Abstract
The human brain is capable of generating new functional neurons throughout life, a phenomenon known as adult neurogenesis. The generation of new neurons is sustained throughout adulthood due to the proliferation and differentiation of adult neural stem cells. This process in humans is uniquely located in the subgranular zone of the dentate gyrus in the hippocampus. Adult hippocampal neurogenesis (AHN) is thought to play a major role in hippocampus-dependent functions, such as spatial awareness, long-term memory, emotionality, and mood. The overall aim of current treatments for cancer (such as radiotherapy and chemotherapy) is to prevent aberrant cell division of cell populations associated with malignancy. However, the treatments in question are absolutist in nature and hence inhibit all cell division. An unintended consequence of this cessation of cell division is the impairment of adult neural stem cell proliferation and AHN. Patients undergoing treatment for cancerous malignancies often display specific forms of memory deficits, as well as depressive symptoms. This review aims to discuss the effects of cancer treatments on AHN and propose a link between the inhibition of the neurogenetic process in the hippocampus and the advent of the cognitive and mood-based deficits observed in patients and animal models undergoing cancer therapies. Possible evidence for coadjuvant interventions aiming to protect neural cells, and subsequently the mood and cognitive functions they regulate, from the ablative effects of cancer treatment are discussed as potential clinical tools to improve mental health among cancer patients.
Keywords: adult hippocampal neurogenesis, cancer treatments, cognition, depression
The process of neurogenesis involves the development and maturation of new neuronal populations from neural progenitor cells, which are, by virtue of their multipotent state, forms of neural stem cells (NSCs). In the adult rodent brain, neurogenesis has been observed to occur in the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus.1 The hippocampus is known to be one of the most important brain structures implicated in memory formation and spatial processing,2–10 and it has hence become evident that the process of hippocampal neurogenesis is of pivotal importance in the maintenance of normal cognitive function.11 It is now also well established that adult hippocampal neurogenesis (AHN) is an important player in the regulation of both mood and anxiety, with decreased rates of AHN being normally accompanied by an increase in depression- and anxiety-related behaviors.12,13 Importantly, it had been proposed that the cell growth patterns observed in rodent and other animal models were analogous to the degree and processes of growth displayed in human subjects.14,15 Indeed, evidence from a recent study dating hippocampal cells with nuclear bomb test–derived carbon-14 confirmed the occurrence of adult neurogenesis in the human hippocampus.16 Moreover, it was revealed that it occurs at rates comparable to those of middle-aged mice, supporting a strong relevance of results from animal models. Given that treatments for cancer are tailored in such a way as to prevent the advent of cell division, it is not surprising that the process of NSC proliferation followed by neurogenesis is inhibited upon administration of various forms of cancer therapy.17
The methods used in the treatment of cancer-based pathologies (namely surgical and radio- and chemotherapies) have often been associated with the induction of memory deficits, cognitive decline, and depressive symptoms in relevant patients,18 with cognitive dysfunction having also been recognized as a long-term problem among pediatric patients.19 At present, few studies have succeeded in determining whether or not a causal link exists among cancer treatments, the impairment of AHN, and the subsequent development of memory and mood dysfunction. The difficulty in establishing such a causal link, especially in the case of depression, may rely on the highly negative cognitions associated with cancer diagnosis, due to the invasive and severe treatments offered and the high morbidity associated with this condition. This psychosocial feature of cancer may confound the assumption that depression could come as a consequence of antiproliferative treatments through a decrease in AHN; it could, in turn, be considered a psychiatric comorbidity established prior to the effects of anticancer interventions as a consequence of other psychobiological mechanisms than hippocampal neurogenesis. In this sense, the use of animal models in the assessment of the possible relationship between cancer therapies and the onset of cognitive deficits and depressive behavior through a decrease in hippocampal neurogenesis is of primary importance. This review aims to summarize the scientific literature relevant to the area of investigation and hypothesize the existence of a tangible causal link among the aforementioned factors.
The Role of the Hippocampus
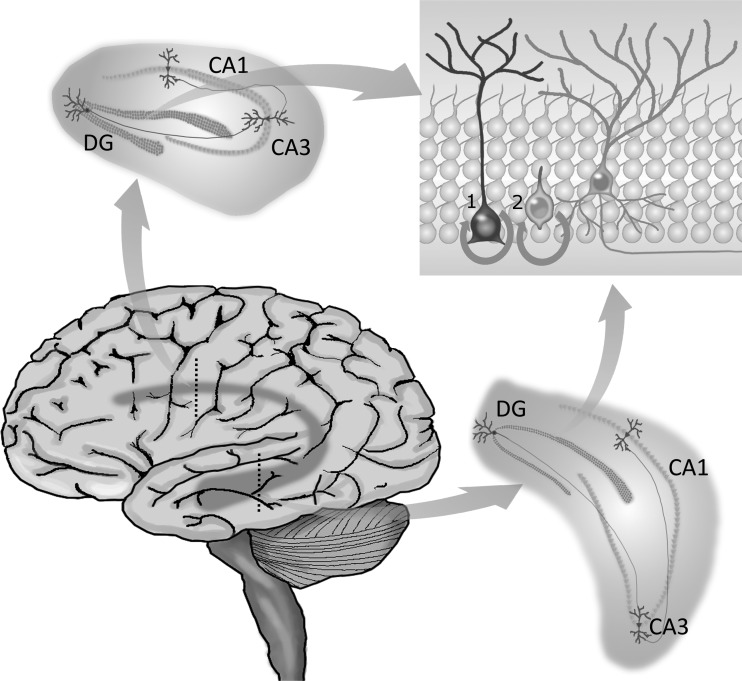
The hippocampus is a structure in the brain that forms part of the limbic system. The hippocampus is implicated in a number of complex cognitive procedures, such as spatial recognition and memory, as well as mood regulation.3,20,21 Anatomically, the hippocampus is divided into 3 main subregions, whose specific cells confer to the system different important stages for information processing (Fig. 1). These main subregions are the cornu ammonis (CA) 1, the CA3, and the DG. The hippocampus processes information through its trisynaptic circuit, starting with the glutamatergic granule cells of the DG receiving input from the entorhinal cortex and sending projections to the CA3, which then connects with pyramidal neurons at the CA1. These, in turn, send the processed information to other parts of the brain through projections to the subiculum and entorhinal cortex.22 Since the hippocampal formation extends longitudinally along the brain, hippocampus-related functions are, in general terms, distributed along its septotemporal axis, with cognitive abilities being more related to the dorsal hippocampus and mood/emotionality being more closely related to pathways within the ventral hippocampus.23–25
Fig. 1.
The hippocampal circuitry. The hippocampus is located within the temporal lobe, extending longitudinally across the brain. It is composed of 3 distinct subregions—the DG, CA1, and CA3—each responsible for specific components of information processing. Granule cells in the DG receive inputs from the entorhinal cortex and send projections to CA3 pyramidal neurons, which connect with those of CA1, from where information is distributed to other parts of the brain. The DG is particularly known for its remarkable characteristic of generating new functional neurons throughout adulthood. The process of adult hippocampal neurogenesis starts with type 1 NSCs generating type 2 progenitors, which can differentiate into neuroblasts. Given appropriate microenvironmental conditions, these cells will mature into neurons and integrate local preexisting circuits. Because the hippocampus extends dorsoventrally, newborn neurons that integrate the dorsal circuitry are believed to regulate functions proper to the dorsal hippocampus, such as spatial memory. In turn, those integrating ventral circuits are thought to play a role in the regulation of ventral hippocampus functions, such as those related to mood and emotionality.
Due to the involvement of the hippocampus in cognitive and mood- and emotion-related processes, it is unsurprising that hippocampal dysfunction has been implicated in the development of a number of psychiatric and neurological pathologies, such as dementia26 and depression.27 Studies into the structure and functioning of the hippocampus have indicated the existence of NSCs in the SGZ of the DG and have asserted that the microenvironment of this so-called neurogenic niche maintains this pool of NSCs under highly regulated proliferative activity. Cell-intrinsic and extracellular factors within the neurogenic niche actively coordinate the generation of new populations of functional granule neurons throughout life.28 These populations and the regulatory processes involved in the coordination of their proliferation and differentiation states are of great scientific interest, in that they are currently therapeutic targets in future treatment paradigms to restore hippocampal function. This becomes especially important in the context of individuals under decreased or ceased cell proliferative activity, as is the case for patients undergoing cancer treatment.
Neurogenesis in the Adult Hippocampus
Hippocampal NSCs are capable of self-renewal via proliferation and differentiation into neurons, oligodendrocytes, and astrocytes throughout the duration of an individual's lifespan. NSCs in the DG, known as type 1 cells, are at a quiescent state and express radial and progenitor markers. Type 1 cells generate type 2 progenitors, which in turn give rise to either astrocytes or neuroblasts, these expressing the immature neuronal marker doublecortin28 (Fig. 1). If given the appropriate conditions, neuroblasts will mature into glutamatergic granule neurons, integrating in the preexisting circuitry of the DG. The whole process is, therefore, dependent on appropriate proliferative activity.
The neurogenetic process is facilitated by the location of NSCs within a neurogenic niche.29 It has been hypothesized that neurogenesis is able to occur in the SGZ due to the facilitative role of the surrounding microenvironment, whereby astrocyte populations, along with autocrine signaling from the NSCs themselves, actively facilitate the process of neurogenesis through the provision of proneurogenetic molecules.28–30 Also, the proximity observed of NSCs to the endothelium of blood vessels facilitates the process, given that these release factors that stimulate self-renewal of NSCs.31
Increasing evidence supports the idea that AHN constitutes an important factor underlying functional plasticity in the adult brain, contributing to the formation of new memories and the regulation of cognitive processes.32 The newly generated neurons appear to have an essential role in the ability of the DG to distinguish contexts, as opposed to simply distinguishing places. This process is known as pattern separation and is believed to confer the uniqueness feature of a certain memory.11,33–35 Of special interest for mental health, disruption in this ability to transform similar experiences into distinguished, nonoverlapping representations has been proposed as an important factor underlying the development of anxiety disorders.36 The development of other aspects of anxiety,37,38 as well as of depression,39,40 has also been shown to be related to low levels of AHN. In this sense, it is plausible to suspect that manipulations that disrupt AHN might also bring deleterious consequences to mental health, specifically in terms of mood, emotionality, and cognition.
