Abstract
Background
Compelling epidemiological evidence indicates that alterations of telomere length are associated with risks of many malignancies in a tumor-specific manner, such as lung cancer, breast cancer, and non-Hodgkin's lymphoma. However, the association between leukocyte telomere length and glioma risk has not been investigated.
Methods
Relative telomere length (RTL) of peripheral blood leukocytes from 467 glioma patients and 467 healthy controls, matched by age and sex, was measured using the real-time PCR-based method in a case-control study. An unconditional multivariate logistic regression model was applied to estimate the association between RTL and glioma risk.
Results
Glioma patients showed notably longer RTL than controls (median, 0.555 vs 0.444; P > .04). RTL was negatively correlated with age in both cases (ρ = −0.430; P < .001) and controls (ρ = −0.388; P < .001). After adjusting for age, sex, smoking status and family history of cancer, multivariate logistic regression analysis showed that there was a U-shaped association between RTL and glioma risk (P for nonlinearity <.001). Compared with individuals in the second tertile of RTL, the odds ratios (95% CI) for participants in the first and third tertiles were 2.16 (range, 1.52–3.09) and 3.51 (range, 2.45–5.00), respectively. Stratified analysis showed that the association between RTL and glioma risk was not modulated by major host characteristics.
Conclusions
Our study demonstrates for the first time that either shorter or longer RTL in peripheral blood leukocytes is associated with increased glioma risk, which warrants further investigation in the future.
Keywords: glioma risk, logistic regression model, real-time PCR, telomere length
Glioma is the most common primary brain tumor in both children and adults.1 Despite the available optimal therapeutic regimen, the prognosis of glioma patients remains dismal, with a median survival time of only 12–15 months in patients with glioblastoma multiform (GBM) and 2–5 years in patients with anaplastic astrocytoma.2 To date, only a few factors have been identified as major risk factors for glioma. Among these are exposure to high-dose ionizing radiation (IR), inherited mutations of highly penetrant genes associated with rare tumor syndromes, and absence of allergy.3–5 Therefore, in order to reduce the disease burden of gliomas, there is an urgent need to identify genetic, behavioral, environmental, and developmental contributors to glioma risk through epidemiological studies.
Telomeres are specified structures located at the ends of linear chromosomes of eukaryote cells that are composed of a tandem TTAGG repeat and associated proteins.6 This structure has 2 major functions: maintenance of complete genetic information and inhibition of unwanted DNA-damage signaling and genomic instability. Telomere length is determined by the balance of processes that shorten and lengthen the telomere, thus leading to telomere variation in individuals at the same age.7 It is estimated that inheritable factors contribute up to 80% of the variation of telomere length,8 whereas environmental factors such as inflammation and oxidative stress accelerate age-related telomere shortening.9,10 The maintenance of telomere length relies on the activity of telomerase, a reverse transcriptase complex that adds DNA sequence repeats (‘TTAGGG’ in all vertebrates) to the 3′ end of DNA strands in the telomere regions. Genetic association studies have indicated that polymorphisms in the telomerase reverse transcriptase-encoding gene TERT and related genes such as TERC, NAF1, OBFC1 and RTEL1 are associated with the variation of telomere length.11 In addition to telomere-associated proteins, growth factor receptor signaling has been reported to affect telomere length through their effects on telomerase activity.12
Telomere length variation is strongly implicated in the process of carcinogenesis, although the current findings are still in debate.6 Due to the 3′-end replication problem of DNA polymerase and insufficiency of telomerase activity, telomeres in normal somatic cells are progressively shortened by 50–200 base pairs with each round of mitosis, eventually leading to a state named “replicative senescence.”13 In checkpoint-competent cells, when one or more telomeres reaches a critically short length, the unprotected chromosome ends are recognized by DNA-damage response factors such as p53 and p16/Rb pathways, inducing a typical senescence state in which the cells continue to live but are irreversibly blocked from further cell division.14,15 This replication-associated attrition of telomeres makes a proliferation barrier of transformed cells in the presence of intact checkpoint mechanisms. However, disrupted checkpoint pathways by oncogenic factors such as SV40 large T antigen allow bypass of replicative senescence that results in genomic instability, including end-to-end fusions and rearrangement of chromosomes, and initiation of malignant transformation in many cancers.16,17 Markedly elevated risk of tumors (about 11 times that of the general population) are observed in patients with dyskeratosis congenita, a disease with very short telomeres caused by germline mutations in the components of telomerase complex.18 Mouse models also support the notion that abnormally short telomere length increases the risk of cancers.19 In comparison, longer telomeres may increase the risk of developing cancer by allowing more cell division cycles during which more oncogenic mutations may occur. In addition, telomere length maintenance is the prerequisite for the infinite proliferative capacity of cells that harbor tumor-promoting mutations. Indeed, a number of cancer cells regain ability of telomere maintenance by reactivation of telomerase.6 These sophisticated mechanisms suggest that proper telomere length is critical to preventing cancer development.
