Figure 2.
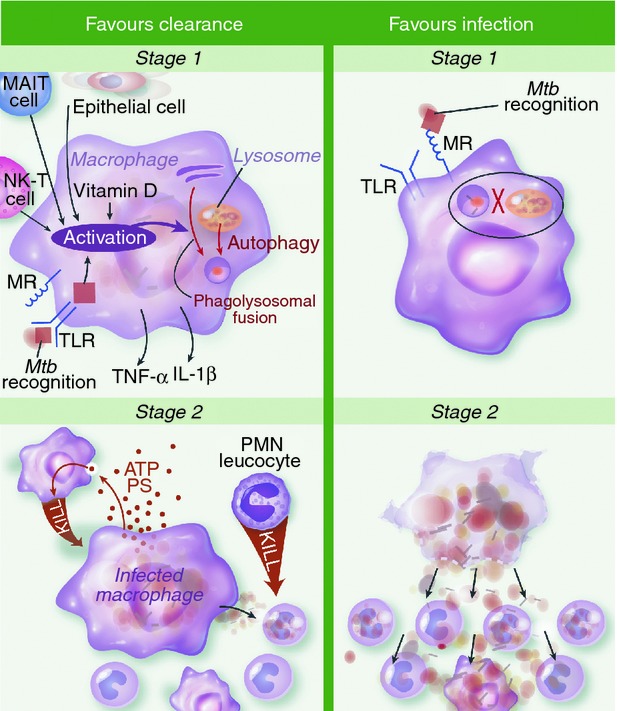
Hypothesized mechanisms of clearance versus infection during first and second stages of Mycobacterium tuberculosis (Mtb) infection. In stage one infection is primarily of alveolar macrophages. Activation of macrophages may be important for early clearance (EC), through recognition of M. tuberculosis via surface, cytosolic or phagosomal pattern recognition receptors (PRRs) and/or tumour necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) secreted by other cells. This promotes killing of intracellular M. tuberculosis by phagosomal acidification, hydrolytic enzymes and generation of reactive nitrogen intermediates. Also, antimicrobial peptides and autophagy promote mycobacterial killing and are both induced by vitamin D. Alternatively, infection is favoured if the macrophage's initial interaction with M. tuberculosis is via ligation of the mannose receptor (MR). This promotes uptake without recognition and inhibition of phagolysosomal fusion. In stage two, infected macrophages undergo apoptosis and express adenosine triphosphate and phosphotidyl serine. This attracts monocytes and neutrophils that engulf the infected cell and deploy oxidative killing mechanisms to achieve clearance. Neutrophils activated in this fashion secrete antimicrobial peptides cathelicidin, human neutrophil peptides and Lipocalin 2 to kill infected monocytes. Sustained infection is most likely when infected macrophages undergo necrotic cell death. The disruption of the macrophage membrane facilitates mycobacterial outgrowth so newly recruited monocytes are infected and logarithmic growth ensues. IL-1β, interleukin-1β; MAIT, mucosa-associated invariant T; NK-T, natural killer T; PMN, polymorphonuclear cells; PS, phosphatidyl serine; TLR, Toll-like receptor
