Abstract
Background
For patients with progressive breast cancer brain metastasis (BCBM) after whole brain radiotherapy (WBRT), few options exist. Patupilone is an epothilone that crosses the blood–brain barrier. We hypothesized that patupilone would produce a 35% 3-month CNS progression-free survival in women with BCBM after WBRT.
Methods
This multicenter phase II trial included 2 cohorts. Group A included women with progressive BCBM after WBRT. Group B was an exploratory cohort of patients with either leptomeningeal metastases or untreated brain metastases. The primary goal was to observe a 35% 3-month CNS progression-free survival in Group A. The sample size was 45 for Group A and 10 for Group B. Patients received patupilone 10 mg/m2 once every 3 weeks until progression. Responses were scored according to the Macdonald criteria.
Results
Fifty-five patients (45 in Group A, 10 in Group B) enrolled. In Group A, the 3-month CNS progression-free survival was 27%, the median overall survival was 12.7 months, and the overall response rate was 9%. In Group B, which enrolled 5 patients with leptomeningeal disease and 5 with no prior WBRT, no responses occurred and 8 patients had CNS progression before 3 months. Systemic responses occurred in 15% of patients, including a complete response in liver metastases. Diarrhea occurred in 87% of patients; 25% had grade 3 and 4 adverse events.
Conclusions
Patupilone in patients with BCBM did not meet the efficacy criteria and had significant gastrointestinal toxicity. Further study of brain-penetrant agents is warranted for patients with CNS metastases from breast cancer.
Keywords: brain metastases, breast cancer, chemotherapy, epothilone, patupilone
Breast cancer brain metastases (BCBMs) are the second most frequent cause of CNS metastases.1 BCBMs are becoming more prevalent with improved systemic therapies for this malignancy.2,3 In addition, patients, especially those with human epidermal growth factor receptor–2+ disease, are surviving longer after the diagnosis of brain metastases.3,4 Novel therapies for this growing patient population are needed.
Patupilone (epothilone B, EPO906) is a naturally occurring member of the epothilone class of microtubule-stabilizing drugs, derived from the myxobacterium Sporangium cellulosum.5 Although epothilones and taxanes both induce microtubule polymerization, important differences exist between these classes of drugs. First, taxanes and epothilones interact with the binding pocket of β-tubulin in distinct manners.6 Patupilone is 3- to 20-fold more potent in vitro than paclitaxel and docetaxel.7 Second, patupilone is a poor substrate for P-glycoprotein and other multidrug transporter proteins that mediate resistance to many cytotoxic agents, including taxanes.8 Thus, patupilone inhibits growth of a broad range of human tumors in vitro, including those that are intrinsically unresponsive to paclitaxel or that have acquired resistance to this and other cytotoxic agents.9 Because the P-glycoprotein efflux pump impairs transit of drug across the blood–brain barrier (BBB), patupilone, unlike taxanes, is able to penetrate the BBB in murine models.10
Patupilone has demonstrated activity against breast cancer with objective responses in several early-phase clinical studies.11,12 Based on this favorable clinical activity, as well as its ability to cross the BBB, a multicenter phase II trial of patupilone for treatment of BCBM was conducted.
Materials and Methods
Study Design
This was a phase II trial for patients with breast cancer and CNS metastases. Two groups were included. Group A subjects, the primary group for which the trial was powered, were patients with progressive, radiographically measurable parenchymal brain metastases after whole brain radiotherapy (WBRT). Group B subjects, an exploratory cohort of 10 patients, had either metastases of leptomeningeal disease (LMD) or unirradiated, asymptomatic BCBM. Although the analysis of Group B was not intended to be statistically robust, this patient population is frequently not included in clinical trials. It was therefore elected to include these patients with the idea that responses in this group might prompt further investigation for these patients. The primary endpoint was the 3-month CNS progression-free survival (PFS) in Group A patients. The primary study objective was to observe a 3-month CNS PFS rate of at least 35% in Group A. Secondary objectives were to describe the toxicity and to determine the CNS response rate, systemic response rate, and overall survival (OS). The exploratory objective was to observe a signal of activity in Group B patients. No effort was made to enroll an equal number of patients with LMD and unirradiated patients.
