Abstract
Regulatory T cells have been well described and the factors regulating their development and function have been identified. Recently, a growing body of evidence has documented the existence of interleukin-10 (IL-10)-producing B cells, which are called regulatory B10 cells. These cells attenuate autoimmune, inflammatory and transplantation reactions, and the main mechanism of their inhibitory action is the production of IL-10. We show that the production of IL-10 by lipopolysaccharide-stimulated B cells is significantly enhanced by IL-12 and interferon-γ and negatively regulated by IL-21 and transforming growth factor-β. In addition, exogenous IL-10 also inhibits B-cell proliferation and the expression of the IL-10 gene in lipopolysaccharide-stimulated B cells. The negative autoregulation of IL-10 production is supported by the observation that the inclusion of anti-IL-10 receptor monoclonal antibody enhances IL-10 production and the proliferation of activated B cells. The effects of cytokines on IL-10 production by B10 cells did not correlate with their effects on B-cell proliferation or on IL-10 production by T cells or macrophages. The cytokine-induced changes in IL-10 production occurred on the level of IL-10 gene expression, as confirmed by increased or decreased IL-10 mRNA expression in the presence of a particular cytokine. The regulatory cytokines modulate the number of IL-10-producing cells rather than augmenting or decreasing the secretion of IL-10 on a single-cell level. Altogether these data show that the production of IL-10 by B cells is under the strict regulatory control of cytokines and that individual cytokines differentially regulate the development and activity of regulatory T cells and IL-10-producing regulatory B cells.
Keywords: autoregulation, B cells, cytokines, interleukin-10 production, immunosuppression
Introduction
The immune response is regulated by a number of phenotypically and functionally different cell types. Among T cells, the populations that suppress1 or negatively regulate2 the immune response have been described and their development and mechanism of action have been well characterized.
More recently, a population of B cells that negatively regulate the immune response in an antibody-independent manner, was described and called regulatory B (Breg) cells.3–5 The main population of Breg cells acts through the production of the suppressive cytokine interleukin-10 (IL-10).6–8 Therefore, some authors have termed these IL-10-producing B cells B10 cells.9 These cells balance the immune response during inflammation, autoimmunity, transplantation and cancer. The mechanism of the regulatory action of B cells now appears to be more complex and other, IL-10 independent, regulatory functions of B cells have been described.10–12 Notably, the expression of Fas ligand, granzyme B or PD-L2 molecules, the production of transforming growth factor-β (TGF-β) or the glucocorticoid-induced tumour necrosis factor ligand-dependent activation of regulatory T (Treg) cells have all been attributed to Breg cells.11,13,14 However, the production of IL-10 remains the main mechanism of action of the heterogeneous Breg population. Furthermore, it has been shown that phenotypically different B-cell subpopulations can produce distinct quantities of IL-10.6,9,14–17 These observations led to the conclusion that the production of IL-10 does not appear to be confined to one B-cell subset, but rather that different subpopulations of B cells can secret IL-10 under appropriate stimulation conditions.18
So far, the regulatory activity of B cells has been described in various models of autoimmunity, transplantation tolerance, anti-tumour immunity and inflammation. In autoimmunity models it has been shown that the elimination of B cells exacerbates the disease and that the transfer of B cells suppresses harmful manifestations of the disease.8,19 In patients, treatment with anti-CD20 antibody often worsened the course of the disease or even induced other autoimmune disorders.20,21 In a transplantation system, Lee et al.22 described a model of anti-CD45RB monoclonal antibody (mAb) induced tolerance of cardiac allografts that showed some dependence on B cells. In transplanted patients, an increased number of B cells displaying an activation phenotype was observed in those recipients with well-tolerated allografts.23,24
Although data on the immunoregulatory role of B cells in various models are extensive and the mechanism of B-cell suppression has been intensively studied (reviewed in refs 5,13), little is known about the activation of Breg cells and the role of cytokines in the regulation of IL-10 production by B cells. Stimulation of B cells through the B-cell receptor and Toll-like receptor has been described as the strongest signal to induce IL-10 production in B cells,9,25 but distinct activation signals determine whether B cells undergo co-stimulation, growth arrest or apoptosis.26 On the basis of our experience with the study of the role of cytokines in Treg cell development,27,28 we studied the role of cytokines in the development of IL-10-producing B cells. We found that IL-10 production by lipopolysaccharide (LPS)-activated B cells is tightly modulated by cytokines and that distinct cytokines regulate the development of Treg cells and IL-10-secreting Breg cells in a positive and negative manner.