Effects of Cancer Treatments on Adult Hippocampal Neurogenesis
Treatments for cancerous conditions are ultimately directed against the proliferation of cancerous tumors, thereby aiming to reduce and arrest the aberrant cell division brought about by the affected cells. Current treatment protocols are usually adjusted in order to arrest the form of cancer that is afflicting the patient. Treatment can involve the use of chemotherapy, radiotherapy, hormone therapy, and surgery. It may be deemed necessary to utilize a number of treatment options, with chemotherapy being frequently used as an adjuvant to radiotherapy. These treatments are fundamentally absolutist in nature, in that they generally act to prevent systemically all forms of cell division. Paradoxically, the treatments, while necessary to curtail the spread and development of the cancer cell population, have undesirable consequences in preventing the division and proliferation of the stable cell populations that are required for the maintenance of generalized homeostasis. Animal studies into the effect of chemotherapeutic agents and radiation on NSC populations involved in AHN have elucidated a number of novel findings. It has been determined that use of therapeutic doses of radiation in the treatment of CNS malignancies results in a decrease in hippocampal NSC proliferation, a concomitant increase in the level of apoptosis of these cells, and an overall >95% decrease in the production of new neurons in adult rodents.41–43 In essence, radiotherapy acts in such a way that ablates the neurogenetic process. Of great importance, however, was the discovery that rodent NSC populations isolated 1 month after exposure to irradiation were relatively unchanged following radiation treatment. This indicates that acute cell death per se does not determine the persistent arrest of neurogenesis subsequent to brain irradiation but that the neurogenic niche could be more profoundly affected by changes caused in the neural microenvironment after radiation exposure, such as the disruption of microvascular angiogenesis within the neurogenic niche.43 Strong evidence points to an association between local angiogenesis and normal AHN, with clusters of precursors found in close anatomical contact with the surrounding microvasculature.44 Enhanced capillary area accompanied by enhanced hippocampal neurogenesis among depressed patients treated with selective serotonin reuptake inhibitors support the coupling of the 2 generational processes.45 Detrimental changes to microangiogenesis could then directly contribute to the decreased capacity for cell proliferation and increased commitment of the remaining neural precursors into a glial rather than a neuronal fate in the irradiated hippocampus. The importance of the surrounding microenvironment for neurogenesis in the context of radiation exposure has been consolidated through the use of transplantation studies in animal models. These experiments have proven successful in demonstrating de novo neurogenesis of irradiated neural precursor cells in vitro, while healthy NSCs transplanted into an irradiated hippocampus did not display distinct evidence of neurogenesis.18 It has generally been found that the neurogenetic niche of the NSCs is altered in 2 ways following radiation therapy. Firstly, the anatomical relationship between the neural progenitor cells and the microvasculature is significantly disrupted, and secondly, the radiation elicits an inflammatory response from the microglia, which is itself sufficient to disrupt the neurovascular relationship with NSCs.18,43
While the effects of radiotherapy appear to be related both to disruption of the cell cycle, with delayed progression through the G1, S, and G2 phases,46 and to the development of a neurotoxic microenvironment triggered by microglial activation, in theory chemotherapy drugs exert their antimitotic effect via well-described intracellular mechanisms. The ultimate goal of these drugs is to disrupt DNA, which can be achieved by mechanisms as diverse as the formation of covalent bonds with, for instance, the amino or phosphate groups of DNA strands (alkylating agents) and the interference of DNA synthesis by structural analogues of metabolites involved in DNA synthesis (antimetabolites, such as 5-fluorouracil [5-FU]).47 Whatever the specific mechanism of action, chemotherapeutics directly or indirectly induce DNA damage of rapidly dividing cells48; their action, however, is not specific to cancer cells, which makes dividing NSCs in the neurogenic zones a vulnerable target of their antimitotic action. Of special concern, both in vivo and in vitro approaches showed that progenitor cell populations are even at more risk than cancer lineages when exposed to different classes of chemotherapy drugs, such as DNA cross-linking agents and antimetabolites.49
Also, as stated previously, one of the main factors that make it possible for the adult hippocampus to generate new functional neurons is the proximity of NSCs to the endothelium of blood vessels. If, on the one hand, the close relationship between neural progenitors and vessel cells facilitates AHN by the local release of self-renewal factors, it also allows for a tight communication with the systemic environment.50 In the context of chemotherapy, this could more likely lead to a decrease in cell proliferation activity via a facilitated contact with antiproliferative agents, thereby impairing the neurogenetic process as a whole. In addition, one of the promising interventions for tumor regression combined with conventional chemotherapy is the administration of antiangiogenic agents. The strategy relies on the prevention of tumor vessel formation mainly through inhibition of the axis of vascular endothelial growth factor (VEGF).51 However, although the combined use of angiogenesis inhibitors with chemotherapy in most cases yields superior outcomes in comparison with single-drug treatment, it could also potentiate the negative consequences of cancer treatment to AHN, given the close—and possibly causal45—relationship between the neuro- and angiogenesis processes.
Cancer Treatments and Cognitive Impairment
In spite of evidence indicating that many normal cell populations can adapt to and subsequently overcome the deleterious effects of cancer treatments, it has been ascertained that the administration of cancer treatment has an important degree of association with long-term cognitive deficits in humans,17,52–54 a theme that because of its extensive complexity and broad approach cannot be fully addressed in this review. Overall, patients may display deficits in long-standing attentional functions,55 motor functions, and executive functions (which may include those related to mental flexibility, planning, rule learning, initiation, monitoring, and maintenance of appropriate action56), which can be attributed to chemotherapeutic agents.57 Interestingly, cognitive deficits resulting from chemotherapy among breast cancer survivors appear to be specific to certain domains, a feature that may guide the design of more targeted neuropsychological interventions to this population. Indeed, deficits presented in a rule-learning task by breast cancer patients who had undergone chemotherapy were selective to decision making under ambiguity, rather than decision making under risk.58 The study compared the performance of chemotherapy treated and nontreated patients, as well as healthy subjects, showing that the cognitive deficits found could be attributed mainly to treatment, and not simply to the psychological stress of having a cancer diagnosis. In addition, the authors interpreted the data as chemotherapy rendering some specific disruption to the limbic circuitry, rather than the prefrontal loop. Although hippocampal neurogenesis cannot so far be directly assessed in vivo in humans, it is plausible to hypothesize that the effects observed could be a consequence of the decreased NSC proliferation and differentiation in the hippocampus as a result of chemotherapy. The fact that the difference found was related to a deficit in dissociating ambiguous stimuli (pattern separation), which is a major function of the hippocampus, strongly supports this hypothesis.
However, it has also been noted that a number of factors have conspired to prevent the development of a directly causal link among the aforementioned variables when considering studies in human populations; these have included “relatively small sample sizes, differences in age of the patients, nature and location of the tumor(s), additional anti-cancer treatments and intensity of the adjuvant treatment.”17 Rodent studies would therefore be of considerable benefit in that they could enable the study of the effects of cancer treatment without confounding factors affecting the results obtained. Consequences of chemotherapy in rodent studies have been examined in a number of investigations utilizing healthy rodents, with many results hinting at a possible link between the use of chemotherapy and subsequent hippocampus-related cognitive deficits59–61 (Table 1).
Table 1.