Eligibility
Two cohorts of patients (Group A and Group B) with histologically proven breast cancer and CNS metastases were eligible for this trial. Eligible patients were neurologically stable with Karnofsky performance scores ≥60 and life expectancy of ≥3 months. Additional eligibility criteria included: absolute neutrophil count >1.5 × 109/L; hemoglobin >9.0 g/dL; platelets >100 × 109/L; total bilirubin <1.5× upper limit of normal (ULN); aspartate aminotransferase, alanine aminotransferase <2.5× ULN; alkaline phosphatase <2.5× ULN; and serum creatinine <1.5× ULN. For patients whose only evaluable CNS lesion(s) had been treated with stereotactic radiosurgery, radiographic (eg, PET, MR spectroscopy) or histologic proof of progressive disease was required to exclude radiation necrosis. Patients were permitted to continue hormone therapy and/or trastuzumab but no other concurrent chemotherapy. Exclusion criteria included prior therapy with epothilones or the concurrent use of warfarin. All patients were required to give institutional review board–approved written informed consent.
Treatment
This was a multicenter, single-arm, phase II clinical trial conducted at the Cleveland Clinic, at Massachusetts General Hospital, at Memorial Sloan-Kettering Cancer Center, and at the University of Michigan. Patients received patupilone 10 mg/m2 as a 20-min intravenous infusion once every 3 weeks until the occurrence of disease progression or unacceptable toxicities. Contrast-enhanced MRI of the brain and CT scans of the chest and abdomen were obtained every 2 cycles of therapy. For patients with LMD and normal MRIs of the brain and spine, CSF cytology was repeated every 2 cycles. Dose modifications were standardized for significant toxicities graded according to the National Cancer Institute's Common Toxicity Criteria v3.0. In addition, an algorithm was established to manage diarrhea promptly, as this was the dose-limiting toxicity in prior studies of this agent.
Response Criteria
Responses were assessed using bidimensional measurements with the following response definitions. These definitions were applied separately to CNS disease and systemic disease: complete response (CR) = resolution of all tumors, with the patient off all steroids or on adrenal maintenance only; partial response (PR) = decrease of >50% in the sum of the products of 2 diameters of all lesions with the patient off all steroids. CR or PR could be assigned only if confirmed by a CT/MR scan performed a minimum of 4 weeks after the initial scan on which the response was identified. Progression = increase of >25% in the sum of the products of 2 diameters of all lesions provided that the patient had not had his/her dose of steroids decreased since the last evaluation period; stable disease (SD) = absence of the criteria for CR, PR, or SD on stable or decreasing doses of steroids. Radiographic responses were not centrally reviewed.
Statistical Considerations
The primary endpoint for the study was the 3-month CNS PFS in Group A patients. Secondary endpoints included toxicity, CNS response rate, systemic disease response rate, and OS. The study was powered for the patient group with radiographically measurable parenchymal brain metastases progressive after WBRT (Group A). For this group, a 3-month CNS PFS of 35% was considered to be worthy of further study, whereas a 15% rate was considered to be not meaningful. The 3-month CNS PFS of 35% as an indicator of efficacy was based on 2 studies of chemotherapy doublets in brain metastases, which demonstrated 3-month CNS PFS rates in the range of 22%–50%.13,14 Forty eligible and evaluable patients were required to yield the maximum half-width of a 95% confidence interval (CI) of 0.16 about a particular quantile (based on the binomial distribution). Therefore, Group A included 40 patients. The exploratory cohort of Group B was set at 10 patients based on estimates that this number of patients could be accrued during the enrollment period of Group A. Assuming a 10% rate of inevaluable patients, the final sample size was 55 patients.
Results
Patient Characteristics
Fifty-five patients were treated on this study between February 2007 and May 2010, all of whom were considered eligible. Table 1 summarizes the patient characteristics. All patients were female; the median age at study entry was 50 years (range, 31–68), and all but 3 patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Table 1.
Patient characteristics
| Characteristic | n |
|---|---|
| Age, y, median (range) | 50 (31–68) |
| ECOG performance status | |
| 0 | 28 (51%) |
| 1 | 24 (44%) |
| 2 | 3 (5%) |
| Group | |
| A: Previously irradiated brain metastases | 45 (82%) |
| B: Leptomeningeal metastases | 5 (9%) |
| B: Unirradiated asymptomatic brain metastases | 5 (9%) |
| Disease characteristics, median (range) | |
| ER and/or PR positive | 24 (44) |
| HER2 positive | 27 (49) |
| ER/PR/HER2 negative | 15 (27) |
| Prior treatment | |
| Taxanes | 50 (91) |
| Median number of cytotoxic regimens | 4 |
| Concurrent treatment | |
| Hormone therapy | 8 (15) |
| Trastuzumab | 17 (31) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Treatment Administration
All patients had discontinued therapy. A median of 2 cycles (range, 1–18) of therapy were delivered. Twenty-three percent (12/53) of patients with data had dose reductions—10 had single 25% decreases and 2 had multiple reductions. Overall 79% (138/174) of cycles were delivered at full dose. Patients discontinued therapy primarily for progressive disease (84%, 46/55). Seven patients withdrew consent (due to diarrhea and/or neuropathy), 1 patient was removed by the physician due to progressive neuropathy, and 1 patient experienced sudden death during cycle 1 of treatment.
