Abstract
Interleukin-12 (IL-12) p70 and IL-23 are bioactive cytokines and their biological functions are becoming clear. Increased expression of IL-7 in the central nervous system as well as in peripheral immune cells is associated with multiple sclerosis and experimental allergic encephalomyelitis. Here, we describe the induction of IL-7 in primary mouse and human microglia, BV-2 microglial cells, mouse peritoneal macrophages and astrocytes by IL-12p70. Interestingly, IL-12 strongly induced the expression of IL-7 whereas IL-23 and other p40 family members remained weak inducers of IL-7 in these cell types. Consistently, IL-12, but not IL-23 and other p40 family members, induced IL-7 promoter-driven luciferase activity in microglial cells. Among various stimuli tested, IL-12 emerged as the most potent stimulus followed by bacterial lipopolysaccharide and HIV-1 gp120 in inducing the activation of IL-7 promoter in microglial cells. Furthermore, increase in IL-7 mRNA expression by over-expression of IL-12p35 subunit, but not p40 and IL-23 p19 subunit, confirm that p35, but not p40 and p19, is responsible for the induction of IL-7. Finally, by using primary microglia from IL-12 receptor β1-deficient (IL-12Rβ1−/−) and IL-12Rβ2−/− mice, we demonstrate that IL-12 induces the expression of IL-7 in microglia and macrophages via both IL-12Rβ2 and IL-12Rβ1. These studies delineate a novel biological function of IL-12 that is absent in IL-23 and other p40 family members.
Keywords: interleukin-12, interleukin-12 receptor, interleukin-23, interleukin-7, microglia, neuroinflammation
Introduction
Interleukin-7 (IL-7) is a well-characterized pleiotropic cytokine of the IL-2/IL-15 family,1 and is expressed by stromal cells (bone marrow, thymus and soft tissue), epithelial cells (liver, gut), endothelial cells, fibroblasts, smooth muscle cells and keratinocytes, and following activation, by dendritic cells.1 Although it was originally described as a growth and differentiation factor for precursor B lymphocytes, subsequent studies have shown that IL-7 is critically involved in T-lymphocyte development and differentiation. Interleukin-7 signalling is essential for optimal CD8 T-cell function, homeostasis and establishment of memory;2 it is required for the survival of most T-cell subsets, and its expression has been proposed to be important for regulating T-cell numbers.1
Production of IL-7 by stromal and other cells has previously been thought to be constitutive and is unaffected by extrinsic stimuli.3,4 Alternatively, IL-7 induction is reported to be driven by interferon-γ (IFN-γ), keratinocyte growth factor, IL-6 and tumour necrosis factor-α (TNF-α).5–7 On the other hand, transforming growth factor-β inhibits the induction of IL-7.5–7 It has been reported that excessive IL-6 signalling increases the amount of IL-7 expression in vivo, ultimately leading to the development of autoimmune arthritis.8,9 Recently the same group has reported that Toll-like receptor ligands induce IL-7 production from liver cells, although the liver does not appear to be a major contributor of IL-7 to T-cell survival; under normal conditions.8
Several reports indicate that IL-7 levels increase in multiple sclerosis (MS) and experimental allergic encephalomyelitis (EAE). Chou et al.10 have demonstrated the profound effects of IL-7 on antigen-specific human T cells isolated from peripheral blood. The IL-7 enhances proliferation and IFN-γ secretion by myelin-activated T cells cultured from both normal controls and MS patients.10 According to Bebo et al.,11 IL-7-treated myelin-specific mouse T cells have an enhanced ability to induce encephalomyelitis. The IL-7 enhances the proliferation, pro-inflammatory cytokine production and adoptive transfer of EAE by myelin-specific T cells. Although IL-7 appears to be an important cytokine in cell-mediated immunity and protection from infectious diseases, inappropriate production of IL-7 may have a role in the pathogenesis of autoimmunity. Together, these findings suggest that IL-7 may be an important factor for the development of autoimmune disorders. However, mechanisms by which IL-7 is produced under autoimmune inflammation are poorly understood.
Multiple sclerosis is generally considered as an autoimmune pathology in which T helper type 1 (Th1) and Th17 cells have a key role.12 In particular, Th17 cells were recently identified as a distinct lineage of CD4+ effector T cells and are associated with various models of autoimmune disorders, such as EAE.13 Interleukin-7 is essential for survival and expansion of pathogenic Th17 cells in EAE;14 IL-7 directly expands Th17 cells in EAE and human Th17 cells from subjects with MS, whereas it is not required for Th17 differentiation. Interleukin-7 receptor (IL-7R) antagonism renders differentiated Th17 cells susceptible to apoptosis through the inhibition of the Janus kinase–signal transducer and activator of transcription-5 (JAK-STAT5) pathway and alters expression of the pro-survival protein Bcl-2 and the pro-apoptotic protein Bax, leading to decreased severity of EAE. Several cytokines, such as transforming growth factor-β, IL-6, IL-1β and IL-21, have been shown to regulate and induce Th17 cell differentiation, contributing crucially to the clinical outcome of autoimmune disease. Interleukin-23 has been implicated to have a major role in the terminal differentiation of Th17 cells potentially through its effect on re-expression of IL-7R on Th17 cells.14
Classically, MS has been presumed to be a CD4-mediated disease, although the role of the different CD4 effector T cells is currently heavily debated.15 Although the role of CD8 T cells in MS is relatively limited, CD8 T cells outnumber CD4 T cells in MS lesions.16 One of the functions of IL-7 is to maintain the homeostasis of CD8 T cells. CD8 effector memory T cells have the capacity to migrate to non-lymphoid tissues. The frequency of CD127+ CD8 effector memory T cells and the expression levels of CD127 on most CD8+ subsets increase in MS patients.17 In the case of MS, they potentially migrate into the central nervous system (CNS), where they re-encounter IL-7 produced by reactive microglia and astrocytes.17,18 This intrathecally produced IL-7 may further activate and increase the cytoxic potential of these T cells by the up-regulation of genes involved in cytoxicity such as granzymes A and B, and induction of αEβ7 integrin. Granzyme A particularly is capable of causing direct myelin damage via degradation of myelin basic protein (MBP).17,19
Interleukin-12 plays a critical role in the early inflammatory response to infection and in the generation of Th1 cells, which favour cell-mediated immunity.20 It has been found that over-production of IL-12 can be dangerous to the host because it is involved in the pathogenesis of a number of autoimmune inflammatory diseases (e.g. MS, arthritis, type 1 diabetes).21,22 Interleukin-12 consists of an H chain (p40) and an L chain (p35) linked covalently by disulphide bonds to give rise to the so-called bioactive heterodimeric (p70) molecule.23,24 The p40 can also pair with p19 to form IL-23, which has biological functions that are similar to as well as distinct from IL-12. Apart from forming heterodimers (IL-12 and IL-23), the p40 subunit is also secreted as a monomer (p40) and a homodimer (p402).23 Because all of these cytokines (IL-12, IL-23, p40 and p402) contain the common p40 subunit, these cytokines can better be grouped into the p40 family of cytokines. Previously, we have reported that both IL-12p70 and p402 induce the expression of inducible nitric oxide synthase (iNOS) and TNF-α in microglia and macrophages.25,26 We have recently demonstrated that p402, but not IL-12p70, induces the expression of IL-16 and lymphotoxin α in various immune cells.27,28
Here we describe that IL-12, but not IL-23 and other p40 family members, induces the expression of IL-7 in microglia, macrophages and astrocytes. Furthermore, we demonstrate that IL-12 induces the expression of IL-7 via both IL-12Rβ1 and IL-12Rβ2.
Materials and methods
Reagents
Fetal bovine serum, Hanks' balanced salt solution and Dulbecco's modified Eagle's medium (DMEM)/F-12 were from Mediatech (Valley Park, MO). Recombinant mouse IL-12 p70, p402 (the p40 homodimer), IL-23 (human), TNF-α and IL-1β were obtained from R&D Systems (Minneapolis, MN). Recombinant mouse IL-12 p40 (the p40 monomer) was obtained from BD Pharmingen (San Jose, CA). Antibodies against IL-7 and glial fibrillary acidic protein (GFAP) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). HIV-1 gp120 was obtained from US Biologicals (Salem, MA). The IL-12Rβ1−/− and IL-12Rβ2−/− mice and their littermate controls were purchased from Jackson Laboratories (Bar Harbor, ME).