Effects of cancer therapies on adult hippocampal stem cells proliferation and neurogenesis in rodents
| Model | Cancer Therapy | Dose | Effect on AHN | Effect on Behavior | Rescuing Intervention | Effect of Rescuing Intervention | Reference |
|---|---|---|---|---|---|---|---|
| In vivo: 3–4-mo-old male Fischer rats | Radiosurgery | 10 Gy | Increased apoptosis in the SGZ paralleled with decreased proliferation of immature progenitor populations responsible for AHN | Not assessed | Not assessed | Not assessed | Peissner et al. 199941 |
| In vivo: 8–10-wk-old male Fischer 344 rats | Irradiation | 1 Gy to 30 Gy | Increased apoptosis in the DG, reaching a plateau at about 3 Gy; reduced cell proliferation in the SGZ after 120 days at doses of 5 Gy or higher | Not assessed | Not assessed | Not assessed | Tada et al. 200042 |
| In vivo: adult female Fischer 344 rats | Irradiation | 2 Gy or 10 Gy | Inhibition of AHN, disruption of AHN-associated angiogenesis, and increased number and activation status of microglia within the neurogenic zone | Not assessed | Not assessed | Not assessed | Monje et al. 200243 |
| In vitro: progenitor cells from the hippocampi of irradiated female rats | |||||||
| In vivo: 2-mo-old female BALB/C mice | MTX + 5-FU | Single i.p. injection of MTX (37.5 mg/kg) + 5-FU (75 mg/kg) each wk for 3 consecutive wk | Not assessed | Impaired spatial (MWM) and nonspatial memory (NMTS and DNMTS) | Not assessed | Not assessed | Winocur et al. 200660 |
| In vivo: 6–8-wk-old CBA mice | BCNU, cisplatin, or cytarabine | 3 consecutive injections of BCNU, cisplatin, or cytarabine (3 × 10 mg/kg, 5 mg/kg, or 250 mg/kg body weight, respectively) | Increased cell death and decreased cell division in the DG | Not assessed | Not assessed | Not assessed | Dietrich et al. 200649 |
| In vitro: rat CNS stem cells, lineage restricted progenitor cells and differentiated cell types | 5-FU | In vitro: 0.5 μM, 1 μM, 5 μM for 24 h; 5 μM for 5 days; 1 mM for 1 h | In vitro: 45%–80% reduction in viability of dividing O-2A/OPCs, nondividing oligodendrocytes, and GRP cells | Not assessed | Not assessed | Not assessed | Han et al. 2008161 |
| In vivo: 6–8-wk-old CBA mice | In vivo: 3 i.p. injections every other day (40 mg/kg) | In vivo: induction of apoptosis and suppression of proliferation in the SVZ, DG, and CC; enhanced loss of DCX + cells from among the BrdU+ population in both the SVZ and DG | |||||
| In vivo: young male C57/BL6 mice | Irradiation | Bilateral cranial irradiation (total dose of 6 Gy/mouse) on postnatal day 9 (P9) | Significant reduction of precursor cell proliferation throughout the entire hippocampal formation; decreased number and altered morphology of DCX+ cells in the DG | Impaired exploratory behavior (OFT) | Voluntary running in adulthood for 4 wk | Voluntary running significantly restored precursor cell and neurogenesis levels, and ameliorated the behavior alterations observed in OFT | Naylor et al. 2008115 |
| In vivo: 150–170 g male, Swiss–Webster mice | MTX | Single i.p. injection of MTX (32 mg/kg); 5-FU (75 mg/kg), or MTX + 5-FU | Not assessed | Impaired memory retrieval in 5-FU mice (autoshaping operant procedure); impaired acquisition and retrieval in MTX + 5-FU mice | Not assessed | Not assessed | Foley et al. 200867 |
| 5-FU | |||||||
| MTX + 5-FU | |||||||
| In vivo: 3-mo-old male Wistar rats | MTX | Experiment I: single i.v. injection (37.5–300 mg/kg) | Experiment I: dose-dependent long-lasting decrease in hippocampal cell proliferation | Experiment II: impaired spatial (MWM) and nonspatial memory (NOR) | Not assessed | Not assessed | Seigers et al. 200865 |
| Experiment II: single i.v. injection (250 mg/kg) | |||||||
| In vivo: 3-mo-old male Wistar rats | MTX | Single i.v. injection (250 mg/kg) | Decreased hippocampal cell proliferation 7 days after treatment; decreased white matter density in the lateral CC 1 day, 1 wk and 3 wk after treatment | Deficits in spatial memory (MWM) and emotional learning (CFC) | Not assessed | Not assessed | Seigers et al. 200968 |
| In vivo: 8–10-wk-old ICR mice | CP | Single i.p. injection (40 mg/kg) | Decreased number of Ki-67- and DCX-positive cells 12 h after treatment, with highest effect 24 h after CP injection; levels were restored between 2 and 10 days | Deficits in emotional (PAT) and nonspatial cognitive memory (NOR) 12 h after treatment | Not assessed | Not assessed | Yang et al. 201063 |
| In vivo: adult Lister hooded rats | 5-FU | 5 i.v. injections every other day over 2 wk of 5-FU (25 mg/kg) with leucovorin (25 mg/kg/day) | Reduced number of proliferating cells in the SGZ of the DG | Impaired emotional (CFC) and cognitive memory (OLR) | Fluoxetine, administered in the drinking water (10 mg/kg/day) over 3 wk | Animals cotreated with fluoxetine had improved performance in the OLR and restored number of proliferating cells | Elbeltagy et al. 201059 |
| In vivo: 48-wk-old female C57BL/6J mice | Irradiation (WBI) | Single dose of 5 Gy | Decreased number of newborn BrdU+/NeuN+ neurons in the DG, decreased hippocampal expression of BDNF and VEGF, and increased expression of IGF-I | Deficits in spatial memory retention (Barnes maze) | Daily voluntary running for 6 wk, starting 1 month after WBI | Daily running prevented decline in spatial memory retention, partially restored AHN, and increased levels of VEGF and IGF-1 | Wong-Goodrich et al. 2010116 |
| In vivo: 5-wk-old male C57BL/6J mice | thioTEPA | 3 daily i.p. injections (10 mg/kg) | Significant immediate and long-term reduction of cell proliferation and survival in the DG | Memory deficits (NOR); no differences in depressive behavior (forced swimming and tail suspension tests) | Not assessed | Not assessed | Mondie et al. 2010110 |
| In vivo: 125–150 g male Lister hooded rats | MTX | 2 i.v. injections (75 mg/kg), a wk apart | Reduced cell proliferation and survival in the SGZ of the DG | Cognitive deficits (OLR) | Fluoxetine (10 mg/kg/day in drinking water for 40 days) | Fluoxetine restored cognitive ability and cell proliferation and survival rates in the SGZ of MTX + fluoxetine rats | Lyons et al. 2011132 |
| In vivo: adult male Fischer 344 rats | WBI | Bilateral irradiation in 2 consecutive half-dose fractions (total doses of 10 Gy and 15 Gy | Reduced progenitor proliferation and neurogenesis within the SGZ following 10 Gy- and 15 Gy-WBI and increased CD68+ activated microglia | Not assessed | RAM administered 24 hours post-WBI and maintained for 12 wk | RAM significantly reduced the effects of radiation on progenitor | Jenrow et al. 2010157 |
| proliferation and neuronal differentiation in the DG following 10 Gy-WBI | |||||||
| In vivo: adult male Fischer 344 rats | WBI | Bilateral irradiation in 2 consecutive half-dose fractions (total doses of 10 Gy and15 Gy | Reduced progenitor proliferation and neurogenesis within the DG following 10 Gy- or 15 Gy-WBI and increased CD68+ activated microglia | Not assessed | AVS administered 24 h post-WBI at doses of 10 and 15 Gy, and daily until 12 wk post-WBI. | Only AVS + RAM mitigated the decrease in neurogenesis following 10 Gy-WBI | Jenrow et al. 2011158 |
| AVS + RAM (same protocol, but following only WBI doses of 10 Gy) | |||||||
| In vivo: 20–25 g male Swiss-Webster mice | Tamoxifen 5-FU MTX 5-FU + MTX | Chronic injection procedure: single i.p injection once a wk for 3 wk: tamoxifen (32 mg/kg); 5-FU (75 mg/kg); MTX (3.2 or 32 mg/kg); 5-FU (75 mg/kg) + MTX (3.2 or 32 mg/kg) | Not assessed | Impaired memory acquisition and retention (autoshaping learning procedure) in chronic treatment with tamoxifen; impaired memory retention in chronic treatment with MTX + 5-FU, which depended on the MTX dose | Not assessed | Not assessed | Walker et al. 201161 |
| Acute injection procedure: single i.p. injection of tamoxifen (32 mg/kg) | |||||||
| In vivo: 3-mo-old, female BALB/C mice | MTX + 5-FU | Single i.p. injection of MTX (50 mg/kg) + 5-FU (75 mg/kg), once a wk for 4 wk | Not assessed | Impaired spatial (MWM) and nonspatial learning and memory (NMTS and DNMTS) | Donepezil (3 mg/kg) | Daily administration of donepezil in conjunction with the chemotherapy drugs prevented deficits in spatial learning and in some measures of the DNMTS | Winocur et al. 2011134 |
| In vivo: 150–200 g male Lister-hooded rats | CP | 7 i.v. doses (30 mg/kg) | Decreased survival of hippocampal cells | No differences in spatial working memory (OLR) | Not assessed | Not assessed | Lyons et al. 201164 |
| In vivo: 4-mo-old female Wistar rats | CMF (CP + MTX + 5-FU) | Single i.p. injection (CP: 40 mg/kg; MTX: 37.5 mg/kg; 5-FU: (75 mg/kg) once a wk for 4 wk | Decreased cell proliferation in the DG; increased acetylation of histone H3 and decreased histone deacetylase activity in the hippocampus | Transient impairment in spatial learning, with persistent disruption in spatial memory (MWM) | Not assessed | Not assessed | Briones & Woods 201169 |
| In vivo: 2-mo-old male athymic nude rats (strain 02N01 Cr:NIH-rnu) | CP | Single i.p. injection (CP: 50 mg/kg; DOX: 2 mg/kg) once a wk for 4 consecutive wk | Decreased AHN (BrdU+/NeuN+); increased activated microglia (ED1-positive) in the hippocampus after CP treatment | Impaired spatial recognition (NPR) and emotional memory (CFC) | Not assessed | Not assessed | Christie et al. 201275 |
| DOX | |||||||
| In vivo: 265–369 g male Wistar rats | OX | Single i.p. injection of OX (12 mg/kg; 8 mg/kg); 5-FU (75 mg/kg), or a combination of 5-FU and OX in 2 i.p. injections | Not assessed | Impaired memory (NOR) in treatment with 5-FU and OX alone; impaired emotional (CFC) and spatial memory (MWM) in combined treatment | Wheel running | Impairments caused by 5FU + OX treatment were prevented by 4 wk of wheel running overnight after drug administration | Fardell et al. 2012114 |
| 5-FU | |||||||
| 5FU + OX | |||||||
| In vivo: 60–75-day-old male Sprague–Dawley rats | TMZ | Single i.p. injection (25 mg/kg) once a day for 3 consecutive days per wk, for 4 wk | Decreased number of BrdU+ cells in the DG 1 wk after BrdU injection | Impaired aversive learning (trace eyeblink response) | Not assessed | Not assessed | Nokia et al. 201274 |
Abbreviations: AVS, atorvastatin; BCNU, carmustine; BDNF, brain-derived neurotrophic factor; BrdU, bromodeoxyuridine; CFC, contextual fear conditioning; DCX, doublecortin; DNMTS, delayed nonmatching-to-sample; DOX, doxorubicin; GRP, glial-restricted precursor cells; IGF, insulin-like growth factor; MWM, Morris water maze; NOR, novel object recognition task; NPR, novel place recognition test; OFT, open-field test; OLR, object location recognition test; O-2A/OPCs, oligodendrocyte type 2 astrocyte progenitor cells; OX, oxaliplatin; PAT, passive avoidance test; RAM, ramipril; SVZ, subventricular zone; WBI, whole-brain irradiation.
