Abstract
Fluoride varnishes play an important role in the prevention of dental caries, promoting the inhibition of demineralization and the increase of remineralization.
Objective
This study aimed to analyze the amount of fluoride released into water and artificial saliva from experimental TiF4 and NaF varnishes, with different concentrations, for 12 h.
Material and Methods
Fluoride varnishes were applied on acrylic blocks and then immersed in 10 ml of deionized water and artificial saliva in polystyrene bottles. The acrylic blocks were divided in seven groups (n=10): 1.55% TiF4 varnish (0.95% F, pH 1.0); 3.10% TiF4 varnish (1.90% F, pH 1.0); 3.10% and 4% TiF4 varnish (2.45% F, pH 1.0); 2.10% NaF varnish (0.95% F, pH 5.0); 4.20% NaF varnish (1.90% F, pH 5.0); 5.42% NaF varnish (2.45% F, pH 5.0) and control (no treatment, n=5). The fluoride release was analyzed after 1/2, 1, 3, 6, 9 and 12 h of exposure. The analysis was performed using an ion-specific electrode coupled to a potentiometer. Two-way ANOVA and Bonferroni's test were applied for the statistical analysis (p<0.05).
Results
TiF4 varnishes released larger amounts of fluoride than NaF varnishes during the first 1/2 h, regardless of their concentration; 4% TiF4 varnish released more fluoride than NaF varnishes for the first 6 h. The peak of fluoride release occurred at 3 h. There was a better dose-response relationship among the varnishes exposed to water than to artificial saliva.
Conclusions
The 3.10% and 4% TiF4 -based varnishes have greater ability to release fluoride into water and artificial saliva compared to NaF varnish; however, more studies must be conducted to elucidate the mechanism of action of TiF4 varnish on tooth surface.
Keywords: Fluoride varnishes, Sodium fluoride, Titanium tetrafluoride
INTRODUCTION
Fluoride products play an important role in the control of dental caries lesions, by reducing demineralization and promoting remineralization. Professional application of topical fluoride leads to a considerably high fluoride acquisition by enamel and dentin, which might favor its effect against dental caries2,3,27.
Fluoride varnishes were developed more than 60 years ago to prolong the contact time between fluoride and tooth surface, increasing fluoride uptake by enamel and the effect on caries control17,26. Clinical evidence has shown that biannual application of fluoride varnishes is effective in the control of caries progression, with a preventive fraction of 30% compared with placebo or non-treatment5,16,19,25. The application of high concentrated NaF, as varnish, is able to promote a CaF2-like layer precipitation on enamel, which can serve as a mineral reservoir, releasing fluoride during the cariogenic challenges22,24.
More recently, another type of fluoride containing a polyvalent metal, titanium tetrafluoride (TiF4), was incorporated into an experimental varnish to improve its effect on enamel de-remineralization13. The effect of TiF4 is related to both fluoride and titanium contents. It is hypothesized that titanium might complex with the phosphate groups producing an acid-resistant surface coating11,20.
The experimental TiF4 varnish has shown to have a superior effect on enamel remineralization in vitro 13 and on the reduction of enamel demineralization in situ compared to NaF varnish8.
Despite studies having shown promising results with the use of TiF4 varnish, there are still no sufficient and insightful data about its mechanism of action from the chemical point of view. Therefore, it would be interesting to test if fluoride release may be different between NaF and TiF4 varnishes using a simple model, without interference of their reaction with tooth, to check if the higher efficacy of TiF4 may be related to the titanium content only or it could be explained by differences in the fluoride release pattern.
Therefore, the aim of the present study was to analyze the amount of fluoride released from experimental TiF4 and NaF varnishes in deionized water and artificial saliva, for a period of 12 h. The null hypothesis was that there is no difference in the amount of fluoride released between the TiF4 and NaF varnishes.