Progression-free and Overall Survival
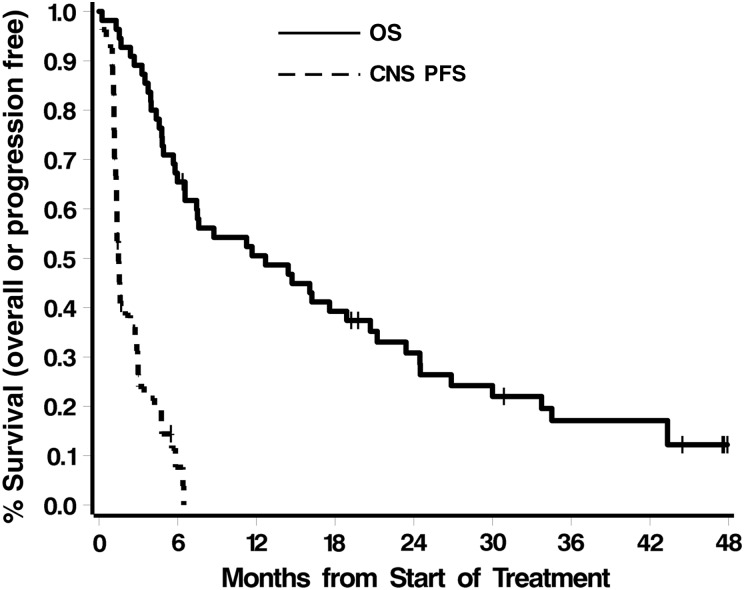
The median time to CNS progression (patients who discontinued therapy for systemic disease progression were censored as of the treatment stop date) was 1.5 months (95% CI: 1.3–2.4). The estimated 3-month PFS was 27% ±7%. The study therefore did not meet its goal of 35% 3-month CNS freedom from progression. The estimated 6-month PFS was 8% ±5%. All patients died. The median survival was 12.7 months (95% CI: 6.6–21). Figure 1 shows Kaplan–Meier curves for both OS and PFS. With the exception of ECOG performance status and concurrent trastuzumab therapy, none of the factors in Table 1 was associated with outcome (all P values ≥.11). Patients with ECOG performance status 0 had significantly better PFS and OS than patients with ECOG performance status 1 or 2 (PFS: median 2.9 vs 1.4, respectively, P = .02; OS: median 20.7 vs 5.7, respectively, P = .04). Improved PFS and OS were also seen in patients who continued to receive trastuzumab while on study (Ps = .08 and .02, respectively), although the continuation of such therapy may reflect a patient subgroup whose systemic disease was under control at study entry.
Fig. 1.
CNS PFS and OS.
Responses
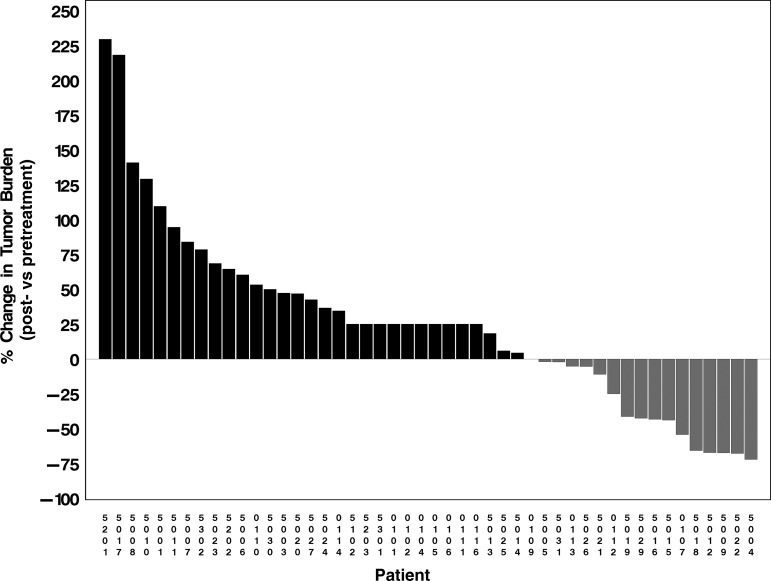
No CRs occurred. Among 38 patients with measurable disease, 5 (13% [9% of all patients]) had confirmed PR of brain metastases that lasted 2.7+ to 5.2 months. The overall response rate was therefore 9%. One patient had an unconfirmed PR. Eleven (29% [20% of all patients]) had short-term (<3 mo) SD, and 39 patients (71%) progressed. The 38 patients with measurable disease had a median (best change) 12% increase in brain tumor burden (range, 72% decrease to 230% increase) with 16 (42% [29% of all patients]) demonstrating at least some reduction (median 43% reduction; Fig. 2). None of the factors in Table 1 was associated with improved response (all P values ≥.30).
