Abstract
Human natural killer (NK) cells have distinct functions as NKtolerant, NKcytotoxic and NKregulatory cells and can be divided into different subsets based on the relative expression of the surface markers CD27 and CD11b. CD27+ NK cells, which are abundant cytokine producers, are numerically in the minority in human peripheral blood but constitute the large population of NK cells in cord blood, spleen, tonsil and decidua tissues. Recent data suggest that these NK cells may have immunoregulatory properties under certain conditions. In this review, we will focus on these new NK cell subsets and discuss how regulatory NK cells may serve as rheostats or sentinels in controlling inflammation and maintaining immune homeostasis in various organs.
Keywords: cell differentiation, human natural killer cells
Introduction
For a long time, natural killer (NK) cells were regarded only as killers but now they are thought not only to have key roles in innate immunity but also to have important functions that shape and influence adaptive immune responses and play immunoregulatory roles. However, NK cells are not a homogeneous cell population and the diversity of NK cells has been demonstrated by the diversity of NK cell receptors and functions. In human peripheral blood, the CD56+ CD3− NK cell subpopulations can be defined on the basis of the relative expression of the markers CD16 and CD56. CD56dim CD16+ NK cells are found predominantly in the peripheral blood and can spontaneously lyse targeted tumour cells, yet CD56bright CD16− NK cells are found mostly in the lymphoid organs and can produce abundant amounts of cytokines but have little ability to kill tumour cell targets.1–3 Recent studies have also reported that CD27 of the tumour necrosis factor receptor family is an important marker for distinguishing between NK cell subsets.4,5 The surface density of CD27 and CD11b divides both human and murine NK cells into four subsets and denotes their level of maturation.6,7
The local microenvironment and unique cellular interactions provide important signals to shape the properties of NK cells. In the microenvironment of a pathological process, NK cells persistently and progressively access local inflammatory factors to induce programmed differentiation and proliferation, ultimately generating NKtolerant, NKcytotoxic and NKregulatory cells. Moreover, recent research highlights the fact that natural killer cells act not only as killers towards tumour or virus-infected cells, but also as regulatory cells to affect the adaptive immune response.8 Here, we review the recent advances mainly concerning human regulatory NK cells and present some data obtained in our laboratory. We will focus on the new NK cell subsets and discuss how regulatory NK cells may be involved in controlling inflammation and maintaining immune homeostasis in different organs.
Human NK subsets divided in phenotype and function
In 1983, Lewis Lanier was the first to divide NK cells into subsets.9 Now, it is widely accepted that human mature NK cells have two subsets: CD56dim NK and CD56bright NK.1,10 However, mouse NK cells do not express the CD56 antigen; hence, translating the biological information in mouse NK cells to human NK cells is problematic. Meanwhile, the development of mouse NK cells has been widely studied using the precursors. The integrin CD11b (Mac-1) has been regarded as a mature marker of both mouse and human NK cells.11,12 CD27 has been indicated as a marker to divide mature NK cells into two subsets.4 NK cells from CD27-deficient mice show normal NK cell differentiation but impaired function upon stimulation.13 Subsequently, the heterogeneity of mature murine NK cells was ultimately represented by four subsets on the basis of CD27 and CD11b.7 These new NK subsets have quickly attracted much attention because human NK cells have also been shown to express CD27, making comparative interpretations of the functionality of the subsets more straightforward.4,5 In the mouse, NK cells can be divided into CD27lo CD11blo, CD27hi CD11blo, CD27hi CD11bhi and CD27lo CD11bhi stages. The differentiation of NK cells has been shown to proceed from CD27hi CD11blo through CD27hi CD11bhi to CD27lo CD11bhi.5 In humans, it has been indicated that approximately 6% of peripheral blood NK cells express CD27, 14% of CD27+ NK cells exist in bone marrow, and > 30% of CD27+ NK cells exist in the spleen and tonsils.5 Our group has characterized four novel populations defined by CD11b and CD27, which can represent the distinct stages of human NK cells from different tissues. More than 90% of NK cells from peripheral blood are of the CD11b+ CD27− population, whereas NK cells from cord blood have populations that are 80% CD11b+ CD27− and 20% CD11b+ CD27+. Compared with these two types of NK cells, decidual NK cells are more immature, having nearly 60% CD11b− CD27− NK cells and > 20% CD27+ NK cells. The NK cells from tumour-infiltrating tissues also showed large populations of the CD11b− CD27− subset,14 indicating the heterogeneity of NK cells (Fig. 1). Each population could be characterized by unique functional and phenotypic attributes: CD11b− CD27+ and CD11b+ CD27+ NK cells show the best ability to secrete cytokines, CD11b+ CD27− NK cells exhibit high cytolytic function, and CD11b− CD27− NK cells display an immature phenotype, expressing high percentages of NKG2A.15
Figure 1.