MATERIAL AND METHODS
Sample preparation and treatments
One hundred and thirty acrylic blocks (10x10x2 mm, emporium Acrílicos, Bauru, SP, Brazil) were manufactured for this protocol. Sixty-five blocks were used for the fluoride release analysis in deionized water and 65 for the fluoride release analysis in artificial saliva. The acrylic blocks were then distributed into 6 treatment groups (n=10) and one control (n=5):
1.55% TiF4 varnish (0.95% F, pH 1.0);
3.10% TiF4 varnish (1.90% F, pH 1.0);
4% TiF4 varnish (2.45% F, pH 1.0);
2.10% NaF varnish (0.95% F, pH 5.0);
4.20% NaF varnish (1.90% F, pH 5.0);
5.42% NaF varnish (2.45% F, pH 5.0);
Control (no treatment).
The varnishes were manufactured by the Brazilian Company FGM (FGM Produtos Odontológicos, Joinville, SC, Brazil). Their pH was measured using pH indicator strips (Whatman®International Ltd., Maidstone, England).
Each acrylic block was fixed with a nylon string to the underside of the plastic lid of a 15 ml polystyrene bottle (Injeplast®, São Paulo, SP, Brazil). The amount of 30 mg of each varnish was painted on a side of the acrylic block using a microbrush (KG Sorensen®, Cotia, SP, Brazil). The weight was checked using an analytical balance (Mettler Toledo®, Greifensee, Switzerland).
The blocks were then immersed into 10 ml of deionized water (pH 7.5) or artificial saliva (0.2 mM glucose, 9.9 mM NaCl, 1.5 mM CaCl2.2H2O, 3 mM NH4Cl, 17 mM KCl, 2 mM NaSCN, 2.4 mM K2HPO4, 3.3 mM urea, 2.4 mM NaH2PO4 and 11 μM ascorbic acid; pH 6.8)12 , inside a polystyrene bottle. All reagents were fromMerck®(Darmstadt, Germany), except NaCl (Synth®, LabSynth, Diadema, SP, Brazil), NaSCN (Sigma-Aldrich®, St. Louis, MO, USA) and ascorbic acid (Quimibrás, Rio de Janeiro, RJ, Brazil).
During the course of the experiment, the bottles lids containing the fixed acrylic blocks were transferred to new bottles with equal volume of deionized water or artificial saliva, at room temperature, after 1/2, 1, 3, 6, 9 and 12 h of the exposure. This procedure was done to check the F release pattern along the time, trying to simulate the saliva clearance in vivo. After the experiment, all bottles were kept at 4°C for analysis of the fluoride content.
Fluoride release analysis
The fluoride release analysis was performed using a fluoride ion-sensitive electrode (Thermo ORION 9609, Beverly, MA, USA) coupled to a potentiometer (Thermo ORION 720A+, Beverly, MA, USA). The electrode was calibrated with standard solutions of sequential fluoride concentrations ranging from 0.10 to 25.6 ppm F-. All standard and experimental bottles were buffered with an equal volume of standard Total Ionic Strength Adjustment Buffer solution (TISAB II) and the readings were performed in volume of 1 ml of each sample bottle, in duplicate.
Each sample bottle was evaluated and the fluoride concentration for each sample was determined by the average of the two readings (mV transformed to µg/ml, standard curve r2=0.99). The duplicate readings agreement was in a range of 98-100%. The average of the 10 samples/group was calculated to obtain the concentration of the fluoride released by the corresponding varnish, in a given time period. Both punctual (in a given time) and cumulative (the sum of the punctual) fluoride release were considered in the statistical analysis.
Statistical analysis
GraphPad InStat and Prism softwares (GraphPad Software, San Diego, CA, USA) were used. All data passed the normality and homogeneity tests (Kolmogorov-Smirnov and Bartlett, respectively). The data from the deionized water and artificial saliva protocols were analyzed individually using two-way ANOVA followed by Bonferroni post hoc test. The treatments and the time points were considered as criteria. The significance level of 0.05 was set for all tests.
RESULTS
Two-way ANOVA revealed significant differences among the treatments, the time points and interaction between the factors for both deionized water and artificial saliva.