Materials and methods
Mice
Mice of both sexes of the inbred strain BALB/c were used in the experiments at the age of 7–9 weeks. The animals were purchased from the Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Prague. The use of the animals was approved by the Local Ethics Committee of the Institute of Experimental Medicine.
B-cell enrichment procedure
Single-cell suspensions of spleen cells were prepared in RPMI-1640 medium (Sigma Corp., St Louis, MO) containing 10% heat-inactivated fetal calf serum (Sigma), antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin), 10 mm HEPES buffer and 5 × 10−5 m 2-mercaptoethanol. The B-cell population was enriched by two cycles of purification on a nylon wool column (Fenwal Labs, Deerfield, IL) according to the techniques originally described by Julius et al.29
The purity and phenotype of the enriched B-cell population were characterized by flow cytometry. The following mAb were used: FITC-labelled anti-CD19 (clone 6D5), Alexa Fluor 647-labelled anti-CD5 (clone 53–73), Alexa Fluor 647-labelled anti-CD22 (clone OX-97), phycoerythrin-labelled anti-CD1d (clone 1B1), allophycocyanin-labelled anti-CD11b (clone M1/70), phycoerythrin-labelled anti-CD14 (clone Sa14-2) and allophycocyanin-labelled anti-CD3 (clone 17A2). All antibodies were purchased from BioLegend (San Diego, CA). Data were collected using an LSRII cytometer (BD Bioscience, Franklin Lakes, NJ) and analysed using FlowJo software (Tree Stars, Askhland, OR).
Production of IL-10
B cells at a concentration 0·9 × 106 cells/ml were incubated in 48-well tissue culture plates (Corning Inc., Corning, NY) in a final volume of 1 ml complete RPMI-1640 medium unstimulated or stimulated with LPS (10 μg/ml; Difco Laboratories, Detroit, MI). After a 72-hr incubation at 37° in an atmosphere of 5% CO2 the supernatants were harvested and tested for the presence of IL-10 and IL-6. To test the effects of cytokines on IL-10 production by B cells, mouse recombinant IL-1β, IL-2, IL-4, IL-6, IL-7, IL-12, IL-13, IL-15, IL-17, IL-21, IL-22, IL-31, IL-33, interferon-γ (IFN-γ) or tumour necrosis factor-α was added to these cultures to final concentrations of 10 ng/ml. Recombinant human TGF-β1 was similarly tested at 2 ng/ml. All cytokines were purchased from PeproTech (Rocky Hill, NJ).
To test the effects of cytokines on IL-10 production by T cells, nylon wool non-adherent cells (>98% of CD3+ cells) at a concentration of 0·6 × 106 cells/ml were stimulated for 72 hr, in the presence of 10% peritoneal macrophages added to the cultures as antigen-presenting cells, with concanavalin A (1·0 μg/ml; Sigma) alone or in the presence of cytokines. The concentrations of IL-10 in the supernatants were measured by ELISA.
Plastic adherent peritoneal exudate cells (as a source of macrophages) were stimulated for 72 hr with LPS (10 μg/ml) and the effects of exogenous cytokines on IL-10 production by macrophages were determined by ELISA.
Proliferation assay
Purified B cells at a concentration 0·75 × 106 cells/ml were cultured in 96-well tissue culture plates (Nunc, Roskilde, Denmark) in a volume of 200 μl complete RPMI-1640 medium unstimulated or stimulated with 10 μg/ml LPS in the presence or absence of individual cytokines (10 ng/ml, only TGF-β1 was used at 2 ng/ml). Cell proliferation was determined by adding [3H]thymidine (1 μCi/well; Nuclear Research Institute, Rez, Czech Republic) for the last 6 hr of the 72-hr incubation period.