The association between telomere length and human cancer has been widely investigated in a range of malignancies.6 Well-documented studies, using cancer tissues from patients, have demonstrated that telomere length in neoplastic tissues altered frequently and showed either longer or shorter than matched adjacent normal tissues.20 Recently, several epidemiological studies have evaluated the associations between telomere length of peripheral blood leukocytes (PBLs) and cancer risk. The majority of these studies showed that shorter telomeres are associated with higher risk of solid tumors (eg, cancers of the bladder,21 lung,22 esophagus,23 stomach,24 head and neck,25 ovary,26 and kidney),27 whereas several studies indicated that longer telomere length is associated with higher risk of cancers such as hepatocellular carcinoma28 and skin melanoma.28 Furthermore, increased risks of lung and breast cancers were also observed in participants with longer telomeres.29,30 These conflicts indicate the complicated role of telomeres in cancer development and suggest that further investigation is warranted in this area.
Nevertheless, the association between leukocyte telomere length and glioma risk has not yet been assessed. To address this question, we conducted a case-control study to evaluate the association of telomere length in PBLs and glioma risk. The relative telomere length (RTL) was measured by real-time PCR, and its association with glioma risk was analyzed using an unconditional multivariate logistic regression model. To the best of our knowledge, this is the first epidemiological study to investigate the role of telomere length of PBLs in glioma etiology.
Materials and Methods
Study Population
In our ongoing case-control study, primary glioma patients who underwent surgical resection were consecutively recruited from the Department of Neurosurgery of Tangdu Hospital (which is affiliated with the Fourth Military Medical University) from February 2010 to August 2012. Eligible cases were histopathologically confirmed, previously untreated, and diagnosed within 1 year of enrollment. There was no restriction on age, sex, and tumor grade or stage for case, and 83% of all new cases were included. Healthy controls without any previous cancer history were recruited from the Medical Examination Center of Tangdu Hospital during the period of case enrollment, with a response rate of about 75%. The controls were frequency-matched to the cases on age (±3 years), sex, and residential areas. All participants were Han Chinese.
Epidemiological and Clinical Data
Epidemiological information, including demographics, smoking history, family history of cancer and IR exposure, was collected using a standardized questionnaire by well-trained staff interviewers. Clinical information was collected from medical records and pathological reports. Individuals who never smoked or who smoked fewer than 100 cigarettes during lifetime were classified as “never smokers,” whereas “ever smokers” were defined as individuals who smoked more than 100 cigarettes. The number of pack-years was calculated as the average number of cigarettes smoked per day divided by 20 cigarettes and then multiplied by smoking years. Data quality was assessed by re-interviewing 5% of all participants. All information exhibited high consistency except IR exposure history, which might stem from inaccurate understanding of IR exposure questionnaires. Therefore, data on IR exposure were not used for further analyses in this study.
After interview, 5 mL venous blood was drawn into coded sodium citrate-coated tubes from each participant. Blood samples were processed and stored according to the procedure described previously.28 The study was approved by the Ethical Committee of the Fourth Military Medical University, and written informed consent was obtained from all participants. All study procedures were carried out in accordance with the ethical standards of the Helsinki Declaration.
Determination of Telomere Length by Real-time Quantitative PCR
High-quality genomic DNA was extracted from PBLs of all participants using the RelaxGene Blood DNA System (TIANGEN) according to the manufacturer's instructions. RTL was measured using a real-time quantitative PCR-based method, as previously described, with the same primers for telomere (Tel-g and Tel-c) and the single-copy nuclear gene HGB (HGB-1 and HGB-2).28 Briefly, 2 pairs of primers were used in the 2 steps of relative quantification of telomere length. In the first step, the ratio of telomere repeat copy number to HGB copy number was determined for each sample using standard curves. The derived ratio was proportional to the overall telomere length. In the second step, the ratio for each sample was normalized to that of a calibrator DNA sample to standardize differences between runs, and the normalized ratio was defined as RTL.
Gene-specific amplification was performed in a LightCycler480 QPCR System (Roche) with a 10 μL PCR mix containing 4 ng of genomic DNA, 1× SYBR Green master mix (Takara), 450 nM Tel-g (or 250 nM HGB-1), and 450 nM Tel-c (or 250 nM HGB-2). The thermal cycling profile for telomere amplification was 1 cycle at 95°C for 30 seconds; 2 cycles at 94°C for 15 seconds and 49°C for 15 seconds; then 32 cycles at 86°C for 15 seconds, 62°C for 10 seconds, and 74°C for 15 seconds with signal acquisition. The thermal cycling conditions for human globulin amplification were at 95°C for 30 seconds, followed by 35 cycles at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 50 seconds with signal acquisition. The same negative and positive controls, a calibrator DNA, and samples for constructing a standard curve were included on each plate. The R2 for each standard curve was ≥0.99, and the acceptable standard deviation was lower than 0.25 (for cycle threshold values). The intra-assay or inter-assay variations were evaluated by assaying one sample in 8 replicates or in 3 different runs, respectively.