Isolation of primary mouse microglia and astrocytes
Microglia were isolated from mixed glial cultures according to the procedure of Giulian and Baker.29 Animal maintenance and experimental protocols were approved by the Rush University Animal Care Committee. Briefly, mixed glial cells were prepared from 7-to 9-day-old mouse pups. On day 9, the mixed glial cultures were washed three times with DMEM/F-12 and subjected to shaking at 240 rpm for 2 hr at 37° on a rotary shaker. Attached cells were positive for GFAP, a marker for astrocytes. The floating cells were washed and seeded onto plastic tissue-culture flasks and incubated at 37° for 1 hr. The attached cells were removed by trypsinization and seeded on to new plates for further studies. These cells were positive for CD11b (BD Pharmingen), a marker for microglia/macrophages. More than 95% of this preparation was found to be positive for CD11b. Twenty-four hours after plating, some of these cells showed a well-spread amoeboid morphology and the rest exhibited an elongated processed morphology.
For the induction of IL-7 expression, astrocytes and microglia were stimulated with IL-12 p70, p402 and other stimuli under serum-free conditions. Mouse BV-2 microglial cells (a gift from V. Bocchini of the University of Perugia, Perugia, Italy) were also maintained and induced as indicated above.
Isolation of primary human microglia
Primary microglia were isolated from mixed glial cultures according to the procedure of Jana et al.30,31 Briefly, brains from 13–17-week-old human fetuses obtained from the Human Embryology Laboratory (University of Washington, Seattle, WA) were dissociated by trituration and trypsinization. Cells were plated on poly-d-lysine pre-coated 75-cm2 flasks and incubated at 37° with 5% CO2 in air. On the 9th day, the cultures were placed on a rotary shaker at 240 rpm at 37° for 2 hr to remove microglia. The cell suspensions were placed on uncoated culture plates for 30 min followed by removal of non-adherent cells by washing. Adherent cells were cultured in DMEM/F-12 containing 10% fetal bovine serum (FBS); > 98% of these cells were CD11b+.31
Isolation of mouse peritoneal macrophages
Macrophages were isolated by peritoneal lavage from mice with sterile RPMI-1640 medium containing 1% FBS and antibiotic–antimycotic mixture (Sigma, St Louis, MO) as described earlier.25,27 Cells were washed three times with the same media at 4° and were maintained at 37° in a humidified incubator containing 5% CO2 in air. Cells were plated in culture dishes in RPMI-1640 medium containing 1% FBS, l-glutamine and antibiotic–antimycotic mixture. After 1 hr, non-adherent cells were removed by washing, and adherent cells were ∼ 95% pure according to immunological and morphological criteria.
Immunostaining of IL-7
Immunostaining was performed as described earlier.27,28,31 Briefly, coverslips containing 200–300 cells/mm2 were fixed with 4% paraformaldehyde for 15 min, followed by treatment with cold ethanol (−20°) for 5 min and two rinses in PBS. Samples were blocked with 3% BSA in PBS containing Tween 20 (PBST) for 30 min and incubated in PBST containing 1% BSA and rabbit anti-IL-7 (1 : 50). After three washes in PBST (15 min each), slides were further incubated with Cy5 and Cy2 (Jackson ImmunoResearch, Inc., West Grove, PA). For negative controls, a set of culture slides were incubated under similar conditions without the primary antibodies. The samples were mounted and observed under a Bio-Rad (Hercules, CA) MRC1024ES confocal laser-scanning microscope.
Semi-quantitative RT-PCR analysis
The expression of different pro-inflammatory molecules was analysed by semi-quantitative RT-PCR using an RT-PCR kit from BD Clontech (Mountain View, CA) as described earlier.32–34 Briefly, total RNA was isolated from stimulated or unstimulated cells by using the Qiagen mini kit (Qiagen GmbH, Qiagen Strasse and Hilden, Germany) followed by digestion with DNase to remove contaminating genomic DNA. Briefly, 1 μg of DNase-digested RNA was reverse transcribed using oligo(dT)12–18 as primer and Moloney murine leukaemia virus reverse transcriptase (BD Clontech) in a 20-μl reaction mixture. The resulting cDNA was appropriately diluted, and diluted cDNA was amplified using Titanium TaqDNA polymerase and the following primers. The following primers were used to amplify mouse IL-7 molecules: IL-7: sense: 5′-TTC ACC AGT GTT TGT GTG CC-3′; antisense: 5′-CCT CCA CTG ATC CTT GTT CTG C-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): sense: 5′-GGT GAA GGT CGG TGT GAA CG-3′, antisense: 5′-TTG GCT CCA CCC TTC AAG TG-3′.
In contrast, the following primers were used to amplify human IL-7.
IL-7: sense: 5′-ATG CCT GAC CTC AAC TCC TC-3′; antisense: 5′-CTC CTG ATG ACA ATC GTG AC-3′; GAPDH: sense: 5′-GGT GAA GGT CGG SGT CAA CG-3′; antisense: 5′-GTG AAG ACG CCA GTG GAC TC-3′.
Amplified products were electrophoresed on 1·8% agarose gels and visualized by ethidium bromide staining. GAPDH was used to ascertain that an equivalent amount of cDNA was synthesized from different samples.
Real-time PCR analysis
Real-time PCR analysis was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems, Foster City, CA) as described earlier27,35 using forward and reverse primers and FAM-labelled probes (Applied Biosystems). The mRNA expression of IL-7 and cytokines was normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection system 1.6 software (IBM, North Castle, NY) and analysed by analysis of variance.
Construction of mouse IL-7 promoter-driven luciferase construct
Mouse genomic DNA isolated from mouse BV-2 microglial cells was used as template during PCR. The 5′ flanking sequence of mouse IL-7 (−1791/+29) gene was isolated by PCR. Primers were designed from GenBank sequences.
IL-7: sense: 5′-acgcgt CCA CAG TAG CTC TTC CAT CC-3′
antisense: 5′-ctcgag CAC CAG AGA GCA GCG CTT ACC-3′.
The sense primer was tagged with MluI restriction enzyme while the antisense primer was tagged with XhoI. The PCR was performed using an Advantage-2 PCR kit (Clontech, Mountain View, CA) according to the manufacturer's instructions. The resulting fragments were gel purified and ligated into the PGEM-TEasy vector (Promega, Madison, WI). These fragments were further subcloned into the PGL-3 Enhancer vector after digestion with corresponding restriction enzymes and verification by sequencing in the automated sequencer of the University of Nebraska at Lincoln Biotechnology Center.
Assay of IL-7 promoter-driven reporter activity
Cells plated at 50–60% confluence in 12-well plates were co-transfected with 0·25 μg of pIL-7-Luc and 25 ng of pRL-TK (a plasmid encoding Renilla luciferase, used as transfection efficiency control; Promega) using Lipofectamine Plus (Invitrogen, Carlsbad, CA). After 24 hr of transfection, cells were stimulated with different stimuli under serum-free conditions for 6 hr. Firefly and Renilla luciferase activities were analysed in cell extracts using the Dual Luciferase kit (Promega) in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA) as described earlier.26,28 Relative luciferase activity of cell extracts was typically represented as the ratio of firefly luciferase value : Renilla luciferase value × 10−3.
Expression of mouse p35, p19 and p40 cDNA in BV-2 microglial cells
Cells at 50–60% confluence were transfected with different amounts of p35, p19 and p40 cDNA expression construct28,36 by LipofectAMINE Plus (Invitrogen) following the manufacturer's protocol.30,36,37 Twenty-four hours after transfection, cells were incubated with serum-free media. After 6 hr of incubation, cells were harvested and RNA was analysed by semi-quantitative and real-time PCR.
ELISA
Astrocytes and microglia were plated on a 24-well plate and were treated with IL-12p70, p402 and IL-23 for a period of 24 hr. Supernatants in each well were collected, and the presence of IL-7 was assayed using high-sensitivity ELISA kits (R&D Systems).
