Abstract
Due to the low radiopacity of Sealer 26, iodoform is frequently empirically added to this sealer. Thus, the interference of this procedure with the physicochemical properties of Sealer 26 must be evaluated.
Objective
This study evaluated the influence of the addition of iodoform on setting time, flow, solubility, pH, and calcium release of an epoxy-based sealer.
Material and Methods
The control group was pure Sealer 26, and the experimental groups were Sealer 26 added with 1.1 g, 0.55 g or 0.275 g of iodoform. Setting time evaluation was performed in accordance with the ASTM C266-03 speciflcation. The analysis of flow and solubility was in accordance with the ISO 6876-2001 speciflcation. For the evaluation of pH and calcium ion release, polyethylene tubes were filled with the materials and immersed in flasks with 10 ml of deionized water. After 24 h, 7, 14, 21, 28, and 45 days pH was measured. In 45 days, the calcium released was evaluated with an atomic absorption spectrophotometer.
Results
The addition of iodoform increased setting time in comparison with pure sealer (P<0.05). As for flow, solubility, and calcium release, the mixtures presented results similar to pure sealer (p>0.05). In the 24 h period, the mixture with 1.1 g and 0.55 g of iodoform showed lower pH than pure sealer and than sealer added with 0.275 g of iodoform (P<0.05).
Conclusions
The iodoform added to Sealer 26 interferes with its setting time and solubility properties. Further studies are needed to address the clinical signiflcance of this interference.
Keywords: Endodontics, Root canal fllling materials, Physical and chemical properties
INTRODUCTION
Endodontic sealers should have an antimicrobial activity28 and the capacity to stimulate the healing of periapical tissue19. These properties are related to the release of hydroxyl and calcium ions13 and the increase of pH15. Furthermore, endodontic sealers should have adequate flow, setting time and low solubility7.
In Brazil, the epoxy-based sealer Sealer 26 is one of the most used in root canal fllling and is widely commercially available6. However, its radiopacity is lower than that of other endodontic sealers such as the methacrylate-based (Epiphany)16, gutta-percha point5 or even another epoxy-based (AH Plus)25. This difference of radiopacity may be inadvertently interpreted as failure in root canal fllling.
A way to improve the radiopacity of Sealer 26 is to add iodoform, in a minimum ratio of 1:3, to the total volume of the sealer14. Materials containing iodoform have been suggested for root canal fllling in primary teeth8. When added to Sealapex, the histological results do not change in relation to pure Sealapex17, and the mixture presented clinical applicability as an alternative radiopacifler for endodontic procedures.
Iodoform (CHI3) is commonly used as radiopacifler for primary tooth root canal fllling8,22,23, but its use in Endodontics is controversial because of its potential for causing toxic effects4,23. Iodoform has a radiopacifying action similar to bismuth subnitrate and higher than zinc oxide, barium sulfate, zirconium oxide, and calcium tungstate10.
Due to the radiopacity of Sealer 26, endodontists have empirically added iodoform to this sealer. But there are no studies evaluating the interference of this procedure with the physicochemical properties of Sealer 26. Since some essential properties such as solubility and setting time depend on the powder/resin ratio of a sealer, preparing the material with varying consistency may result in variations in the properties of the sealer7. This may have a clinical consequence, such as difflculty in handling and/or interference in the antimicrobial action7,20.
Flow is important as it determines the ability of the sealer to penetrate into small irregularities, ramiflcations and lateral canals1. When the flow of the sealer is not in accordance with ISO (6876) or ADA (57) norms, its use in root canal obturation is contraindicated. The preservation of alkalization potential and calcium ion release properties of the sealer after its manipulation are important to their biological and microbiological actions9.
Therefore, studies that assess the interference of the addition of several proportions of iodoform with the properties of Sealer 26 are necessary. This study aims to evaluate the hypothesis that the addition of iodoform interferes negatively with its physicochemical properties.
The aim of this study was to evaluate the initial and flnal setting time, flow, solubility, pH and calcium release from an epoxy-based sealer (Sealer 26), both pure and added with 1.1 g; 0.55 g or 0.275 g of iodoform.