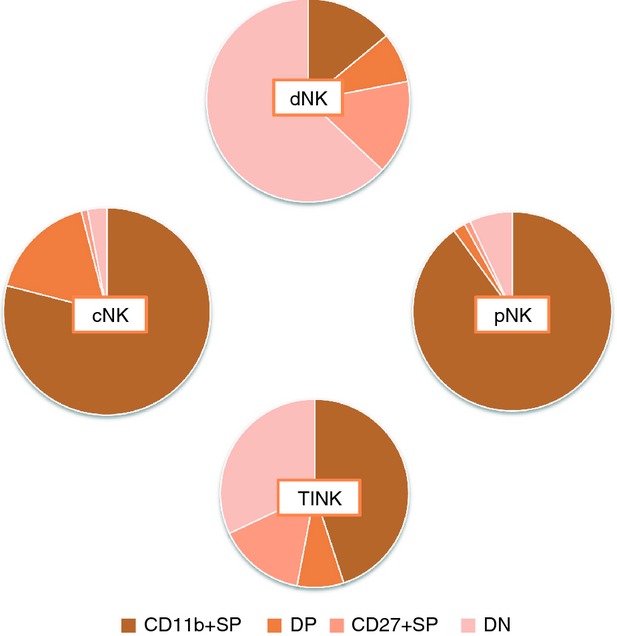
Four natural killer (NK) subsets defined by CD11b and CD27 in humans. Human NK cells can be divided into four subsets on the basis of the relative expressions of the markers CD11b and CD27, including CD11b+ CD27− (CD11b+ SP), CD11b+ CD27+ (DP), CD11b− CD27+ (CD27+ SP) and CD11b− CD27− (DN). More than 90% of NK cells from peripheral blood (pNK) are of the CD11b+ CD27− population, whereas NK cells from cord blood (cNK) have 80% CD11b+ CD27− and 20% CD11b+ CD27+ subset. Decidual NK cells (dNK) are nearly 60% CD11b− CD27− and > 20% CD27+ subset. NK cells from tumour-infiltrating tissues (TINK) also show a large population of the CD11b−CD27− subset.
Affected by various microenvironments and signals, NK cells can be divided into three functional subsets: NKtolerant (NK cells with dominant inhibitory signals), NKcytotoxic (NK cells with dominant activating signals, target cells with a high expression of pressure stimulus-induced ligand) and NKregulatory (NK cells with dominant activating signals, target cells with a high expression of inflammatory molecules) (Fig. 2). From the phenotype, the NKcytotoxic subset is mainly CD56dim NK cells or CD11b+ CD27− NK cells defined on the basis of the relative expression of the markers CD11b and CD27. The NKtolerant subset is mainly CD56bright NK cells or CD27− CD11b− NK cells. The NKregulatory subset is mainly CD56bright NK cells or CD27+ NK cells. Furthermore, these different NK subsets exist in a variety of tissues or organs, reflecting their functional diversity.16 For example, liver NK cells can mediate immune tolerance or immune injury,17–19 decidual NK cells can mediate maternal–fetal immune regulation or vascular remodelling,20 and tumour-infiltrating NK (TINK) cells can mediate tumour immune escape or direct killing.21
Figure 2.
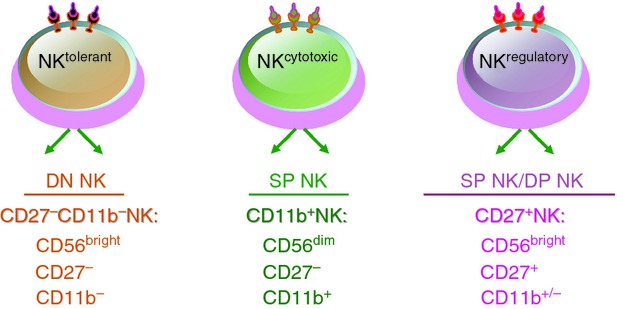
Human natural killer (NK) subsets presented according to phenotype and function. Human NK cells can be divided into three functional subsets: NKtolerant, which is mainly CD56bright NK cells or CD27− CD11b− NK cells; NKcytotoxic, which is mainly CD56dim NK cells or CD11b+ CD27− NK cells; NKregulatory, which is mainly CD56bright NK cells or CD27+ NK cells.