Statistical Analysis
All statistical analyses were performed using the IBM SPSS Statistics 19.0 software (IBM). Normally distributed continuous data were expressed as mean ± SD, while abnormally distributed continuous variables were expressed as median with a bracketed range. The chi-square test was used to examine differences of categorical variables between cases and controls. The Student t test was used to analyze the differences in age and pack-years of smoking, and the Mann–Whitney U test was used for RTL comparison. Spearman correlation analysis was conducted to explore the relationship between RTL and age. The association between RTL and glioma risk was estimated by odds ratio (OR) and 95% confidence interval (CI) using an unconditional multivariate logistic regression model with adjustments for age, sex, smoking status, and family history of cancer. A restricted cubic spline curve was plotted in the logistic regression model to evaluate the shape of the association. All statistical tests were 2-sided, and P < .05 was considered significant.
Results
A total of 467 glioma cases and 467 healthy controls were included in this study. The epidemiological and clinical characteristics of the participants were summarized in Table 1. The cases and controls were well matched in age (P = .93) and sex (P > .99). The ages of controls and cases were 42.9 ± 16.1 years and 42.8 ± 15.7 years (P = .93), respectively. There was a higher percentage of men who suffered from glioma than women (61.5% vs 38.5%) in our study, which was consistent with previous epidemiological data on glioma.1 There was no significant difference in either smoking status or family history of cancer between cases and controls. Among the total of 467 cases, 209 were diagnosed with low-grade glioma (WHO grade I/II) including 143 astrocytomas, 44 oligoastrocytomas, 5 oligodendrocytomas, 16 ependymomas and 1 pleomorphic xanthoastrocytoma, and 258 were diagnosed with high-grade glioma (WHO grade III/IV) including 95 anaplastic astrocytomas, 12 anaplastic oligoastrocytomas, 6 anaplastic oligodendrocytomas, 3 anaplastic ependymomas, and 142 GBMs.
Table 1.
Clinical and epidemiological characteristics of glioma cases and controls
| Variables | Cases (n = 467) | Controls (n = 467) | P value |
|---|---|---|---|
| Age in years, mean ± SD | 42.9 ± 16.1 | 42.8 ± 15.7 | .93 |
| Pack-years of smokinga, mean ± SD | 26.2 ± 14.9 | 24.9 ± 13.1 | <.12 |
| Sex (%) | |||
| Male | 287 (61.5) | 287 (61.5) | >.99 |
| Female | 180 (38.5) | 180 (38.5) | |
| Smoking status (%) | |||
| Ever smokers | 112 (24.0) | 97 (26.2) | <.24 |
| Never smokers | 355 (76.0) | 370 (73.8) | |
| Family history of cancer (%) | |||
| Yes | 48 (10.3) | 35 (7.5) | <.14 |
| No | 419 (89.7) | 432 (92.5) | |
| WHO grade (%) | |||
| I/II | 209 (44.8) | ||
| III/IV | 258 (55.2) | ||
aOnly for ever smokers.
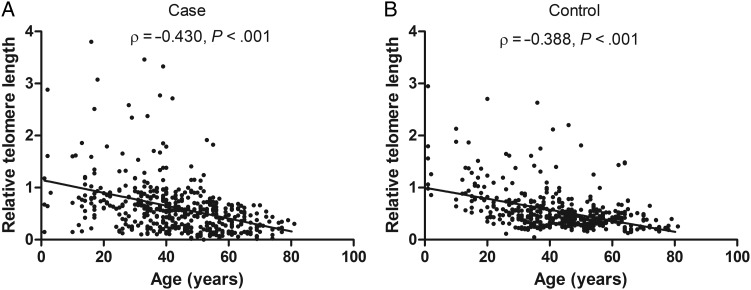
We performed real-time quantitative PCR to measure the RTL of PBLs from cases and controls. The mean inter-assay coefficient variation (CV) of real-time PCR reaction was 6.2% (range, 3.6%–9.5%), whereas intra-assay CV was 5.3% (range, 2.8%–7.1%). Our results indicated that glioma patients had notably longer median RTL than healthy controls (0.555 vs 0.444; P > .04; Table 2). Moreover, we compared RTL according to host characteristics (Table 2). Our data showed that glioma patients younger than aged 43 years had longer median RTL than corresponding healthy controls (0.665 vs 0.553; P < .02), whereas no significant case-control difference was observed in other stratified subgroups. In addition, no significant modulating effect of selected characteristics (except for age) on RTL was found in both cases and controls, with P values ranging from <.10 to >.59. Similarly, no significant difference in RTL was found between patients diagnosed with low-grade glioma and those with high-grade glioma (0.549 vs 0.563, P = .65). In both cases and controls, we observed that older individuals had significantly shorter RTL than younger individuals (both P < .001). Spearman' correlation analysis showed that RTL was negatively correlated with age in both groups, with a correlation coefficient (ρ) of −0.388 (P < .001) and −0.430 (P < .001) in controls and cases, respectively (Fig. 1).