MATERIAL AND METHODS
The epoxy-based sealer used for this study was Sealer 26 (Dentsply, Maillefer Instruments SA, Ballaigues, Switzerland). The main constituents of this material, according to the manufacturer, are: a. powder (bismuth trioxide, calcium hydroxide, hexamethylenetetramine, titanium dioxide); b. resin (Bisphenol-epoxy resin).
To manipulate Sealer 26, 1.1 g of powder was added to 1.0 g of resin and mixed during 30 s. This mixture was considered as the control group (G1). For the experimental groups, the mixture was prepared by adding iodoform (Biodinâmica Ind. Com., Ibiporã, PR, Brazil) in proportions of 1.1 g (G2), 0.55 g (G3), and 0.275 g (G4).
Determination of setting time
The initial and flnal setting times ofthe mixtures were determined according to the International Standards Organization (ISO) 6876 speciflcation18 and the ASTM C266-03 (Philadelphia: ASTM; 2008) speciflcation2 . All specimens were measured under controlled temperature and humidity: 37±1°C and 95±5% relative humidity. The sealers were mixed and inserted into metallic ring molds with 10 mm of diameter and 2 mm of thickness (n=3, each group).
At 180 seconds, each specimen was indented with a Gilmore needle (113.5 g) until the initial setting time was determined. Then, a Gilmore needle weighing 456.5 g was used to determine the flnal setting time. Setting times were determined as being the time elapsed from the beginning of the mixing to the time at which no indentation was detected on the surface of the mixtures. The initial and flnal setting times were determined when the Gilmore needles, with 113.5 g and 456.5 g respectively, failed to leave a deflnite mark on the surface of the sample. Data obtained was subjected to one-way ANOVA and Dunn tests, at a signiflcance level of 5%.
Flow
All procedures were performed according to ISO 6876:20011,7,18 . The sealers were mixed according to the conditions described before, and 0.05±0.005 ml of mixture was placed on the center of a glass plate with a graduated syringe. After 3 min, a second plate weighing 20 g and a weight of 100 g were placed on the sealer. After seven additional minutes, the mean of the biggest and smallest diameter of the disc of sealer was recorded in mm using a digital caliper with a resolution of 0.01 mm (Mitutoyo MTI Corporation, Tokyo, Japan). The mean of three measurements for each sealer was taken as the flow ofthe material and the data submitted to Kruskal-Wallis test at a signiflcance level of 5%.
Solubility
Teflon ring molds (n = 10, each group) measuring 1.5 mm thickness and 20 mm internal diameter were filled with the mixtures. Two glass plates, covered with colorless cellophane, were placed under and on the mold. The samples were stored for 45 days in 37±1°C and 95±5% relative humidity to set. After the removal of residues or loose particles, the specimens were placed in a desiccator with silica for 24 h. Then, they were weighed on a precision scale (Shimadzu AW-220; Shimadzu, São Paulo, SP, Brazil) and immersed individually in 50 ml of deionized water in closed flasks. Special care was taken to keep the specimens hung in the water. All specimens were again stored for 7 days in 37±1°C. After this period, the specimens were again placed in a desiccator for 24 h and subsequently weighed, in similar conditions as described above. The solubility and disintegration between the original mass of the specimen and its final mass were calculated to deduce the percentage of original mass loss of the specimen. All procedures were in accordance with Vivan, et al.27 (2010). The data obtained was submitted to Kruskal-Wallis test at a significance level of 5%.
pH and calcium release
Forty polyethylene tubes measuring 0.5 cm in length and 1.0 mm in internai diameter (n = 10, each group) were filled with the mixtures by using a Lentulo spiral (Dentsply, Maillefer Instruments SA, Ballaigues, Switzerland).
The tubes filled with fresh mixtures were weighed in order to check the standardization of the amount of sealer. They were placed in polypropylene fiasks (Injeplast, São Paulo, SP, Brazil) containing 10 mL of deionized water and kept at 37°C. Prior to immersion of the specimens, both pH and calcium concentration of the deionized water were verified, with pH being 6.8 and calcium being totally absent. Evaluations of pH and calcium ion release were carried out after 24 h, 7, 14, 21, 28, and 45 days. The specimens were kept in the same distilled water during all the experiment. Measurement of pH was performed with a pHmeter (model Q400I; Quimis, Diadema, SP, Brazil), previously calibrated with solutions of known pH (4, 7, and 14), at constant temperature (25°C). The flasks were placed in a shaker (Farmen, São Paulo, SP, Brazil) for 5 seconds before pH measurement. The control procedure included measuring the pH of the water in which no specimens had been immersed.