The main checkpoint in the differentiation of NK subsets
The differentiation of NK cells depends on extrinsic regulation within the physiological microenvironment and the pathological microenvironment in addition to intrinsic regulation by various transcription factors.
Under the effect of early haematopoietic growth factors, such as FLT-3 ligand and c-kit ligand, CD34+ haematopoietic stem cells (HSCs) up-regulate the expression of interleukin-2 (IL-2)/IL-15Rβ (CD122) and gradually differentiate into the CD34+ CD122+ CD56− NK precursor cells.22 Via the CD122 molecule, these NK precursor cells obtain the ability to respond to IL-15, which is produced mainly by bone marrow stromal cells and plays a key role in the ultimate expression of CD56 to promote the formation of mature CD3− CD56+ NK cells.23–25 However, several observations also suggested that bone marrow is not the only important site for NK cell development. One clue is that NK cells can also develop from other secondary lymphoid tissue such as the lymph nodes and tonsils.26 Most of these haematopoietic precursor cells become CD56bright NK cell subsets when stimulated by IL-15 or IL-2 or activated lymph node T cells.27,28 In human intestinal mucosa, CD34+ CD45RA+ NK precursor cells expressing CD38, CD33, IL-2Rα and IL-7Rα, with the abundant expression of Id2, PU.1 and SpiB1, may differentiate into CD56+ c-kitdim cells during in vitro culture.29,30 In addition to bone marrow, lymph nodes and the small intestine, NK cells can also develop in the liver, spleen and thymus.31
The main checkpoints that lead to the generation of different NK subsets appear to depend on the pathological microenvironment, local-specific chemokines and cytokines, as well as unique cellular interactions. Natural killer cells express a variety of chemokine receptors, which are affected by the local tissue microenvironment. CD56dim CD16+ NK cells at a resting state highly express CXCR1, CXCR2, CXCR3, CXCR4 and CX3CR1, whereas CD56bright CD16− NK cells highly express CCR5 and CCR7. These receptors interact with their corresponding chemokines and regulate the migration of NK cells to various tissues, thereby playing different biological functions.32 For example, during pregnancy, human CD56bright CD16− NK cells in peripheral blood can be recruited by chemokine CXCL12 and migrate to the uterus.33 In B16 metastatic melanoma, CX3CR1 plays an important role for DX5+ CD3− cells accumulating in the lung.34 Moreover, CXCL16, constitutively presented by the liver endothelium, plays an important role in maintaining the CXCR6+ NK subset in the liver.35
Cytokines from accessory cells in the microenvironment have been revealed to have an important impact on the maturation and function of NK cells. In patients with systemic lupus erythematosus, interferon-α (IFN-α) produced by plasmacytoid dendritic cells mediate the activation-induced cell death of NK cells.36 In persistent hepatitis B virus liver infection, transforming growth factor-β1 (TGF-β1) exhibits an important role in reducing the expression of NKG2D/DAP10 and 2B4/SAP to impair NK cell function and induce tolerant NK cells.37 It has been indicated that CD56bright NK cells are present in human lymph nodes and are co-stimulated by CD4+ T-cell-derived IL-2 to secrete IFN-γ.28 In the tumour microenvironment, regulatory T cells can effectively suppress NK cell-mediated tumour rejection via a TGF-β-dependent mechanism.38,39 Interfering with such a negative impact, tumour-infiltrating NK cells induce a substantial CD11b− CD27− NK cell population that exhibits profound defects in degranulation and IFN-γ production in humans.14 Moreover, in the pathological microenvironment of cancer, monocytes have been shown to mediate the terminal differentiation of peripheral NK cells and to sustain their transition from the CD11b+ CD27+ to CD11b+ CD27− stage.40 Interestingly, another study has further reported that members of the commensal microbiota are necessary for the priming of NK cells by mononuclear phagocytes.41 Mature neutrophils have recently been shown to be required both in the bone marrow and in the periphery for proper NK cell development, and neutrophil deficiency impairs the maturation of CD11b+ CD27+ NK to CD11b+ CD27− NK in mice. The role of neutrophils as key regulators of NK cell functions was confirmed in patients with severe congenital neutropenia and autoimmune neutropenia.42 Hence, the pathological microenvironment including specific cytokines, chemokines and several immune responses shapes NK cells, emphasizing the plasticity and the adaptive nature of these innate immune cells.
