Abstract
Background
Ipilimumab is a novel FDA-approved recombinant human monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4 and has been used to treat patients with metastatic melanoma. Immune-related neurological adverse effects include inflammatory myopathy, aseptic meningitis, posterior reversible encephalopathy syndrome, Guillain-Barré syndrome, myasthenia gravis–type syndrome, sensorimotor neuropathy, and inflammatory enteric neuropathy. To date, there is no report for ipilimumab-induced chronic inflammatory demyelinating polyneuropathy (CIDP), transverse myelitis (TM), or concurrent myositis and myasthenia gravis–type syndrome. Our objective is to raise early recognition of atypical neurological adverse events and to share our therapeutic approach.
Methods
We report 3 cases of metastatic melanoma treated with ipilimumab in which the patients developed CIDP, TM, and concurrent myositis and myasthenia gravis–type syndrome, respectively, at the MD Anderson Cancer Center between July 2012 and June 2013. Patients consented to release of medical information for publication/educational purposes.
Results
Our 3 cases of metastatic melanoma treated with ipilimumab developed CIDP, TM, and concurrent myositis and myasthenia gravis–type syndrome, respectively. The median time to onset of immune-related adverse events following ipilimumab treatment ranged from 1 to 2 weeks. Ipilimumab was discontinued due to the severe neurological symptoms. Plasmapheresis was initiated in the patients with CIDP and concurrent myositis and myasthenia gravis–type syndrome; high-dose intravenous steroids were given to the patient with TM, and significant clinical response was demonstrated.
Conclusions
Ipilimumab could induce a wide spectrum of neurological adverse effects. Our findings support the standard treatment of withholding or discontinuing ipilimumab. Plasmapheresis or high-dose intravenous steroids may be considered as the initial choice of treatment for severe ipilimumab-related neurological adverse events. Improvement of neurological symptoms may be seen within 2 weeks.
Keywords: chronic inflammatory demyelinating polyneuropathy, immune-related adverse events, ipilimumab, metastatic melanoma, transverse myelitis
Melanoma is an immunogenic cancer, and numerous immunotherapies have been used to control its progression, including α-interferon, interleukin (IL)-2, and monoclonal antibodies. Ipilimumab is a novel FDA-approved immunotherapy agent that has been used to treat patients with unresectable and/or metastatic melanoma since May 2011. Ipilimumab is a recombinant human monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4, which is an inhibitor of T-cell activation; thereby, ipilimumab potentiates an immune response. Immune-related adverse events (irAEs) may occur in patients treated with ipilimumab, however. Reported neurological irAEs include inflammatory myopathy,1 aseptic meningitis, posterior reversible encephalopathy syndrome,2 Guillain-Barré syndrome, myasthenia gravis–type syndrome, sensorimotor neuropathy,2,3–5 and inflammatory enteric neuropathy.6
We report 3 cases of metastatic melanoma treated with ipilimumab in which the patient developed rare ipilimumab-related adverse events: chronic inflammatory demyelinating polyneuropathy (CIDP), transverse myelitis (TM), and concurrent myositis and myasthenia gravis–type syndrome, respectively. We believe these events were due to immunological pathogenesis involving various human leukocyte antigen subgroups and immunotherapy regimens. We report 3 cases of ipilimumab-related irAEs presented with rare neurological symptoms to raise awareness of the wide spectrum of neurological side effects of this medication and provide our experience on their treatment.
Case #1
A 44-year-old man with stage IV metastatic melanoma to the brain had an initial diagnosis at age 41 and was treated with systemic chemotherapy, including 6 cycles of cisplatin, vinblastine, dacarbazine, and paclitaxel. He was then treated with ipilimumab owing to the progression of the disease, completing 4 cycles over 9 weeks. One week after initiation of ipilimumab treatment, he started to develop intermittent numbness and tingling in the right upper and lower extremities and on the right side of the face; these symptoms progressed to involve the contralateral side. Five weeks later, his symptoms had become constant, and he began having difficulty climbing stairs and getting up from a chair. He required a cane for ambulation within the house and a wheelchair for long distances.