In 45 days, a Spectra 55B atomic spectrophotometer (Varian, Inc.,Palo Alto, CA, USA) was used to determine the calcium release concentration (in ppm) in the deionized water. Lanthanum oxide was added to all samples to eliminate ionic interference. Solutions containing calcium concentrations of 0, 1, 2, 3, 4 and 5 ppm were used to create a standard calibration curve23 .
RESULTS
The initial and final setting times between the groups were statistically different (P>0.05). The mean and standard deviation of the initial and final setting times in minutes for Sealer 26 and experimental mixtures are showed in Table 1.
Table 1.
Mean and standard deviation of the initial and final setting times in minutes for Sealer 26 and experimental mixtures
| G1 | G2 | G3 | G4 | |
|---|---|---|---|---|
| (1.1:0.0:1.0) | (1.1:1.1:1.0) | (1.1:0.55:1.0) | (1.1:0.275:1.0) | |
| Initial | 470 (8.66)a | 11.486 (0.1)b | 9.136 (199)c | 5.595 (0.1)d |
| Final | 2.569 (2.88)a | 15.536 (0.1)b | 11.952 (509)c | 7.675 (103)d |
a,b,c,d Different letters in each line indicate significant difference (P<0.05). The proportion is in the sequence powder:iodoform:resin (in weight)
Regarding the flow test, the experimental mixtures and the pure Sealer 26 presented similar results (P>0.05) (Table 2). Thefiow ofall mixtures is in accordance with ISO 6876:200118.
Table 2.
Mean and standard deviation of flow and calcium release for Sealer 26 and experimental mixtures
| G1 | G2 | G3 | G4 | |
|---|---|---|---|---|
| (1.1:0.0:1.0) | (1.1:1.1:1.0) | (1.1:0.55:1.0) | (1.1:0.275:1.0) | |
| Flow (mm) | 22.99 (±0.83) | 21.91 (±1.87) | 22.00 (±0.58) | 22.01 (±1.01) |
| Calcium release (ppm) | 1.37 (±0.20) | 1.12 (±0.27) | 1.23 (±0.13) | 1.180 (0.20) |
* No statistical difference was observed between the groups (P>0.05). The proportion is in the sequence powder:iodoform:resin (in weight)
The experimental groups presented higher solubility than Sealer 26 (P<0.05), but no differences were observed between them (P>0.05). All the sealers released calcium and were similar (P>0.05) (Table 3).
Table 3.
Mean of percentage of original mass loss for Sealer 26 and experimental mixtures
| G1 | G2 | G3 | G4 | |
|---|---|---|---|---|
| (1.1:0.0:1.0) | (1.1:1.1:1.0) | (1.1:0.55:1.0) | (1.1:0.275:1.0) | |
| Mass loss | 2.0a | 10.98b | 10.89b | 9.38b |
a,b Different letters in each line indicate significant difference (P<0.05). The proportion is in the sequence powder:iodoform:resin (in weight)
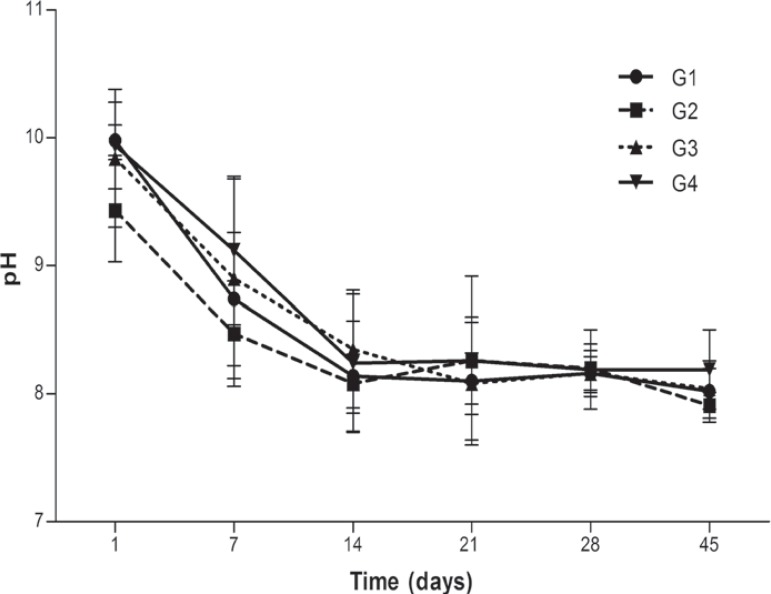
The mean and standard deviation for pH values, in several periods, of Sealer 26 and the three experimental mixtures are presented in figure 1. In the 24 h period, the addition of 1.1 g (G2) or 0.55 g (G3) of iodoform promoted reduction of pH values. In addition, these values were lower than the ones presented by G1 and G4 (P<0.05). In the other periods, pH values between the groups were similar (P>0.05). The pH values reduced in the course of time.