In several mouse studies, it has been demonstrated that cyclophosphamide (CP), an alkylating agent that inhibits DNA synthesis, decreases the capacity for memory retention,62,63 although it has also been suggested to be less neurotoxic than other chemotherapy drugs.64 Antimetabolites such as methotrexate (MTX) and 5-FU are chemotherapeutic agents used to impair the biosynthesis of nucleic acids and hence arrest movement of cells through the cell cycle. Rats receiving MTX, 5-FU, or a treatment combining the 2 display cognitive dysfunction, performing poorly in spatial learning tasks65,66 and displaying memory consolidation impairment.67,68 Combined treatment of CP, MTX, and 5-FU has also been shown to significantly impair hippocampus-dependent memory, in association with decreased cell proliferation in the DG and altered histone remodeling in the hippocampus.69
Radiation therapy for malignancies of the CNS has likewise been associated with the induction of “progressive deficits in learning and memory.”53 A wide range of cognitive deficits have been reported in studies examining the effect of radiotherapy on neuropsychological cognition, and it is perhaps appropriate to attribute the wide range of deficits observed to differences in the nature and extent of the core pathology, the brain volume where radiotherapy was applied, or the means by which the cognitive deficit was measured.18 In patient groups, early-delayed reactions to radiotherapy, usually occurring from the first to the sixth month of treatment, often manifest as deficits in memory and word retrieval, in addition to slowed information processing speed.70 Late-delayed side effects such as a decrease in quantitative skills and further memory deficits may present months or years after the initiation or even the cessation of treatment and are often progressive in form. The dose of radiation administered to the medial temporal lobe—which contains the hippocampus—is also, according to one study, directly proportional to the extent of subsequent cognitive dysfunction.71 Interestingly, no correlation has been observed between mild to moderate cognitive impairment and subsequent radiological findings, with affected patients often displaying normal findings on neuroimaging scans.72 In certain cases, radiation may induce vascular and demyelinative changes, but memory deficits have nevertheless been observed in numerous subjects who do not display any evidence of such pathological development.52 These findings perhaps suggest that the pathological development of cognitive decline induced by radiotherapy may not be amenable to visualization through neuroimaging techniques.
In spite of a large number of studies documenting the existence and presentation of neurotoxicity following radiotherapy, it is generally recognized that only little is known about the precise cellular and metabolic processes underpinning the development of the neurotoxic state. Thus, 2 separate hypotheses have been advanced as to how this cancer treatment intervention leads to neurotoxicity. A “vascular hypothesis” suggests that vascular injury induced by the radiation leads to vascular insufficiency and infarction, which in turn contribute to the neurotoxic environment. An alternative “glial hypothesis” posits that an arrest of gliogenesis caused by the treatment ultimately leads to a demyelinative necrosis.52 However, experimental evidence suggesting that gliogenesis is relatively well preserved following radiation therapy would tend to repudiate the glial hypothesis,43 while neither hypothesis can account for the conspicuous lack of gross pathology evident on the neuroimaging of patients displaying cognitive deficits posttreatment. The effect of cancer treatment—both chemo- and radiotherapy—is of particular note when considering how neurogenesis and self-renewing lineage-committed neural progenitor cells are altered as a result of the development of neurotoxicity.18
Radiation administration is associated with an extensive microglial inflammatory response in the regions of the hippocampus associated with neurogenesis.43 This leads to an inappropriate increase in the amount of proinflammatory interleukin (IL)-6, which acts to inhibit neurogenesis.52 Of special interest for translational neuro-oncology, it has been recently demonstrated that administration of MW-151, a selective inhibitor of proinflammatory cytokine production, in adult rats was able to mitigate the impaired neurogenesis and cognitive decline induced by whole brain irradiation.73
In addition to the chemotherapy studies described previously, it has been shown that chronic treatment with the antimitotic temozolomide (TMZ) is associated with poor performance in the acquisition of hippocampus-dependent trace eyeblink conditioning in rats, a specific task that requires the association of events across a temporal gap.74 No differences were observed when the association was to be accomplished with temporally overlapping stimuli. This suggests that the effects of antimitotic drugs, like TMZ, might be highly specific to certain types of memory, and interestingly, that the types of memory affected are likely to relate to those encoded by newly generated neurons. Indeed, this same study identified a TMZ-induced reduction in early survival of young cells in the DG, without disruption of memories formed prior to treatment, which are independent of newly born neurons. This is in accordance with other findings in rats showing impaired cognitive and emotional memory following treatment with CP and doxorubicin, where an actual decrease in AHN (shown by the number of bromodeoxyuridine-positive cells colocalizing with the mature neuron marker NeuN) was also observed.75 In addition, it was verified that TMZ leads to disrupted theta oscillations in the hippocampus.74 Synchronized activity within the theta range (3–12 Hz) is believed not only to predict learning rates76 but also to be one of the means by which immature neurons differentially regulate temporal aspects of hippocampal encoding.77 Thus, a possible mechanistic explanation for part of the effects of some chemotherapy drugs on learning and memory could be that as a direct consequence of the decrease in cell proliferation and neurogenesis in the DG, a reduction in overall theta activity of immature neurons disrupts the temporal information they are due to encode for appropriate stimuli association and, therefore, for optimal cognition.
Cancer Treatments and Depression
Growing evidence indicates an important relationship between depression and survival among cancer patients. For instance, it has been shown that high levels of cortisol and depressive symptoms link to increased expression of proinflammatory and prometastatic genes in circulating leukocytes of renal cell carcinoma patients, suggesting that the dysregulation of cortisol and inflammatory markers of depression is an important predictor of survival among these patients.78
Depression is believed to have its origin in a dysfunctionality of multiple biological factors. Here, we highlight 2 of the mechanisms that emerge as candidates potentially underlying depressive symptoms and lower recovery among cancer patients undergoing classic treatments, such as chemo- and radiotherapy. These mechanisms are the decrease or transient ablation of hippocampal neurogenesis and the onset of inflammatory responses in the brain.
Due to its plastic nature, the hippocampus is especially vulnerable to changes in brain homeostasis. This is clearly evident in the case of chronic stress. Stressful events are known to cause the adrenal glands to release glucocorticoid hormones, such as cortisol, whose receptors (glucocorticoid receptor [GR] and mineralocorticoid receptor) are abundantly expressed in the hippocampus.79 Adrenal hormones play an important role in the regulation of the stress response,80 but chronic glucocorticoid signaling has been associated with decreased levels of trophic factors, cell death, dendritic retraction, and hippocampal neurogenesis impairment.81 Interestingly, it has been proposed that the newly generated neurons in the mouse DG have a role in buffering the stress response, via modulation of the hypothalamic-pituitary-adrenal axis.40 Although a causal relationship between cell genesis and the onset of or vulnerability to depression still remains a subject of constant debate,82 some studies have succeeded in showing a link between the ablation of neurogenesis and the emergence of a depressive behavioral profile.83,84 A recent study also showed that rats repeatedly treated with corticosterone had decreased number of surviving immature neurons in the DG, a feature accompanied by increased depressive-like behaviors.85 A causal link between AHN and depression must still be addressed with caution, though, and one must consider that it may exist within the context of certain and not all kinds of stress conditions.21 Another factor that seems to play a role for the establishment of decreased AHN as a mechanism underlying depression is the time course of analysis after exposure of the individual to the cytogenesis suppression.86 While animal studies performing behavioral analysis immediately after the ablation of hippocampal cell genesis failed to identify depressive-like behaviors,87 those where cytogenesis suppression was prolonged88 or in which behavior was analyzed 4 weeks after the cessation of cell genesis suppression40 reported a late manifestation of mood deficits. A plausible way to interpret this recent data is that sustained mood may require the appropriate integration of new neurons in the DG, a process that starts with appropriate rates of cell proliferation.
If on the one hand a causal link between AHN and mood is not yet of consensus, there seems to be less controversy when it comes to considering normal levels of AHN as a prerequisite for remission from depression.39,89 Indeed, a reduction in depressive behavior and improvement of cognitive function with increased hippocampal neurogenesis has been observed following chronic antidepressant treatment.90 Suppression of AHN, in turn, was shown to reduce the efficacy of antidepressants in restoring depressive and anxious behavior in a number of animal studies.91,92 Among the possible arguments to explain this phenomenon is the proposition that the newly generated neurons resulting from stimulation by antidepressants could play a role in maintaining the inhibitory connections that target areas of the hypothalamic-pituitary-adrenal axis.93 In addition, they could integrate into memory circuits that regulate appropriate responses to contextual changes, exerting a protective effect against chronic stressors.94
With regard to the effects of cancer therapies on depression, it is not clear yet whether a decreased rate of AHN caused by chemo- and radiotherapy would lead to depressive behaviors, or whether depression would come as a consequence of a vulnerable phenotype in principle independently of AHN, which being decreased after radio- or chemotherapy would contribute to depressive symptoms and/or hamper remission. Therefore, behavioral studies using rodent models to assess the particularities of mood-related decline after chemo- and radiotherapy treatments, as well as the use of cellular and molecular approaches in in vitro models of depression in the hippocampus,95 should add vital information on how cancer treatments, depression, and AHN are linked. As a consequence, specific neuroprotective strategies could be more effectively designed.
Although not yet established as clearly causal, a link between inflammation and depression has been increasingly suggested. A number of studies have proposed a potential role of the immune system in the development of major depression.96,97 Increased concentration of a number of inflammatory markers, such as C reactive protein and the proinflammatory cytokines IL-6 and tumor necrosis factor α, has been consistently found in serum and cerebrospinal fluid of depressed patients.98,99 In addition, patients receiving immunotherapy with cytokines are at increased risk for depression,100 and immunotherapy has been increasingly used as a form of oncological treatment.101 The exact mechanisms by which an increase in the level of proinflammatory agents (as a consequence of different stressors, such as medical illness, medication, and psychological aspects) participate in the onset or progression of depressive symptoms are still unknown, but some possible and well-grounded explanations have arisen.102 Cytokines are cell signaling molecules, and it is not surprising that they therefore exert their effects in a wide range of pathways and mechanisms. These appear to include a disruption in serotonergic transmission,103 by which classic antidepressants exert their effects; increased activation of the glutamatergic receptor N-methyl-d-aspartate, resulting in increased excitoxicity and calcium-mediated cell death104; decreased neurogenesis52; and increased glucocorticoid resistance.105 In turn, a reduction in GR function has been suggested to trigger hyperactivity of the hypothalamic-pituitary-adrenal axis40 as well as inflammatory responses.97 Dysfunctionality of the GR could then be pointed to as a potential substrate through which stress contributes to depression, in that decreased GR activity leads to a vicious cycle of increased release of cortisol and inflammation, both mechanisms contributing to a decrease in neurogenesis and to the development of depression. In addition, increased serum levels of IL-6 and tumor necrosis factor α were associated with decreased hippocampal volume among breast cancer survivors.106 Although the latter study found that the concentrations of cytokines and altered volume of the hippocampus were particularly associated with verbal memory, previous studies have proposed a possible relationship between reduced hippocampal volume and depression.89,107,108 Whether decreased hippocampal volume could also be part of the events underlying depressive symptoms among cancer patients, as either a risk factor or a consequence of depression, remains to be further investigated.