Fig. 2.
Waterfall plot of best change in CNS tumor burden—patients who progressed rapidly and therefore may not have had repeat scans are indicated as having had a 25% increase in tumor size.
Exploratory Cohort
Ten patients, 5 with no prior radiation and 5 with LMD, were enrolled on the study. No radiographic or cytologic responses occurred. In each group, only 1 patient survived 3 months free of CNS progression. The remaining 8 patients had CNS progression at 1–2 months.
Systemic Responses
Radiographic responses in systemic disease occurred in 8 patients (15%), with 1 CR (liver metastases); 7 PR (lung 3; liver 2; lymph node 1; breast 1). Stable systemic disease occurred in 18 patients (33%), while the remainder (53%) had either progression or incomplete data to judge response.
Adverse Events/Toxicity
Table 2 summarizes adverse events considered at least possibly related to treatment. Toxicities on this trial were predominantly gastrointestinal (GI). Diarrhea was the most common toxicity, occurring in 87% of patients. Twenty-five percent of patients on the study experienced grade 3 diarrhea. Nausea and vomiting occurred in 60% (grade 3 in 5%), and fatigue occurred in 82% of patients (grade 3 in 9%). Overall, 3 patients (5%) experienced grade 4 events, including anemia, neutropenia, and hypokalemia, the latter of which was likely due to diarrhea. No toxic deaths occurred. One patient died suddenly at home within 1 week of starting treatment.
Table 2.
Adverse events considered at least possibly related to treatment
| Toxicity | Grade, n (%) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Overall | |
| GI | |||||
| Diarrhea | 15 (27) | 19 (35) | 14 (25) | 0 | 48 (87) |
| Nausea/vomiting | 19 (35) | 11 (20) | 3 (5) | 0 | 33 (60) |
| Anorexia | 12 (22) | 6 (11) | 1 (2) | 0 | 19 (35) |
| Abdominal pain | 8 (15) | 3 (5) | 3 (5) | 0 | 14 (25) |
| Weight loss | 9 (16) | 2 (4) | 0 | 0 | 11 (20) |
| Heartburn/dyspepsia | 4 (7) | 4 (7) | 0 | 0 | 8 (15) |
| Constipation | 7 (13) | 0 | 0 | 0 | 7 (13) |
| Dehydration | 1 (2) | 2 (4) | 3 (5) | 0 | 6 (11) |
| Upper GI bleed | 0 | 0 | 1 (2) | 0 | 1 (2) |
| Other | 0 | ||||
| Fatigue | 14 (25) | 26 (47) | 5 (9) | 0 | 45 (82) |
| Neuropathy | 7 (13) | 3 (5) | 1 (2) | 0 | 11 (20) |
| Alopecia | 7 (13) | 1 (2) | 0 | 0 | 8 (15) |
| Joint pain | 4 (7) | 4 (7) | 0 | 0 | 8 (15) |
| Mucositis | 7 (13) | 0 | 0 | 0 | 7 (13) |
| Anemia | 2 (4) | 2 (4) | 1 (2) | 1 (2) | 6 (11) |
| Hypokalemia | 3 (5) | 0 | 2 (4) | 1 (2) | 6 (11) |
| Neutropenia | 0 | 1 (2) | 0 | 1 (2) | 2 (4) |
Discussion
This clinical trial achieved a 3-month CNS PFS of 27% but unfortunately failed to meet its primary objective of 35% 3-month CNS PFS. Several factors may contribute to this result. A significant number of patients withdrew consent prior to 3 months, a reflection of poor tolerance of therapy in this heavily pretreated patient population. The toxicity profile, however, appears comparable to that observed in other trials of patupilone.12,15,16 Despite preclinical evidence of BBB penetration, it is not known whether concentrations within the metastases reached cytotoxic levels. One presurgical trial of patupilone in recurrent glioblastoma, however, did document tumor drug levels 30 times that of plasma.17