The differentiation and maturation of NK cells are accompanied by the intrinsic signals from transcription factors. Recent studies in mice have afforded great progress in our understanding of the transcription factors involved in NK cell development.3 For example, PU.1, E4pb4, Ikaros and Ets-1 are involved in the generation of NK precursor cells.43–46 Although Id2 is expressed in pre-pro-NK cells, its activity is required later during NK development.47 T-bet expression is required for the maintenance and homeostasis of immature NK cells, whereas the induction of Ly49 receptors and DX5 requires cooperation with Eomes.48 Later, GATA-3 plays an important role in NK cell expression of the mature marker CD11b and IFN-γ production.49 The final maturation of NK cells involves the reduction of CD27, and the proliferative potential requires Blimp-1.50 These transcription factors provide important intrinsic signals that impact the differentiation of NK cells and shape the cytotoxicity or immunoregulatory effects of NK cell activation.
In summary, the physiological microenvironment provides conditions for the development and differentiation of NK cells, and the pathological microenvironment induces NK cell activation, programmed proliferation and function polarization, whereas transcription factors mediate intrinsic signals for NK cell maturation and function (Fig. 3). Although several cytokines, such as type I IFN, IL-2, IL-12, IL-15, IL-18 and insulin-like growth factor-1, are potent activators of the NK cell effector function,51–53 very limited information is available to demonstrate the key threshold required to induce regulatory NK cells. Nevertheless, several cytokines may have impacts on the generation of regulatory NK cells. Transforming growth factor-β has key impact on NK cells and promotes the conversion of CD16+ peripheral blood NK cells into CD56bright NK cells.54 Evidence has also shown that IL-7 is necessary for promoting the survival of the regulatory CD56bright NK cell subset.55 The way of inducing regulatory NK cells and the mechanism involved remain to be explored further.
Figure 3.
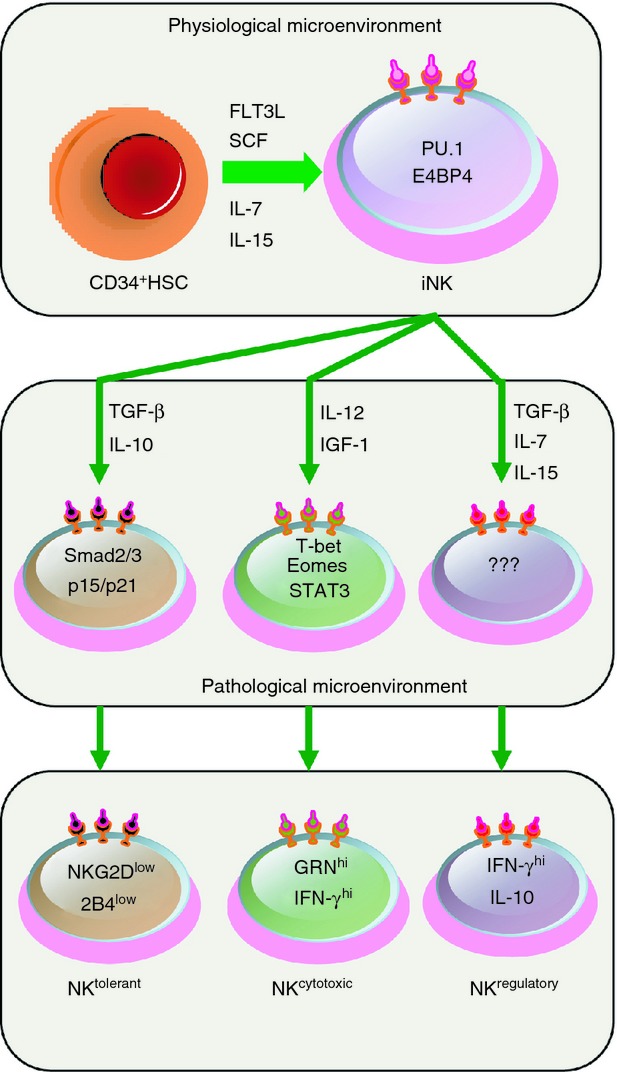
The programmed differentiation of natural killer (NK) cells and the generation of NKtolerant, NKcytotoxic and NKregulatory cells. The programmed differentiation of NK cells can be divided into three steps. First, NK cells predominantly develop from CD34+ haematopoietic stem cells (HSCs) in the physiological microenvironment of bone marrow or lymph nodes, producing immature NK (iNK) cells. Second, under the effect of chemokines, NK cells are recruited into different pathological microenvironments, such as the uterus and brain, and then develop under the control of specific cytokines and transcription factors. Third, these differentiated NK cells may act as NKtolerant, NKcytotoxic or NKregulatory cells. IFN-γ, interferon-γ; IGF-1, insulin-like growth factor 1; IL-7, interleukin-7; SCF, stem cell factor; STAT3, signal transducer and activator of transcription 3; TGF-β, transforming growth factor-β. ??? refers to unknown transcription factors that provide important intrinsic signals to impact the differentiation of regulatory NK cells.