Considering this body of evidence, it is reasonable to suspect that cancer patients are at high vulnerability to depression, given the cytogenesis suppression and proinflammatory nature of current cancer treatments, as well as the high levels of psychosocial stress in which this population is immersed. Altogether, these factors could maintain the vicious cycle of higher levels of depression mediated by increased inflammatory responses and decreased neurogenesis/response to antidepressants, bringing serious consequences to quality of life and, ultimately, to survival among cancer patients.
Potential Strategies to Protect/Improve AHN and Reduce the Deleterious Consequences of Cancer Treatments on Mood and Cognition
Whether depression among cancer patients as primarily caused by a treatment-induced decrease in AHN is a topic yet to be fully unraveled, it seems clear that depression and cancer are somehow related in humans. It is known, for instance, that depressive breast cancer patients are less likely to adhere to treatment plans, particularly to postradiotherapy medications,109 which could become an additional obstacle to recovery. It seems, then, essential that effective strategies to tackle depression within this patient population be developed as part of the cancer treatment itself, an endeavor that could benefit from the growing evidence relating AHN induction and mood improvement. Some chemotherapeutic agents, however, were shown to exert detrimental effects on cognition without changes on depressive behavior in mice,110 suggesting that—if such effect is also true for humans—specific interventional strategies designed in accordance with the drug used could maximize results in different patients.
Given that the development of novel treatments and strategies will be necessary to prevent the progression of mood- and cognition-based disorders resulting from the various treatments for cancer, it is here proposed that interventions known to increase AHN could be seen as important factors in coadjuvant cancer treatment (Fig. 2). A number of diverse environmental factors have been shown to have effects on hippocampal neurogenesis. Unpredictable chronic stress, for instance, has been associated with the production of decreased AHN and the amplification/development of depressive-like behavior.111 Although this evidence is based on animal models, it is likely that these kinds of stress paradigms could also play a deleterious role in mental health among cancer patients.
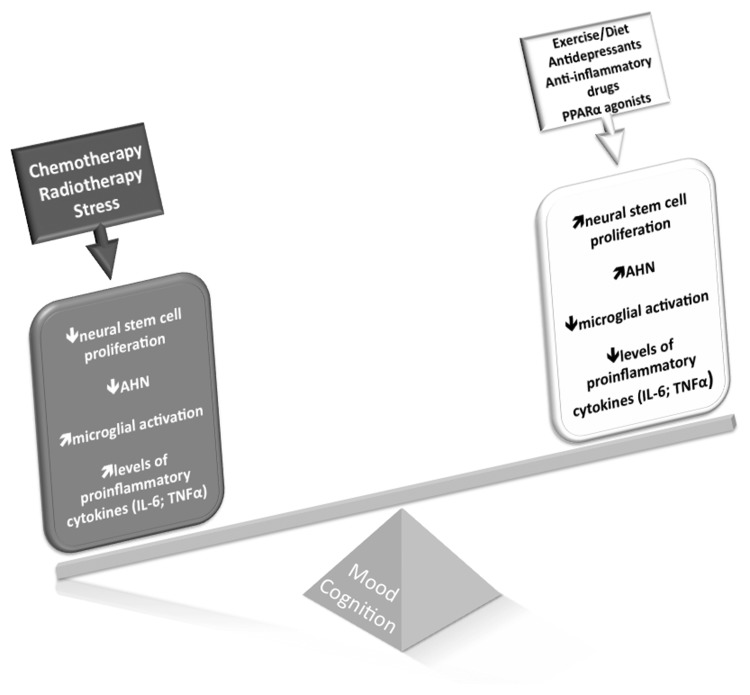
Fig. 2.
Consequences of cancer treatments to mental health. Cancer treatments, such as chemotherapy and radiotherapy, aim at ablating the proliferative activity of tumorous cells. However, given their unspecific action, these treatments also arrest proliferation of desirable cell populations, such as those maintaining the process of neurogenesis in the postnatal/adult brain. Adult hippocampal neurogenesis is thought to underlie an important portion of neural plasticity throughout life, with newly born neurons integrating memory and mood circuits. AHN can be downregulated directly by the antimitotic action of cancer treatments, or indirectly by the pro-inflammatory responses triggered by these agents. Chronic stress is significantly present in the routine of cancer patients and is another important contributor to lower levels of AHN. Decreased AHN, in turn, has been consistently linked with cognitive decline and depression. On the other hand, environmental factors like exercise, diet, and use of antidepressants and anti-inflammatory drugs, as well as peroxisomal proliferator-activated receptor alpha agonists, are known to increase AHN and restore cognitive abilities and mood states. They could, therefore, positively contribute to mental health among cancer patients. Other strategies, such as hippocampal sparing, cognitive stimulation, and treatment with memantine, stimulants, and ramipril, have been shown to exert positive effects on mental health in the context of cancer treatments, a phenomenon that probably occurs via protection or increase of hippocampal neurogenesis.
Voluntary running, in turn, has been shown to have a tangible beneficial link with hippocampal neurogenesis. Studies have again illustrated that running in rodent groups is associated with an increase in the extent of neurogenesis in the hippocampus and that this is associated with an increased capacity for hippocampus-dependent learning while also partially rescuing memory impairment resulting from a genetically low basal rate of neurogenesis.112,113 It has furthermore been demonstrated that the process of running also helps to prevent cognitive decline, even following combination chemotherapy with 5-FU and oxaliplatin.114 The positive effects of voluntary running were seen in a range of exploratory behaviors of adult mice whose brains had been irradiated in early postnatal development.115 Interestingly, the behavior amelioration observed was followed by a recovery in both precursor cell and neurogenesis levels in the hippocampus. Similar results were obtained from rats exposed to daily voluntary running a month after irradiation, where an increase in the hippocampal expression of brain-derived vascular endothelial growth factor was observed, in addition to the restoration of both AHN and cognitive function.116 It is believed, therefore, that exercise might be a potential adjuvant in tackling the deleterious effects of radiotherapy on mental health, for it contributes to the development of a proneuroplastic milieu, enhancing both trophic factors and cell proliferation levels in the brain.117
The importance of diet in the maintenance and facilitation of hippocampal neurogenesis and appropriate cognitive function has also been investigated. It has been empirically observed that a calorific reduction of 30%–40% results in an increase in hippocampal neurogenesis, while extending the time between meals also acts to improve the extent to which neurogenesis occurs.118 Certain dietary components (such as flavonoids) have been shown to improve both depressive symptoms119 and spatial working memory in rodents.120–122 Of special interest in the context of diet and cancer therapies, there is evidence that the activation of the peroxisomal proliferator-activated receptor alpha—a nuclear receptor that regulates gene transcription upon activation by, for instance, long chain fatty acids and eicosanoids123—can inhibit the microglial inflammatory responses induced by radiation,124,125 without protecting tumor cells.126
Cognitive stimulation can be pointed to as an important factor favoring cognitive amelioration among cancer-treated patients. A recent randomized controlled trial using a 12-week computerized cognitive training program with long-term breast cancer survivors successfully showed significant improvements in aspects such as cognitive flexibility, processing speed, and verbal fluency.127 Breast cancer survivors benefited from a group rehabilitation program, showing significant improvement in neurocognitive tests.128 Patients who received treatment (chemotherapy, radiotherapy, or surgery) for other types of cancer, including bladder, prostate, colon, and uterine, presented amelioration on perceived cognition abilities and quality of life after a group-based cognitive rehabilitation intervention.129 Hypothetically, it could be argued that in some cases depressive symptoms could arise or worsen if not only via decreased hippocampal neurogenesis as a psychological consequence of cognitive disruption. In this sense, cognitive stimulation through cognitive rehabilitation programs could, indirectly, help prevent and/or reduce mood-related symptomatology.
