogeneities, GBM invariably resists conventional chemo- and radiotherapies. Several chromosome alterations and gene mutations were detected in GBM. Simian virus 40 (SV40), a small DNA tumor virus, has been found in GBM specimens by some studies, while other investigations have not confirmed the association.
Methods
An indirect enzyme-linked immunosorbent assay with 2 synthetic peptides mimicking SV40 antigens of viral capsid proteins 1–3 was employed to detect specific antibodies against SV40 in serum samples from GBM-affected patients, together with controls represented by patients affected by breast cancer and normal subjects of the same median age.
Results
Our data indicate that in serum samples from GBM-affected patients (n = 44), the prevalence of antibodies against SV40 viral capsid protein antigens is statistically significantly higher (34%, P = .016 and P = .03) than in the control groups (15%), represented by healthy subjects (n = 101) and patients affected by breast cancer (n = 78), respectively.
Conclusion
Our data indicate that SV40, or a closely related yet undiscovered human polyomavirus, is associated with a subset of GBM and circulates in humans. Our study can be transferred to the clinical oncology application to discriminate different types of heterogeneous GBM, which in turn may address an innovative therapeutic approach to this fatal cancer.
Keywords: glioblastoma multiforme, immunglobulin G, simian virus 40
Glioblastoma multiforme (GBM) is the most severe form of cancer among gliomas. Indeed, GBM is a World Health Organization grade IV tumor of the CNS, with a mean survival time of 0.5 years.1 Its incidence is ∼1/100 000, which represents 30% of all CNS malignancies.2 According to the recent Eurocare 3 report,3 the mean age of patients affected by glioblastoma is 66 years. The extremely grim prognosis of GBM patients probably explains why such a rare tumor accounts for 2% of all cancer-related deaths in both Europe and the United States.4 GBM is characterized by high genotypic and phenotypic heterogeneities, but it is invariably and homogeneously resistant to conventional chemo/radiotherapy.1 The vast majority (95%) of GBM is represented by primary de novo glioblastomas.5
GBM is considered, like other tumors, a genetic disease of the somatic cell due to an impressive series of alterations of the cellular genome, such as point mutations, gene amplifications, translocations, and deletions, as well as epigenetic modifications, including methylation of specific genes. A limited percentage (5%) of GBM is linked to genetic predispositions, which are mainly associated with rare hereditary syndromes, such as Li–Fraumeni syndrome, Turcot syndrome, Peutz–Jegher syndrome, and neurofibromatosis 1 and 2.2
Exposure to chemical and physical carcinogenic agents, the genetic predisposition, and/or infections by oncogenic viruses, including simian virus 40 (SV40), have been repeatedly advocated in the literature.6,7 SV40 is a nonenveloped small DNA virus of ∼5.2 kb in size. SV40 was recognized in the 1960s as a contaminant of both inactivated (Salk) and live attenuated (Sabin) antipoliomyelitis vaccines. As a consequence, SV40 was inadvertently administered to human populations worldwide mainly by antipoliomyelitis vaccines, produced in SV40 naturally infected monkey kidney cells. Soon after its isolation, SV40 was characterized as a transforming and tumorigenic viral agent.6,7 Cell transformation and tumorigenesis by SV40 is induced by 2 oncoproteins, the large T antigen (Tag) and the small t antigen (tag), which target key cellular proteins, such as the tumor suppressor p53 and retinoblastoma family proteins, inactivating their functions.6,8 This small DNA tumor virus was found to be associated with some human cancers, including GBM. SV40 footprints were detected at high prevalence in GBM specimens, but SV40 sequences have also been revealed, although at lower prevalence, in blood specimens from healthy donors.9–11 In this context it is worth recalling that other investigations did not find SV40 footprints in human brain tumor specimens12,13 or higher SV40 antibodies in serum samples from brain tumor patients than in normal individuals.14 It has been reported that SV40-negative data on human tumor samples obtained by some groups were affected by technical flaws that invalidate the conclusions.15–17 A considerable debate, because of these contrasting reports, has developed in the scientific community.6,7 SV40 antibody detection had been performed in most cases using serologic methods with SV40 virus-like particles, but due to the high protein homology among the 3 main polyomaviruses—SV40, BKV, and JCV—final results were always influenced by some cross-reactivity.18–24 Altogether these reports prompted us to use an indirect ELISA with synthetic peptides employed as specific mimotopes, mimicking SV40 viral capsid antigens. Indeed, recent investigations have reported on the development of a specific and sensitive serologic test for SV40. The test is based on an indirect ELISA employing synthetic peptides as mimotopes/antigens of SV40 viral capsid proteins (VPs), without cross-reactivity with other closely related human polyomaviruses, such as BKV and JCV.25–28 This immunologic assay was used to detect specific serum antibodies against SV40 VPs in normal individuals of different ages and oncologic patients affected by malignant pleural mesothelioma and breast cancer (BC).25–28
In this investigation, serum samples from GBM patients were comparatively analyzed for the presence and titer of antibodies against SV40 infection, with serum samples from healthy individuals and from other reliable controls. No other slow virus infections were investigated.
Materials and Methods
Patients and Serum Samples
A total of 223 serum samples were collected in different Italian institutions, from GBM (n = 44) and BC patients (n = 78) and a wide spectrum of adult healthy subjects (n = 101). All patients/subjects were vaccinated against poliomyelitis. The sera of our collection were obtained from discarded clinical laboratory analysis samples, anonymously collected, and coded with indications of age, gender, and pathology, if any. The project was approved by the County Ethical Committee, Ferrara, Italy.