Table 2.
Distributions of relative telomere length by host characteristics in all participants
| Variables | Relative telomere length, median (range) |
P value | |
|---|---|---|---|
| Cases (n = 467) | Controls (n = 467) | ||
| Total | 0.555 (0.003–3.802) | 0.444 (0.048–2.950) | >.04 |
| Age | |||
| ≤43 years | 0.665 (0.102–3.802) | 0.553 (0.048–2.950) | <.02 |
| >43 years | 0.422 (0.003–1.914) | 0.394 (0.129–2.201) | >.93 |
| P value | <0.001 | <0.001 | |
| Sex | |||
| Male | 0.534 (0.003–3.462) | 0.429 (0.117–2.705) | <.11 |
| Female | 0.575 (0.033–3.802) | 0.464 (0.048–2.950) | <.21 |
| P value | <.43 | <.10 | |
| Smoking status | |||
| Ever smokers | 0.520 (0.003–3.762) | 0.431 (0.063–2.541) | <.12 |
| Never smokers | 0.581 (0.047–3.802) | 0.467 (0.048–2.950) | <.32 |
| Pvalue | >.59 | >.14 | |
| Family history of cancer | |||
| Yes | 0.541 (0.133–3.802) | 0.428 (0.048–2.841) | >.12 |
| No | 0.568 (0.003–3.756) | 0.459 (0.073–2.950) | >.19 |
| P value | <.51 | <.35 | |
| WHO grade | |||
| I/II | 0.549 (0.003–3.802) | ||
| III/IV | 0.563 (0.016–3.462) | ||
| P value | .65 | ||
Fig. 1.
Spearman's correlation analysis between relative telomere length and age in glioma cases (A) and healthy controls (B). There were negative correlations between RTL and age in participants of both groups.
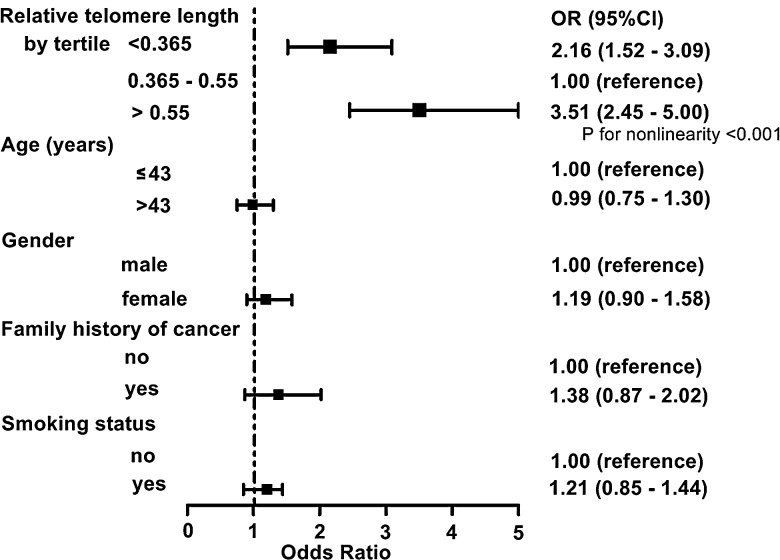
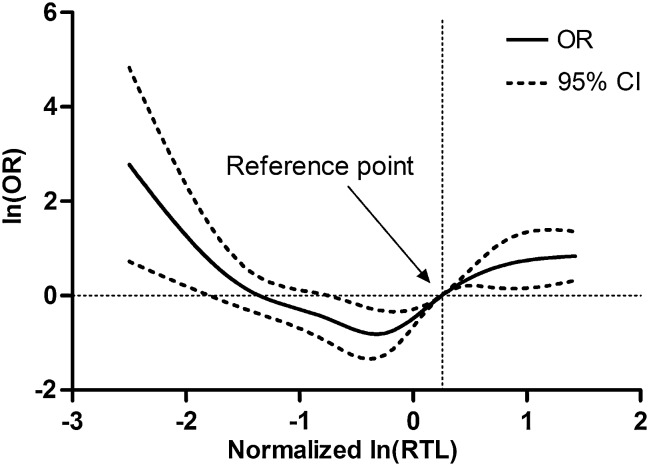
To assess the association between RTL and glioma risk, we performed an unconditional multivariate regression analysis adjusted for age, sex, smoking status, and family history of cancer. The participants were categorized into 3 groups based on the tertile values of RTL in controls. When using the second tertile as reference, the ORs (95% CI) for participants in the first and third tertiles of RTL were 2.16 (range, 1.52–3.09) and 3.51 (range, 2.45–5.00), respectively (Fig. 2), which indicated a nonlinear relation between RTL and glioma risk. We further used a restricted cubic spline function in the logistic regression model to evaluate the shape of association (Fig. 3). Our result exhibited a typical U-shaped association between RTL and risk of glioma; that is, either shorter or longer RTL was associated with increased risk (P for nonlinearity <.001). Moreover, major host characteristics, such as smoking status and family history of cancer, had no effect on the risk of glioma. To further remove the confounding effects of host characteristics, we conducted a stratified analysis and found that all of the host characteristics, including age, sex, smoking status, and family history of cancer and grade, had no impact on the association between RTL and glioma risk except for a borderline P value (P < .07) in the male participants (Table 3).