Figure 1.
pH changes of Sealer 26 and mixtures according to different periods of time
DISCUSSION
Studies ofthe initial and the final setting time, flow, solubility, pH and calcium releases of the pastes and endodontic sealers are as important as the ones that evaluate antimicrobial activity3,20, radiopacity16,25 biological behavior23 and toxic effect4 of these materials. The decision making for clinical use of these materials have to be based on these evidences.
The results of the present study, particularly of setting time and solubility tests, allow us to accept the hypothesis that the addition of iodoform, independently of the proportion added, interferes with the properties of Sealer 26. Therefore, this procedure should be avoided.
The addition of iodoform to the powder of the sealer in proportions of 1.1 g, 0.55 g, and 0.275 g provides total mass increase of 52.38%, 26.19%, and 13.09%, respectively. Despite this, the flow was similar in all groups. When 10% calcium hydroxide was added to another epoxy-based sealer (AH Plus), the flow reduced significantly in comparison with the original consistency11. However, AH Plus is a paste/paste system and Sealer 26 is a powder/paste system and the amount, in volume, of iodoform aggregated was insufficient to promote alterations in the sealer flow.
Several studies reported the importance that endodontic sealers present alkaline pH value and promote calcium ion release9,15,21. In the 24 h period, the addition of iodoform to G2 and G3 groups promoted reduction of pH value probably because the amount of calcium hydroxide present in the powder sealer decreased in relation to the sealer original proportion (0.37 g/g). In all the other periods, there was reduction of the pH values. However, in each of these periods, the values among different groups were similar. The presence of some acid hydrogen components in the sealer, such as bisphenol-epoxy resin, may have caused a reaction with hydroxyl ion causing pH decrease24. The setting reaction of the substances that compose the sealer, with the consequent prevention of hydroxyl ion release, may have also contributed to the prevention of hydroxyl ion release24.
In this study, the assessment of calcium release by the mixtures was only carried out at the end of the experiment and the values corresponded to the release throughout the experiment. It is possible that the same chemical reactions may have been involved, as partial neutralization of hydroxyl ions also occurred in the calcium chelation24. Other studies with Sealer 26 were conducted in different conditions, which makes the comparison with our results impossible9,26.
The addition of iodoform into sealer powder, in all proportions used, promoted increase of initial and final setting time. The setting of the sealer occurs because of the reaction between the bisphenol-epoxy resin and hexamethylenetetramine9. The addition of iodoform decreased the amount of hexamethylenetetramine, significantly affecting the setting time of the sealer. Probably, as a consequence of the interference with setting time and of powder/resin ratio modification, the solubility of experimental mixtures increased when compared with the original Sealer 26 sealer. Similarly, when calcium hydroxide was added into AH Plus (epoxy-based sealer), there was an increase in the solubility of the sealer11. The powder/liquid ratio infiuences the physicochemical properties of endodontic sealers7.
Thus, the addition of iodoform in Sealer 26 interferes with the physicochemical properties of the material. Other studies should be conducted to select a radiopacifier that does not interfere with the epoxy-based sealer properties.