Induction of EAE
Specific pathogen-free female SJL/J mice and C57/BL6 (4–6 weeks old) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). For active immunization, B6 females were immunized with myelin oligodendrocyte glycoprotein 35–55 (MOG35–55; 100 μg) as described.38 Relapsing–remitting EAE was induced by adoptive transfer of MBP-primed T cells in SJL mice as described earlier.25,34 Briefly, donor mice were immunized subcutaneously with 400 μg bovine MBP (Invitrogen) and 60 μg Mycobacterium tuberculosis (H37RA; Difco Labs, Detroit, MI) in incomplete Freund's adjuvant (Calbiochem, Billerica, MA). Animals were killed 10–12 days post-immunization and the draining lymph nodes were harvested. Single cell suspensions were cultured at a concentration of 4 × 106 to 5 × 106 cells/ml in six-well plates in RPMI-1640 supplemented with 10% FBS, 50 μg/ml MBP, 50 μm 2-mercaptoethanol, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. On Day 4, cells were harvested and resuspended in Hanks' balanced salt solution. Then 2 × 107 viable cells in a volume of 200 μl were injected into the tail vein of naive mice. Pertussis toxin (150 ng/mouse; Sigma) was injected once intraperitoneally (intraperitoneal route on day 0 post-transfer of cells). Cells isolated from donor mice immunized with complete Freund's adjuvant or incomplete Freund's adjuvant alone were not viable after 4 days in culture with 50 μg/ml bovine MBP and therefore were not transferred. MBP reactivity of lymph node cells was measured by [3H]thymidine (New England Nuclear, Plymouth, MA) incorporation assay of parallel microplate cultures.25,34 Animals were observed daily for clinical symptoms. Experimental animals were scored by a masked investigator as follows: 0, no clinical disease; 0·5, piloerection; 1, tail weakness; 1·5, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 3·5, forelimb weakness; 4, forelimb paralysis; 5, moribund or death.
Histological and immunofluorescence microscopy
On day 17 post-transfer (acute phase), six mice from each of the following groups (control and EAE) were anaesthetized. Mice were perfused with PBS (pH 7·4) and then with 4% (weight/volume) paraformaldehyde solution in PBS followed by dissection of the cerebellum and spinal cord from each mouse for immunofluorescence microscopy.25,34,39 For histological analysis, routine histology was performed to obtain perivascular cuffing and morphological details of spinal cord tissues of EAE mice. Paraformaldehyde-fixed tissues were embedded in paraffin, and serial sections (4 μm) of spinal cords were cut. For cerebellum, briefly, samples were incubated in PBST (containing 0·05% Tween 20), 10% sucrose for 3 hr and then 30% sucrose overnight at 4°. Cerebellum was then embedded in O.C.T (Tissue Tech, Miami, FL) at −80°, and processed for conventional cryosectioning. Frozen sections (10 μm) were treated with cold ethanol (−20°) followed by two rinses in PBS. Samples were blocked with 3% BSA in PBST and incubated in PBST-BSA and primary antibodies. For double-labelling, tissue sections were incubated with rabbit anti-IL-7 (Santa Cruz Biotechnology) (1 : 200) along with one of the following antibodies: rat anti-mouse Iba1 (Chemicon, Temecula, CA) (1 : 200) and goat anti-GFAP (Santa Cruz Biotechnology) (1 : 200. After three washes in PBST, sections were further incubated with Cy2 and Cy5 (Jackson ImmunoResearch, Inc.). The samples were mounted and observed under an Olympus fluorescent microscope. Inflammation was scored using the following scale as described. For meninges and parenchyma: 0, no infiltrating cells; 1, few infiltrating cells; 2, numerous infiltrating cells; and 3, widespread infiltration. For vessels: 0, no cuffed vessel; 1, one or two cuffed vessels per section; 2, three to five cuffed vessels per section; and 3, more than five cuffed vessels per section. At least six serial sections of each spinal cord from each of five mice per group were scored and statistically analysed by analysis of variance.
Results
IL-12p70, but not IL-23, induces the expression of IL-7 in mouse and human primary microglia
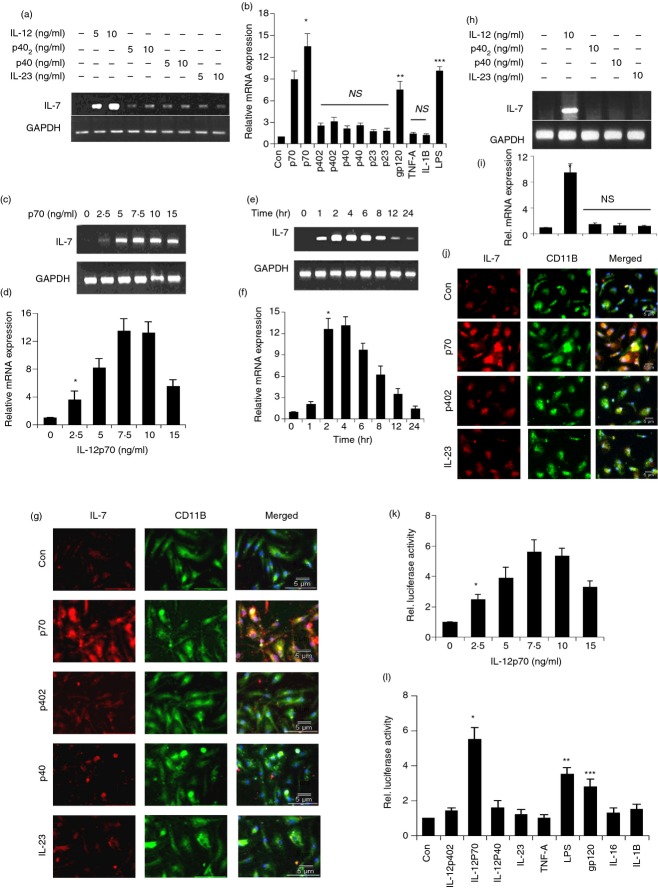
Molecules or agents that stimulate the expression of IL-7, an important factor for T-cell development and differentiation, in microglia/macrophages are not known. To determine whether the p40 family of cytokines (p40, IL-23, p402 and IL-12p70) plays any role in the induction of IL-7 in microglia, we treated the mouse microglia with 5 and 10 ng/ml of p40, IL-23 (p23), p402 and IL-12 for 4 hr. It is clearly evident from Fig. 1(a) that IL-12 markedly induced the mRNA expression of IL-7 in microglia. On the other hand, p402, p40 and IL-23 were unable to induce the expression of IL-7 (Fig. 1a). Real-time PCR results also confirmed that only IL-12, lipopolysaccharide (LPS) and gp120 among other stimuli were capable of inducing the mRNA expression of IL-7 (Fig. 1b).
Figure 1.
Time-and dose-dependent induction of interleukin-7 (IL-7) expression by IL-12p70, p402, p40, and IL-23. Mouse microglia cells were stimulated with different concentration (5 and 10 ng/ml) of IL-12p70, p402, p40, IL-23 (p23), and other stimuli under serum-free conditions. After 4 hr of stimulation, the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (a) and quantitative real-time PCR (b). Results are means ± SD of three different experiments.*P < 0·001 versus control IL-7 mRNA; **P < 0·01 versus control; ***P < 0·01 versus control IL-7 mRNA; NS, non significant. Cells were stimulated with (10 ng/ml) of IL-12p70 under serum-free conditions. At different time-points of stimulation, total RNA was analysed for the expression of IL-7 by semi-quantitative RT-PCR (c) and quantitative real-time PCR (d). Results are means ± SD of three different experiments.*P < 0·001 versus control IL-7 mRNA. Cells were stimulated with different concentration of IL-12p70 under serum-free conditions. After 4 hr of stimulation, total RNA was analysed for the expression of IL-7 by semi-quantitative RT-PCR (e) and quantitative real-time PCR (f). *P < 0·001 versus control IL-7 mRNA; **P < 0·01 versus control; ***P < 0·01 versus control. After 18 hr of stimulation, the expression of IL-7 protein was monitored by immunofluorescence (g). Human primary microglia cells were stimulated with (10 ng/ml) of p402, IL-12p70, p40 and IL-23 under serum-free conditions. After 4 hr of stimulation, the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (h) and quantitative real-time PCR (i). *P < 0·001 versus control IL-7 mRNA. NS, non significant. After 18 hr of stimulation, the expression of IL-7 protein was monitored by immunofluorescence (j). DAPI was used to visualize the nucleus. Cells were plated at 50–60% confluence in 12-well plates and transfected with 0·25 μg pIL-7-Luc and 25 ng pRL-TK (Renilla luciferase control) by Lipofectamine Plus (Invitrogen) as described earlier.30,33 Twenty-four hours after transfection, cells were stimulated with different concentrations of IL-12p70 (k) or different stimuli in mouse BV-2 (l) for 6 hr under serum-free condition. Firefly and Renilla luciferase activities were determined by Dual Luciferase Kit (Promega) following the manufacturer's protocol. Data are mean ± SD of three separate experiments. *P < 0·001 versus control (k); *P < 0·001 versus control and**P < 0·01 versus control (l). Concentrations of different stimuli are as follows: p402, 10 ng/ml; lipopolysaccharide (LPS), 1 μg/ml; IL-12p70, 10 ng/ml; IL-23, 10 ng/ml; tumour necrosis factor-α (TNF-α), 20 ng/ml; gp120, 200 pg/ml; IL-16, 10 ng/ml; IL-1β, 10 ng/ml; p40, 10 ng/ml.