In some experiments, the effects of neutralization anti-cytokine antibodies on the proliferation of LPS-stimulated B cells or on the production of IL-10 were determined. In these studies, mAb anti-IL-10 (clone JES55-16E3; BioLegend) or anti-IL-10R (clone 1B1.3a; BioLegend) were tested, and mAb anti-IL-6 (clone MP5-20F3; BioLegend) was used as a control antibody. The antibodies were included into wells containing LPS-stimulated B cells to a final antibody concentration of 5 μg/ml.
Cytokine detection by ELISA
The production of IL-10 and IL-6 by B cells was determined by ELISA using as capture and detection antibodies anti-IL-10 (purchased from R & D Systems, Minneapolis, MN) or anti-IL-6 (obtained from PharMingen, San Diego, CA) and following the instructions of the manufacturers. The reactions were quantified by spectrophotometry using a Sunrise Remote ELISA Reader (Gröding, Austria).
ELISPOT for IL-10
The complete IL-10 ELISPOT kit was purchased from U-CyTech (Utrecht, the Netherlands) and the assay was carried out according to the instructions of the manufacturer. In brief, purified B cells were stimulated for 24 hr or 48 hr with LPS (10 μg/ml; Difco) with or without added cytokines. After this pre-incubation, the cells were harvested, washed and diluted to the appropriate concentration. The cells (1·0 × 105 cells/well and their two-fold dilutions) were incubated at 37° for another 24 hr in an ELISPOT plate pre-coated with mAb anti-IL-10. The cells were incubated in culture medium or in the medium supplemented with LPS and cytokines corresponding to the pre-incubation conditions. After washing of the plates, the presence of bound IL-10 protein was visualized using a combination of secondary biotinylated anti-IL-10 detection mAb, streptavidin-horseradish peroxidase and β-amini-9-ethylcarbazole to yield a coloured zone. The zones were calculated using ELISPOT Reader CTL-ImmunoSpot S5 UV Analyzer (Shaker Heights, OH).
Intracellular detection of IL-10
Intracellular IL-10 expression was analysed by flow cytometry. The isolated B-cell population was stimulated with LPS (10 μg/ml) in the absence or presence of IL-12, IL-21, IFN-γ or TGF-β, and PMA (20 ng/ml; Sigma), ionomycin (500 ng/ml; Sigma) and Brefeldin A (5 μg/ml; eBioscience, San Diego, CA) were added to the cultures for the last 5 hr of the 48-hr incubation period. Dead cells were stained with a Live/Dead Fixable Violet Dead Cell Stain Kit (Molecular Probes, Eugene, OR) before intracellular staining. Cells were stained with mAb anti-CD19 (clone 6D5; BioLegend) and then permeabilized using a Fixation and Permeabilization Kit (eBioscience) according to the manufacturer's instructions. The cells were intracellularly stained with allophycocyanin-conjugated anti-IL-10 mAb (clone JES5-16E3; eBioscience) and analysed using an LSRII flow cytometer (BD Bioscience).
Detection of IL-10 gene expression
The expression of the gene for IL-10 was detected using reverse transcription–polymerase chain reaction (RT-PCR) as we have described elsewhere.30 In brief, B cells were cultured for 48 hr unstimulated or stimulated with LPS in the presence of cytokines (IL-12, IL-21, IFN-γ, TGF-β). Total RNA was isolated using TRIreagent (Molecular Research Center, Cincinnati, OH). One microgram of total RNA was reverse transcripted into cDNA using SuperScript III (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. The quantities of the cDNA samples were first normalized to yield equal amounts of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sense 5′-GGGTGTGAACCACGAGAAAT-3′, antisense 5′-ACACATTGGGGGTAGGAACA-3′). Subsequently, the samples were hybridized with 5′ and 3′ primers for IL-10 (sense 5′-GTGAAGACTTTCTTTCAAACAAAG-3′, antisense 5′-CTGCTCCACTGCCTTGCTCTTATT-3). The PCR products were analysed by 2% agarose gel electrophoresis.