Pharmacological treatment can provide some effective protection to neuronal cells and, consequently, exert positive effects on behavior. Administration of anti-inflammatory agents helps to restore the relationship between the NSC population and the microvasculature, hence facilitating the neurogenetic process. However, the full extent of normal neurogenesis is not realized with anti-inflammatory therapy alone,130 and it is evident that additional therapies will have to be developed to achieve full restoration of the neurogenetic process. In the context of antidepressants, fluoxetine reversed both the reduction in cell proliferation in the DG and the cognitive deficit observed in mice treated with only 5-FU,131 as well as survival in rats treated with MTX.132 Not all antidepressants should be seen as potential coadjuvants for cancer patients, though. Some antidepressants can interact with antineoplastic agents, resulting in less efficacy of the drug or increased side effects.133 Drugs such as escitalopram, citalopram, venlafaxine, mirtazapine, and milnacipran have been pointed to as presenting a safer profile for use among cancer patients,133 yet specific studies investigating their potential role in improving mood in the context of chemo- and radiotherapy are still lacking. Other types of drugs may exert positive effects to buffer the deleterious consequences of antiproliferative treatments in the context of mental health. In this sense, administration of cognitive enhancers such as the cholinesterase inhibitor donepezil in conjunction with chemotherapy drugs 5-FU and MTX was shown to prevent some of the cognitive impairments observed in rats treated with the chemotherapeutics alone134 (other cognitive enhancers have been reviewed elsewhere135). On the other hand, the psychostimulant modafinil did not exceed placebo effects on cognition among primary brain tumor patients,136 suggesting that other categories of drugs could be more effective for this population. Interestingly, modafinil was found to suppress hippocampal cell proliferation in rodents.137
Other stimulants, such as the amphetamine-like agent methylphenidate, improved cognition in patients with advanced cancer and hypoactive delirium,138 although no effects were found in 2 placebo-controlled trials where methylphenidate was administered to patients during chemotherapy.139,140 At least 3 hypotheses can be drawn to explain the controversy over the use of this psychostimulant drug among cancer patients: (i) its effects could be linked with the delirium, and no result should be expected in cancer patients without this condition; (ii) its effects could be linked with the time course and severity of cancer, since the study that showed cognitive improvement after treatment with methylphenidate was conducted with advanced cancer patients; (iii) the effects of methylphenidate could be observed when administered long after completion of cancer treatment, in this case, chemotherapy. Indeed, some interventions are designed to take place only after chemo- or radiotherapy or surgery, as they require some neural stabilization and recovery in order to exert beneficial effects toward cognitive enhancement.127–129 On the other hand, a retrospective analysis of the effects of methylphenidate showed that depression and fatigue in advanced cancer patients improved after treatment with this stimulant,141 and a small pilot randomized trial showed that patients with primary brain tumor presented improved executive functions after a 4-week treatment with methylphenidate or modafinil.142 The potential of methylphenidate as a cognition and mood enhancer may go beyond its classic mechanism of action as a dopamine and noradrenaline reuptake inhibitor; interestingly, this drug was shown to enhance cell proliferation and neuroblast differentiation in the SGZ of the DG of adolescent rats, likely through an increase in levels of brain-derived neurotrophic factor.143 Future studies unraveling the specific effects and side effects of these different modalities of drugs on the various populations of cancer patients are thus highly desirable in that they can be translated into clinical practice as potent tools toward profound consequences in overall quality of life of adult cancer survivors, and in the psychosocial and educational development of pediatric patients. Still in the pharmacological context, it goes without saying that the search for chemotherapy agents that are selectively more effective in reducing tumor cell outgrowth while sparing neuronal progenitor populations is a highly desirable endeavor. Aligned with that, recent findings show that 2 newer drugs—the proteasome inhibitor bortezomib and the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib—were effective against glioma stemlike cells and delivered less toxic effects to NSCs, at least in comparison with the conventional chemotherapeutics TMZ and cisplatin.144
In the context of cognitive dysfunction among patients receiving whole-brain radiotherapy (WBRT), an important randomized double-blind placebo trial with memantine by the Radiation Therapy Oncology Group (the RTOG 0614 study) showed that this drug is effective in preserving cognition of patients with brain metastasis under WBRT.145 Memantine is a noncompetitive antagonist of the glutamatergic N-methyl-d-aspartate receptor, which leads to excitotoxicity when overstimulated by, for instance, proinflammatory cytokines.104 In the trial, patients received a total of 37.5 Gy WBRT (15 fractions of 2.5 Gy); then, no later than 3 days after initiating WBRT, a 24-week oral treatment with either memantine or placebo started. The results were promising, with memantine improving cognition, delaying cognitive decline, and reducing the rate of decline in several cognitive functions, such as memory and processing speed. It is also noteworthy that the toxicity and tolerance of memantine were equivalent to those of the placebo, making its prescription to be highly recommended for WBRT patients. Interestingly, cotreatment with memantine in rats receiving MTX chemotherapy was neuroprotective, decreasing the spatial memory deficits induced by the latter.146 This finding could help expand the use of memantine to chemotherapy patients, a hypothesis that needs to be addressed by human studies.
In order to prevent WBRT-related cognitive decline, modern radiotherapy appliances and protocols are designed in such a way as to contour the hippocampus and use intensity-modulated radiotherapy (IMRT) to direct the radiation beam, avoiding the contoured area, a process known as hippocampal sparing. Indeed, increasing radiotherapy doses to the hippocampus and temporal lobes have been found to be associated with neurocognitive decline following cranial irradiation in children.147 Using modern IMRT techniques, an important study conducted with 5 patients with brain metastases showed that it is possible to achieve acceptable target coverage and homogeneity and still reduce the mean dose per fraction of radiation to the hippocampus by 87% using helical tomotherapy, and by 81% to 0.73 Gy2 using linear accelerator–based IMRT.148 The potentially neuroprotective effects of this intervention in the context of cognition are now under analysis by the phase II clinical trial (RTOG 0933). The sparing of NSC niches during irradiation of intracranial tumors has also been found to be feasible without compromising the quality of coverage in a retrospective planning study.149 These findings are encouraging, especially considering that sparing the hippocampus can be considered a safe procedure,150 as the hippocampal formation is rarely involved with metastases.151 In addition, stem cell therapies point to effective prevention of the radiation-induced decrease in neurogenesis, but considerable additional research is still needed for such an approach to be considered for translation into clinical practice.
Another aspect that deserves special consideration is the potential role of renin-angiotensin inhibitors. Although primarily acknowledged to play a systemic role in regulating blood pressure, local renin-angiotensin systems have been identified.152 In the brain, components of these systems, such as angiotensin (Ang) I, II, and III, are believed to participate as neuromodulators in brain regions that regulate homeostatic functions153; in addition, evidence points to a role in neurogenesis, with Ang II increasing NSC proliferation, possibly via Ang II type 2 receptor.154 However, in the context of tissue damage, Ang II has been associated with proinflammatory and pro-apoptotic processes that can lead to long-term detrimental effects.155 Blockade of Ang II type 1 receptor has been proposed to ameliorate brain injury conditions, including brain inflammation.156 Interestingly, in rats given 10 Gy WBRT, the angiotensin converting enzyme inhibitor ramipril could reduce the deleterious effects of radiation on progenitor cell proliferation and neuronal differentiation in the DG, possibly through a decrease in apoptosis and inflammation.157 Ramipril in combination with atorvastatin has been shown to mitigate the radiation-induced reduction of hippocampal neurogenesis158 and to prevent alone the perirhinal cortex-dependent cognitive impairment observed following WBRT.159
Also of potential clinical value, important evidence has recently emerged for a role of neuroprotective agents such as glial cell–derived neurotrophic factor or 4-methylcatechol to prevent the loss of catecholaminergic nerves in the bone marrow of mice given cisplatin chemotherapy.160 While providing obvious beneficial effects to cancer recovery itself, these neuroprotectors could also have a positive effect on AHN and mental health, a hypothesis that remains to be addressed. Further studies investigating the effects of specific neuroprotective agents in the contexts of cancer therapies, AHN, cognition, and mood may constitute, therefore, a promising field for future clinical application in the scope of cancer and mental health.
Conclusion
It is evident that current therapies for cancer, while effective in curtailing cancer cell proliferation, also cause deleterious cell loss and dysfunction in normal cell populations. This currently unsatisfactory treatment paradigm contributes significantly to the development of serious cognitive deficits in patients receiving these forms of treatment. If the central postulate of this review has a significant degree of logical veracity (ie, that cancer treatments—both chemo- and radiotherapy—contribute meaningfully to these cognitive deficits through inhibition of AHN), treatment protocols can be developed that avoid or at least reduce the deleterious aspects of current treatments of cancer. Identifying the precise mechanisms by which each type of treatment has consequences to AHN and to the microenvironment surrounding neural cells holds great promise for the establishment of strategies to protect the CNS from long-lasting injury. On that, it has been found that interventions as diverse as anti-inflammatory and antidepressant medication, voluntary running, and diet alteration were all able to exert beneficial effects in animal models. That opens a promising avenue of possible interventional studies to be conducted in humans, with the ultimate goal to reduce chemo- and radiotherapy-induced cognitive and mood decline among cancer patients. As the number of cancer survivors increases due to advances in treatment, more extensive research is required to categorically determine the benefit of particular interventions. It is noteworthy, though, that such endeavor will achieve translational significance only if studies are conducted with large samples and appropriate control conditions, essential components not always present in clinical trials.
As a whole, the present understanding garnered from investigations conducted thus far points the way to a more complete understanding of the process of AHN and in turn how it affects other cellular and behavioral processes. Advances in this understanding will evidently be of benefit to the patient populations that are currently in receipt of the treatments, as well as those who will undergo these interventions at junctures in the future.
Funding
Funding for this work was provided by the Research Council UK; the Medical Research Council; the Psychiatry Research Trust; the Brazilian Council for Scientific and Technological Development (CNPq, Science without Borders Program [244420/2012-2; 246873/2012-4]); FAPERJ/PRONEX [E-26/110.058/2011; E-26/102.753/2012; E-26/110.098/2013); and INCT/CNPq/National Institute of Translational Medicine.
Conflict of interest statement. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript, apart from those disclosed.