The patient underwent a further neuro-oncologic evaluation. Brain MRI revealed no changes in the known brain metastases and no evidence of ischemic or hemorrhagic events. Initial neurological examination revealed bilateral decreased sensation to light touch and a pinprick throughout the face. Strength examination showed bilateral proximal muscle weakness involving the deltoids (3/5), hip flexors (4/5), and knee flexors and extensors (4/5). Sensory examination showed length-dependent small- and large-fiber neuropathy in both the upper and lower extremities, with a gradient to the knees and forearms, respectively. The patient also showed decreased sensation to a pinprick in the midsternal region but did not have a defined sensory level. His reflexes were absent throughout. The patient's gait was wide-based, and he was unable to walk on his toes.
The results of pertinent serum and CSF laboratory tests are listed in Table 1. Cerebrospinal fluid was negative for malignant cells. Serum immunoelectrophoresis showed oligoclonal gammopathy without paraproteinemia. Serum paraneoplastic antibodies were negative. Nerve conductions were suggestive of CIDP and met the European Federation of Neurological Societies/Peripheral Nerve Society diagnostic criteria.7,8 All potentials of the blink response were markedly delayed, suggesting demyelinating polyneuropathy. Pertinent findings of an electrodiagnostic study are listed in Table 2.
Table 1.
Pertinent laboratory values in a patient with chronic inflammatory polyradiculoneuropathy
| Laboratory Test | Value |
Normal Value | Units | ||
|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3 | |||
| CBC | |||||
| WBC | 3.7 | 12.0 | 10.5 | 4–11 | K/uL |
| ANC | 2.24 | 10.37 | 7.13 | 1.7–7.3 | K/uL |
| TSH | 2.93 | 2.32 | 0.91 | 0.27–4.2 | UIU/mL |
| CK | 31 | 60 | 1268 | 55–170 | U/L |
| CSF | |||||
| WBC | 1 | 28 | n/a | 0–5 | Cells/uL |
| RBC | 5 | 0 | n/a | 0 | Cells/uL |
| Protein | 44 | 50 | n/a | 11–55 | Mg/dL |
| Glucose | 84 | 137 | n/a | 40–70 | Mg/dL |
| Cytology |
|
Negative for melanoma or a bacterial, fungal, viral infection | n/a | ||
| SIEP, IFE |
|
No abnormal patterns indicative of a monoclonal gammopathy | n/a | ||
| Paraneoplastic panel | (−) | n/a | (−) | ||
| Ganglioside antibody panel | (−) | n/a | (−) | ||
| MAG antibody | (−) | n/a | n/a | ||
| SGPG antibody | (−) | n/a | n/a | ||
| Other antibody | n/a | n/a |
|
||
Abbreviations: CBC, complete blood count; WBC, white blood cell; ANC, absolute neutrophil count; TSH, thyroid stimulating hormone; CK, creatine kinase; RBC, red blood cell; SIEP, serum immunoelectrophoresis; IFE, immunofixation electrophoresis; MAG, myelin-associated glycoprotein; SGPG, sulfate-3-glucuronyl paragloboside; (−), negative; n/a, not applicable; Ach-R Ab, acetylcholine receptor antibody.
Table 2.