For a quantitative characterization of IL-10 gene expression, real-time PCR was used as recently described in detail elsewhere.31
Statistics
The results are expressed as the mean ± SD. Comparisons between two groups were analysed by Student's t-test, and multiple comparisons were calculated by analysis of variance. A value of P < 0·05 was considered statistically significant.
Results
Characterization of the enriched B-cell population
The enriched B-cell population was phenotypically characterized by flow cytometry. As demonstrated in Fig. 1, the isolated B-cell population contained >87% CD19+ cells. This population included about 86% CD19+ CD22+ cells, 83% CD19+ CD1d+ cells and approximately 6% CD19+ CD5+ cells. Less than 1·0% of the cells were CD3+, around 1·5% of the cells were CD11b+ and < 3% were CD14+. To exclude the possibility that the B cells could be overgrown by minority cell populations during their 72-hr incubation with IL-12, IL-21, IFN-γ or TGF-β, the flow cytometry analysis was also performed on B cells cultured with cytokines. The flow cytometry data confirmed that the proportion of contaminating cells did not increase during the cultivation of purified B cells with cytokines (data not shown).
Figure 1.
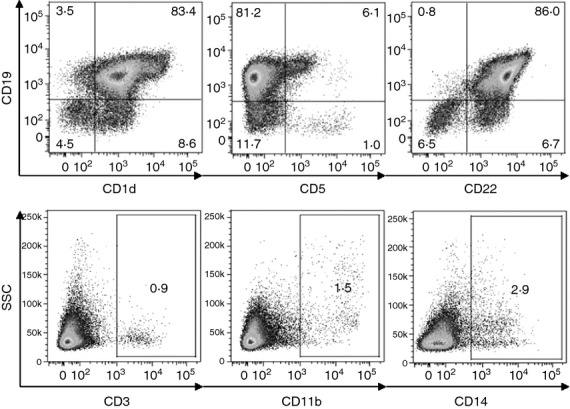
Phenotypic characterization of an enriched B-cell population. B cells were purified by two cycles of purification on nylon wool and the purity of the cells was characterized using flow cytometry. Representative dot plots indicate the percentage of CD19+ CD1d+, CD19+ CD5+, CD19+ CD22+, CD3+, CD11b+ and CD14+ cells. One typical of three similar experiments is shown.
Effects of cytokines on the development of IL-10-producing B cells
The B-cell-enriched cell population was cultured without LPS in the presence of a panel of cytokines and the concentrations of IL-10 in the supernatants were determined by ELISA. As demonstrated in Figure 2(a), none of the cytokines by itself induced a significant IL-10 secretion during the 72-hr incubation period. When B cells were stimulated with LPS, a significant production of IL-10 was detected (Fig. 2b). Furthermore, IL-12 and IFN-γ significantly enhanced LPS-induced IL-10 production, whereas IL-21 and TGF-β had a negative effect on the development of IL-10-producing B cells. In the preliminary experiments (data not shown), a wider range of cytokine concentrations (from 1 to 100 ng/ml) was tested and the results were consistent with those in Fig. 2. To exclude the possibility that the effects of cytokines on IL-10 production are mediated by minority contaminating cell populations (T cells, macrophages), in a separate experiment the enriched B-cell population was depleted of residual contaminating T cells by treatment with cytotoxic anti-CD3 mAb and complement or pre-incubated on plastic to remove possible contamination with plastic-adherent macrophages, and the remaining cells were stimulated with LPS. The preservation of IL-10 production and the consistent effects of cytokines in these T-cell-depleted or macrophage-depleted B-cell populations confirmed that IL-10 was really produced by a population of B cells and that the effects of the cytokines were not mediated through non-B-cell populations.
Figure 2.