CONCLUSIONS
Under the conditions of this study, it seems that the addition of iodoform to the powder of Sealer 26 has infiuence on its physicochemical properties. Two properties are significantly altered: solubility and setting time. Only in the 24 h period, the addition of iodoform, in proportions of 1.1 g and 0.55 g, decreased the pH value of the sealer. The flow and calcium release were not affected. Therefore, these alterations of the properties of Sealer 26 are likely to be clinically relevant, and these mixtures are not recommended for clinical use.
REFERENCES
- 1.Almeida JF, Gomes BP, Ferraz CC, Souza-Filho FJ, Zaia AA. Filling of artificial lateral canals and microleakage and flow of five endodontic sealers. Int Endod J. 2007;40:692–699. doi: 10.1111/j.1365-2591.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- 2.American Society for Testing and Materials . ASTM C266-03: Standard test method for time and setting of hydraulic-cement paste by Gilmore needles. Philadelphia: ASTM; 2000. [Google Scholar]
- 3.Amorim LF, Toledo OA, Estrela CR, Decurcio DA, Estrela C. Antimicrobial analysis of different root canal filling pastes used in pediatric dentistry by two experimental methods. Braz Dent J. 2006;17:317–322. doi: 10.1590/s0103-64402006000400010. [DOI] [PubMed] [Google Scholar]
- 4.Araki K, Hirakawa N, Kosugi T, Higashimoto I, Kakiuchi Y, Nakashima M. Iodoform intoxication: a case report of prolonged consciousness disturbance in a patient with a high plasma iodine level. Fukuoka Igaku Zasshi. 2007;98:397–401. [PubMed] [Google Scholar]
- 5.Aznar FD, Bueno CE, Nishiyama CK, Martin AS. Radiopacity of seven endodontic sealers evaluated using digitized radiograph. RGO. 2010;58:181–184. [Google Scholar]
- 6.Bodanezi A, Munhoz EA, Bernardineli N, Capelozza AL, Moraes IG, Bramante CM. Radiographic analysis of root canal fillings: infuence of two sealers on the perception of voids. Braz Dent J. 2010;21:142–147. doi: 10.1590/s0103-64402010000200009. [DOI] [PubMed] [Google Scholar]
- 7.Camps J, Pommel L, Bukiet F, About I. Influence of the powder/liquid ratio on the properties of zinc oxide-eugenol-based root canal sealers. Dent Mat. 2004;20:915–923. doi: 10.1016/j.dental.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Cerqueira DF, Mello-Moura AC, Santos EM, Guedes-Pinto AC. Cytotoxicity, histopathological, microbiological and clinical aspects of an endodontic iodoform-based paste used in pediatric dentistry: a review. J Clin Pediatr Dent. 2008;32:105–110. doi: 10.17796/jcpd.32.2.k1wx5571h2w85430. [DOI] [PubMed] [Google Scholar]
- 9.Duarte MA, Demarchi AC, Giaxa MH, Kuga MC, Fraga SC, Souza LC. Evaluation of pH and calcium ion release of three root canal sealer. J Endod. 2000;26:389–390. doi: 10.1097/00004770-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Duarte MA, El Kadre GD, Vivan RR, Tanomaru JMG, Tanomaru-Filho M, Moraes IG. Radiopacity of Portland cement associated with different radiopacifying agents. J Endod. 2009;35:737–740. doi: 10.1016/j.joen.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Duarte MA, Ordinola-Zapata R, Bernardes RA, Bramante CM, Bernardineli N, Garcia RB, et al. Influence of calcium hydroxide association on the physical properties of AH Plus. J Endod. 2010;36:1048–1051. doi: 10.1016/j.joen.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Duarte MA, Weckwerth PH, Moares IG. Analysis of the antimicrobial action of sealers and pastes used in endodontic practice. Rev Odontol Univ São Paulo. 1997;11:299–305. [Google Scholar]
- 13.Estrela C, Pesce HF. Chemical analysis of the liberation of calcium hydroxyl ions from calcium hydroxide pastes in connective tissue in the dog - Part I. Braz Dent J. 1996;7:41–46. [PubMed] [Google Scholar]