References
- 1.Kempermann G, Jessberger S, Steiner B, Kronenberg G., et al. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Squire L. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Squire L, Ojemann JG, Miezin FM, et al. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci U S A. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squire L. The hippocampus and spatial memory. Trends Neurosci. 1993;16:56–57. doi: 10.1016/0166-2236(93)90016-f. [DOI] [PubMed] [Google Scholar]
- 5.Suthana N, Ekstrom A, Moshirvaziri S, et al. Dissociations within human hippocampal subregions during encoding and retrieval of spatial information. Hippocampus. 2011;21:694–701. doi: 10.1002/hipo.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard G, Titiz A, Tyler A, et al. Speed modulation of hippocampal theta frequency correlates with spatial memory performance. Hippocampus. 2013;23:1269–1279. doi: 10.1002/hipo.22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsien J, Li M, Osan R, et al. On initial brain activity mapping of associative memory code in the hippocampus. Neurobiol Learn Mem. 2013;105:200–210. doi: 10.1016/j.nlm.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 9.Sweatt J. Hippocampal function in cognition. Psychopharmacology. 2004;174:99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- 10.Rolls E. A computational theory of episodic memory formation in the hippocampus. Behav Brain Res. 2010;215:180–196. doi: 10.1016/j.bbr.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisch A, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez-David I, Hen R, Gardier AM, et al. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71:143–149. doi: 10.1016/j.pharma.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson P, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 15.Gould E, Gross CG. Neurogenesis in adult mammals: some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spalding K, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich J, Monje M, Wefe J, et al. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–1295. doi: 10.1634/theoncologist.2008-0130. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez J, Scully RE, Miller TL, et al. Long-term effects of treatments for childhood cancers. Curr Opin Pediatr. 2007;19:23–31. doi: 10.1097/MOP.0b013e328013c89e. [DOI] [PubMed] [Google Scholar]
- 20.Ekstrom A, Kahana MJ, Caplan JB, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 21.Hanson N, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacol. 2011;36:2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. The Hippocampus Book. New York: Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- 23.Fanselow M, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheirbek M, Hen R. Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood. Neuropsychopharmacol. 2011;36:373–374. doi: 10.1038/npp.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanti A, Rainer Q, Minier F, et al. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology. 2012;63:374–384. doi: 10.1016/j.neuropharm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Tampellini D, Capetillo-Zarate E, Dumont M, et al. Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer's disease transgenic mice. J Neurosci. 2010;30:14299–14304. doi: 10.1523/JNEUROSCI.3383-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Encinas J, Hamani C, Lozano AM, et al. Neurogenic hippocampal targets of deep brain stimulation. J Comp Neurol. 2011;519:6–20. doi: 10.1002/cne.22503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu Y, Lee SW, Gage FH. Signalling in adult neurogenesis. Curr Opin Neurobiol. 2010;20:416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gage F. Molecular and cellular mechanisms contributing to the regulation, proliferation and differentiation of neural stem cells in the adult dentate gyrus. Keio J Med. 2010;59:79–83. doi: 10.2302/kjm.59.79. [DOI] [PubMed] [Google Scholar]
- 30.Lie D, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 31.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 32.Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci U S A. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehl M, Abrous DN. A new chapter in the field of memory: adult hippocampal neurogenesis. Eur J Neurosci. 2011;33:1101–1114. doi: 10.1111/j.1460-9568.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- 34.Burghardt N, Park EH, Hen R, et al. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garthe A, Kempermann G. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci. 2013;7:63. doi: 10.3389/fnins.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kheirbek M, Klemenhagen KC, Sahay A, et al. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Revest J, Dupret D, Koehl M, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 38.Nishijima T, Llorens-Martín M, Tejeda GS, et al. Cessation of voluntary wheel running increases anxiety-like behavior and impairs adult hippocampal neurogenesis in mice. Behav Brain Res. 2013;15:34–41. doi: 10.1016/j.bbr.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 40.Snyder J, Soumier A, Brewer M, et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peissner W, Kocher M, Treuer H, et al. Ionizing radiation-induced apoptosis of proliferating stem cells in the dentate gyrus of the adult rat hippocampus. Brain Res Mol Brain Res. 1999;71:61–68. doi: 10.1016/s0169-328x(99)00170-9. [DOI] [PubMed] [Google Scholar]
- 42.Tada E, Parent JM, Lowenstein DH, et al. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 43.Monje M, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 44.Palmer T, Wilhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Boldrini M, Hen R, Underwood MD, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernhard E, Maity A, Muschel RJ, et al. Effects of ionizing radiation on cell cycle progression. A review. Radiat Environ Biophys. 1995;34:79–83. doi: 10.1007/BF01275210. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra V, Perry MC. Classical chemotherapy: mechanisms, toxicities and the therapeutic window. Cancer Biol Ther. 2003;2:S2–S4. [PubMed] [Google Scholar]
- 48.Woods D, Turchi JJ. Chemotherapy induced DNA damage response: convergence of drugs and pathways. Cancer Biol Ther. 2013;14:379–389. doi: 10.4161/cbt.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietrich J, Han R, Yang Y, et al. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villeda S, Luo J, Mosher KI, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cesca M, Bizzaro F, Zucchetti M, et al. Tumor delivery of chemotherapy combined with inhibitors of angiogenesis and vascular targeting agents. Front Oncol. 2013;3:259. doi: 10.3389/fonc.2013.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monje M, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 53.Monje M, Vogel H, Masek M, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 54.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 55.Buizer A, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr Blood Cancer. 2005;45:281–290. doi: 10.1002/pbc.20397. [DOI] [PubMed] [Google Scholar]
- 56.Chan R, Shum D, Toulopoulou T, et al. Assessment of executive functions: review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Anderson-Hanley C, Sherman ML, Riggs R, et al. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9:967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 58.Chen X, Zhu C, Li J, et al. Dissociation of decision making under ambiguity and decision making under risk in breast cancer patientsreceiving adjuvant chemotherapy: a neuropsychological study. Brain Res. 2013;1533:63–72. doi: 10.1016/j.brainres.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 59.Elbeltagy M, Mustafa S, Umka J, et al. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav Brain Res. 2010;208:112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 60.Winocur G, Vardy J, Binns MA, et al. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Walker E, Foley JJ, Clark-Vetri R, et al. Effects of repeated administration of chemotherapeutic agents tamoxifen, methotrexate, and 5-fluorouracil on the acquisition and retention of a learned response in mice. Psychopharmacology. 2011;217:539–548. doi: 10.1007/s00213-011-2310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiriz A, Reolon GK, Preissler T, et al. Cancer chemotherapy and cognitive function in rodent models: memory impairment induced by cyclophosphamide in mice. Clin Cancer Res. 2006;12:5000. doi: 10.1158/1078-0432.CCR-06-0138. [DOI] [PubMed] [Google Scholar]
- 63.Yang M, Kim JS, Song MS, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93:487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Lyons L, Elbeltagy M, Bennett G, et al. The effects of cyclophosphamide on hippocampal cell proliferation and spatial working memory in rat. PLoS One. 2011;6:e21445. doi: 10.1371/journal.pone.0021445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Mustafa S, Walker A, Bennett G, et al. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28:323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- 67.Foley J, Raffa RB, Walker EA. Effects of chemotherapeutic agents 5-fluorouracil and methotrexate alone and combined in a mouse model oflearning and memory. Psychopharmacology. 2008;199:527–538. doi: 10.1007/s00213-008-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seigers R, Schagen SB, Coppens CM, et al. Methotrexate decreases hippocampal cell proliferation and induces memory deficits in rats. Behav Brain Res. 2009;201:279–284. doi: 10.1016/j.bbr.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 69.Briones T, Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12:124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crossen J, Garwood D, Glatstein E, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 71.Abayomi O. Pathogenesis of cognitive decline following therapeutic irradiation for head and neck tumors. Acta Oncol. 2002;41:346–351. doi: 10.1080/028418602760169389. [DOI] [PubMed] [Google Scholar]
- 72.Dropcho E. Central nervous system injury by therapeutic irradiation. Neurol Clin. 1991;9:969–988. [PubMed] [Google Scholar]
- 73.Jenrow K, Brown SL, Lapanowski K, et al. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res. 2013;179:549–556. doi: 10.1667/RR3026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nokia M, Anderson ML, Shors TJ. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci. 2012;36:3521–3530. doi: 10.1111/ejn.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christie L, Acharya MM, Parihar VK, et al. Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin Cancer Res. 2012;18:1954–1965. doi: 10.1158/1078-0432.CCR-11-2000. [DOI] [PubMed] [Google Scholar]
- 76.Nokia M, Sisti HM, Choksi MR, et al. Learning to learn: theta oscillations predict new learning, which enhances related learning and neurogenesis. PLoS One. 2012;7:e31375. doi: 10.1371/journal.pone.0031375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rangel L, Quinn LK, Chiba AA, et al. A hypothesis for temporal coding of young and mature granule cells. Front Neurosci. 2013;7:75. doi: 10.3389/fnins.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han F, Ozawa H, Matsuda K, et al. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res. 2005;51:371–381. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Sarabdjitsingh R, Meijer OC, Schaaf MJ, et al. Subregion-specific differences in translocation patterns of mineralocorticoid and glucocorticoid receptors in rat hippocampus. Brain Res. 2009;1249:43–53. doi: 10.1016/j.brainres.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 81.McEwen B. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 82.Tanti A, Belzung C. Hippocampal neurogenesis: a biomarker for depression or antidepressant effects? Methodological considerations and perspectives for future research. Cell Tissue Res. 2013;354:203–219. doi: 10.1007/s00441-013-1612-z. [DOI] [PubMed] [Google Scholar]
- 83.Malberg J, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacol. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 84.Pollak D, Monje FJ, Zuckerman L, et al. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lussier A, Lebedeva K, Fenton EY, et al. The progressive development of depression-like behavior in corticosterone-treated rats is paralleled by slowed granule cell maturation and decreased reelin expression in the adult dentate gyrus. Neuropharmacology. 2013;71:174–183. doi: 10.1016/j.neuropharm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 86.Mateus-Pinheiro A, Patrício P, Bessa JM, et al. Cell genesis and dendritic plasticity: a neuroplastic pas de deux in the onset and remission from depression. Mol Psychiatry. 2013;18:748–750. doi: 10.1038/mp.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.David D, Samuels BA, Rainer Q, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mateus-Pinheiro A, Pinto L, Bessa JM, et al. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl Psychiatry. 2013;3:e210. doi: 10.1038/tp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vollmayr B, Mahlstedt MM, Henn FA. Neurogenesis and depression: what animal models tell us about the link. Eur Arch Psychiatry Clin Neurosci. 2007;257:300–303. doi: 10.1007/s00406-007-0734-2. [DOI] [PubMed] [Google Scholar]