Synthetic Peptides
Computer-assisted analyses allowed us to select 2 specific SV40 peptides from the late viral region by comparing the 3 capsid proteins, VP1, VP2, and VP3, from SV40, with the amino acids of the human BKV and JCV polyomaviruses, which are highly homologous to SV40,26 as well as with other, less homologous, polyomaviruses.25 Previous ELISA results indicated that the 2 SV40 peptides did not cross-react with the BKV and JCV hyperimmune sera employed as controls.26 The 2 peptides belong to VP1/VP2/VP3 (http://www.ncbi.nlm.nih.gov/nuccore). The amino acid (aa) sequences of the 2 peptides, named VP1 B and VP2/3 C, are as follows:
VP1 B: NH2- NPDEHQKGLSKSLAAEKQFTDDSP- COOH
VP2/3 C: NH2- IQNDIPRLTSQELERRTQRYLRD- COOH
VP1 B and VP2/3 C mimotopes were selected because they react specifically in indirect ELISA with the rabbit hyperimmune serum, which had been experimentally immunized with SV40 (positive control serum). BKV and JCV hyperimmune sera did not react with VP1 B and VP2/3 C peptides (negative control sera). The aa residues of the 2 specific SV40 VP peptides show low homology with the VPs of BKV and JCV.26 Human neutrophil peptide, aa sequence SFRNGVGTGMKKTSFQRAKS, was employed as a negative control peptide.29 The synthetic peptides were synthesized by standard procedures and were purchased from UFPeptides.
Control Immune Sera
Hyperimmune sera against SV40 and BKV were obtained from rabbits inoculated with purified viral stocks as previously reported.26 The serum against JCV was kindly provided by Dr Major of the National Institutes of Health.30 The immune serum anti-BKV was titered by the hemagglutination inhibition test employing human erythrocytes from the O Rh+ group.26 Anti-SV40 serum was titered by a plaque-reduction neutralization assay.26,28
Indirect Enzyme-linked Immunosorbent Assay
An indirect ELISA was developed and standardized to detect specific antibodies against SV40 in human sera using synthetic peptides. Peptide coating: plates were coated with 5 μg of the selected peptide for each well, diluted in 100 μL of Coating Buffer (Candor Bioscience). Peptide blocking: blocking was made with 200 μL/well of Blocking Solution (Candor Bioscience) at 37°C for 90 min. Primary antibody adding: different wells were covered with 100 μL containing the following sera: positive control, represented by the immune rabbit serum containing anti-SV40 antibodies, negative controls represented by the immune sera anti-BKV and anti-JCV, and human serum samples under analysis diluted at 1:20 in Low Cross Buffer (Candor Bioscience). Each sample was analyzed 3 times. Secondary antibody adding: the solution contained a goat anti-human IgG heavy and light chain specific peroxidase conjugate (Calbiochem-Merck) diluted 1:10 000 in Low Cross Buffer. Dye treatment and spectrophotometric reading: samples were treated with 100 μL of 2,2′-azino-bis 3-ethylbenzthiazoline-6-sulfonic acid (ABTS) solution (Sigma-Aldrich) and then read at the spectrophotometer (Thermo Electron Multiskan EX) at a wavelength (λ) of 405 nm. This approach detects the color intensity in wells where the immunocomplexes were formed by optical density (OD).
Cutoff Determination
The cutoff was determined in each assay by an OD reading of 2 negative controls, added to the standard deviation and multiplied 3 times. Sera with antibodies against SV40 were considered VP positive upon reacting to both peptides from the late region. The reproducibility of the results was assessed with 3 replica experiments carried out by independent operators with no data variability.
SV40 Neutralization Assay With Human Serum Samples
Permissive CV-1 monkey kidney cells were used for a plaque-reduction neutralization assay on SV40 infectivity, described and modified slightly as follows: CV-1 cells were grown and propagated in Dulbecco's modified Eagle's medium (DMEM) containing 2X vitamins and amino acids, supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere with 5% CO2. SV40 infectivity neutralization was carried out by incubating each human serum, diluted at 1:20 in phosphate buffered saline (PBS) with 5 × 104 plaque forming units of SV40 at 37°C for 30 min. Then the suspension was added to the CV-1 cell monolayer for 2 h at 37°C. The inoculum was removed and the monolayer washed 3 times with DMEM and overlaid with the medium containing 2% FBS. Each sample was tested in duplicate. The neutralization assay included the following controls: (i) SV40 only in PBS, (ii) SV40 mixed with rabbit or human nonimmune serum, (iii) SV40 mixed with hyperimmune rabbit serum, and (iv) cells only in PBS. Cultures were observed using a light microscope for the presence of cytopathic effect for 3 weeks.
Statistical Analyses
The prevalence of SV40-positive serum samples from oncologic patients was compared with the prevalence detected in healthy individuals. All data are expressed as percentage (%). To determine significances between 2 groups, we used a 2-sided chi-square test with Yates' correction. All computational analyses were performed by Prism 4.0 software (GraphPad). For all tests, we considered P < .05 to be statistically significant. The serologic profile of serum antibody reactivity to SV40 mimotopes was statistically analyzed using Student's unpaired t-test.