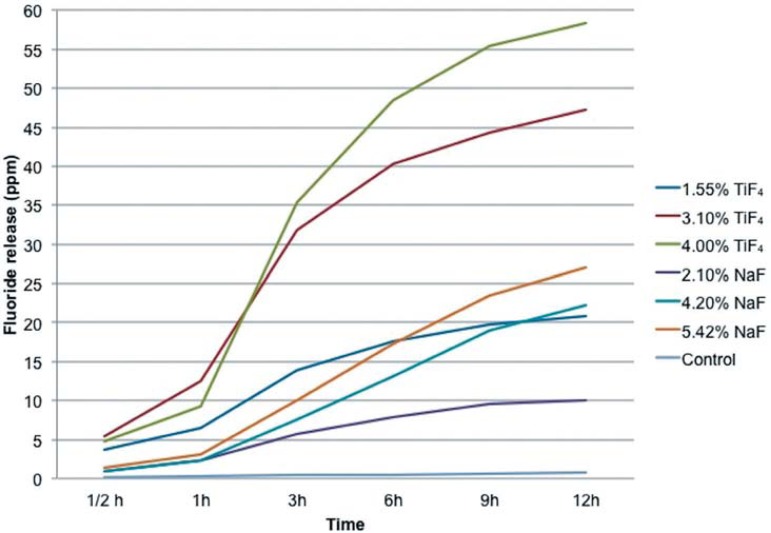
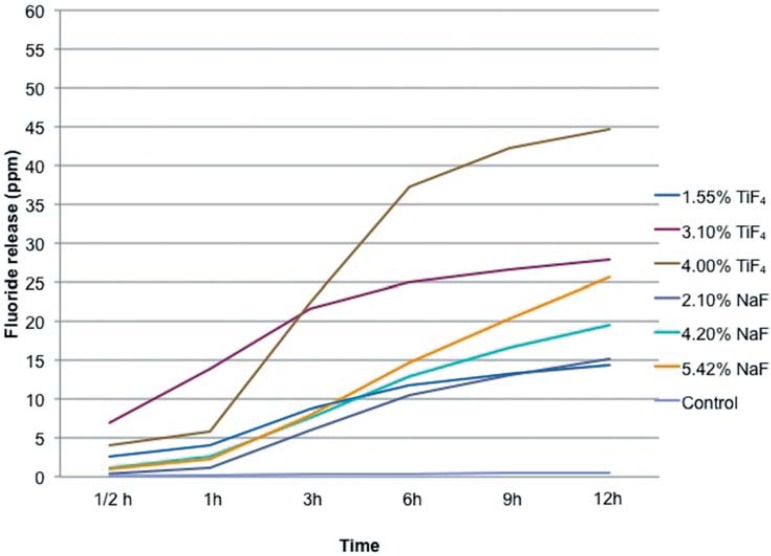
In respect to the cumulative fluoride release, there was some dose-dependency relationship between the fluoride concentration of the experimental varnishes and the amounts of released fluoride, which were better defined for deionized water compared to artificial saliva, especially after 6 h. The TiF4 varnishes released more fluoride into the water than NaF varnishes for all tested concentrations for 12 h (Figure 1). In the artificial saliva, the TiF4 varnishes released more fluoride than the respective NaF varnishes for 6, 9 and 12 h, respectively (Figure 2).
Figura 1.
Mean of the cumulative fluoride release (ppm) from the experimental vernishes in deionized water according to the different periods
Figure 2.
Mean of the cumulative fluoride release (ppm) from the experimental vernishes in artificial saliva according to the different periods
Tables 1 and 2 show the punctual fluoride release into the water and saliva, respectively. The peak of the fluoride release occurred in the first 3 h of contact, but the varnishes were still releasing fluoride up to 12 h of exposure. The TiF4 (except for 1.55%) releasing pattern was significantly higher than the NaF varnishes along 6 h in water and 1/2, 3 and 6 h in artificial saliva.