Dose-dependent studies also showed that IL-12 induced the expression of IL-7 mRNA in microglia (Fig. 1c,d). Although the induction was observed at a concentration of 2·5 ng/ml IL-12, the maximum increase was recorded at 7·5 or 10 ng/ml IL-12 (Fig. 1c,d). On the other hand, the expression of IL-7 mRNA decreased at a concentration of 15 ng/ml IL-12.
Time-dependent studies showed that the mRNA level of IL-7 increased significantly within 1 hr of stimulation, the maximum increase was observed at 2–6 hr of challenge followed by a gradual decrease after a further hour of stimulation (Fig. 1e,f). Immunofluorescence analysis in Fig. 2(g) also showed that IL-12 markedly increased the expression of IL-7 protein in primary microglia. On the other hand, p402, p40 and IL-23 had no significant effect on the level of IL-7 protein in microglia compared with untreated controls (Fig. 1g). These results show that IL-12, but not other p40 family members, is capable of inducing the expression of IL-7 in primary mouse microglia.
Figure 2.
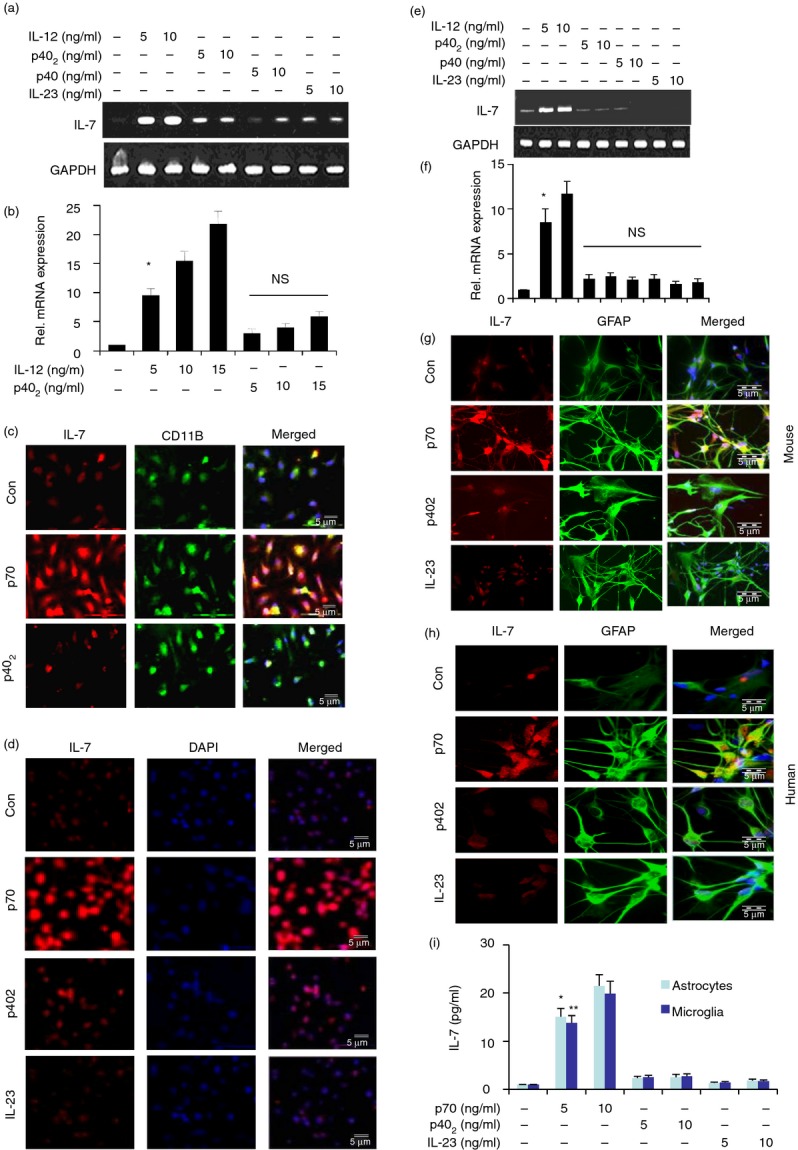
Effect of p402, p40, interleukin-23 (IL-23) and IL-12p70 on the expression of IL-7 in mouse primary macrophages, RAW cells, human and mouse primary astrocytes. Cells were stimulated with different concentrations (5 and 10 ng/ml) of p402, p40, IL-12p70 and IL-23 separately under the serum-free condition. After 4 hr of stimulation, the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (a and e) and real-time PCR (b and f). Results are means ± SD of three different experiments.*P < 0·001 versus control IL-7 mRNA. NS, non-significant. After 18 hr of stimulation by 10 ng/ml p402, IL-12p70 and IL-23, the expression of IL-7 protein was monitored by immunofluorescence in peritoneal macrophages (c), RAW cells (d), mouse astrocytes (g) and human astrocytes (h). DAPI was used to visualize the nucleus. Results represent three independent experiments. Mouse primary microglia and astrocytes (5 × 105) were stimulated with IL-12p70, p402 and IL-23 under serum-free conditions. After 12 hr, supernatants were collected. Concentration of IL-7 was measured in culture supernatants by a high-sensitivity ELISA (i). Two separate wells from each treatment group have been processed and shown. Results represent three independent experiments. *P < 0·001 versus control; **P < 0·001 versus control.
Results from mouse cells are not often replicated in the human system. Therefore, next, we examined the effect of IL-12, p40, IL-23 and p402 on the expression of IL-7 in primary human microglia isolated from 13-to 17-week-old human fetal brains. As evident from semi-quantitative RT-PCR results in Fig. 1(h), only IL-12 increased the expression of IL-7 mRNA in human fetal microglia. On the other hand, under similar conditions, p402 and IL-23 were very weak inducers of IL-7. These results were also corroborated by real-time PCR (Fig. 1i). Next, we examined the protein level of IL-7 by immunofluorescence analysis. Similar to that found in mouse microglia, Fig. 1(j) shows that IL-12, but not p402 and IL-23, stimulated the expression of IL-7 protein in human microglia.
To understand the mechanism of IL-12-mediated stimulation of IL-7 mRNA, we examined the effect of IL-12 on the activation of IL-7 promoter in BV-2 microglial cells. Cells were transfected with the reporter plasmid PGL3-1820 containing IL-7 promoter fragment [PGL-3-IL-7 (−1791/+29)]. As evident from Fig. 1(k), IL-12 dose-dependently increased IL-7 promoter-driven luciferase activity in microglial cells. Although IL-12 induced the activation of IL-7 promoter significantly at a concentration of 2·5 ng/ml, the activation of IL-7 promoter was maximum at a dose of 7·5 pg/ml IL-12 and decreased at higher concentrations (Fig. 1k). Next we examined the effect of other pro-inflammatory stimuli on the activation of IL-7 promoter. It was found that most common pro-inflammatory cytokines such as p402, IL-23, p40, TNF-α, IL-1β and IL-16 were ineffective in inducing the activation of IL-7 promoter in BV-2 microglial cells (Fig. 1l). Of all these pro-inflammatory stimuli tested, only LPS and HIV-1 coat protein gp120 were capable of inducing IL-7 promoter-driven luciferase activity (Fig. 1l). Incidentally, among all the stimuli tested, IL-12 has been found to be the strongest inducer of IL-7 promoter activation in microglia (Fig. 1l).
IL-12, but not IL-23, induces the expression of IL-7 in mouse macrophages, RAW cells and astrocytes
The results described above clearly indicate that IL-12 induces the expression of IL-7 mRNA and protein in CNS microglia. In addition to controlling CNS inflammatory disease, IL-7 is also known to be involved in the development of several peripheral inflammatory and autoimmune disorders. It is not known whether peripheral macrophages produce IL-7 or not. Therefore, we investigated if IL-12 was also capable of increasing the expression of IL-7 in macrophages. Similar to observations in microglia, the mRNA expression of IL-7 was stimulated by IL-12, but not by p402 and p23, in mouse peritoneal macrophages (Fig. 2a,b). Immunofluorescence analysis also demonstrates marked increase in IL-7 protein expression by IL-12 in mouse peritoneal macrophages and RAW cells (Fig. 2c,d).