Fig. 2.
Risk of glioma as estimated by selected characteristics. Odds radios (ORs) were calculated by logistic regression analysis with adjustments for age (years, continuous variable), sex, smoking status, family history of cancer, and relative telomere length, where appropriate. Solid squares indicated study-specific ORs; horizontal lines indicated 95% confidence intervals; dotted vertical line showed the OR value of reference.
Fig. 3.
Dose-dependent effect of RTL on glioma risk. RTL was transformed into natural logarithm and then standardized using the mean and standard deviation of RTL in control group. There was a U-shaped relationship between RTL and glioma risk (P for nonlinearity <.001).
Table 3.
Association between relative telomere length and glioma risk stratified by selected characteristics
| RTL | Cases (%) | Controls (%) | Adjusted OR (95% CI)a | P value |
|---|---|---|---|---|
| Age ≤43b | ||||
| ≤0.364 | 52 (11.1) | 54 (11.6) | 2.06 (1.15–3.69) | <.02 |
| 0.365–0.550 | 29 (6.2) | 61 (13.1) | ref | |
| >0.550 | 162 (34.7) | 118 (25.3) | 2.96 (1.79–4.89) | <.001 |
| Age >43b | ||||
| ≤0.364 | 104 (22.3) | 100 (21.3) | 2.25 (1.44–3.53) | <.001 |
| 0.365–0.550 | 45 (9.6) | 98 (21.0) | ref | |
| >0.550 | 75 (10.9) | 36 (7.7) | 4.38 (2.58–7.43) | <.001 |
| Male | ||||
| ≤0.364 | 97 (20.8) | 104 (22.3) | 1.52 (0.97–2.36) | <.07 |
| 0.365–0.550 | 51 (10.9) | 87 (18.6) | ref | |
| >0.550 | 139 (29.8) | 96 (20.6) | 2.60 (1.66–4.08) | <.001 |
| Female | ||||
| ≤0.364 | 59 (12.6) | 50 (10.7) | 4.00 (2.18–7.33) | <.001 |
| 0.365–0.550 | 23 (4.9) | 72 (15.4) | ref | |
| >0.550 | 98 (21.0) | 58 (12.4) | 5.73 (3.15–10.40) | <.001 |
| Ever smokers | ||||
| ≤0.364 | 41 (8.8) | 28 (6.0) | 3.39 (1.59–7.23) | <.02 |
| 0.365–0.550 | 16 (3.4) | 37 (7.9) | ref | |
| >0.550 | 55 (11.8) | 35 (7.5) | 3.63 (1.76–7.49) | <.001 |
| Never smokers | ||||
| ≤0.364 | 115 (24.6) | 126 (27.0) | 1.92 (1.28–2.87) | .001 |
| 0.365–0.550 | 58 (12.4) | 122 (26.1) | ref | |
| >0.550 | 182 (39.0) | 119 (25.5) | 2.18 (1.73–4.75) | <.001 |
| Positive family history of cancer | ||||
| ≤0.364 | 18 (3.9) | 9 (1.9) | 4.80 (1.29–17.88) | <.02 |
| 0.365–0.550 | 5 (1.1) | 12 (2.6) | ref | |
| >0.550 | 25 (5.4) | 14 (3.0) | 4.29 (1.25–14.68) | >.02 |
| Negative family history of cancer | ||||
| ≤0.364 | 138 (29.6) | 145 (31.0) | 2.03 (1.40–2.93) | <.001 |
| 0.365–0.550 | 69 (14.7) | 147 (31.5) | ref | |
| >0.550 | 212 (45.3) | 140 (30.0) | 3.23 (2.26–4.61) | <.001 |
| WHO grade I/II | ||||
| ≤0.364 | 69 (14.8) | 68 (14.6) | 1.90 (1.12–3.22) | <.02 |
| 0.365–0.550 | 36 (7.7) | 69 (14.8) | ref | |
| >0.550 | 104 (22.3) | 72 (15.4) | 3.04 (1.78–5.18) | <.001 |
| WHO grade III/IV | ||||
| ≤0.364 | 87 (18.6) | 86 (18.4) | 2.41 (1.49–3.90) | <.001 |
| 0.365–0.550 | 38 (8.1) | 90 (19.3) | ref | |
| >0.550 | 133 (28.5) | 82 (17.5) | 4.02 (2.48–6.52) | <.001 |
aORs were adjusted for age, sex, smoking status, and family history of cancer, where appropriate.
bMedian age (43 years) in control was used to dichotomize our study population into young and old subgroups.