Results
High Prevalence of Antibodies Against SV40 in Serum Samples From GBM Patients
Serum samples from oncologic patients affected by GBM were analyzed by indirect ELISA for the presence of IgG class antibodies against SV40 VP mimotopes/epitopes.
In the first phase of this investigation, indirect ELISA testing was employed to assay serum samples from GBM patients (median age = 61 y), which had been diluted at 1:20, for reactivity to SV40 epitopes from VP1, VP1 B peptide. Serum samples that reacted with the SV40 VP1 B mimotope reached an overall prevalence of 43% (Table 1).
Table 1.
Prevalence of serum IgG antibodies reacting with SV40 VP mimotopes
| Human Sera | n | Median Age, y | Male, % | Number of Positive Samples (%) |
||
|---|---|---|---|---|---|---|
| VP B | VP C | VPs B + C | ||||
| GBM | 44 | 61 | 68 | 19 (43) | 18 (41) | 15 (34) |
| Healthy individuals | 101 | 61 | 64 | 19 (19) | 17 (17) | 15 (15) |
| BC | 78 | 42 | 0 | 14 (18) | 13 (17) | 12 (15) |
Human sera were from GBM patients, healthy blood donors, and BC patients. The different prevalence of SV40 antibodies between the cohorts of GBM-affected patients was statistically significant compared with cohorts of healthy blood donors (P = .016) and BC patients (P = .03). Statistical analysis was performed with the χ2 test with Yates' correction.
The same assay was then addressed to detect IgG class serum antibodies against SV40 VP2/3 epitopes, which are known as VP2/3 C. It turned out that serum samples reacted with the SV40 VP2/3 C peptide with a prevalence of 41%, which is similar to that detected previously for the VP1 B peptide (Table 1). Conversely, seronegative samples for the SV40 VP1 B peptide failed to react with SV40 VP2/3 C epitopes. The exceptions were negligible and were represented by a few serum samples found to be negative for VP1 B while testing positive for VP2/3 C peptide, and vice versa. The difference was not statistically significant (P > .05; Table 1). Sera, diluted at 1:20, had a general value of ∼0.16 OD.
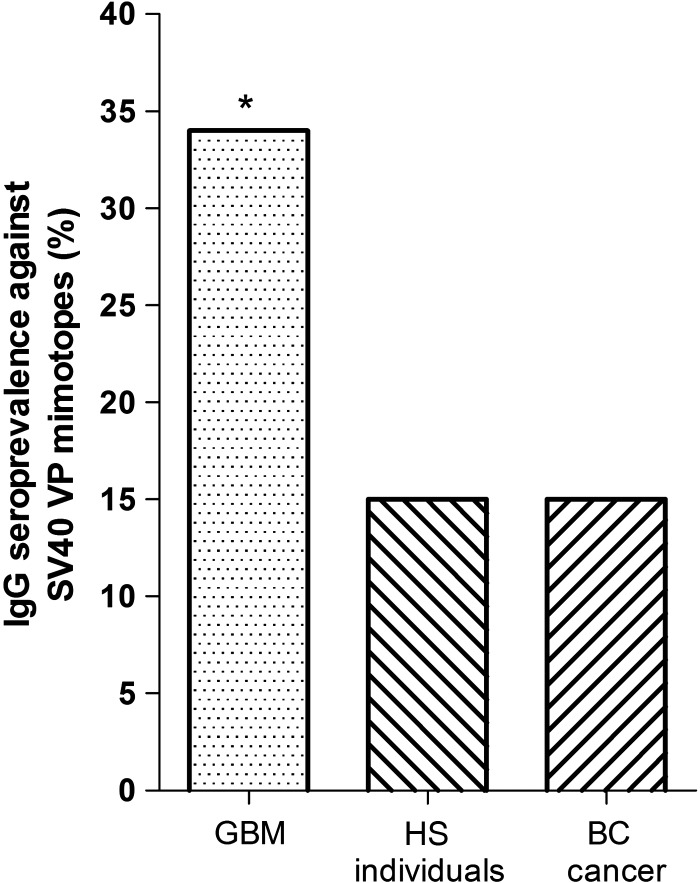
Combining the SV40-positive sera, both for the VP1 B and VP2/3 C peptides, the overall prevalence was 34% (Table 1, Fig. 1).
Fig. 1.
Prevalence of SV40-positive serum samples from GBM and BC patients and healthy subjects (HS). To compare the SV40 prevalence of GBM with that detected in HS employed as controls, HS were chosen with the same median age and sex. Statistical analyses revealed significant differences in SV40 prevalence between GBM patients and the relative cohort of HS (P = .016) and between GBM and BC patients (P = .03). Statistical analysis was performed by the χ2 test with Yates' correction. *P < .05.
No positive results were obtained with human peptide used as a control, which had an OD of <0.1. SV40-positive sera tested by indirect ELISA diluted at 1:20 had a general cutoff, by spectrophotometric reading, in the range of 0.17–0.19 OD. This cutoff represents the value that discriminates SV40-negative (sample bellow OD 0.17–0.19) from SV40-positive samples (OD >0.17–0.19).26 The positive control, represented by the SV40 hyperimmune serum, had an OD of up to 1.8, while the 2 JCV and BKV hyperimmune sera, which were employed as negative controls, had an OD of <0.1.