Electrodiagnostic study in a patient with chronic inflammatory polyradiculoneuropathy
| Motor Nerve Conduction | |||||
|---|---|---|---|---|---|
| Nerve | Latency |
Amplitude | CV | F-waves | |
| (ms) | (mV) | (ms) | (mV) | Latency (ms) | |
| R. peroneal (EDB) | 75.3 (≤57) | ||||
| Ankle | 9.5 | (<5.5) | 2.8 (>3) | (>40) | |
| Ankle–fibula head | 29 | ||||
| L. peroneal | 79.5 | ||||
| Ankle | 10.6 | 2.4 | |||
| Ankle–fibula head | 32 | ||||
| R. tibial (AHL) | 66.2 (≤57) | ||||
| Ankle | 5.5 | (<6) | 1.4 (>8) | (≥40) | |
| Ankle–popliteal fossa | 31 | ||||
| L. tibial | NR | ||||
| Ankle | 7 | 2.2 | |||
| Ankle–popliteal fossa | 33 | ||||
| R. median (APB) | NR (≤32) | ||||
| Wrist | 23.6 | (<3.9) | 2.8 (>6) | (≥50) | |
| L. median | 41.6 | ||||
| Wrist | 17.8 | 1.9 | |||
| Elbow | 39 | ||||
| R. ulnar (ADM) | 42.3 (≤32) | ||||
| Wrist | 7 | (<3.1) | 4.5 (>7) | (>50) | |
| Wrist–B.elbow | 47 | ||||
| L. ulnar | 48.7 | ||||
| Wrist | 5.6 | 3.9 | |||
| Wrist–B.elbow | 52 | ||||
| Blink reflex | |||||
| Nerve |
Ipsilateral | Ipsilateral | Contralateral | ||
| R1 Lat (ms) |
R2 Lat (ms) |
R2 Lat (ms) |
|||
| Trigeminal | (<13) | (<41) | (<44) | ||
| R | 21.2 | 46.6 | 48.2 | ||
| L | 22.6 | 45.3 | 45 | ||
| Electromyography | |||||
| Limited needle examination was performed in the right lower extremity. Tibialis anterior, vastus lateralis, iliopsoas, midcervical, and lumbar paraspinal muscles did not reveal any abnormal spontaneous activity. | |||||
Abbreviations: CV, conduction velocity; NR, no response; EDP, extensor digitorum brevis; AHL, adductor hallucis longus; APB, abductor pollicis brevis; ADM, abductor digiti minimi.
Treatment with ipilimumab was withheld. The patient was treated with 5 sessions of plasmapheresis within 1 week and discharged with significant improvement in both motor and sensory symptoms. He received outpatient physical and occupational therapy. Neurological examination at a 2-week follow-up visit revealed bilateral normal strength (5/5) in the proximal and distal muscles of the upper and lower extremities. Paresthesia improved significantly, although it was not completely resolved. He was able to ambulate independently inside his house and used a cane for long distances. He underwent 2 more sessions of plasmapheresis as an outpatient and continued to improve. His regimen was switched to vemurafenib, a B-Raf enzyme inhibitor, without recurrence of neurological symptoms but with melanoma progression.
Case #2
A 62-year-old man with a history of right uveal melanoma, diagnosed in 1998, was treated with transpupillary laser therapy, along with radioactive plaque therapy. He was apparently doing well until August 2011, when he was found to have metastatic lesions in the liver and extensive lymphadenopathy. In December 2011, CT-guided biopsy of the liver lesions confirmed metastatic choroidal melanoma. The patient did not undergo radiofrequency ablation; instead, the patient underwent 4 cycles of systemic chemotherapy with carboplatin and paclitaxel. He subsequently underwent 5 treatments of transarterial chemoembolization with doxorubicin LC Beads (Biocompatibles) in May 2012, but scans showed progression of the disease. He was then treated with ipilimumab on February 26 and March 19, 2013. After receiving another dose of ipilimumab on April 8, 2013, he developed a diffuse inflammatory process, which included uveitis, dermatitis, colitis, genitourinary symptoms, acute renal failure, and generalized weakness.
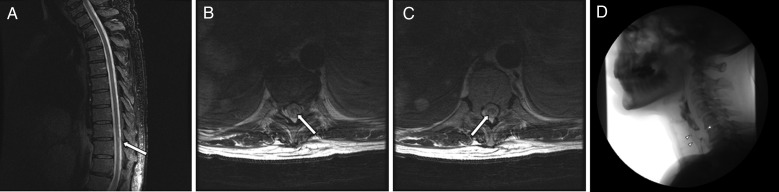
On April 14, 2013, he was transferred to MD Anderson Cancer Center for further management of metastatic melanoma and irAEs due to ipilimumab. Two days before admission, lower extremity weakness and paresthesias were noticed, as well as intermittent urinary retention and fecal incontinence. Neurological examination showed bilateral anterior uveitis and decreased strength in the proximal and distal muscles of the upper (4+/5) and lower (3/5) extremities, affecting hip flexion, knee flexion/extension, and dorsiflexion/plantar flexion. The sensory level was determined to be at T10, with overlapping length-dependent reduced temperature and touch sensation in all extremities (the patient had a history of diabetic and chemotherapy-induced neuropathy). Deep tendon reflexes were 2+ in the biceps, triceps, and brachioradialis bilaterally but were absent in the lower extremities. The Babinski sign was present bilaterally. Thoracic spine MRI without contrast showed a focal T2 signal abnormality at T9-10 and T10 without expansion of the cord. At that time, leptomeningeal disease could not be excluded without MRI with intravenous contrast (Fig. 1A–C).