Table 1.
Mean ± standard deviation (s.d.) of fluoride release (ppm) from the experimental varnishes in deionized water at each time point
| ½h | 1h | 3h | 6h | 9h | 12h | |
|---|---|---|---|---|---|---|
| 1.55% TiF4 | 3.68(0.55)bB | 2.73(0.62)aAB | 7.59(1.85)cC | 3.74(1.35)aB | 2.16(0.27)aAB | 1.37(0.21)aA |
| 3.10% TiF4 | 5.42(1.52)bBC | 7.05(2.72)bCD | 20.12(3.94)dE | 8.58(1.20)bD | 4.09(0.70)bAB | 2.92(1.04)aA |
| 4.00% TiF4 | 4.75(0.87)bA | 4.48(2.00)bA | 26.85(5.26)eD | 13.12(2.22)cC | 6.95(1.17)cB | 3.33(0.57)bA |
| 2.10% NaF | 0.93(0.47)aA | 1.32(0.83)aA | 3.60(1.88)aB | 2.20(0.78)aAB | 1.65(0.71)aAB | 1.01(0.54)aA |
| 4.20% NaF | 0.92(0.30)aA | 1.39(0.27)aA | 5.42(1.73)abBC | 5.59(1.63)aBC | 5.95(1.58)bcC | 3.77(0.99)bB |
| 5.42% NaF | 1.36(0.35)aA | 1.79(0.65)aAB | 7.20(1.50)bcC | 7.55(1.23)bC | 6.21(1.06)bcC | 3.63(0.83)bB |
| Control | 0.16(0.01) | 0.15(0.02) | 0.11(0.00) | 0.09(0.00) | 0.14(0.00) | 0.15(0.01) |
Different lowercase letters show significant differences among the treatments for each time point (comparison per column), whereas capital letters show significant differences among the different time points for each treatment (comparison per line). Two-way ANOVA(p<0.0001). The control group was included to test any possible contamination.
Table 2.
Mean ± standard deviation (s.d.) of fluoride release (ppm) from the experimental varnishes in artificial saliva at each time point
| ½h | 1h | 3h | 6h | 9h | 12h | |
|---|---|---|---|---|---|---|
| 1.55% TiF4 | 2.55(0.55)bAB | 1.45(0.53)aA | 4.72(0.64)aC | 3.08(0.41)aB | 1.40(0.14)aA | 1.26(0.28)aA |
| 3.10% TiF4 | 6.97(1.73)dC | 6.92(1.51)bC | 7.69(1.22)bC | 3.54(0.40)aB | 1.58(0.16)aA | 1.17(0.08)aA |
| 4.00% TiF4 | 4.03(0.55)cB | 1.84(0.32)aA | 16.63(4.79)cD | 14.78(4.46)dC | 5.06(1.56)bcB | 2.42(0.58)abA |
| 2.10% NaF | 0.40(0.13)aA | 0.74(0.17)aAB | 4.93(1.55)aC | 4.43(0.53)abC | 2.64(0.29)abB | 1.99(0.19)abB |
| 4.20% NaF | 1.09(0.39)abA | 1.51(0.36)aAB | 5.04(1.11)aC | 5.36(0.94)bcC | 3.71(0.43)bB | 2.83(0.44)bB |
| 5.42% NaF | 0.92(0.27)aA | 1.34(0.45)aA | 5.69(0.58)aB | 6.80(1.19)cB | 5.56(0.82)cB | 5.44(0.50)cB |
| Control | 0.10(0.00) | 0.10(0.0) | 0.10(0.00) | 0.08(0.00) | 0.08(0.00) | 0.08(0.00) |
Different lowercase letters show significant differences among the treatments for each time point (comparison per column), whereas capital letters show significant differences among the different time points for each treatment (comparison per line). Two-way ANOVA (p<0.0001). The control group was included to test any possible contamination.