Although patupilone is not a substrate for P-glycoprotein, other mechanisms of drug resistance exist, particularly in a patient population generally heavily pretreated whose tumors often demonstrate resistance to multiple agents. One resistance mechanism against patupilone in particular is the Gli1 protein, a transcription activator in the hedgehog pathway.18 Various beta-tubulin isotypes can also mediate resistance to patupilone.19
Somewhat surprisingly and disappointingly, those patients who had not received prior WBRT fared poorly, with no responses. Although this cohort was too small to allow statistically sound conclusions, the response rates of chemotherapy in previously unirradiated brain metastases have generally appeared to be comparable to rates achieved in non-CNS metastases and greater than those achieved in patients post-WBRT.20–22 The poor results with LMD patients is less surprising because these patients typically have disease that is poorly responsive to therapy, perhaps due to failure to achieve cytotoxic concentrations in the CSF. Cerebrospinal concentrations of patupilone have not been measured.
Patupilone showed some activity in systemic disease. Although the trial was not designed to compare CNS with systemic disease responses, the number of patients (26 [47%]) with responses or stable systemic disease appears to be greater than that in the CNS (16 [29%]).
Patupilone has been tested in other clinical trials of CNS malignancies. A phase I trial of patupilone with concurrent radiotherapy included 17 patients with brain metastases.23 A trial of patupilone in patients with brain metastases from non-small-cell lung cancer demonstrated a response rate of 36%.24 Other epothilones have been explored in brain metastases. Although ixabepilone is the only approved epothilone for patients with breast cancer, it has not been studied specifically for brain metastases. A trial of sagopilone, a second-generation epothilone, in women with BCBM demonstrated modest activity, with a CNS response rate of 13% and PFS of 1.4 months.25 Additional trials of chemotherapy in patients with recurrent BCBM after radiation therapy have reported response rates of 6%–20%.13,26,27 The results of the current trial are in the range of those previously reported, although response criteria differ among the trials.
This trial has several limitations. The patients, despite having a uniform primary site, had a variety of molecular subtypes. Although overexpression of tubulin-β-III has been associated with efficacy of patupilone and other epothilones in gynecological malignancies, these markers were not assayed in this trial because this association was not known at the time of trial design.28 The trial setting—progression after WBRT—selects a heavily pretreated population whose tolerance of chemotherapy may be impaired. Although the small numbers of previously unirradiated patients in this study fared poorly, a number of pre-irradiation chemotherapy trials suggest that this setting would offer a better opportunity to observe activity of an agent of interest.
In conclusion, patupilone in this setting was ineffective in the CNS and caused significant GI toxicity. As CNS metastases become a more prevalent manifestation of breast cancer, further study of agents with BBB penetration should be conducted in biomarker-enriched populations of patients.
Funding
This work was supported by Novartis Pharmaceuticals.
Conflict of interest statement. None declared.