Fig. 1.
Case 2, A–C Magnetic resonance imaging of the thoracic spine without contrast shows a focal T2 signal abnormality at the T9-10 and T10 levels within the cord. These are confirmed on the axial T2-weighted images. These appear very focal, with no expansion of the cord. Case 3, D Videofluoroscopy shows a very prominent cricopharyngeus muscle at the C5-6 level, which prevents the passage of contrast and is thought to represent the patient's cause of dysphagia.
A lumbar puncture was performed on April 16, 2013, which revealed protein (50 mg/dL), glucose (137 mg/dL), and white blood cells (28 cell/dL) with predominantly lymphocytoid cells. Cytology showed rare atypical cells favoring reactive histiocytes. Immunoperoxidase and Gomori-Grocott methenamine silver stain results were negative, which is not consistent with melanoma, a viral condition, or a bacterial or fungal infection. Cerebrospinal fluid analyses were negative for West Nile virus antibody (Ab), Epstein-Barr virus PCR, herpes simplex virus 1 and 2 PCR, human herpesvirus 6 PCR, cytomegalovirus PCR, varicella zoster virus PCR, cryptococcal antigen, Lyme Ab, neuromyelitis optica Ab, and fungal/bacterial culture. A myelopathy workup was negative, including serum and urine protein electrophoresis, serum vitamin B12, vitamin E, copper, folate, HIV Ab, West Nile virus Ab, rapid plasma reagin, and Lyme serology. Transverse myelitis induced by irAEs was suspected and ipilimumab was withheld. A course of high-dose i.v. steroids was given. The patient's symptoms improved throughout a 2-week hospitalization. Two weeks after discharge, the patient had gradual improvement of strength in lower extremities (4/5), but melanoma progressed.
Case #3
A 70-year-old female with left uveal melanoma, diagnosed in February 2012, subsequently had left eye enucleation in April 2012. The patient had a remote history of non-Hodgkin's lymphoma that was treated in the 1990s with surgery and intrathecal and systemic chemotherapy. In April 2013, she underwent a liver biopsy, which confirmed metastatic melanoma. On April 14, 2013, she was started on ipilimumab, receiving 2 doses, on May 3 and May 24, respectively. On June 2, she developed generalized myalgias and dysphagia/odynophagia. The head and neck surgery team evaluated her using nasal endoscopy and identified posterior laryngitis but no physical obstruction. The source of the inflammation was unknown. A modified barium swallow study showed a prominent cricopharyngeus muscle at the C5-C6 level with a moderate amount of residue remaining after swallowing (Fig. 1D).
Neurological examination showed submandibular/paratracheal lymphadenopathy; bilateral ptosis; fatigability/weakness of the neck flexion and extension (4/5); proximal muscle weakness in both arms and legs (4+/5); distal muscle strength (5/5); and diffuse tenderness to palpation in all limbs, but mainly in the proximal muscles. Examinations of sensory, coordination, and deep tendon reflexes were unremarkable. Electrophysiological studies demonstrated mild diffuse myopathic findings with no muscle membrane instability. Repetitive nerve stimulation showed mild postneuromuscular junction dysfunction. There was 12% decrement at baseline for spinal axillary/trapezius pair testing with repair following exercise and return of exhaustion after 2 min rest. There was 18% decrement on immediate postexercise for facial nerve/nasalis pair. There were no compelling findings for acute inflammatory demyelinating polyradiculoneuropathy (Guillain-Barré syndrome). Lumbar spine MRI showed degenerative disease in the spine, with both a small annular tear and a larger, more chronic posterior disc osteophyte complex at the L4-5 and L1-2 levels, respectively. There was no evidence of metastatic disease to the spine. The consensus was that the patient probably had myositis, as was evident by high levels of creatine kinase (1200 IU/L), and that this was a result of the recent treatment with ipilimumab. Evaluation for myasthenia gravis and myositis reported elevated acetylcholine receptor-binding Ab (2.09 nmol/L), acetylcholine receptor-modulating Ab (93%), and antistriated muscle Ab (1:983 040). Other serum paraneoplastic Abs were negative. CT chest with contrast was revised and negative for thymoma.