- 91.Wang J, David DJ, Monckton JE, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu X, Yan HC, Zhang J, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30:12653–12663. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anacker C, Pariante CM. Can adult neurogenesis buffer stress responses and depressive behaviour? Mol Psychiatry. 2012;17:9–10. doi: 10.1038/mp.2011.133. [DOI] [PubMed] [Google Scholar]
- 94.Sahay A, Scobie KN, Hill AS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anacker C, Zunszain PA, Cattaneo A, et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16:738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller A. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24:1–8. doi: 10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hepgul N, Cattaneo A, Zunszain PA, et al. Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med. 2013;11:28. doi: 10.1186/1741-7015-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heimberger A, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13:3–13. doi: 10.1093/neuonc/noq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2013;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- 103.Cavanagh J, Paterson C, McLean J, et al. Tumour necrosis factor blockade mediates altered serotonin transporter availability in rheumatoid arthritis: a clinical, proof-of-concept study. Ann Rheum Dis. 2010;69:1251–1252. doi: 10.1136/ard.2009.107912. [DOI] [PubMed] [Google Scholar]
- 104.Bernardino L, Xapelli S, Silva AP, et al. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005;25:6734–6744. doi: 10.1523/JNEUROSCI.1510-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pace T, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2009;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30:S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 108.Spalletta G, Piras F, Caltagirone C, et al. Hippocampal multimodal structural changes and subclinical depression in healthy individuals. J Affect Disord. 2013;152–154:105–112. doi: 10.1016/j.jad.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 109.Manning M, Bettencourt BA. Depression and medication adherence among breast cancer survivors: bridging the gap with the theory of planned behaviour. Psychol Health. 2011;26:1173–1187. doi: 10.1080/08870446.2010.542815. [DOI] [PubMed] [Google Scholar]
- 110.Mondie C, Vandergrift KA, Wilson CL, et al. The chemotherapy agent, thioTEPA, yields long-term impairment of hippocampal cell proliferation and memory deficits but not depression-related behaviors in mice. Behav Brain Res. 2010;209:66–72. doi: 10.1016/j.bbr.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Warner-Schmidt J, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 112.Thuret S, Toni N, Aigner S, Yeo G, Gage F., et al. Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus. 2009;19:658–669. doi: 10.1002/hipo.20550. [DOI] [PubMed] [Google Scholar]
- 113.Lafenetre P, Leske O, Ma-Hogemeie Z, et al. Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Front Behav Neurosci. 2010;3:34. doi: 10.3389/neuro.08.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fardell J, Vardy J, Shah JD, et al. Cognitive impairments caused by oxaliplatin and 5-fluorouracil chemotherapy are ameliorated by physical activity. Psychopharmacology. 2012;220:183–193. doi: 10.1007/s00213-011-2466-2. [DOI] [PubMed] [Google Scholar]
- 115.Naylor A, Bull C, Nilsson MK, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008;105:14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wong-Goodrich S, Pfau ML, Flores CT, et al. Voluntary running prevents progressive memory decline and increases adult hippocampal neurogenesis and growth factor expression after whole-brain irradiation. Cancer Res. 2010;70:9329–9338. doi: 10.1158/0008-5472.CAN-10-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodgers S, Trevino M, Zawaski JA, et al. Neurogenesis, exercise, and cognitive late effects of pediatric radiotherapy. Neural Plast. 2013;2013:698528. doi: 10.1155/2013/698528. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dias G, Cavegn N, Nix A, et al. The role of dietary polyphenols on adult hippocampal neurogenesis: molecular mechanisms and behavioural effects on depression and anxiety. Oxid Med Cell Longev. 2012;2012:541971. doi: 10.1155/2012/541971. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams C, El Mohsen MA, Vauzour D, et al. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neuro-trophic factor (BDNF) levels. Free Radic Biol Med. 2008;45:295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 121.Dimpfel W. Rat electropharmacograms of the flavonoids rutin and quercetin in comparison to those of moclobemide and clinically used reference drugs suggest antidepressive and/or neuroprotective action. Phytomedicine. 2009;16:287–294. doi: 10.1016/j.phymed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 122.Zainuddin M, Thuret S. Nutrition, adult hippocampal neurogenesis and mental health. Br Med Bull. 2012;103:89–114. doi: 10.1093/bmb/lds021. [DOI] [PubMed] [Google Scholar]
- 123.Forman B, Chen J, Evans RM. The peroxisome proliferator-activated receptors: ligands and activators. Ann N Y Acad Sci. 1996;804:266–275. doi: 10.1111/j.1749-6632.1996.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 124.Ramanan S, Zhao W, Riddle DR, et al. Role of PPARs in radiation-induced brain injury. PPAR Res. 2010;2010:234975. doi: 10.1155/2010/234975. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schnegg C, Kooshki M, Hsu FC, et al. PPARδ prevents radiation-induced proinflammatory responses in microglia via transrepression of NF-κB and inhibition of the PKCα/MEK1/2/ERK1/2/AP-1 pathway. Free Radic Biol Med. 2012;52:1734–1743. doi: 10.1016/j.freeradbiomed.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kesler S, Hadi Hosseini SM, Heckler C, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13:299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ercoli L, Castellon SA, Hunter AM, et al. Assessment of the feasibility of a rehabilitation intervention program for breast cancer survivors with cognitive complaints. Brain Imaging Behav. 2013;7:543–553. doi: 10.1007/s11682-013-9237-0. [DOI] [PubMed] [Google Scholar]
- 129.Cherrier M, Anderson K, David D, et al. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sci. 2013;93:617–622. doi: 10.1016/j.lfs.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Monje M, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 131.Elbeltagy M, Mustafa S, Umka J, et al. The effect of 5-fluorouracil on the long term survival and proliferation of cells in the rat hippocampus. Brain Res Bull. 2012;88:514–518. doi: 10.1016/j.brainresbull.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 132.Lyons L, Elbeltagy M, Umka J, et al. Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology. 2011;215:105–115. doi: 10.1007/s00213-010-2122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miguel C, Albuquerque E. Drug interaction in psycho-oncology: antidepressants and antineoplastics. Pharmacology. 2011;88:333–339. doi: 10.1159/000334738. [DOI] [PubMed] [Google Scholar]
- 134.Winocur G, Binns MA, Tannock I. Donepezil reduces cognitive impairment associated with anti-cancer drugs in a mouse model. Neuropharmacology. 2011;61:1222–1228. doi: 10.1016/j.neuropharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 135.Wigmore P. The effect of systemic chemotherapy on neurogenesis, plasticity and memory. Curr Top Behav Neurosci. 2013;15:211–240. doi: 10.1007/7854_2012_235. [DOI] [PubMed] [Google Scholar]
- 136.Boele F, Douw L, de Groot M, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol. 2013;15(10):1420–1428. doi: 10.1093/neuonc/not102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kochman L, Fornal CA, Jacobs BL. Suppression of hippocampal cell proliferation by short-term stimulant drug administration in adult rats. Eur J Neurosci. 2009;29:2157–2165. doi: 10.1111/j.1460-9568.2009.06759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gagnon B, Low G, Schreier G. Methylphenidate hydrochloride improves cognitive function in patients with advanced cancer and hypoactive delirium: a prospective clinical study. J Psychiatry Neurosci. 2005;30:100–107. [PMC free article] [PubMed] [Google Scholar]
- 139.Mar Fan H, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 140.Lower E, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38:650–662. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 141.Lasheen W, Walsh D, Mahmoud F, et al. Methylphenidate side effects in advanced cancer: a retrospective analysis. Am J Hosp Palliat Care. 2010;27:16–23. doi: 10.1177/1049909109345145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gehring K, Patwardhan SY, Collins R, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. J Neurooncol. 2012;107:165–174. doi: 10.1007/s11060-011-0723-1. [DOI] [PubMed] [Google Scholar]
- 143.Lee T, Lee CH, Kim IH, et al. Effects of ADHD therapeutic agents, methylphenidate and atomoxetine, on hippocampal neurogenesis in the adolescent mouse dentate gyrus. Neurosci Lett. 2012;524:84–88. doi: 10.1016/j.neulet.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 144.Gong X, Schwartz PH, Linskey ME, et al. Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology. 2011;76:1126–1134. doi: 10.1212/WNL.0b013e318212a89f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brown P, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cole P, Vijayanathan V, Ali NF, et al. Memantine protects rats treated with intrathecal methotrexate from developing spatial memory deficits. Clin Cancer Res. 2013;19:4446–4454. doi: 10.1158/1078-0432.CCR-13-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Redmond K, Mahone EM, Terezakis S, et al. Association between radiation dose to neuronal progenitor cell niches and temporal lobes and performance on neuropsychological testing in children: a prospective study. Neuro Oncol. 2013;15:360–369. doi: 10.1093/neuonc/nos303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oehler J, Brachwitz T, Wendt TG, et al. Neural stem cell sparing by LINAC based intensity modulated stereotactic radiotherapy in intracranial tumors. Radiat Oncol. 2013;8:187. doi: 10.1186/1748-717X-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: safety profile for RTOG 0933. Radiother Oncol. 2010;95:327–331. doi: 10.1016/j.radonc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shaw M, Ball DL. Treatment of brain metastases in lung cancer: strategies to avoid/reduce late complications of whole brain radiation therapy. Curr Treat Options Oncol. 2013;14:553–567. doi: 10.1007/s11864-013-0258-0. [DOI] [PubMed] [Google Scholar]
- 152.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 153.McKinley M, Albiston AL, Allen AM, et al. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 154.Chao J, Yang L, Buch S, et al. Angiotensin II increased neuronal stem cell proliferation: role of AT2R. PLoS One. 2013;8:e63488. doi: 10.1371/journal.pone.0063488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Robbins M, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64:6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 156.Saavedra J, Sánchez-Lemus E, Benicky J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: therapeutic implications. Psychoneuroendocrinology. 2011;36:1–18. doi: 10.1016/j.psyneuen.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jenrow K, Brown SL, Liu J, et al. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:6. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jenrow K, Liu J, Brown SL, et al. Combined atorvastatin and ramipril mitigate radiation-induced impairment of dentate gyrus neurogenesis. J Neurooncol. 2011;101:449–456. doi: 10.1007/s11060-010-0282-x. [DOI] [PubMed] [Google Scholar]
- 159.Lee T, Greene-Schloesser D, Payne V, et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178:46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lucas D, Scheiermann C, Chow A, et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. 2013;19:695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han R, Yang YM, Dietrich J, et al. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7:12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]