Regulatory NK cells in organs
In the first trimester of pregnancy, nearly 70% of human decidual lymphocytes are NK cells with a CD56bright CD16− phenotype, making deciduas a typical model to use when researching regulatory NK cell subsets. These accumulated NK cells may migrate from the peripheral blood through a CXCR4-and CXCL12-dependent mechanism33 or may develop in situ from CD34+ haematopoietic precursors56 or endometrial NK cells.57 We and others have provided evidence that human decidual NK cells comprise a large population of the CD27+ CD11b− and CD27− CD11b− subset, express the activation markers CD69 and killer cell immunoglobulin-like receptors and are granulated but of low cytotoxicity.15,58 Decidual NK cells, capable of producing IL-22, have been found to resemble the unique early developmental stages of human NK cell differentiation.59 Multiple tetraspanin family members, such as CD9 and CD151, have also been found to be exclusively expressed on decidual NK cells but not on peripheral blood NK cells. Two secreted proteins, galectin-1 and progestogen-associated protein 14, which are known to have immunomodulatory functions, are selectively expressed in decidual NK cells.58 These characteristics make decidual NK cells a unique subset of NK cells with immunomodulatory potential, sharing the properties of, but not identical to, peripheral blood NK cells.
Decidual NK cells exist at the unique maternal–fetal interface, whereby a pregnant mother recognizes her semi-allogeneic fetus, and her immune system has to retain tolerance and not reject the fetus. Recent studies have characterized that decidual NK cells play a key role in this adaptation. Croy and colleagues reported landmark research in which decreased NK cells in mouse deciduas led to the disordered adaption of blood vessels in the uterine mucosa. Decidual NK cell-derived IFN-γ is required for vascular modifications to occur during pregnancy, and it is now evident that NK cell depletion or disruption of the IFN-γ signal in mice results in altered vascular remodelling.60–62 Human decidual NK cells have also been shown to control trophoblast invasion and vascular remodelling through their ability to secrete an array of angiogenesis-regulating molecules, cytokines and chemokines, such as vascular endothelial growth factor, IL-8, IFN-inducible protein-10 and placental growth factor.63 Hence, in addition to simply killing cells, a new paradigm of NK cell function has emerged through the pregnancy model, whereby these cells also promote the regulation of tissue homeostasis.
Moreover, invasion from allogenic fetal cells or spiral arteries may cause inflammation at the maternal–fetal interface. Indeed, the prevention of strong inflammatory responses is essential to ensure normal pregnancy.64 Our group recently showed that CD56bright CD27+ decidual NK cells function as key regulatory cells at the maternal–fetal interface by suppressing T helper type 17-mediated local inflammation via IFN-γ-dependent pathways. This NK cell-mediated regulatory response is lost in women with recurrent spontaneous abortions, resulting in a prominent T helper type 17 response, extensive local inflammation and eventual loss of maternal–fetal tolerance.65 These findings provided evidence that decidual NK cells act as sentinel cells to control local inflammation, which is clearly critical for maintaining tolerance at the fetal–maternal interface. A recent study also demonstrates that resident decidual NK cells have close contact with particular myelomonocytic CD14+ cells, which results in the induction of regulatory T cells.66 Interestingly, seminal studies of human maternal and fetal genotypes have suggested that the interactions between fetal HLA-C molecules and killer cell immunoglobulin-like receptors on uterine NK cells are important for reproductive success, showing that the regulation of NK cell activation is crucial for normal placentation and hence a successful pregnancy.67 Hence, both mouse and human studies suggest that decidual NK cells act as key regulatory cells at the maternal–fetal interface by regulating trophoblast invasion and vascular remodelling, promoting tolerogenic DCs and monocytes and suppressing T helper type 17-mediated local inflammation (Fig. 4).
Figure 4.