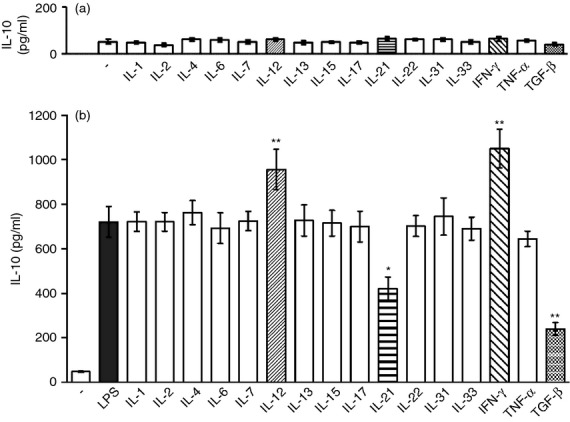
The effect of cytokines on interleukin-10 (IL-10) production by B cells. Purified B cells were cultured for 72 hr (a) in the presence of exogenous cytokines without stimulation or (b) stimulated with 10 μg/ml of lipopolysaccharide (LPS) in the absence or in the presence of cytokines and the concentrations of IL-10 in the supernatants were determined by ELISA. Each bar represents the mean ± SD from five independent determinations. Values with asterisks are significantly (*P < 0·01, **P < 0·001) different from the control (the cells stimulated in the absence of added cytokines).
Effects of cytokines on B-cell proliferation
A panel of cytokines was tested for their effects on the proliferation of LPS-stimulated B cells. Figure 3 shows that more cytokines augmented or inhibited B-cell proliferation, and there was no correlation between the effects of the cytokines on LPS-induced IL-10 production (Fig. 2) and B-cell proliferation (Fig. 3).
Figure 3.
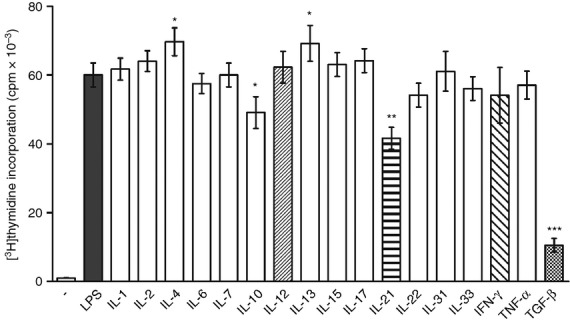
The effect of exogenous cytokines on the proliferation of B cells. Purified B cells were stimulated with 10 μg/ml lipopolysaccharide (LPS) in the absence or presence of exogenous cytokines. Cell proliferation was determined by [3H]thymidine added to the cultures for the last 6 hr of the 72-hr incubation period. Each bar represents the mean ± SD from five independent determinations. Values with asterisks are significantly (*P < 0·05, **P < 0·01, ***P < 0·001) different from the control (the cells stimulated in the absence of added cytokines).
Distinct effects of IL-12, IL-21, IFN-γ and TGF-β on IL-10 production by B cells, T cells and macrophages
The effects of IL-12, IL-21, IFN-γ and TGF-β on IL-10 production by B cells, T cells and macrophages were compared. Consistent with the results shown in Fig. 2, IL-12 and IFN-γ significantly increased, and IL-21 and TGF-β decreased, ΙL-10 production by LPS-stimulated B cells (Fig. 4a). When the effects of the above cytokines were tested on IL-10 production by Concanavalin A-stimulated T cells, the production of IL-10 was strongly enhanced by IL-12, whereas IL-21 and IFN-γ had no effect and TGF-β significantly increased IL-10 production (Fig. 4b). As concerns the effects of cytokines on IL-10 production by LPS-stimulated macrophages, IL-12 had no significant effect, IL-21 slightly increased IL-10 secretion and IFN-γ and TGF-β decreased IL-10 production (Fig. 4c). In addition, the effects of the tested cytokines on IL-6 production from LPS-stimulated B cells were also determined. As shown in Fig. 4(d), neither IL-12 nor IFN-γ significantly enhanced IL-6 production, but production of IL-6 was inhibited by IL-21 and TGF-β.
Figure 4.