The 2 indirect ELISA tests, with the 2 distinct VP B and C peptides, gave overlapping results, thus confirming the presence of anti-SV40 VP antibodies in human sera from patients with GBM (Table 1). Altogether our immunologic data indicate that these sera (n = 44) had a high prevalence of antibodies (34%) reacting with SV40 VP peptides.
Prevalence of SV40 Antibodies in Serum Samples From Healthy Individuals
To verify whether human sera from healthy individuals contained IgG antibodies that reacted to SV40 antigens/peptides, indirect ELISA was set up employing synthetic peptides corresponding to SV40 VP epitopes. Serum samples diluted at 1:20, from adults of the same median age (61 y) as GBM patients, were tested for reactivity to 2 SV40 peptides from VP1/VP3 capsid proteins VP B and VP C. The SV40 antibodies of IgG class had a prevalence of 15% detected in the cohort of healthy individuals (median age 61 y) (Table 1, Fig. 1).
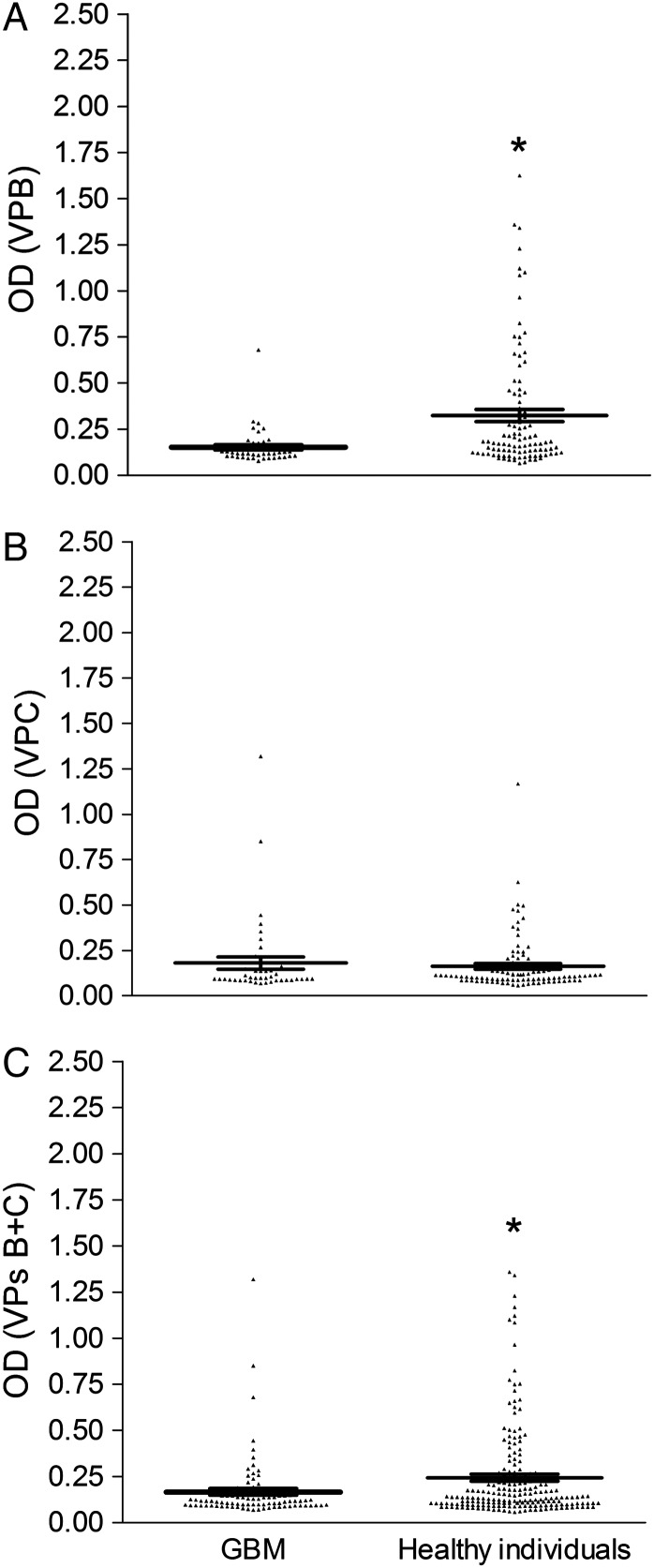
It turned out that seropositive samples of SV40 VP2/3 C peptide were the same samples as were found positive for the SV40 VP1 B peptide. Conversely, seronegative samples of SV40 VP1 B peptide failed to react with SV40 VP2/3 C peptide. A few serum samples were found negative for VP1 B while testing positive for VP2/3 C peptide. Sera, diluted at 1:20, had a general value of ∼0.24 OD. Serologic profiles of serum antibody reactivity to SV40 mimotopes are presented in Fig. 2. The difference in OD mean value of sera from GBM and healthy subjects for both SV40 B and C peptides or for the peptide B alone was statistically significant, P = .01 and P = .0009, respectively, but was not significant for the peptide C (P > .05; Fig. 2).
Fig. 2.
Serologic profile of serum antibody reactivity to SV40 mimotopes VP1 B (A) and VP2/3 C (B) and VPs B +C (C). Immunologic data are from patients affected by GBM and from healthy individuals. Data are OD values at 405 nm of serum samples diluted 1:20, detected in indirect ELISA. In scatter dot plotting, each plot represents the dispersion of OD values to a mean level indicated by the line inside the scatter with SEM for each group of subjects analyzed (mean OD ± SEM). The difference in OD mean value for reactivity with VP1 B and VPs B +C is statistically significant in sera from patients with GBM. *P < .05, unpaired t-test.