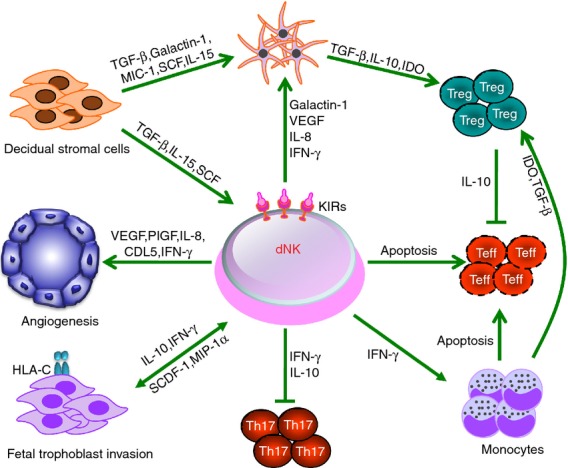
Key pathways of the regulatory decidual natural killer (dNK) cells involved in immune tolerance during the first trimester of human pregnancy. Decidual NK cells with a CD56bright CD16− phenotype can control trophoblast invasion and vascular remodelling, inhibit inflammatory T helper type 17 cells, promote the generation of indoleamine 2,3-dioxygenase (IDO)-producing monocytes and regulatory T cells and induce the apoptosis of effector T cells. Meanwhile, dNK cells are maintained and educated by the decidual microenvironment including stromal cells, trophoblast cells and hormones such as progesterone. IFN-γ, interferon-γ; IL, interleukin; MIC-1, macrophage inhibitory cytokine-1; MIP-1α, macrophage inflammatory protein-1α; PlGF, placental growth factor; SCDF-1, stromal cell-derived factor-1; SCF, stem cell factor; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
In other organs, regulatory NK cells can also inhibit immune reactions. In the central nervous system (CNS), depletion of NK cells from Lewis rats, SJL mice and C57BL/6 mice exacerbated demyelination in different experimental autoimmune encephalomyelitis models.68–70 Importantly, the effects of NK cells on CNS pathology are dependent on the activity of CNS-resident but not peripheral NK cells,69,71 demonstrating that CNS-specific NK cells control inflammation during experimental autoimmune encephalomyelitis in mice. In humans, the administration of daclizumab, a humanized monoclonal antibody against the IL-2 receptor α-chain (CD25), consistently reduces CNS lesions and inflammation in multiple sclerosis patients.72–74 Daclizumab therapy was associated with a significant expansion of regulatory CD56bright NK cells in vivo and a gradual decline in circulating CD4+ and CD8+ T cells, providing supporting evidence for the existence of an immunoregulatory pathway through which activated CD56bright NK cells inhibit T-cell survival.75 Defined by the differential expression of a combination of CD27 and CD11b, analysis of NK cell subsets indicated that the immature subset was dominant in the liver and that the immature CD27+ CD11b− hepatic NK cell subset was protective against liver metastasis,76 indicating that the liver maintains a special local immune tolerogenic microenvironment and educates NKtolerant cells.77
Concluding remarks
Herein, we review the NK subsets and the regulatory effect of NK cells, and provide examples of how these cells may serve as rheostats or sentinels in controlling inflammation and in maintaining immune homeostasis in different organs. We also discuss the three differentiated functions of NK cells in different microenvironments: NKtolerant, NKcytotoxic and NKregulatory cells. It is interesting that regulatory CD56bright CD16− NK cells predominate in extensive disease models, such as in deciduas during pregnancy,65 rheumatoid arthritis joints,78 the CNS after daclizumab treatment75 and patients with hepatitis B virus after pegylated IFN-α therapy.79 However, many aspects of regulatory NK cells remain to be unveiled. The persisting questions include the following. Which subpopulation of NK cells plays the key role as regulatory NK cells? What is the relationship between CD56bright NK and CD27+ NK cells? How does the organ-specific pathological microenvironment direct NK cells into different directions? Which transcription factors are involved in the regulatory effect of NK cells? Additionally, few studies have been undertaken to explore regulatory NK cells in humans. Although many observations and the mechanisms involved remain to be explored, the regulatory ability of NK cells deserves further attention, as the improved understanding of regulatory NK cells may pave the way for new immunotherapeutic approaches for alleviating or preventing many diseases.
Acknowledgments
We sincerely apologize to colleagues whose work could not be adequately discussed or cited owing to space limitations. Our work is supported by Natural Science Foundation of China Grants 81330071 and 81202367; the Ministry of Science and Technology of China 973 Basic Science Project Grants 2013CB530506 and 2012CB519004;China Postdoctoral Science Foundation 2012M511424.