- 14.Figueiredo JA, Vidor MM, Oliveira FF, Filipini HP, Gomes MS. Evaluation of the radiopacity of sealers called Sealapex and Sealer-26, with addition iodineform, by Accu-Ray digital image. Rev Fac Odontol Porto Alegre. 1997;38:11–18. [Google Scholar]
- 15.Gonçalves JL, Viapiana R, Miranda CE, Borges AH, Cruz-Filho AM. Evaluation of physico-chemical properties of Portland cements and MTA. Braz Oral Res. 2010;24:277–283. doi: 10.1590/s1806-83242010000300004. [DOI] [PubMed] [Google Scholar]
- 16.Guerreiro-Tanomaru JM, Duarte MA, Gonçalves M, Tanomaru-Filho M. Radiopacity evaluation of root canal sealers containing calcium hydroxide and MTA. Braz Oral Res. 2009;23:119–123. doi: 10.1590/s1806-83242009000200005. [DOI] [PubMed] [Google Scholar]
- 17.Holland R, Mello W, Souza V, Nery MJ, Bernabé PF, Otoboni JA., Filho Behaviour of the periapical tissues of dog's teeth to root canal filling with Sealapex with or without iodoform. Rev Odontol UNESP. 1990;19:97–104. [PubMed] [Google Scholar]
- 18.International Standardization Organization . ISO 6876: Dental root canal sealing materials. Geneva: ISO; 2001. [Google Scholar]
- 19.Mutoh N, Tani-Ishii N. A biocompatible model for evaluation of the responses of rat periapical tissue to a new zinc oxide-eugenol sealer. Dent Mater J. 2011;30:176–182. doi: 10.4012/dmj.2010-095. [DOI] [PubMed] [Google Scholar]
- 20.Pizzo G, Giammanco GM, Cumbo E, Nicolosi G, Gallina G. In vitro antibacterial activity of endodontic sealers. J Dent. 2006;34:35–40. doi: 10.1016/j.jdent.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Santos AD, Moraes JC, Araújo EB, Yukimitu K, Valério-Filho WV. Physico-chemical properties of MTA and a novel experimental cement. Int Endod J. 2005;38:443–447. doi: 10.1111/j.1365-2591.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 22.Sarigol CG, Cogulu D, Oncag O, Deliloglu IG. Cytotoxic effects of primary tooth root canal filling materials on L929 cell line. J Dent Child (Chic) 2010;77:72–76. [PubMed] [Google Scholar]
- 23.Silva LA, Leonardo MR, Oliveira DS, Silva RA, Queiroz AM, Hernández PG. Histopathological evaluation of root canal filling materials for primary teeth. Braz Dent J. 2010;21:38–45. doi: 10.1590/s0103-64402010000100006. [DOI] [PubMed] [Google Scholar]
- 24.Silva LA, Leonardo MR, Silva RS, Assed S, Guimarães LE. Calcium hydroxide root canal sealers: evaluation of pH, calcium ion concentration and conductivity. Int Endod J. 1997;30:205–209. doi: 10.1046/j.1365-2591.1997.00079.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanomaru JM, Cezare L, Gonçalves M, Tanomaru-Filho M. Evaluation of the radiopacity of root canal sealers by digitization of radiographic images. J App Oral Sci. 2004;12:355–357. doi: 10.1590/s1678-77572004000400019. [DOI] [PubMed] [Google Scholar]
- 26.Tanomaru-Filho M, Saçaki JN, Faleiros FB, Guerreiro-Tanomaru JM. pH and calcium ion release evaluation of pure and calcium hydroxide-contained Epiphany for use in retrograde fllling. J Appl Oral Sci. 2011;19:1–5. doi: 10.1590/S1678-77572011000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivan RR, Zapata RO, Zeferino MA, Bramante CM, Bernardineli N, Garcia RB, et al. Evaluation of the physical and chemical properties of two commercial and three experimental root-end fllling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:250–256. doi: 10.1016/j.tripleo.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Shen Y, Ruse ND, Haapasalo M. Antibacterial activity of endodontic sealers by modifled direct contact test against Enterococcus faecalis. J Endod. 2009;35:1051–1055. doi: 10.1016/j.joen.2009.04.022. [DOI] [PubMed] [Google Scholar]