Prevalence of SV40 Antibodies in Serum Samples From Breast Cancer Patients
As an additional control, sera from oncologic patients affected by BC (n = 78) were analyzed by indirect ELISA for VP epitopes. Indeed, BC is a human neoplasm that is not associated with SV40. Serum samples from breast carcinoma–affected patients reacted to SV40 VP epitopes with a prevalence of 15%. A statistically significant difference was detected between the prevalence of GBM and BC in patients (P = .03; Table 1, Fig. 1). The differing prevalence of SV40 antibodies between the cohorts of BC patients and healthy subjects was not statistically significant (P > .05; Table 2).29
Table 2.
Prevalence of IgG antibodies reacting with SV40 viral peptide (VP) mimotopes
| Human Sera | n | Median Age, y | Male, % | Number of Positive Samples (%) |
||
|---|---|---|---|---|---|---|
| VP B | VP C | VPs B + C | ||||
| BC patients | 78 | 42 | – | 14 (18) | 13 (17) | 12 (15) |
| Healthy women blood donors | 87 | 42 | – | 18 (21) | 20 (23) | 16 (18) |
Human sera were from BC patients and healthy blood donors. The different prevalence of SV40 antibodies between the cohorts of BC patients was not significant compared with the healthy women blood donors (P > .05). Statistical analysis was performed using the χ2 test with Yates’ correction.
In summary, a higher prevalence of SV40 antibodies was detected in serum samples of GBM than in controls represented by healthy subjects (P = .016) and BC patients (P = .03) (Table 1, Fig. 1).
Antibody Titer
To verify the antibody titer, 24 SV40-positive sera—12 from normal individuals and 12 from GBM patients—were serially diluted from 1:20 to 1:640 and further investigated by indirect ELISA. Analyzed sera remain positive at a 1:160 or 1:80 dilution, indicating that the titer of SV40 antibodies does not greatly differ in normal individuals and GBM patients.
Neutralization Activity to SV40
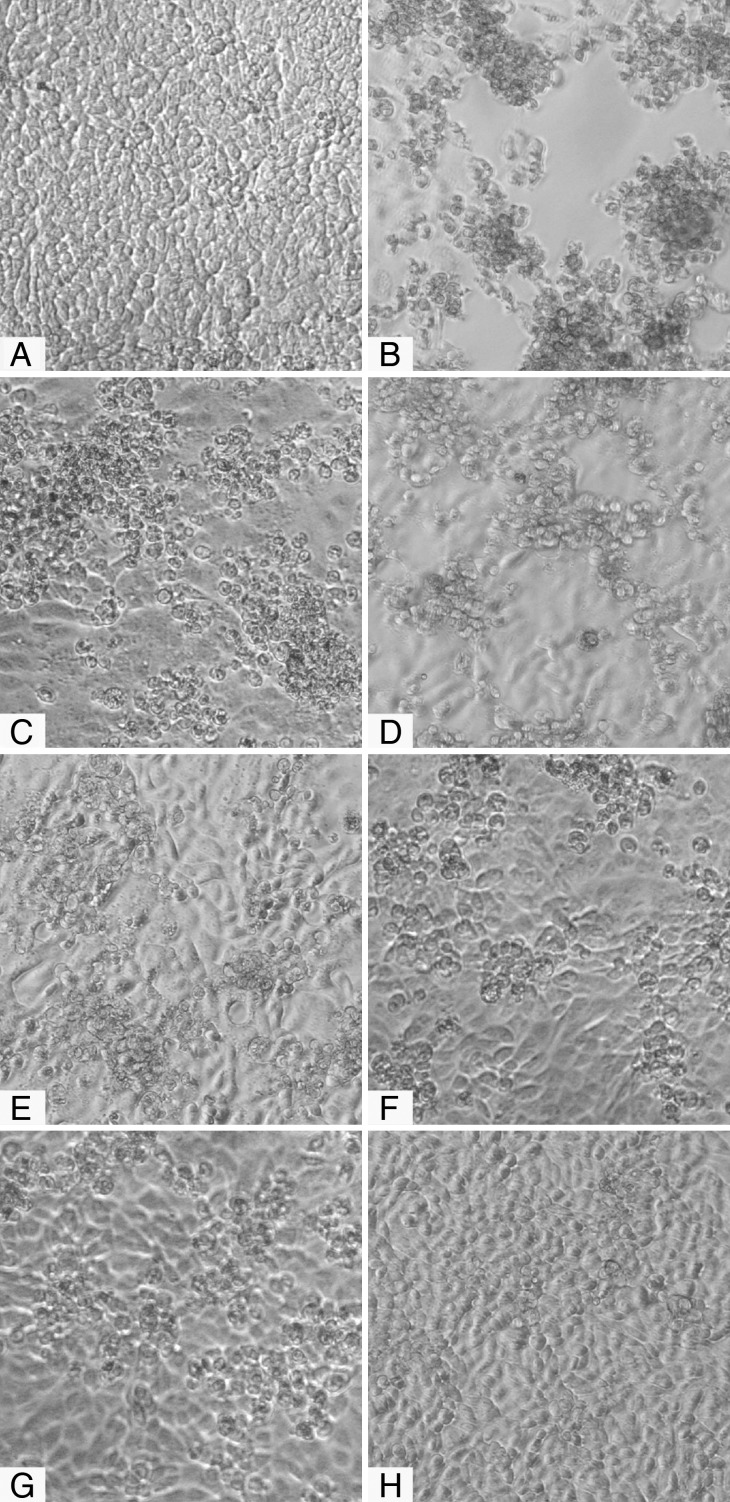
To verify the neutralization activity of SV40 immune sera, an inhibition test was performed. Six SV40 sera with a high OD (range 0.310–0.877, diluted 1:20) were challenged to inhibit the SV40 cytopathic effect in permissive CV1-infected cells. These sera were BKV and JCV negative, as determined by a hemagglutination inhibition test. The SV40 cytopathic effect was abolished or hampered, suggesting that immune sera were positive for neutralizing antibodies (Fig. 3). We infer that exposure to SV40 occurred in those immunized individuals and that the immune serum was elicited by SV40 infection and not by BKV or JCV.