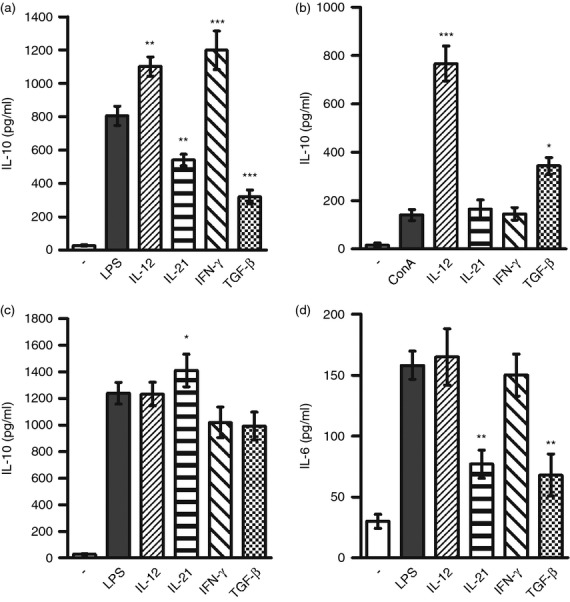
A comparison of the effects of interleukin-12 (IL-12), IL-21, interferon-γ (IFN-γ) and transforming growth factor-β (TGF-β) on IL-10 production by B cells, T cells and macrophages. Purified B cells (a) were stimulated with LPS (10 μg/ml), purified T cells (b) were stimulated with Concanavalin A (1·5 μg/ml) and peritoneal macrophages (c) were stimulated with lipopolysaccharide (LPS) (10 μg/ml), in the absence or presence of IL-12, IL-21, IFN-γ or TGF-β, and the concentrations of IL-10 in the supernatants were determined after a 72-hr incubation period by ELISA. In addition, the concentrations of IL-6 in the supernatants from stimulated B cells were determined (d). Each bar represents the mean ± SD from four independent determinations. Values with asterisks are significantly (*P < 0·05, **P < 0·01, ***P < 0·001) different from the control (the cells stimulated in the absence of added cytokines).
Autoregulation of IL-10 production
The results in Fig. 3 demonstrating the effects of exogenous cytokines on B-cell proliferation suggested a regulatory function of IL-10. To prove an autoregulatory effect of endogenously produced IL-10 on B-cell proliferation, B cells were stimulated with LPS in the presence of neutralization anti-IL-10 or anti-IL-10R mAb. As demonstrated in Figure 5(a), the neutralization of endogenously synthesized IL-10 or the blocking of its binding to IL-10R resulted in a significant augmentation of B-cell proliferation. In contrast, anti-IL-6 mAb used as a control antibody, did not modulate B-cell proliferation (Fig. 5a). To test the effect of IL-10 on IL-10 synthesis, B cells were stimulated with LPS in the presence of added IL-10 and the expression of the gene for IL-10 was determined by real-time PCR. As shown in Fig. 5b, exogenous IL-10 significantly inhibited, in a dose-dependent manner, the expression of the IL-10 gene.
Figure 5.