Astrocytes, the predominant cell type in the CNS, play an important role in different neurodegenerative diseases. Therefore, we examined the effect of IL-12, p402, p40 and IL-23 on the expression of IL-7 in primary mouse and human astrocytes. It was found that IL-12 markedly increased the expression of IL-7 in astrocytes compared with the other three stimuli tested (Fig. 2e,f). Immunofluorescence analysis also demonstrated an increase in IL-7 protein expression by IL-12 in primary mouse (Fig. 2g) and human astrocytes (Fig. 2h).
To understand the role of IL-12 in the induction of IL-7, we examined the effect of IL-12 on the production of IL-7 protein in mouse astrocytes and microglia. Results in Fig. 2(i) clearly showed that mouse IL-12, but not other stimuli, markedly induced the production of IL-7. Together, these results demonstrate that IL-12, but not other p40 family members, is a strong inducer of IL-7 in microglia, macrophages and astrocytes.
Does IL-12-induced expression of IL-7 in microglial cells depend on new protein synthesis?
Recently Sawa et al.8 demonstrated that TLR ligands induce the production of IFN-β from undefined cells from liver, which in turn acted on hepatocytes eliciting IL-7 production. IL-7 induction was also reported to be driven in vitro by IFN-γ or TNF-α and in vivo by keratinocyte growth factor or IL-6.9 Therefore, to understand if new protein synthesis was required for IL-12-induced expression of IL-7, BV-2 microglial cells pre-treated with different concentrations of cycloheximide, a protein synthesis inhibitor, were challenged by IL-12. It is clearly evident from Fig. 3(a,b) that cycloheximide at a dose of even 15 μm was unable to suppress the mRNA expression of IL-7 in IL-12-stimulated microglial cells, suggesting that IL-12 does not require any new protein synthesis for the induction of IL-7 in microglial cells. On the other hand, as expected, actinomycin D, an inhibitor of transcription, markedly inhibited the mRNA expression of IL-7 (Fig. 3a,b).
Figure 3.
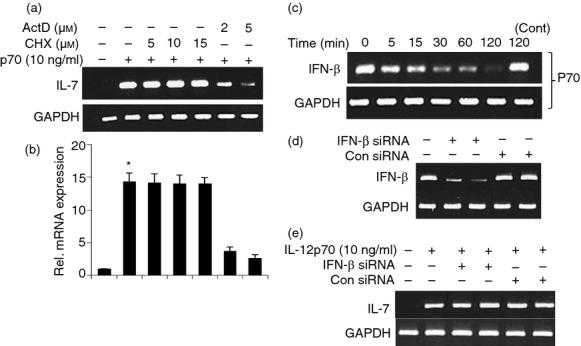
Effect of cycloheximide (CHX) and actinomycin D (ActD) on interleukin-12 p70 (IL-12p70)-induced expression of IL-7 in mouse BV-2 microglial cells. Cells pre-incubated with different concentrations of cycloheximide and actinomycin D for 30 min were stimulated with IL-12p70 (10 ng/ml) under serum-free conditions. After 4 hr of stimulation, the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (a) and real-time PCR (b). Results are means ± SD of three different experiments.*P < 0·001 versus control IL-7 mRNA. Cells were stimulated with 10 ng/ml IL-12p70, for different time periods under the serum-free condition. The mRNA expression of interferon-β (IFN-β) was monitored by semi-quantitative RT-PCR (c). Microglial cells were transfected with IFN-β-siRNA. After 48 hr of transfection, expression of IFN-β was monitored by PCR (d). After 48 hr of transfection, cells were treated with IL-12 for 4 hr and the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (e). Results represent three independent experiments.
To investigate the role of IL-12 in the induction of IFN-β production, we treated the microglial cells with IL-12 for different time periods. Figure 3(c) shows that IL-12 suppressed IFN-β production in a time-dependent manner. Next, we investigated if IL-12 required IFN-β for the up-regulation of IL-7 in microglia. At first, we examined if antisense knockdown of IFN-β was capable of suppressing the expression of IFN-β mRNA in BV-2 microglia. As shown in Fig. 3(d), IFN-β siRNA, but not control siRNA, decreased the expression of IFN-β in microglia and was unable to inhibit the IL-12-mediated up-regulation of IL-7 mRNA (Fig. 3e). These results indicate that IFN-β does not play any role in IL-7 production in microglial cells.
Expression of mouse p35 cDNA induces the expression of IL-7 in BV-2 microglial cells
We used recombinant mouse p402 and IL-12 for the stimulation of microglia. It is possible that any contaminant in IL-12 preparation is actually inducing the expression of IL-7. To investigate the validity of this possibility, we examined the effect of transient expression of mouse p40, p19 and p35 cDNA on the expression of IL-7 in BV-2 microglial cells.27 As shown by semi-quantitative RT-PCR analysis (Fig. 4a) and quantitative real-time PCR analysis (Fig. 4b), expression of p35 cDNA but not empty vector, p19 and p40, induced the expression of IL-7 mRNA. We also found that in the presence of p40 neutralizing antibody, p35 was unable to stimulate the IL-7 expression.
Figure 4.
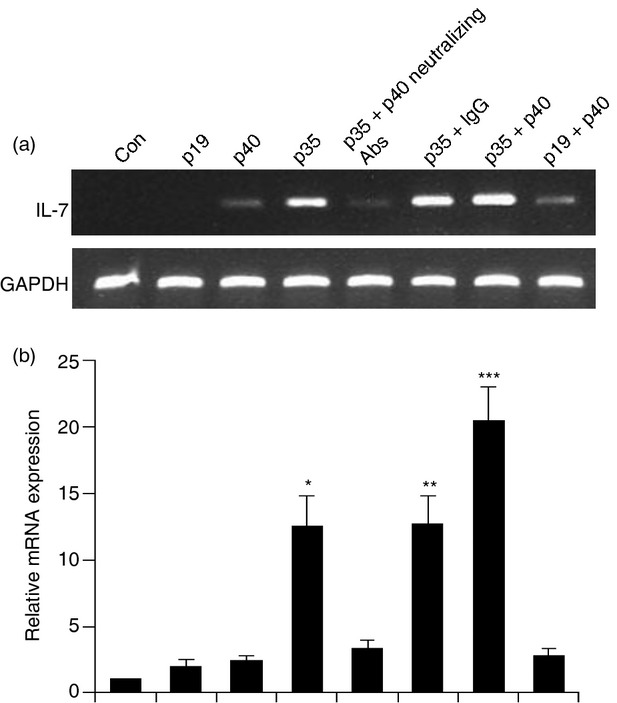
Expression of p35, but not p40 and p19, cDNA induces the expression of interleukin-7 (IL-7) in BV-2 microglial cells. Microglial cells plated in 12-well plates were transfected with different amounts of either p40, p19 or p35 cDNA by LipofectAMINE Plus (Invitrogen). The p35 cDNA well was pre-treated with (100 ng/ml) P40 neutralizing antibody. Empty vector (pCIneo mammalian expression vector from Promega) was used as control. After 24 hr of transfection, cells were incubated in serum-free media. After 4 hr, the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (a) and quantitative real-time PCR (b). Results represent three independent experiments. *P < 0·001 versus control; **P < 0·001 versus control; ***P < 0·001 versus control.
These results confirm that the transfected IL-12p35 dimerizes with the IL-12p40 expressed (perhaps induced by the transfection process) by the cells, and signalling through the IL-12R complex. Furthermore, these results suggest that the induction of IL-7 by IL-12 is real, not due to any contamination by LPS.
IL-12 induces the expression of IL-7 via both IL-12Rβ1and IL-12Rβ2
Next, we investigated mechanisms by which IL-12 induced the expression of IL-7 in microglia. Previous studies have demonstrated that IL-12 interacts with both IL-12Rβ1 and IL-12Rβ2 in T cells and microglia,23,30 and microglia/macrophages express both IL-12Rβ1 and IL-12Rβ2.40 However, it is not known if IL-12 is using these two receptors to induce the expression of IL-7 in microglia.