Disclosures
All authors declare no competing financial interests.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flodstrom-Tullberg M, Bryceson YT, Shi FD, Hoglund P, Ljunggren HG. Natural killer cells in human autoimmunity. Curr Opin Immunol. 2009;21:634–40. doi: 10.1016/j.coi.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Narni-Mancinelli E, Ugolini S, Vivier E. Tuning the threshold of natural killer cell responses. Curr Opin Immunol. 2013;25:53–8. doi: 10.1016/j.coi.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 5.Vossen MT, Matmati M, Hertoghs KM, et al. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol. 2008;180:3739–45. doi: 10.4049/jimmunol.180.6.3739. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–96. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Zhang J, Tian Z. The regulatory effect of natural killer cells: do “NK-reg cells” exist? Cell Mol Immunol. 2006;3:241–54. [PubMed] [Google Scholar]
- 9.Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural-killer cells defined by expression of the Leu-7 (Hnk-1) and Leu-11 (Nk-15) antigens. J Immunol. 1983;131:1789–96. [PubMed] [Google Scholar]
- 10.Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56 (bright) natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–65. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–8. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 12.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Colvenaer V, Taveirne S, Delforche M, et al. CD27-deficient mice show normal NK-cell differentiation but impaired function upon stimulation. Immunol Cell Biol. 2011;89:803–11. doi: 10.1038/icb.2010.171. [DOI] [PubMed] [Google Scholar]
- 14.Jin J, Fu B, Mei X, Yue T, Sun R, Tian Z, Wei H. CD11b− CD27− NK cells are associated with the progression of lung carcinoma. PLoS ONE. 2013;8:e61024. doi: 10.1371/journal.pone.0061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 2011;133:350–9. doi: 10.1111/j.1365-2567.2011.03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11:658–71. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654–62. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun HY, Sun C, Tian ZG, Xiao WH. NK cells in immunotolerant organs. Cell Mol Immunol. 2013;10:202–12. doi: 10.1038/cmi.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng H, Jiang X, Chen Y, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–56. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med. 2013;19:548–56. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 21.Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4:84–95. doi: 10.7150/jca.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–97. [PubMed] [Google Scholar]
- 23.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–37. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Miller JS, Alley KA, Mcglave P. Differentiation of natural-killer (Nk) cells from human primitive marrow progenitors in a stroma-based long-term culture system – identification of a Cd34+7+ Nk progenitor. Blood. 1994;83:2594–601. [PubMed] [Google Scholar]
- 25.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–40. [PubMed] [Google Scholar]
- 26.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 27.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 29.Lynch L, O'Donoghue D, Dean J, O'Sullivan J, O'Farrelly C, Golden-Mason L. Detection and characterization of hemopoietic stem cells in the adult human small intestine. J Immunol. 2006;176:5199–204. doi: 10.4049/jimmunol.176.9.5199. [DOI] [PubMed] [Google Scholar]
- 30.Chinen H, Matsuoka K, Sato T, et al. Lamina propria c-kit+ immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterology. 2007;133:559–73. doi: 10.1053/j.gastro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Huntington ND, Vosshenrich CAJ, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–14. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 32.Bernardini G, Gismondi A, Santoni A. Chemokines and NK cells: regulators of development, trafficking and functions. Immunol Lett. 2012;145:39–46. doi: 10.1016/j.imlet.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna J, Wald O, Goldman-Wohl D, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16– human natural killer cells. Blood. 2003;102:1569–77. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 34.Yu YR, Fong AM, Combadiere C, Gao JL, Murphy PM, Patel DD. Defective antitumor responses in CX3CR1-deficient mice. Int J Cancer. 2007;121:316–22. doi: 10.1002/ijc.22660. [DOI] [PubMed] [Google Scholar]
- 35.Paust S, Gill HS, Wang BZ, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian Z, Wei H. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:3421–31. doi: 10.4049/jimmunol.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8:e1002594. doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+-CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 39.Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol. 2013;10:222–9. doi: 10.1038/cmi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soderquest K, Powell N, Luci C, et al. Monocytes control natural killer cell differentiation to effector phenotypes. Blood. 2011;117:4511–18. doi: 10.1182/blood-2010-10-312264. [DOI] [PubMed] [Google Scholar]
- 41.Ganal SC, Sanos SL, Kallfass C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–86. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Jaeger BN, Donadieu J, Cognet C, et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012;209:565–80. doi: 10.1084/jem.20111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gascoyne DM, Long E, Veiga-Fernandes H, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–24. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 44.Colucci F, Samson SI, DeKoter RP, Lantz O, Singh H, Di Santo JP. Differential requirement for the transcription factor PU.1 in the generation of natural killer cells versus B and T cells. Blood. 2001;97:2625–32. doi: 10.1182/blood.v97.9.2625. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez K, Chandler KJ, Spaulding C, Zandi S, Sigvardsson M, Graves BJ, Kee BL. Gene deregulation and chronic activation in natural killer cells deficient in the transcription factor ETS1. Immunity. 2012;36:921–32. doi: 10.1016/j.immuni.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boggs SS, Trevisan M, Patrene K, Geogopoulos K. Lack of natural killer cell precursors in fetal liver of Ikaros knockout mutant mice. Nat Immun. 1998;16:137–45. doi: 10.1159/000069438. [DOI] [PubMed] [Google Scholar]