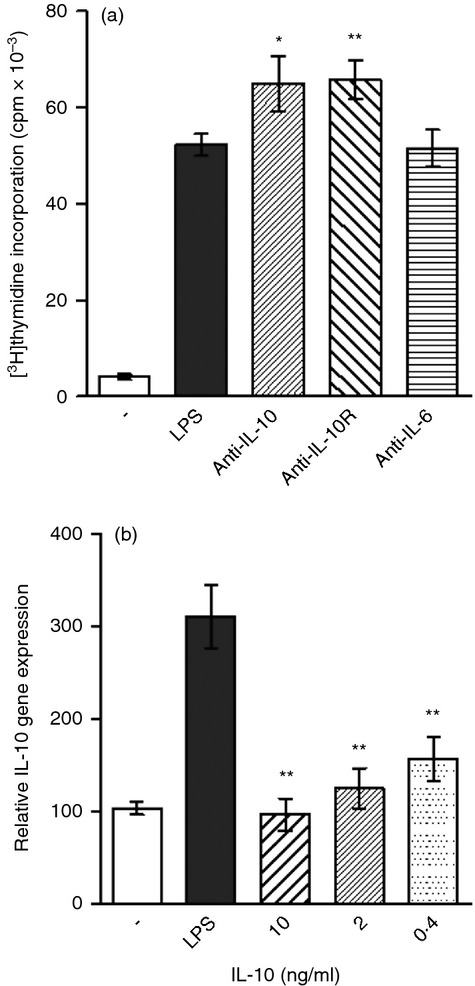
The regulatory effects of interleukin-10 (IL-10) on B-cell proliferation and IL-10 gene expression in B cells. (a) Purified B cells were stimulated with lipopolysaccharide (LPS; 10 μg/ml) in the presence (5 μg/ml) of monoclonal antibody anti-IL-10, anti-IL-10R or anti-IL-6, and cell proliferation was determined by [3H]thymidine added to the cultures for the last 6 hr of the 72-hr incubation period. (b) Purified B cells were stimulated for 72 hr with LPS (10 μg/ml) in the presence of the indicated concentrations of exogenous IL-10 and the expression of the IL-10 gene was determined by real-time PCR. Each bar represents the mean ± SD from three or four independent determinations. Values with asterisks are significantly (*P < 0·05, **P < 0·01) different from the control (the cells stimulated in the absence of added cytokines).
IL-12, IL-21, IFN-γ and TGF-β modulate IL-10 production on the level of IL-10 gene expression
To test whether the regulatory effects of IL-12, IL-21, IFN-γ and TGF-β on IL-10 production by B cells occur already on the level of IL-10 gene expression, B cells were stimulated with LPS in the presence of the cytokines and the expression of the IL-10 gene was determined by RT-PCR. It was observed that IL-12 and IFN-γ enhanced IL-10 mRNA expression, whereas IL-21 and TGF-β decreased IL-10 mRNA levels (Fig. 6a). To exclude the possibility that the different quantities of IL-10 protein detected by ELISA are due to a different absorption of IL-10 by IL-10R in cultures with cytokines, B cells were stimulated with LPS in the presence of anti-IL-10R blocking mAb. As shown in Fig. 6(b), the differences in the level of IL-10 protein observed in B-cell cultures in the presence of cytokines remained the same when anti-IL-10R antibody was included in the cultures (Fig. 6b).
Figure 6.
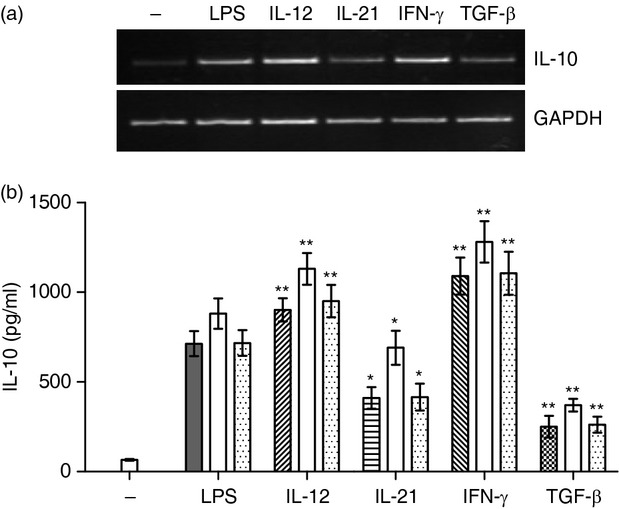
The cytokine-mediated regulation of interleukin-10 (IL-10) production occurs on the level of gene expression and is not caused by differences in IL-10 absorption. (a) Purified B cells were stimulated for 48 hr with lipopolysaccharide (LPS; 10 μg/ml) in the presence of IL-12, IL-21, interferon-γ (IFN-γ) or transforming growth factor-β (TGF-β) and the expression of the IL-10 gene was determined by RT-PCR. One representative experiment of four similar ones is shown. (b) Purified B cells were stimulated with 10 μg/ml LPS in the presence of IL-12, IL-21, IFN-γ or TGF-β in cultures without antibody or with 5 μg/ml of anti-IL-10R neutralization monoclonal antibody (open bars) or 5 μg/ml of control unrelated monoclonal antibody anti-IL-6 (dotted bars). The concentrations of IL-10 in the supernatants were determined by ELISA. Each bar represents the mean ± SD from three independent determinations. Values with asterisks are significantly (*P < 0·05, **P < 0·01) different from the control (the cells stimulated with LPS and a particular cytokine in the absence of added monoclonal antibody).