Therefore, primary microglia isolated from wild-type, IL-12Rβ1−/− and IL-12Rβ2−/− mice were challenged with IL-12 and p402. As expected, IL-12, but not p402, induced the expression of IL-7 (Fig. 5a,b) in microglia isolated from wild-type mice. In contrast, IL-12 was unable to induce the expression of IL-7 mRNA (Fig. 5a,b) in microglia isolated from both IL-12Rβ1−/− and 12Rβ2−/− mice (Fig. 5a,b). These results were corroborated further by immunofluorescence analysis (Fig. 5c–e). All of these results clearly suggest that IL-12 requires both IL-12Rβ2 and IL-12Rβ1, for the expression of IL-7. On the other hand, p402 remained unable to induce the expression of IL-7 in microglia isolated from wild-type, IL-12Rβ1−/− and IL-12Rβ2−/− mice (Fig. 5a,b).
Figure 5.
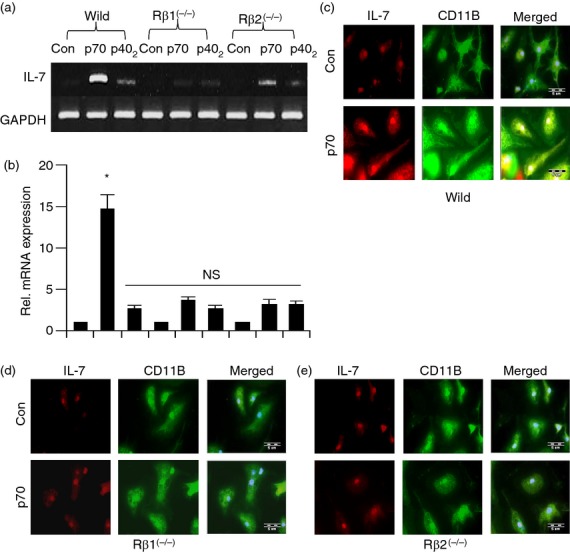
Effect of p402 and IL-12p70 on the expression of interleukin-7 (IL-7) in primary microglia isolated from wild-type, IL-12Rβ1−/− and IL-12Rβ2−/− mice. Microglia isolated from B6.129 wild-type, IL-12Rβ1−/− and IL-12Rβ2−/− mice were stimulated with 10 ng/ml of p402 and IL-12p70, under serum-free condition. After 4 hr of stimulation, the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (a) and real-time PCR (b). After 18 hr of stimulation by 10 ng/ml IL-12p70, the expression of IL-7 protein was monitored by immunofluorescence (c–e). DAPI was used to visualize the nucleus. Results represent three independent experiments. *P < 0·001 versus control; NS, non significant.
How does IL-12Rβ2 and IL-12Rβ1 regulate IL-12-induced expression of IL-7 in mouse BV-2 microglial cells?
Earlier, we showed that activation of nuclear factor-κB (NF-κB) is involved in the expression of iNOS in activated microglia following either neuroantigen primed T-cell contact or CD40 ligation.34,36 Recently we have also reported that activation of NF-κB is important for p402-and IL-12-induced microglial expression of iNOS.30 A search of the Genomatix data base shows that the IL-7 gene promoter does not contain any NF-κB binding sites. To investigate whether NF-κB plays any role in the induction of IL-7, mouse primary microglia were pre-treated with NF-κB essential modifier-binding domain (NBD) peptide34 before the IL-12 followed by IL-7 mRNA analysis. Results showed that NBD peptide was unable to inhibit the IL-12-induced IL-7 in mouse primary microglia (Fig. 6a). This result was specific as NBD peptide strongly inhibited IL-12-induced expression of TNF-α in microglia (Fig. 6a).
Figure 6.
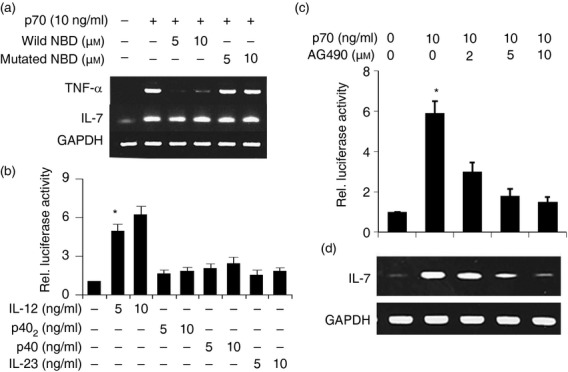
Role of nuclear factor-κB (NF-κB) and GAS in interleukin-12 p70 (IL-12p70)-induced expression of IL-7 in mouse BV-2 microglial cell. Cells were treated with NF-κB essential modifier-binding domain (NBD) peptide 30 min before the IL-12p70 under serum-free conditions. After 4 hr of stimulation, the mRNA expression of IL-7 was monitored by semi-quantitative RT-PCR (a). Cells were co-transfected with pGAS-Luc and pRL-TK. After 24 hr of transfection, cells were treated with different concentrations (5 and 10 ng/ml) of IL-12p70, p402, p40 and IL-23. After 6 hr of stimulation, luciferase activities were analysed (b). *P < 0·001 versus control. Cells were transfected with pIL-7-Luc. After 24 hr of transfection, cells were treated with different concentrations of AG490 for 30 min followed by stimulation with 10 ng/ml IL-12p70. After 6 hr of stimulation, luciferase activities were analysed (c). *P < 0·001 versus control. Cells pre-incubated with different concentrations of AG490 for 30 min were stimulated with 10 ng/ml IL-12p70. After 4 hr of stimulation, the expression of IL-7 was monitored by RT-PCR (d). Results represent three independent experiments.
Besides NF-κB, many others transcription factors such as interferon regulatory factor 1 (IRF-1) binding to IFN-stimulated response element (ISRE) and STAT binding to interferon-gamma-activated sequence (GAS) may play a role in the transcription of IL-7. Recently we have shown that IL-12, but not p402, induces GAS-dependent luciferase activity in BV-2 microglial cells.30 Here we observed that IL-12 markedly increased the GAS luciferase activity in BV-2 microglial cells whereas other stimuli had little effect (Fig. 6b).
To further confirm the role of STAT/GAS in IL-12-induced expression of IL-7, we examined the effect of AG490, an inhibitor of JAK, on IL-12-induced activation of IL-7 promoter activity and expression of IL-7. As evident from luciferase activities in Fig. 6(c) and RT-PCR analysis in Fig. 6(d), AG490 dose-dependently inhibited IL-12-induced activation of IL-7 promoter activity and expression of IL-7 mRNA, suggesting the involvement of JAK/STAT in the activation of GAS and the expression of IL-7 in IL-12-stimulated microglial cells.
Infiltration of mononuclear cells and IL-7 production increases in EAE mouse brain
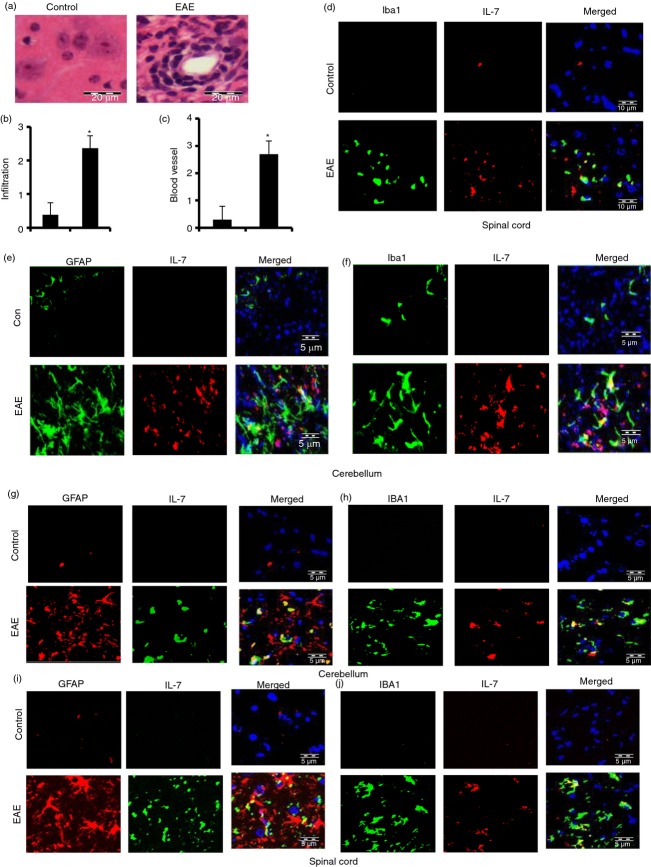
Both EAE and MS are caused by infiltration of autoreactive T cells and associated mononuclear cells, like macrophages, into the CNS, followed by broad-spectrum inflammatory events.10,29 We examined whether infiltration was increased in adoptively transferred EAE mice. During the acute phase of EAE, mice were killed. Haematoxylin & eosin staining showed widespread infiltration of inflammatory cells into the spinal cord (Fig. 7a,b) of EAE mice. We also observed the appearance of cuffed vessels (Fig. 7c) in the spinal cord of mice with relapsing–remitting EAE.