Fig. 3.
Inhibition of SV40 cytopathic effect (CPE) in CV-1 infected cells by human immune sera. Inhibition of SV40 CPE in infected cells by human serum samples. (A) Negative control represented by uninfected CV-1 cells. (B) Positive control represented by the CPE induced by SV40 in CV-1 infected cells and (C–H) different degrees of CPE inhibition. There was a correlation between the OD reading and the CPE inhibition activity of the tested immune sera. Among the 6 SV40-positive samples, one serum sample (H) completely inhibited SV40 CPE. This sample had an OD of 0.725 for the VP mimotopes, whereas 5 other serum samples that partially inhibited the SV40 CPE had ODs of 0.310–0.655 (C–G).
Discussion
In our investigation, serum samples from GBM patients (n = 44) and normal individuals (n = 101) of the same median age (61 y) were analyzed for their reactivity to SV40 peptides of VPs by indirect ELISA. No additional investigations were carried out in GBM patients, while immunologic data became available after the brain tumor excision and therapies. Sera from GBM patients reacted against SV40 VP antigens with a higher prevalence (34%) compared with the control cohort represented by healthy individuals (15%), the difference being statistically significant. This result indicates that SV40 infection is associated with a subset of GBM and that SV40 is also present in the healthy adult population, although at a lower prevalence. The high prevalence of SV40 antibodies in serum samples from GBM seems to confirm earlier data obtained in investigations carried out by PCR and/or immunohistochemistry techniques, which indicated a high prevalence of SV40 footprints in GBM.9,10,31 However, other teams did not find the association between SV40 and GBM or other human tumors. This discrepancy could be ascribed to the different approaches employed by different investigators or to technical artifacts.
In our investigation, serum samples from oncologic patients affected by BC were introduced as an additional control. Immunologic data from indirect ELISAs indicate that the prevalence of SV40 VP mimotopes in serum samples from the cohort of BC patients (n = 78) of a median age of 42 years was 15%. The comparative analysis indicated that the prevalence of SV40-positive sera was higher in GBM patients (34%) than in women affected by BC (15%). This difference was statistically significant (P = .03; Table 1, Fig. 1). It is worth mentioning that the difference in prevalence of SV40 antibodies between patients affected by BC and healthy individuals (n = 87, 18%) of the same median age (42 y) was not statistically significant (P > .05; Table 2).
The data indicate that the overall prevalence of the IgG class of SV40 antibodies is in the range of 15%–18% in healthy individuals. This result suggests that in the human population, natural SV40 infection occurs with a lower prevalence compared with the ubiquitous BKV and JCV.32,33 It is possible that SV40 is transmitted through contact in the familial environment and communities.34 Indeed, it has been shown that SV40 is present in the urine, stool, tonsil, and blood specimens of children and adults, suggesting that different routes of transmission are responsible for SV40 infection.6,7,9–11,32,34–37 Our data seem to confirm the results obtained by earlier studies on subjects administered SV40-contaminated vaccines by different routes. In these individuals, SV40 was detected/isolated after a number of weeks or days, either in stools or from the throat, depending on oral or nasal spray administration of contaminated vaccines.7,38,39 In recent years, many studies have detected the presence of SV40 footprints in individuals too young or too old to be vaccinated with SV40-contaminated vaccines, suggesting that this virus spreads in the human population independently from the contaminated vaccines.
Data on the inhibition of SV40 infectivity obtained with immune sera from normal subjects and GBM patients are of interest. There is a correlation between the grade of the inhibitory effect of SV40 infection by immune sera and the level of antibodies as determined by OD readings. Since the selected SV40 immune sera were BKV and JCV negative, there is no possible cross-inhibition effect. The highest endpoint titer was observed at a 1:160 dilution in immune sera from normal individuals.
The onset/progression of GBM, like other cancers, is associated with specific gene mutations. However, the agents responsible for the occurrence of mutations/chromosome alterations are poorly understood. SV40 was found to be associated (investigating its sequences mainly by PCR) with GBM, indicating that this small DNA tumor virus could be involved in the onset/progression of GBM malignancy. Alternatively, SV40 could be only a passenger virus that multiplies better in some transformed cells than in normal cells. The high prevalence of SV40 antibodies in sera from GBM patients is not proof of cause/effect in inducing human tumors by SV40.