References
- 1.Lim E, Lin NU. New insights and emerging therapies for breast cancer brain metastases. Oncology (Williston Park) 2012;26:652–659, 663. [PubMed] [Google Scholar]
- 2.Melisko ME, Moore DH, Sneed PK, et al. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol. 2008;88:359–365. doi: 10.1007/s11060-008-9578-5. [DOI] [PubMed] [Google Scholar]
- 3.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 4.Karam I, Hamilton S, Nichol A, et al. Population-based outcomes after brain radiotherapy in patients with brain metastases from breast cancer in the pre-trastuzumab and trastuzumab eras. Radiat Oncol. 2013;8:12. doi: 10.1186/1748-717X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Nettles JH, Li H, Cornett B, et al. The binding mode of epothilone A on alpha,beta-tubulin by electron crystallography. Science. 2004;305:866–869. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 7.Altmann KH, Wartmann M, O'Reilly T. Epothilones and related structures—a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim Biophys Acta. 2000;1470:M79–M91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 8.Cheng KL, Bradley T, Budman DR. Novel microtubule-targeting agents—the epothilones. Biologics. 2008;2:789–811. doi: 10.2147/btt.s3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol®) J Biol Chem. 1997;272:2534–2541. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 10.O'Reilly T, Wartmann M, Brueggen J, et al. Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother Pharmacol. 2008;62:1045–1054. doi: 10.1007/s00280-008-0695-9. [DOI] [PubMed] [Google Scholar]
- 11.Rubin EH, Rothermel J, Tesfaye F, et al. Phase I dose-finding study of weekly single-agent patupilone in patients with advanced solid tumors. J Clin Oncol. 2005;23:9120–9129. doi: 10.1200/JCO.2005.03.0981. [DOI] [PubMed] [Google Scholar]
- 12.Tsimberidou AM, Takimoto CH, Moulder S, et al. Effects of patupilone on the pharmacokinetics and pharmacodynamics of warfarin in patients with advanced malignancies: a phase I clinical trial. Mol Cancer Ther. 2011;10:209–217. doi: 10.1158/1535-7163.MCT-10-0774. [DOI] [PubMed] [Google Scholar]
- 13.Rivera E, Meyers C, Groves M, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107:1348–1354. doi: 10.1002/cncr.22127. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto FM, Omuro AM, Raizer JJ, et al. A phase II trial of vinorelbine and intensive temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol. 2008;87:85–90. doi: 10.1007/s11060-007-9491-3. [DOI] [PubMed] [Google Scholar]
- 15.Chi KN, Beardsley E, Eigl BJ, et al. A phase 2 study of patupilone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel: Canadian Urologic Oncology Group study P07a. Ann Oncol. 2012;23:53–58. doi: 10.1093/annonc/mdr336. [DOI] [PubMed] [Google Scholar]
- 16.Bystricky B, Chau I. Patupilone in cancer treatment. Expert Opin Investig Drugs. 2011;20:107–117. doi: 10.1517/13543784.2011.542148. [DOI] [PubMed] [Google Scholar]
- 17.Oehler C, Frei K, Rushing EJ, et al. Patupilone (epothilone B) for recurrent glioblastoma: clinical outcome and translational analysis of a single-institution phase I/II trial. Oncology. 2012;83:1–9. doi: 10.1159/000339152. [DOI] [PubMed] [Google Scholar]
- 18.Mozzetti S, Martinelli E, Raspaglio G, et al. Gli family transcription factors are drivers of patupilone resistance in ovarian cancer. Biochem Pharmacol. 2012;84:1409–1418. doi: 10.1016/j.bcp.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Gan PP, McCarroll JA, Byrne FL, et al. Specific beta-tubulin isotypes can functionally enhance or diminish epothilone B sensitivity in non-small cell lung cancer cells. PLoS One. 2011;6:e21717. doi: 10.1371/journal.pone.0021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franciosi V, Cocconi G, Michiara M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: a prospective study. Cancer. 1999;85:1599–1605. [PubMed] [Google Scholar]
- 21.Rosner D, Nemoto T, Lane WW. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832–839. doi: 10.1002/1097-0142(19860815)58:4<832::aid-cncr2820580404>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Siena S, Crino L, Danova M, et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: a multicenter phase II study. Ann Oncol. 2010;21:655–661. doi: 10.1093/annonc/mdp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogh S, Machtay M, Werner-Wasik M, et al. Phase I trial using patupilone (epothilone B) and concurrent radiotherapy for central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2010;77:1009–1016. doi: 10.1016/j.ijrobp.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 24.Nayak L, Abrey L, DeAngelis L, et al. San Diego, CA: An open-label, multi-center, phase II study of patupilone (EPO906), in the treatment of recurrent or progressive brain metastases in patients with non-small cell lung cancer (NSCLC) Paper presented at: American Academy of Neurology Annual Meeting; March 16, 2013. [Google Scholar]
- 25.Freedman RA, Bullitt E, Sun L, et al. A phase II study of sagopilone (ZK 219477; ZK-EPO) in patients with breast cancer and brain metastases. Clin Breast Cancer. 2011;11:376–383. doi: 10.1016/j.clbc.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 28.Roque DM, Bellone S, English DP, et al. Tubulin-beta-III overexpression by uterine serous carcinomas is a marker for poor overall survival after platinum/taxane chemotherapy and sensitivity to epothilones. Cancer. 2013;119:2582–2592. doi: 10.1002/cncr.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]