DISCUSSION
It is acknowledged that the formation of fluoride reservoirs on the tooth is related to the F amount released from the dental product9,22,24. This study showed a higher amount of fluoride released from TiF4 varnish compared to NaF varnish, which might induce greater fluoride uptake by enamel. This finding might help explaining the best anti-caries effect found for TiF4 compared to NaF in previous studies8,13. Therefore, the null hypothesis of this study was rejected.
The fact that TiF4-based varnishes released more fluoride than NaF varnishes- considering that both were adjusted to contain the same fluoride concentration and the same resin base-might be related to its low pH allowing the dissociation of the fluoride from the resin base.
It is suggested that the maintenance of low levels of fluoride in saliva for long-term periods can control the carious lesions progression4,24. The increase of salivary levels after the application of a topical fluoride agent may be an indicative of the fluoride available for interaction with the teeth surfaces18. Low salivary fluoride level, around 0.04 ppm, has been shown to be correlated with a significant protective effect on dental caries10. Therefore, salivary fluoride levels are considered important parameters to predict the effectiveness of fluoride agents15,24. Accordingly, the results of the present study demonstrate that all the experimental fluoride varnishes might have a good effect on the prevention of dental caries (>0.40 ppm F release), and this effect may be even better for the most concentrated TiF4 varnish (>2.42 ppm F release).
It is important to discuss that the present study was designed to evaluate the amount of fluoride released into water (in the absence of any ionic interaction) and artificial saliva (simulating the ions level in saliva) in vitro. For both media, we could see some relationship between F varnish concentration and F release among the tested products, which were more consistent in water due to the absence of ionic interaction.
The periods of exposition of 12 h were chosen based on previous clinical studies. A previous study showed significant levels of fluoride in saliva within 1 h after application of two fluoride (6% and 2.26% F, respectively) varnishes, which lasted up to 6 h in vivo 23. However, the fluoride level returns to the baseline after 24 h in vivo 9.
In the present study, the peak of fluoride release occurred in the first 3 h. TiF4 releasing pattern was significantly higher than NaF for the first 6 h. These results are similar to a recent study21, in which the short-term fluoride release from four different 5% NaF varnishes was evaluated. The authors observed that over a 48 h period the fluoride release from three NaF varnishes reached a plateau at the first 4 h. One of these varnishes (containing both amorphous calcium phosphate and fluoride) was still showing the highest rate of fluoride release for 8 h.
In a clinical scenario, it is important to determine the fluoride release plateau after an application of fluoride varnish since it can be a parameter to be applied to compare the efficacy of different products. Furthermore, it is also important to establish critical time point considering the instructions that should be given for the patients after a professional fluoride application21.
We have to keep in mind that there are differences between the laboratory study and clinical trials. Oral functions-such as salivation, swallowing and chewing-in addition to brushing and flossing are not present in vitro; thus, the fluoride release and the return to the baseline levels may be faster in a clinical setting6,7. An in situ model showed that, within the first day after fluoride varnish application, KOH-soluble fluoride uptake occurred in enamel samples located only in the vicinity close to the fluoridation site, so the fluoride transfer via saliva was strictly limited to the close neighborhood1. Clinically, it may mean that all sites in the oral cavity that require fluoridation have to be directly treated with the varnish.
Therefore, fluoride release pattern from the experimental varnishes should be confirmed in vivo. Considering that the lowest F concentrated varnish was unable to point out differences between both fluoride salts, it would be suggested to compare only 3% and 4% TiF4 varnishes with the correspondent NaF varnishes in a further clinical trial. There are two hypotheses that could explain this result: TiF4 varnish is only effective in releasing fluoride from the resin when the concentration is higher than 1.55% or NaF at high concentration (>5%, as 5.42%) reaches the fluoride solubility into the varnish, leading to a reduction in fluoride bioavailability. Further studies should be conducted on this field.
Finally, the present results help to explain previous findings showing that 4% TiF4 varnish presents higher efficacy than NaF to reduce demineralization and increase remineralization of carious enamel lesions in vitro and in situ 8,13, in addition to being effective in dental erosion control14. However, further studies should focus on the analysis of weak fluoride and fluoride structurally bound enamel after treatment with the experimental varnishes, in order to better answer this issue.