Our ELISA gave reliable results, which can be obtained for many samples in a short period of time at affordable cost. This test can be shared with the scientific community and may represent a standardized assay for studying SV40 infection and its association with specific human tumors. At present, there is not a direct proof that SV40 plays a role in the onset/progression of human malignancies. However, if SV40 is a tumor agent in humans, new antiviral strategies could be applied with drugs or vaccines, which should be administered very early in life. One may postulate that after infecting the host, SV40 may exert its tumorigenic potential when the immune system is impaired, as in the case of oncologic patients. We should also consider, as an alternative explanation, that another, not yet discovered human polyomavirus, closely related to SV40, may be responsible for our immunologic data.
Funding
This study was supported, in part, by grants from ASLEM, Repubblica di San Marino; Regione Emilia Romagna, Bologna, Regione Friuli Venezia Giulia LR 22/2001; ISS, Roma; Fondazione Cassa di Risparmio di Cento, Cento; University of Verona and University of Ferrara, FAR projects, Ferrara, Italy.
Acknowledgments
Dr Elisa Mazzoni is a postdoctoral fellow of the Fondazione Veronesi, Milan, Italy. We would like to thank Dr Eugene O. Major, the Laboratory of Molecular Medicine and Neuroscience, and the National Institute of Neurological Disorders and Stroke, Bethesda, Maryland, for the hyperimmmune serum against JCV.
Conflict of interest statement. None declared.
References
- 1.Gulati S, Jakola AS, Johannesen TB, Solheim O. Survival and treatment patterns of glioblastoma in the elderly: a population-based study. World Neurosurg. 2012;78(5):518–526. doi: 10.1016/j.wneu.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 3.Sant M, Minicozzi P, Lagorio S, Borge Johannesen T, Marcos-Gragera R, Francisci S. Survival of European patients with central nervous system tumors. Int J Cancer. 2012;131(1):173–185. doi: 10.1002/ijc.26335. [DOI] [PubMed] [Google Scholar]
- 4.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4(4):278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smoll NR, Schaller K, Gautschi OP. The cure fraction of glioblastoma multiforme. Neuroepidemiology. 2012;39(1):63–69. doi: 10.1159/000339319. [DOI] [PubMed] [Google Scholar]
- 6.Martini F, Corallini A, Balatti V, Sabbioni S, Pancaldi C, Tognon M. Simian virus 40 in humans. Infect Agent Cancer. 2007;2:13. doi: 10.1186/1750-9378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbanti-Brodano G, Sabbioni S, Martini F, Negrini M, Corallini A, Tognon M. Simian virus 40 infection in humans and association with human diseases: results and hypotheses. Virology. 2004;318(1):1–9. doi: 10.1016/j.virol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Saenz-Robles MT, Sullivan CS, Pipas JM. Transforming functions of simian virus 40. Oncogene. 2001;20(54):7899–7907. doi: 10.1038/sj.onc.1204936. [DOI] [PubMed] [Google Scholar]
- 9.Martini F, Iaccheri L, Lazzarin L, et al. SV40 early region and large T antigen in human brain tumors, peripheral blood cells, and sperm fluids from healthy individuals. Cancer Res. 1996;56(20):4820–4825. [PubMed] [Google Scholar]
- 10.Martini F, Lazzarin L, Iaccheri L, et al. Different simian virus 40 genomic regions and sequences homologous with SV40 large T antigen in DNA of human brain and bone tumors and of leukocytes from blood donors. Cancer. 2002;94(4):1037–1048. [PubMed] [Google Scholar]
- 11.Pancaldi C, Balatti V, Guaschino R, et al. Simian virus 40 sequences in blood specimens from healthy individuals of Casale Monferrato, an industrial town with a history of asbestos pollution. J Infect. 2009;58(1):53–60. doi: 10.1016/j.jinf.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Engels EA, Sarkar C, Daniel RW, et al. Absence of simian virus 40 in human brain tumors from northern India. Int J Cancer. 2002;101(4):348–352. doi: 10.1002/ijc.10621. [DOI] [PubMed] [Google Scholar]
- 13.Rollison DE, Helzlsouer KJ, Alberg AJ, et al. Serum antibodies to JC virus, BK virus, simian virus 40, and the risk of incident adult astrocytic brain tumors. Cancer Epidemiol Biomarkers Prev. 2003;12(5):460–463. [PubMed] [Google Scholar]
- 14.Rollison DE, Utaipat U, Ryschkewitsch C, et al. Investigation of human brain tumors for the presence of polyomavirus genome sequences by two independent laboratories. Int J Cancer. 2005;113(5):769–774. doi: 10.1002/ijc.20641. [DOI] [PubMed] [Google Scholar]
- 15.Klein G, Powers A, Croce C. Association of SV40 with human tumors. Oncogene. 2002;21(8):1141–1149. doi: 10.1038/sj.onc.1205173. [DOI] [PubMed] [Google Scholar]
- 16.Carbone M, Bocchetta M, Cristaudo A, et al. SV40 and human brain tumors. Int J Cancer. 2003;106(1):140–142. doi: 10.1002/ijc.11189. author reply 143–145. [DOI] [PubMed] [Google Scholar]
- 17.Carbone M, Rdzanek MA, Rudzinski JJ, et al. SV40 detection in human tumor specimens. Cancer Res. 2005;65(21):10120–10121. doi: 10.1158/0008-5472.CAN-05-1911. [DOI] [PubMed] [Google Scholar]