The patient completed a 3-day course of daily plasmapheresis and 125 mg of i.v. methylprednisolone. She had significant clinical response within 2 weeks and was subsequently maintained on i.v. Ig infusions and low-dose pyridostigmine. Patient ability to climb flight of stairs has improved. Patient swallowing has improved with occasional need for small sip of water for solids. For her persistent but stable melanoma, she is receiving additional chemotherapy (cisplatin and pegylated arginine deiminase); and for her persistent myasthenic symptoms, she is receiving additional i.v. Ig infusion.
Discussion
Melanoma is an immunogenic cancer that can mount an immune response in the host. Melanocytes and Schwann cells are derived from the neural crest and share some similar antigens. An immune response against melanocyte antigens, called molecular mimicry, also can attack similar antigens of the Schwann cells as a result of cross-reaction. Gangliosides such as GM2, GM3, GD2, and GD3 expressed on melanocytes are highly immunogenic in humans,9 with subsequent antibody formation that may also be responsible for initiating CIDP. Although CIDP and melanoma are only rarely seen concurrently, when they occur together they manifest at the onset of melanoma.9
Some studies showed that IL-6 levels were markedly elevated in the spinal fluid of TM patients. IL-6 is secreted by astrocytes and microglia and binds to oligodendroglia and axons. High levels of IL-6 can cause indirect damage by inducing nitric oxide synthetase in microglia.10 Some monoclonal antibody immunotherapy medications have been found to increase levels of IL-6, but no studies have yet focused on the association between IL-6 and ipilimumab. It is known that melanoma cells also induce elevated IL-6 levels.11 However, in our TM case, the onset and improvement of symptoms correlated with the initiation and discontinuation of ipilimumab, respectively. Thus, melanoma was unlikely to be the single driving factor inducing TM in this patient. The other possibility is that ipilimumab induces TM through a pathway other than elevated IL-6 level.
In the literature, only 9 cases of myopathy have been reported that have manifested as myositis, polymyositis, or ocular myositis.12 In 2009, Hunter et al1 reported the first case of myositis with diffuse involvement of facial muscles, pharyngeal muscles, and all extremities. Ipilimumab was discontinued, and the patient received i.v. Ig and high-dose i.v. steroids, with near-complete resolution of symptoms within 3 weeks. In our case, the patient's symptoms responded well to discontinuation of ipilimumab and/or plasmapheresis in regard to symptoms related to myositis. However, symptoms of myasthenia gravis–type syndrome persisted 2 months after cessation of ipilimumab. This, along with high serum anti–acetylcholine receptor Ab titers, indicates the possibility that ipilimumab uncovered underlying myasthenia gravis. To our knowledge this is the first case report of concurrent neurological entities related to ipilimumab therapy.
Metastatic melanoma therapy is based on immunotherapy and the inhibition of the mitogen-activated protein kinase pathway. Ipilimumab is a human monoclonal antibody that blocks cytotoxic T-lymphocyte antigen-4, thereby resulting in T-cell activation; ipilimumab indirectly mediates the T-cell immune response against tumors but induces irAEs as well. Reported irAEs include dermatitis and mucositis (47%–68%), colitis (31%–46%), hepatitis (3%–9%), hypophysitis (4%–6%), pancreatitis (1.5%), uveitis (1%), and lymphadenopathy (1%). The incidence of neurological irAEs in ipilimumab phase II and III trials was reported to be 0.1%.2 It is possible that the true incidence of neurological irAEs related to ipilimumab has been underestimated due to lack of recognition and/or report of these syndromes; these are typically transient peripheral sensorimotor neuropathies. A single case of Guillain-Barré syndrome, several cases of myasthenia gravis–type syndrome,3–5 a case of inflammatory enteric neuropathy,7 posterior reversible encephalopathy syndrome, and aseptic meningitis2 have been described, but to our knowledge, this is the first report of CIDP or TM related to ipilimumab therapy.