Figure 7.
Expression of interleukin-7 (IL-7) in cerebellum and spinal cord of adoptively transferred experimental autoimmune encephalomyelitis (EAE) in SJL mice (d–f) and myelin oligodendrocyte glycoprotein-induced chronic EAE in C57BL/6 mice (h–k). Longitudinal sections of the spinal cord isolated from control and EAE mice were stained with haematoxylin and eosin (a). Digital images were collected under a bright-field setting using a ×40 objective. Infiltration (b) and cuffed vessel (c) were represented quantitatively by using a scale as described in the Materials and methods. Data are expressed as the mean ± SD of five different mice; P < 0·001 versus control. On day 17 post-transfer, EAE animals were killed, cerebellum and spinal cord sections were double-immunolabelled with IL-7 and either Iba1 (d, f, h and j) or GFAP (e, g and i) and observed under a fluorescence microscope. Results represent three independent experiments.
Expression of IL-7 in cerebellum and spinal cord of adoptively transferred EAE mice was increased compared with control mice without EAE (Fig. 7d–f). Similarly, the increased expression of IL-7 was also found in cerebellum and spinal cord of mice with MOG-induced chronic EAE mice (Fig. 7g–j). Double-labelling analysis of IL-7 with cell-specific markers showed that induced IL-7 co-localized with microglial Iba1 (Fig. 7d,f,h,j), and astroglial GFAP (Fig. 7e,g,i). These results clearly suggest that IL-7 production increases in the CNS of EAE mice.
Discussion
Interleukin-7 is an important factor for the regulation of B-cell and T-cell development, myelopoiesis, and activating monocytes/macrophages, natural killer cells and cytoxic T cells.5 Several reports indicate that IL-7 is expressed in kidney, liver, bone marrow stromal cells, spleen and keratinocytes.41 Recent study has shown that IL-7 is over-expressed in brain lesions of MS patients.18 It is detected in the cytoplasm of reactive astrocytes and in inflammatory mononuclear cells in proximity to blood vessels.18 However, the pro-inflammatory cytokines TNF-α and IL-1β, which play an important role in EAE and MS, do not induce IL-7. Therefore, stimuli that could induce the production of IL-7 from immune cells in MS and EAE are not well characterized and identifying such stimuli and understanding underlying mechanisms for the regulation of IL-7 are important areas of investigation.
Several lines of evidence presented in this study clearly support the conclusion that IL-12p70, but not p402, p40 and IL-23, markedly induce the expression of IL-7 in microglia, macrophages and astrocytes. The conclusion was based on the following observations: First, IL-12 induced the expression of IL-7 mRNA and protein in microglia, macrophages and astrocytes. Second, IL-12 markedly increased the activation of IL-7 promoter as indicated by IL-7 promoter-driven luciferase activity. On the other hand, p402, IL-23 and p40 remained very weak inducers of IL-7 promoter activity. Only two other stimuli (gp120 and LPS) were capable of stimulating the activation of IL-7 promoter activity. Third, only IL-12 was capable of inducing the activation of GAS luciferase activity. Other stimuli had very little effect on the GAS–luciferase activity in microglial cells. Fourth, the expression of mouse p35, but not mouse p40, cDNA induced the expression of IL-7, suggesting that p35, but not p40, subunit of IL-12 is involved in the induction of IL-7 in microglia.
Recently, it has been demonstrated that triggering of several different TLRs induces type 1 IFN expression via the TIR-domain-containing adapter inducing interferon-β (TRIF) signalling pathway.8 This type 1 IFN acts on hepatocytes, inducing IL-7 expression and release, which then prolongs the survival of naive and memory cells. In this study, we provide compelling evidence that IL-12 directly induces the expression of IL-7 from microglia. We have found that cycloheximide, a protein synthesis inhibitor, is unable to inhibit IL-7 production whereas transcriptional inhibitor actinomycin D markedly inhibits the IL-7 expression. Surprisingly we have also observed that IL-12 inhibits the expression of type I IFN in a time-dependent manner. We also observed that IL-12 induces IL-7 expression in IFN-β-deficient microglial cells. These results further confirm that IL-12 directly regulates the IL-7 production in microglia independent of type I IFN production.
Signalling events transduced by pro-inflammatory cytokines and LPS for the expression of IL-7 are not clearly known.42 In murine keratinocytes, IFN-γ induces IL-7 via activation of an ISRE located in the 5′ upstream region of the IL-7 gene.42 In human intestinal epithelial cells, IRF-1 and IRF-2 distinctively up-regulate gene expression and production of IL-7 in response to IFN-γ. On the other hand, other stimuli such as IL-1, TNF-α and transforming growth factor-β have no influence on the levels of IL-7 production.43 Accordingly, we have demonstrated that both IL-12 and p402 are unable to induce ISRE-dependent luciferase activity, suggesting that IRF-1 may not be involved in IL-12-and p402-induced expression of iNOS in microglia.30 In this study, we also observe the possible involvement of the JAK-STAT pathway in IL-12-but not p402-induced microglial expression of IL-7. The mouse IL-7 promoter contains two STAT binding sites. In our previous study, we reported that siRNA knockdown of IL-12Rβ2, but not IL-12Rβ1, abrogates p70-induced activation of GAS, suggesting the involvement of IL-12Rβ2, but not IL-12Rβ1, in IL-12-mediated activation of GAS.30 We also observe that the STAT inhibitor attenuates IL-7 promoter activity and IL-7 expression in BV-2 microglial cells. On the other hand, from a Genomatix data base search, we have not found any NF-κB-binding site in IL-7 promoter, suggesting that NF-κB may not play a role in the transcription of IL-7. We have also demonstrated that wild-type, but not mutated, NBD peptide is unable to inhibit the IL-12-induced IL-7 expression in microglial cells. IL-12 is unable to induce the production of IL-7 from both IL-12 Rβ1−/− and IL-12Rβ2−/− microglial cells. Earlier we demonstrated that IL-12 employed IL-12 Rβ1, but not IL-12Rβ2, to induce the activation of extracellular signal-regulated kinase and p38. These mitogen-activated protein kinases ultimately couple to NF-κB and CCAAT/enhancer-binding protein-β for the transcription of iNOS in microglia.30 It is possible that IL-12 employs IL-12Rβ1 to induce the activation extracellular signal-regulated kinase and p38; and activate some unknown factor that regulates the IL-7 production.
It has been demonstrated that IL-12Rβ2, but not IL-12Rβ1, is capable of binding STAT4.44 It has also been shown that a defective STAT4 activation is involved in the impaired IL-12-dependent T-cell functions with aging.45 Taken together, these results suggest that IL-12Rβ2 couples IL-12 p70 to IL-7 in microglia by way of the JAK-STAT signalling pathway.
Microglia are considered as CNS-resident professional macrophages and sensor cells that function as the principal immune effector cells of the CNS responding to any pathological event. Activation of microglia has been implicated in the pathogenesis of a variety of neurodegenerative diseases, including MS, Alzheimer's disease and HIV-associated dementia.46,47 It has been found that activated microglia accumulate at sites of demyelination in MS patients. Although the in vitro situation of mouse and human microglia in culture and its treatment with IL-12 may not resemble the in vivo situation of microglia in the brain of patients with MS, our results identify IL-12 as a possible candidate for controlling IL-7.
Acknowledgments
This study was supported by grants from National Multiple Sclerosis Society (RG4170-A-1) to MJ and from the National Institutes of Health (AT6681) and Veteran Affairs Merit Award (I01BX002174) to KP.
Disclosure
The authors declare no conflict of interest.