- 18.Viscidi RP, Rollison DE, Viscidi E, et al. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin Diagn Lab Immunol. 2003;10(2):278–285. doi: 10.1128/CDLI.10.2.278-285.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter JJ, Madeleine MM, Wipf GC, et al. Lack of serologic evidence for prevalent simian virus 40 infection in humans. J Natl Cancer Inst. 2003;95(20):1522–1530. doi: 10.1093/jnci/djg074. [DOI] [PubMed] [Google Scholar]
- 20.Barbanti-Brodano G, Corallini A, Accolla RS, Martini F, Tognon M. Re: lack of serologic evidence for prevalent simian virus 40 infection in humans. J Natl Cancer Inst. 2004;96(10):803–804. doi: 10.1093/jnci/djh151. author reply 804–805. [DOI] [PubMed] [Google Scholar]
- 21.Lundstig A, Eliasson L, Lehtinen M, Sasnauskas K, Koskela P, Dillner J. Prevalence and stability of human serum antibodies to simian virus 40 VP1 virus-like particles. J Gen Virol. 2005;86(6):1703–1708. doi: 10.1099/vir.0.80783-0. [DOI] [PubMed] [Google Scholar]
- 22.Kjaerheim K, Roe OD, Waterboer T, et al. Absence of SV40 antibodies or DNA fragments in prediagnostic mesothelioma serum samples. Int J Cancer. 2007;120(11):2459–2465. doi: 10.1002/ijc.22592. [DOI] [PubMed] [Google Scholar]
- 23.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5(3):e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro T, Fleury MJ, Granieri E, et al. Investigation of the prevalence of antibodies against neurotropic polyomaviruses BK, JC and SV40 in sera from patients affected by multiple sclerosis. Neurol Sci. 2010;31(4):517–521. doi: 10.1007/s10072-010-0353-y. [DOI] [PubMed] [Google Scholar]
- 25.Taronna A, Mazzoni E, Corallini A, et al. Serological evidence of an early seroconversion to simian virus 40 in healthy children and adolescents. PloS One. 2013;8(4):e61182. doi: 10.1371/journal.pone.0061182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corallini A, Mazzoni E, Taronna A, et al. Specific antibodies reacting with simian virus 40 capsid protein mimotopes in serum samples from healthy blood donors. Hum Immunol. 2012;73(5):502–510. doi: 10.1016/j.humimm.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni E, Tognon M, Martini F, et al. Simian virus 40 (SV40) antibodies in elderly subjects. The Journal of infection. 2013;67(4):356–358. doi: 10.1016/j.jinf.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Mazzoni E, Corallini A, Cristaudo A, et al. High prevalence of serum antibodies reacting with simian virus 40 capsid protein mimotopes in patients affected by malignant pleural mesothelioma. Proc Natl Acad Sci U S A. 2012;109(44):18066–18071. doi: 10.1073/pnas.1213238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martini F, Mazzoni E, Corallini A, et al. Breast cancer and simian virus 40 infection. Epidemiology. 2013;24(3):464–465. doi: 10.1097/EDE.0b013e31828d3ae6. [DOI] [PubMed] [Google Scholar]
- 30.Guerrini R, Salvadori S, Rizzi A, Regoli D, Calo G. Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor. Med Res Rev. 2010;30(5):751–777. doi: 10.1002/med.20180. [DOI] [PubMed] [Google Scholar]
- 31.Major EO, Miller AE, Mourrain P, Traub RG, de Widt E, Sever J. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci U S A. 1985;82(4):1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martini F, De Mattei M, Iaccheri L, et al. Human brain tumors and simian virus 40. J Natl Cancer Inst. 1995;87(17):1331. doi: 10.1093/jnci/87.17.1331. [DOI] [PubMed] [Google Scholar]
- 33.Barbanti-Brodano G, Martini F, De Mattei M, Lazzarin L, Corallini A, Tognon M. BK and JC human polyomaviruses and simian virus 40: natural history of infection in humans, experimental oncogenicity, and association with human tumors. Adv Virus Res. 1998;50:69–99. doi: 10.1016/s0065-3527(08)60806-4. [DOI] [PubMed] [Google Scholar]
- 34.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71(1):115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 35.Vanchiere JA, Abudayyeh S, Copeland CM, Lu LB, Graham DY, Butel JS. Polyomavirus shedding in the stool of healthy adults. J Clin Microbiol. 2009;47(8):2388–2391. doi: 10.1128/JCM.02472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel NC, Vilchez RA, Killen DE, et al. Detection of polyomavirus SV40 in tonsils from immunocompetent children. J Clin Virol. 2008;43(1):66–72. doi: 10.1016/j.jcv.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanchiere JA, White ZS, Butel JS. Detection of BK virus and simian virus 40 in the urine of healthy children. J Med Virol. 2005;75(3):447–454. doi: 10.1002/jmv.20287. [DOI] [PubMed] [Google Scholar]
- 38.Morris JA, Johnson KM, Aulisio CG, Chanock RM, Knight V. Clinical and serologic responses in volunteers given vacuolating virus (SV-40) by respiratory route. Proc Soc Exp Biol Med. 1961;108:56–59. doi: 10.3181/00379727-108-26843. [DOI] [PubMed] [Google Scholar]
- 39.Melnick JL, Stinebaugh S. Excretion of vacuolating SV-40 virus (papova virus group) after ingestion as a contaminant of oral poliovaccine. Proc Soc Exp Biol Med. 1962;109:965–968. doi: 10.3181/00379727-109-27392. [DOI] [PubMed] [Google Scholar]