In clinical studies, the median time to onset of irAEs was ∼6 weeks, with the majority occurring during the induction phase.3,4 In our patients, the time to onset ranged from 1 to 2 weeks. Most irAEs resolve around 4 to 7 weeks after onset,3,4 which also differs from the 2-week period seen in our patients. The mainstay of treatment is withholding or permanently discontinuing ipilimumab therapy, depending on the severity of the irAE.4 In our 3 cases, ipilimumab was discontinued due to the severe neurological symptoms. Plasmapheresis was initiated in 2 patients, those with CIDP and myositis. These patients demonstrated significant clinical responses, but it is unclear whether the improvement was owing to discontinuation of ipilimumab, initiation of plasmapheresis, or both. The patient with TM showed significant clinical improvement after discontinuation of ipilimumab, high-dose i.v. steroids, or both. Myasthenia gravis–type syndrome in our patient persisted 2 months after cessation of ipilimumab. This, along with high serum anti–acetylcholine receptor Ab titers, indicates the possibility that ipilimumab uncovered underlying myasthenia gravis.
We conclude that our patients had irAEs manifested as CIDP, TM, concurrent myositis, and possible uncovered underlying myasthenia gravis, given the absence of any possible etiology other than ipilimumab and the timing of the symptomatology with ipilimumab treatment. Neurological irAEs should be suspected in patients on ipilimumab who develop new sensory and/or motor symptoms during the induction phase. This case series highlighted the fact that ipilimumab-related irAEs could involve the CNS, peripheral nervous system, neuromuscular junction, and muscle. Some patients may have simultaneous occurrence of 2 or more neurological complications. Recognition of ipilimumab-related irAEs is critical to the early institution of appropriate management. For moderate to severe neuropathy, CIDP, TM, or myositis, ipilimumab therapy should be withheld. Plasmapheresis may be considered as the initial choice of treatment for ipilimumab-related neurological adverse events. Improvement or resolution of neurological symptoms may be seen within 2 weeks.
Funding
The authors report no funding. No core grant was used for any work related to this manuscript.
Conflict of interest statement. None declared.
References
- 1.Hunter G, Voll C, Robinson CA. Autoimmune inflammatory myopathy after treatment with ipilimumab. Can J Neurol Sci. 2009;36(4):518–520. doi: 10.1017/s0317167100007939. [DOI] [PubMed] [Google Scholar]
- 2.Tarhini A. Immune-mediated adverse events associated with ipilimumab CTLA-4 blockage therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. doi: 10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel SP, Woodman SE. Profile of ipilimumab and its role in treatment of metastatic melanoma. Drug Des Devel Ther. 2011;5:489–495. doi: 10.2147/DDDT.S10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews S, Holden R. Characteristics and management of immune-related adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma. Cancer Manag Res. 2012;4:299–307. doi: 10.2147/CMAR.S31873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 6.Wilgenhof S, Neyns B. Anti–CTLA-4 antibody-induced Guillain-Barré syndrome in a melanoma patient. Ann Oncol. 2011;22(4):991–993. doi: 10.1093/annonc/mdr028. [DOI] [PubMed] [Google Scholar]
- 7.Koski CL, Baumgarten M, Magder LS, et al. Derivation and validation of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. 2009;277(1–2):1–8. doi: 10.1016/j.jns.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. Eur J Neurol. 2010;17(3):356–363. doi: 10.1111/j.1468-1331.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- 9.Anthoney DA, Bone I, Evans TR. Inflammatory demyelinating polyneuropathy: a complication of immunotherapy in malignant melanoma. Ann Oncol. 2000;11(9):1197–1200. doi: 10.1023/a:1008362714023. [DOI] [PubMed] [Google Scholar]
- 10.Awad A, Stüve O. Idiopathic transverse myelitis and neuromyelitis optica: clinical profiles, pathophysiology and therapeutic choices. Curr Neuropharmacol. 2011;9(3):417–428. doi: 10.2174/157015911796557948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoejberg L, Bastholt L, Schmidt H. Interleukin-6 and melanoma. Melanoma Res. 2012;22(5):327–333. doi: 10.1097/CMR.0b013e3283543d72. [DOI] [PubMed] [Google Scholar]
- 12.Yervoy (ipilimumab) injection, prescribing information. Princeton, NJ: Bristol-Myers Squibb; 2011. Available at: http://packageinserts.bms.com. Accessed January 18, 2012. [Google Scholar]