CONCLUSIONS
The 3.10% and 4% TiF4-based varnishes have greater ability to release fluoride into water and artificial saliva compared to the correspondent NaF varnishes.
ACKNOWLEDGMENTS
The present study was supported by scholarships (LPC #2011/11263-7 and BMS #2010/07001-4) from the São Paulo Research Foundation (FAPESP) and grant(s) from FAPESP (#2012/20698-0) and the National Council for Scientific and Technological Development (CNPq) (#305035/2011-8).
REFERENCES
- 1.Attin T, Lennon AM, Yakin M, Becker K, Buchalla W, Attin R, et al. Deposition of fluoride on enamel surfaces released from varnishes is limited to vicinity of fluoridation site. Clin Oral Investig. 2007;11(1):83–88. doi: 10.1007/s00784-006-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attin T, Schaller HG, Hellwig E. Fluoride uptake in dentin with and without simulating dentinal fluid flow. Clin Oral Investig. 1997;1(3):125–130. doi: 10.1007/s007840050023. [DOI] [PubMed] [Google Scholar]
- 3.Buchalla W, Attin T, Schulte-Monting J, Hellwig E. Fluoride uptake, retention, and remineralization efficacy of a highly concentrated fluoride solution on enamel lesions in situ. J Dent Res. 2002;81(5):329–333. doi: 10.1177/154405910208100508. [DOI] [PubMed] [Google Scholar]
- 4.Buzalaf MA, Pessan JP, Honório HM, ten Cate JM. Mechanisms of action of fluoride for caries control. Monogr Oral Sci. 2011;22:97–114. doi: 10.1159/000325151. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho DM, Salazar M, Oliveira BH, Coutinho ES. Fluoride varnishes and decrease in caries incidence in preschool children: a systematic review. Rev Bras Epidemiol. 2010;13(1):139–149. doi: 10.1590/s1415-790x2010000100013. [DOI] [PubMed] [Google Scholar]
- 6.Castillo JL, Milgrom P, Kharasch E, Izutsu K, Fey M. Evaluation of fluoride release from commercially available fluoride varnishes. J Am Dent Assoc. 2001;132(1):1389–1392. doi: 10.14219/jada.archive.2001.0053. [DOI] [PubMed] [Google Scholar]
- 7.Castillo JL, Milgrom P. Fluoride release from varnishes in two in vitro protocols. J Am Dent Assoc. 2004;135(12):1696–1699. doi: 10.14219/jada.archive.2004.0121. [DOI] [PubMed] [Google Scholar]
- 8.Comar LP, Wiegand A, Moron BM, Rios D, Buzalaf MA, Buchalla W, et al. In situ effect of sodium fluoride or titanium tetrafluoride varnish and solution on carious demineralization of enamel. Eur J Oral Sci. 2012;120(4):342–348. doi: 10.1111/j.1600-0722.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- 9.Eakle WS, Featherstone JD, Weintraub JA, Shain SG, Gansky SA. Salivary fluoride levels following application of fluoride varnish or fluoride rinse. Community Dent Oral Epidemiol. 2004;32(6):462–469. doi: 10.1111/j.1600-0528.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 10.Featherstone JDB. Prevention and reversal of dental caries: role of low level of fluoride. Community Dent Oral Epidemiol. 1999;27(1):31–40. doi: 10.1111/j.1600-0528.1999.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 11.Gu Z, Li J, Söremark R. Influence of tooth surface conditions on enamel fluoride uptake after topical application of TiF4 in vitro. Acta Odontol Scand. 1996;54(5):279–281. doi: 10.3109/00016359609003538. [DOI] [PubMed] [Google Scholar]
- 12.Klimek J, Hellwig E, Ahrens G. Fluoride taken up by plaque, by the underlying enamel and by clean enamel from three fluoride compounds in vitro. Caries Res. 1982;16(2):156–161. doi: 10.1159/000260592. [DOI] [PubMed] [Google Scholar]