Abbreviations: CI, confidence interval; ref, reference; RTL, relative telomere length.
Discussion
In this case-control study, we examined the RTL in PBLs from glioma patients and healthy controls using real-time PCR-based method. Our findings indicated that RTL was notably longer in patients with glioma than in healthy controls. Most importantly, logistic regression analysis demonstrated that either longer or shorter RTL was significantly associated with an increased risk of glioma, indicating a U-shaped association between RTL and glioma risk. These data suggest that RTL might serve as a potential susceptibility biomarker for early preventive screening of glioma.
To date, a number of studies have examined telomere length in PBLs and its association with cancer risks.31 However, the results are inconsistent with positive, negative, or null associations between telomere length and cancer risks. The majority of these studies have shown that short telomere length is significantly associated with increased risks of cancers such as bladder,21 lung,22 stomach,24 ovarian,26 kidney,27 and breast32 cancers as well as osteosarcoma33 and non-Hodgkin's lymphoma.34 On the contrary, longer telomere has also been found to be associated with increased risks of hepatocellular carcinoma,28 skin melanoma,35 non-Hodgkin's lymphoma,36 lung,30 and breast29 cancers. Interestingly, our findings indicate that both longer and shorter RTL are associated with higher risk of glioma, suggesting a U-shaped association between RTL in PBLs and glioma risk consistent with the report of Skinner H et al., who observed a U-shaped association of telomere length in PBLs and pancreatic adenocarcinoma.37 Moreover, data from the Shanghai Women's Health Study also demonstrated a U-shaped association between telomere length and the risks of breast and colorectal cancers in women.38,39 These discrepancies may be partially explained by the dual role of telomere in the development of specific cancers. Certainly, differences in study design, specific cancer site, limited statistical power, variability in confounding factors, and laboratory measurement of telomere length may be contributing factors to these discrepancies. In addition, it remains unclear whether the timing of sample collection plays a role in the variability of findings across studies. Therefore, large studies, particularly in prospective settings, are needed to confirm these findings.
The detailed molecular mechanisms underlying these findings need to be further investigated. Recent studies have suggested that the length of telomere plays a crucial role, like a double-edged sword, in the carcinogenesis.6 On the one hand, telomere shortening may trigger genomic instability, which leads to gene rearrangement and cancer initiation.40 On the other hand, long telomeres may abolish the replicative barrier of cells which harbors tumorigenic mutations, thus leading to an elevated cancer risk.41 Moreover, it is conceivable that there may be a balance of telomere length to maintain normal physiological homeostasis. Either excessively short or long telomere length could contribute to cancer development once the balance is broken. The phenomena that both longer and shorter RTL are associated with higher cancer risk might reflect different mechanisms of carcinogenesis caused by telomere dysfunction in different subtypes of cancers. Indeed, genomic and molecular pathological heterogeneity do exist in malignancies, including glioma.2 Therefore, the detailed mechanisms of telomere-associated tumorigenesis need to be further explored.
In addition to the TERT, regulator of telomere elongation helicase 1 (RTEL1) also plays an important role in the stability, protection, and elongation of telomeres.49 Therefore, polymorphisms in these genes may affect the predisposition to telomere dysfunction-related malignancies, including glioma. Indeed, genome-wide association studies (GWAS) have reported that SNPs rs2736100 and rs2853676 in TERT and SNPs rs6010620, rs2297440, and rs4809324 in RTEL1 are significantly associated with the risk of glioma.33 In a case-control study of Chinese population, we have validated the GWAS findings (except for rs2736100) and observed that there is a significant association between rs2853676, rs6010620 or rs2297440, but not rs4809324, and glioma risk.42,43 In this study, we collected all genotyping data for these 5 SNPs and evaluated their associations with RTL. Consistent with previous GWAS findings,13 our results indicated that SNP rs2736100, but not others, was significantly associated with telomere length (data not shown). These findings suggest that telomere length , as a quantitative trait, can be affected by many genetic and environmental factors as well as their interaction. The mechanisms underlying the effects of SNPs in telomere length-regulating genes on glioma risk need to be elucidated in future studies. Moreover, Walsh et al have reported that risk alleles on rs2736100 and rs6010620 are associated with older age at glioma diagnosis, indicating the important role of interaction between SNPs and aging in gliomagenesis.44 Our stratified analysis has also identified a more evident association between RTL and glioma risk in older patients (>43 years) than those in younger patients (≤43 years).