References
- 1.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 3.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 4.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 5.van Roon JA, Glaudemans KA, Bijlsma JW, Lafeber FP. Interleukin 7 stimulates tumour necrosis factor α and Th1 cytokine production in joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:113–9. doi: 10.1136/ard.62.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariizumi K, Meng Y, Bergstresser PR, Takashima A. IFN-γ-dependent IL-7 gene regulation in keratinocytes. J Immunol. 1995;154:6031–9. [PubMed] [Google Scholar]
- 7.Tang J, Nuccie BL, Ritterman I, Liesveld JL, Abboud CN, Ryan DH. TGF-β down-regulates stromal IL-7 secretion and inhibits proliferation of human B cell precursors. J Immunol. 1997;159:117–25. [PubMed] [Google Scholar]
- 8.Sawa Y, Arima Y, Ogura H, et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–57. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Sawa S, Kamimura D, Jin GH, et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med. 2006;203:1459–70. doi: 10.1084/jem.20052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou YK, Bourdette DN, Barnes D, et al. IL-7 enhances Ag-specific human T cell response by increasing expression of IL-2R α and γ chains. J Neuroimmunol. 1999;96:101–11. doi: 10.1016/s0165-5728(99)00002-8. [DOI] [PubMed] [Google Scholar]
- 11.Bebo BF, Jr, Schuster JC, Adlard K, Vandenbark AA, Offner H. Interleukin 7 is a potent co-stimulator of myelin specific T cells that enhances the adoptive transfer of experimental autoimmune encephalomyelitis. Cytokine. 2000;12:324–31. doi: 10.1006/cyto.1999.0564. [DOI] [PubMed] [Google Scholar]
- 12.Traggiai E, Biagioli T, Rosati E, Ballerini C, Mazzanti B, Ben Nun A, Massacesi L, Vergelli M. IL-7-enhanced T-cell response to myelin proteins in multiple sclerosis. J Neuroimmunol. 2001;121:111–9. doi: 10.1016/s0165-5728(01)00433-7. [DOI] [PubMed] [Google Scholar]
- 13.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Leung S, Wang C, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16:191–7. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 15.El-behi M, Rostami A, Ciric B. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:189–97. doi: 10.1007/s11481-009-9188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassmann H, Ransohoff RM. The CD4-Th1 model for multiple sclerosis: a critical [correction of crucial] re-appraisal. Trends Immunol. 2004;25:132–7. doi: 10.1016/j.it.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Kreft KL, Verbraak E, Wierenga-Wolf AF, van Meurs M, Oostra BA, Laman JD, Hintzen RQ. The IL-7Ralpha pathway is quantitatively and functionally altered in CD8 T cells in multiple sclerosis. J Immunol. 2012;188:1874–83. doi: 10.4049/jimmunol.1102559. [DOI] [PubMed] [Google Scholar]
- 18.Kremlev SG, Gaurnier-Hausser AL, Del Valle L, Perez-Liz G, Dimitrov S, Tuszynski G. Angiocidin promotes pro-inflammatory cytokine production and antigen presentation in multiple sclerosis. J Neuroimmunol. 2008;194:132–42. doi: 10.1016/j.jneuroim.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Malmestrom C, Lycke J, Haghighi S, Andersen O, Carlsson L, Wadenvik H, Olsson B. Relapses in multiple sclerosis are associated with increased CD8+ T-cell mediated cytotoxicity in CSF. J Neuroimmunol. 2008;196:159–65. doi: 10.1016/j.jneuroim.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 21.Constantinescu CS, Goodman DB, Hilliard B, Wysocka M, Cohen JA. Murine macrophages stimulated with central and peripheral nervous system myelin or purified myelin proteins release inflammatory products. Neurosci Lett. 2000;287:171–4. doi: 10.1016/s0304-3940(00)01184-8. [DOI] [PubMed] [Google Scholar]
- 22.Zipris D, Greiner DL, Malkani S, Whalen B, Mordes JP, Rossini AA. Cytokine gene expression in islets and thyroids of BB rats. IFN-gamma and IL-12p40 mRNA increase with age in both diabetic and insulin-treated nondiabetic BB rats. J Immunol. 1996;156:1315–21. [PubMed] [Google Scholar]
- 23.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Kweon MN, Kuwata H, Schreiber RD, Kiyono H, Takeda K, Akira S. Toll-like receptor-dependent production of IL-12p40 causes chronic enterocolitis in myeloid cell-specific Stat3-deficient mice. J Clin Investig. 2003;111:1297–308. doi: 10.1172/JCI17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003;86:519–28. doi: 10.1046/j.1471-4159.2003.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahan K, Sheikh FG, Liu X, Hilger S, McKinney M, Petro TM. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J Biol Chem. 2001;276:7899–905. doi: 10.1074/jbc.M008262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jana M, Pahan K. IL-12 p40 homodimer, but not IL-12 p70, induces the expression of IL-16 in microglia and macrophages. Mol Immunol. 2009;46:773–83. doi: 10.1016/j.molimm.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jana M, Pahan K. Induction of lymphotoxin-alpha by interleukin-12 p40 homodimer, the so-called biologically inactive molecule, but not IL-12 p70. Immunology. 2009;127:312–25. doi: 10.1111/j.1365-2567.2008.02985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–78. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jana M, Dasgupta S, Pal U, Pahan K. IL-12 p40 homodimer, the so-called biologically inactive molecule, induces nitric oxide synthase in microglia via IL-12R beta 1. Glia. 2009;57:1553–65. doi: 10.1002/glia.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jana M, Jana A, Pal U, Pahan K. A simplified method for isolating highly purified neurons, oligodendrocytes, astrocytes, and microglia from the same human fetal brain tissue. Neurochem Res. 2007;32:2015–22. doi: 10.1007/s11064-007-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jana M, Jana A, Liu X, Ghosh S, Pahan K. Involvement of phosphatidylinositol 3-kinase-mediated up-regulation of I kappa B alpha in anti-inflammatory effect of gemfibrozil in microglia. J Immunol. 2007;179:4142–52. doi: 10.4049/jimmunol.179.6.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–64. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-kappaB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–54. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- 35.Jana M, Pahan K. Redox regulation of cytokine-mediated inhibition of myelin gene expression in human primary oligodendrocytes. Free Radic Biol Med. 2005;39:823–31. doi: 10.1016/j.freeradbiomed.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jana M, Liu X, Koka S, Ghosh S, Petro TM, Pahan K. Ligation of CD40 stimulates the induction of nitric-oxide synthase in microglial cells. J Biol Chem. 2001;276:44527–33. doi: 10.1074/jbc.M106771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Jana M, Dasgupta S, Koka S, He J, Wood C, Pahan K. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric-oxide synthase in human astroglia. J Biol Chem. 2002;277:39312–9. doi: 10.1074/jbc.M205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber MS, Benkhoucha M, Lehmann-Horn K, et al. Repetitive pertussis toxin promotes development of regulatory T cells and prevents central nervous system autoimmune disease. PLoS One. 2010;5:e16009. doi: 10.1371/journal.pone.0016009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor-alpha. Mol Pharmacol. 2007;72:934–46. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]
- 40.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Ueno Y, Yajima T, et al. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998;187:389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aragane Y, Schwarz A, Luger TA, Ariizumi K, Takashima A, Schwarz T. Ultraviolet light suppresses IFN-gamma-induced IL-7 gene expression in murine keratinocytes by interfering with IFN regulatory factors. J Immunol. 1997;158:5393–9. [PubMed] [Google Scholar]
- 43.Oshima S, Nakamura T, Namiki S, et al. Interferon regulatory factor 1 (IRF-1) and IRF-2 distinctively up-regulate gene expression and production of interleukin-7 in human intestinal epithelial cells. Mol Cell Biol. 2004;24:6298–310. doi: 10.1128/MCB.24.14.6298-6310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–8. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Tortorella C, Stella I, Piazzolla G, Cappiello V, Simone O, Pisconti A, Antonaci S. Impaired interleukin-12-dependent T-cell functions during aging: role of signal transducer and activator of transcription 4 (STAT4) and suppressor of cytokine signaling 3 (SOCS3) J Gerontol A Biol Sci Med Sci. 2006;61:125–35. doi: 10.1093/gerona/61.2.125. [DOI] [PubMed] [Google Scholar]
- 46.Martin J, LaBranche CC, Gonzalez-Scarano F. Differential CD4/CCR5 utilization, gp120 conformation, and neutralization sensitivity between envelopes from a microglia-adapted human immunodeficiency virus type 1 and its parental isolate. J Virol. 2001;75:3568–80. doi: 10.1128/JVI.75.8.3568-3580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]