- 47.Boos MD, Yokota Y, Eberl G, Kee BL. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–30. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, Reiner SL. The transcription factors t-bet and eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36:55–67. doi: 10.1016/j.immuni.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samson SI, Richard O, Tavian M, et al. GATA-3 promotes maturation, IFN-γ production, and liver-specific homing of NK cells. Immunity. 2003;19:701–11. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- 50.Kallies A, Carotta S, Huntington ND, Bernard NJ, Tarlinton DM, Smyth MJ, Nutt SL. A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood. 2011;117:1869–79. doi: 10.1182/blood-2010-08-303123. [DOI] [PubMed] [Google Scholar]
- 51.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l'union fait la force”. Blood. 2005;106:2252–8. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 52.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 53.Ni F, Sun R, Fu B, Wang F, Guo C, Tian Z, Wei H. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat Commun. 2013;4:1479. doi: 10.1038/ncomms2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL. TGF-β promotes conversion of CD16+ peripheral blood NK cells into CD16– NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA. 2007;104:3378–83. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michaud A, Dardari R, Charrier E, Cordeiro P, Herblot S, Duval M. IL-7 enhances survival of human CD56bright NK cells. J Immunother. 2010;33:382–90. doi: 10.1097/CJI.0b013e3181cd872d. [DOI] [PubMed] [Google Scholar]
- 56.Vacca P, Vitale C, Montaldo E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci USA. 2011;108:2402–7. doi: 10.1073/pnas.1016257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manaster I, Mizrahi S, Goldman-Wohl D, et al. Endometrial NK cells are special immature cells that await pregnancy. J Immunol. 2008;181:1869–76. doi: 10.4049/jimmunol.181.3.1869. [DOI] [PubMed] [Google Scholar]
- 58.Koopman LA, Kopcow HD, Rybalov B, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–12. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. 2010;185:3913–18. doi: 10.4049/jimmunol.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashkar AA, Di Santo JP, Croy BA. Interferon-γ contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–70. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Croy BA, He H, Esadeg S, et al. Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction. 2003;126:149–60. doi: 10.1530/rep.0.1260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang JH, Chen ZL, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanna J, Goldman-Wohl D, Hamani Y, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12:1065–74. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 64.Sargent IL, Borzychowski AM, Redman CWG. NK cells and human pregnancy – an inflammatory view. Trends Immunol. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, Wei H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci USA. 2013;110:E231–40. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vacca P, Cantoni C, Vitale M, et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci USA. 2010;107:11918–23. doi: 10.1073/pnas.1001749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharkey AM, Gardner L, Hiby S, et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition gestational age. J Immunol. 2008;181:39–46. doi: 10.4049/jimmunol.181.1.39. [DOI] [PubMed] [Google Scholar]
- 68.Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;163:24–30. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Zhang BN, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–87. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR γδ T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–8. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 71.Hao J, Liu R, Piao W, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–21. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rose JW, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol. 2004;56:864–7. doi: 10.1002/ana.20287. [DOI] [PubMed] [Google Scholar]
- 73.Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis – MRI and clinical results. Neurology. 2007;69:785–9. doi: 10.1212/01.wnl.0000267662.41734.1f. [DOI] [PubMed] [Google Scholar]
- 74.Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol-Chicago. 2009;66:483–9. doi: 10.1001/archneurol.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2R α-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ballas ZK, Buchta CM, Rosean TR, Heusel JW, Shey MR. Role of NK cell subsets in organ-specific murine melanoma metastasis. PLoS ONE. 2013;8:e65599. doi: 10.1371/journal.pone.0065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li FL, Tian ZG. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10:292–302. doi: 10.1038/cmi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pridgeon C, Lennon GP, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158negative phenotype. Rheumatology. 2003;42:870–8. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
- 79.Micco L, Peppa D, Loggi E, et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-α therapy of chronic hepatitis B. J Hepatol. 2013;58:225–33. doi: 10.1016/j.jhep.2012.09.029. [DOI] [PubMed] [Google Scholar]