Due to its age-dependent shortening in most somatic cells, telomere length can serve as a biomarker for biological age.45 Our observation supports this biological phenomenon by showing that RTL was negatively correlated with age in both cases and controls. In addition, we did not find a significant association between the telomere length and major host characteristics such as sex, smoking, and family history of cancer in both cases and controls. These observations are in line with some of the previous reports.46
Our study has several strengths and limitations. Our population was enrolled from Xi'an and its adjacent areas, a region with high geographical stability, which could greatly reduce the potential confounding effects of the heterogeneous participants in most case-control studies. However, because the participant cases are from hospital-based patients rather than the general population, there might be some risk of selection bias if they had any differences in terms of the studied exposures. Moreover, due to inaccurate understanding of the IR-exposure questionnaire by participants, we did not obtain acceptable consistency for IR exposure data when cross-check was performed by 2 interviewers. Thus, we were unable to adjust for this important risk factor in our association study, and the potential interaction between RTL and IR exposure cannot be examined. However, the frequency of IR exposure in Chinese glioma cases and healthy controls is always too low (<5%) to generate a meaningful analysis, as previously reported.47 Therefore, the lack of IR exposure data would not significantly affect our results. To elucidate the specific telomere-environment interaction would be of significant interest in future studies. Furthermore, because the current study is a retrospective one in which blood samples were collected after diseases were diagnosed, it is not clear whether the variation of RTL happened before or after the onset of glioma. Therefore, future prospective epidemiological studies are warranted to further validate our findings.
In conclusion, our study, for the first time, provides epidemiological evidence for the association between telomere length in PBLs and risk of glioma, with a U-shaped dose-dependent manner in a hospital-based case-control study. Once confirmed, RTL in PBLs may serve as a novel biomarker for glioma risk predication, and preventive strategies could be developed based on the preservation of telomere length. In future studies, further efforts are needed to explore the biological mechanisms of telomere alteration in the development of glioma and its clinical significance in diagnosis and treatment of glioma.
Funding
This work was supported by Program for New Century Excellent Talents in University (to J.X.), National Natural Science Foundation (81171966 to J.X.), and National Key Technologies R&D Program (2011ZX09307-001-04 to J.X.) of China.
Conflict of interest statement. None declared.
References
- 1.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Gu J, Liu Y, Kyritsis AP, Bondy ML. Molecular epidemiology of primary brain tumors. Neurotherapeutics. 2009;6(3):427–435. doi: 10.1016/j.nurt.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99(20):1544–1550. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 5.Calboli FC, Cox DG, Buring JE, et al. Prediagnostic plasma IgE levels and risk of adult glioma in four prospective cohort studies. J Natl Cancer Inst. 2011;103(21):1588–1595. doi: 10.1093/jnci/djr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Li S, Stohr BA. The role of telomere biology in cancer. Annu Rev Pathol. 2013;8:49–78. doi: 10.1146/annurev-pathol-020712-164030. [DOI] [PubMed] [Google Scholar]
- 7.Hug N, Lingner J. Telomere length homeostasis. Chromosoma. 2006;115(6):413–425. doi: 10.1007/s00412-006-0067-3. [DOI] [PubMed] [Google Scholar]
- 8.Nordfjall K, Larefalk A, Lindgren P, Holmberg D, Roos G. Telomere length and heredity: Indications of paternal inheritance. Proc Natl Acad Sci U S A. 2005;102(45):16374–16378. doi: 10.1073/pnas.0501724102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 10.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 11.Codd V, Nelson CP, Albrecht E, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeg S, Hirt N, Queisser A, et al. EGFR overexpression induces activation of telomerase via PI3 K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesis. Cancer Sci. 2011;102(2):351–360. doi: 10.1111/j.1349-7006.2010.01796.x. [DOI] [PubMed] [Google Scholar]
- 13.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg RA, Chin L, Femino A, et al. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell. 1999;97(4):515–525. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 15.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11(5):461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velicescu M, Yu J, Herbert BS, Shay JW, Granada E, Dubeau L. Aneuploidy and telomere attrition are independent determinants of crisis in SV40-transformed epithelial cells. Cancer Res. 2003;63(18):5813–5820. [PubMed] [Google Scholar]