- 13.Magalhães AC, Comar LP, Rios D, Delbem AC, Buzalaf MA. Effect of a 4% titanium tetrafluoride varnish on demineralisation and remineralisation of bovine enamel in vitro. Dent. 2008;36(2):158–162. doi: 10.1016/j.jdent.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Magalhães AC, Wiegand A, Rios D, Buzalaf MA, Lussi A. Fluoride in dental erosion. Monogr Oral Sci. 2011;22:158–170. doi: 10.1159/000325167. [DOI] [PubMed] [Google Scholar]
- 15.Margolis HC, Moreno EC. Physiochemical perspectives on the cariostatic mechanism of systemic and topical fluorides. J Dent Res. 1990;69:606–613. doi: 10.1177/00220345900690S119. [DOI] [PubMed] [Google Scholar]
- 16.Marinho VC, Higgins JP, Logan S, Sheiham A. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2002;3: doi: 10.1002/14651858.CD002279. [DOI] [PubMed] [Google Scholar]
- 17.Marinho VC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2013;7: doi: 10.1002/14651858.CD002279.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveby A, Ekstrand J, Lagerlof F. Effect of salivary flow rate on salivary fluoride clearance after use of a fluoride-containing chewing gum. Caries Res. 1987;21(5):393–401. doi: 10.1159/000261045. [DOI] [PubMed] [Google Scholar]
- 19.Petersson LG, Twetman S, Dahlgren H, Norlund A, Holm AK, Nordenram G, et al. Professional fluoride varnish treatment for caries control: a systematic review of clinical trials. Acta Odontol Scand. 2004;62(3):170–176. doi: 10.1080/00016350410006392. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro CC, Gibson I, Barbosa MA. The uptake of titanium ions by hydroxyapatite particles-structural changes and possible mechanisms. Biomaterials. 2006;27(9):1749–1761. doi: 10.1016/j.biomaterials.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Ritwik P, Aubel JD, Xu X, Fan Y, Hagan J. Evaluation of short-term fluoride release from fluoride varnishes. J Clin Pediatr Dent. 2012;36(3):275–278. doi: 10.17796/jcpd.36.3.q304488478w52334. [DOI] [PubMed] [Google Scholar]
- 22.Saxegaard E, Rölla G. Fluoride acquisition on and in human enamel during topical application in vitro. Scand J Dent Res. 1988;96(6):523–535. doi: 10.1111/j.1600-0722.1988.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 23.Twetman S, Sköld-Larsson K, Modéer T. Fluoride concentration in whole saliva and separate gland secretions after topical treatment with three different fluoride varnishes. Acta Odontol Scand. 1999;57(7):263–266. doi: 10.1080/000163599428670. [DOI] [PubMed] [Google Scholar]
- 24.Vogel GL. Oral fluoride reservoirs and the prevention of dental caries. Monogr Oral Sci. 2011;22:146–157. doi: 10.1159/000325166. [DOI] [PubMed] [Google Scholar]
- 25.Weintraub JA, Ramos-Gomez F, Jue B, Shain S, Hoover CI, Featherstone JD, et al. Fluoride varnish efficacy in preventing early childhood caries. J Dent Res. 2006;85(2):172–176. doi: 10.1177/154405910608500211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weyant RJ, Tracy SL, Anselmo TT, Beltrán-Aguilar ED, Donly KJ, Frese WA, et al. Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc. 2013;144(11):1279–1291. doi: 10.14219/jada.archive.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiegand A, Krieger C, Attin R, Hellwig E, Attin T. Fluoride uptake and resistance to further demineralisation of demineralised enamel after application of differently concentrated acidulated sodium fluoride gels. Clin Oral Investig. 2005;9(1):52–57. doi: 10.1007/s00784-005-0306-7. [DOI] [PubMed] [Google Scholar]