- 17.O'Hagan RC, Chang S, Maser RS, et al. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell. 2002;2(2):149–155. doi: 10.1016/s1535-6108(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 18.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361(24):2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artandi SE, Chang S, Lee SL, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406(6796):641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 20.Svenson U, Roos G. Telomere length as a biological marker in malignancy. Biochim Biophys Acta. 2009;1792(4):317–323. doi: 10.1016/j.bbadis.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 21.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers. 2007;16(4):815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 22.Jang JS, Choi YY, Lee WK, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99(7):1385–1389. doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing J, Ajani JA, Chen M, et al. Constitutive short telomere length of chromosome 17p and 12q but not 11q and 2p is associated with an increased risk for esophageal cancer. Cancer Prev Res. 2009;2(5):459–465. doi: 10.1158/1940-6207.CAPR-08-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou L, Savage SA, Blaser MJ, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiology, Biomarkers and Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2009;18(11):3103–3109. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95(16):1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 26.Mirabello L, Garcia-Closas M, Cawthon R, et al. Leukocyte telomere length in a population-based case-control study of ovarian cancer: a pilot study. Cancer Causes Control. 2010;21(1):77–82. doi: 10.1007/s10552-009-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao L, Wood CG, Zhang D, et al. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol. 2007;178:1492–1496. doi: 10.1016/j.juro.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Yang Y, Zhang H, et al. Longer leukocyte telomere length predicts increased risk of hepatitis B virus-related hepatocellular carcinoma: a case-control analysis. Cancer. 2011;117(18):4247–4256. doi: 10.1002/cncr.26015. [DOI] [PubMed] [Google Scholar]
- 29.Gramatges MM, Telli ML, Balise R, Ford JM. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol Biomarkers. 2010;19(2):605–613. doi: 10.1158/1055-9965.EPI-09-0896. [DOI] [PubMed] [Google Scholar]
- 30.Lan Q, Cawthon R, Gao Y, et al. Longer Telomere Length in Peripheral White Blood Cells Is Associated with Risk of Lung Cancer and the rs2736100 (CLPTM1L-TERT) Polymorphism in a Prospective Cohort Study among Women in China. PloS One. 2013;8(3):e59230. doi: 10.1371/journal.pone.0059230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou L, Zhang X, Gawron AJ, Liu J. Surrogate tissue telomere length and cancer risk: shorter or longer? Cancer Lett. 2012;319(2):130–135. doi: 10.1016/j.canlet.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Shen J, Terry MB, Gurvich I, Liao Y, Senie RT, Santella RM. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res. 2007;67(11):5538–5544. doi: 10.1158/0008-5472.CAN-06-3490. [DOI] [PubMed] [Google Scholar]
- 33.Mirabello L, Richards EG, Duong LM, et al. Telomere length and variation in telomere biology genes in individuals with osteosarcoma. Int J Mol Epidemiol Genet. 2011;2(1):19–29. [PMC free article] [PubMed] [Google Scholar]
- 34.Widmann TA, Herrmann M, Taha N, Konig J, Pfreundschuh M. Short telomeres in aggressive non-Hodgkin's lymphoma as a risk factor in lymphomagenesis. Exp Hematol. 2007;35(6):939–946. doi: 10.1016/j.exphem.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Han J, Qureshi AA, Prescott J, et al. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol. 2009;129(2):415–421. doi: 10.1038/jid.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan Q, Cawthon R, Shen M, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(23):7429–7433. doi: 10.1158/1078-0432.CCR-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skinner HG, Gangnon RE, Litzelman K, et al. Telomere length and pancreatic cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2095–2100. doi: 10.1158/1055-9965.EPI-12-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu S, Wen W, Shu XO, et al. Association of Leukocyte Telomere Length With Breast Cancer Risk: Nested Case-Control Findings From the Shanghai Women's Health Study. Am J Epidemiol. 2013;177(7):617–624. doi: 10.1093/aje/kws291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui Y, Cai Q, Qu S, et al. Association of leukocyte telomere length with colorectal cancer risk: nested case-control findings from the Shanghai Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1807–1813. doi: 10.1158/1055-9965.EPI-12-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey SM, Murnane JP. Telomeres, chromosome instability and cancer. Nucleic Acids Res. 2006;34(8):2408–2417. doi: 10.1093/nar/gkl303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Suarez E, Flores JM, Blasco MA. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol Cell Biol. 2002;22(20):7291–7301. doi: 10.1128/MCB.22.20.7291-7301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Jin TB, Wei XB, et al. Selected polymorphisms of GSTP1 and TERT were associated with glioma risk in Han Chinese. Cancer Epidemiol. 2012;36(6):525–527. doi: 10.1016/j.canep.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Li G, Jin T, Liang H, et al. RTEL1 tagging SNPs and haplotypes were associated with glioma development. Diagn Pathol. 2013;8:83. doi: 10.1186/1746-1596-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walsh KM, Rice T, Decker PA, et al. Genetic variants in telomerase-related genes are associated with an older age at diagnosis in glioma patients: evidence for distinct pathways of gliomagenesis. Neuro Oncol. 2013;15(8):1041–1047. doi: 10.1093/neuonc/not051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxwell F, McGlynn LM, Muir HC, et al. Telomere attrition and decreased fetuin-A levels indicate accelerated biological aging and are implicated in the pathogenesis of colorectal cancer. Clini Cancer Res. 2011;17(17):5573–5581. doi: 10.1158/1078-0432.CCR-10-3271. [DOI] [PubMed] [Google Scholar]
- 46.Fu X, Wan S, Hann HW, et al. Relative telomere length: a novel non-invasive biomarker for the risk of non-cirrhotic hepatocellular carcinoma in patients with chronic hepatitis B infection. Eur J Cancer. 2012;48(7):1014–1022. doi: 10.1016/j.ejca.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou K, Liu Y, Zhang H, et al. XRCC3 haplotypes and risk of gliomas in a Chinese population: a hospital-based case-control study. Int J Cancer. 2009;124(12):2948–2953. doi: 10.1002/ijc.24307. [DOI] [PubMed] [Google Scholar]