IL-12, IL-21, IFN-γ and TGF-β regulate the number of IL-10-producing B cells
Theoretically, the difference in the concentrations of IL-10 produced by B cells in the presence of cytokines can be due to a difference in IL-10 production by the same number of IL-10-producing cells, or the cytokines can modulate the number of cells secreting IL-10. To distinguish between these two possibilities, B cells were stimulated with LPS in the presence of cytokines and the number of IL-10-producing cells was determined by ELISPOT and by intracellular IL-10 staining. Figure 7(a) shows a typical ELISPOT experiment and the results are summarized in Fig. 7(b). Figure 7(a,b) shows that IL-12 and IFN-γ significantly increased the number of IL-10-producing cells, whereas the number of cells producing IL-10 was significantly decreased in cultures containing IL-21 or TGF-β. The regulatory effects of cytokines on IL-10 production were confirmed by the visualization of individual IL-10-producing B cells (CD19+ IL-10+ cells) using intracellular staining and flow cytometry analysis (Fig. 7c).
Figure 7.
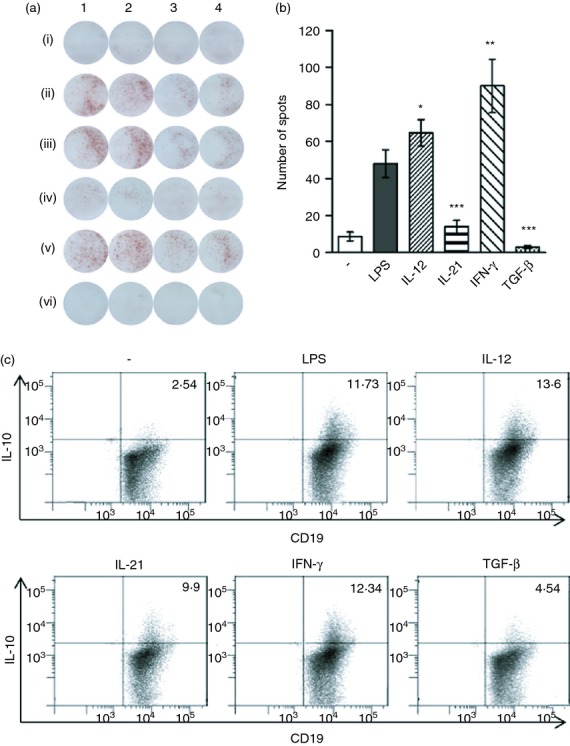
Detection of interleukin-10 (IL-10)-producing B cells by ELISPOT and intracellular staining. Purified B cells were cultured unstimulated, stimulated with 10 μg/ml lipopolysaccharide (LPS) or stimulated with LPS in the presence of IL-12, IL-21, interferon (IFN-γ) or transforming growth factor-β (TGF-β). After a 48-hr pre-incubation, the number of IL-10-producing cells was determined by ELISPOT or by flow cytometry. (a) A typical ELISPOT experiment (one of five similar ones) is shown: (i) unstimulated cells, (ii) cells stimulated with LPS, or cells stimulated with LPS in the presence of (iii) IL-12, (iv) IL-21, (v) IFN-γ or (vi) TGF-β. Lanes 1 and 2: 1·0 × 105 cells per well, lanes 3 and 4: 0·5 × 105 cells/well. (b) An average number of IL-10-producing cells as detected by ELISPOT in cultures containing cytokines is demonstrated. Each bar represents the mean ± SD from five independent determinations. Values with asterisks are significantly (*P < 0·01, **P < 0·001 ***P < 0·0001) different from the control (the cells stimulated with LPS in the absence of added cytokines). (c) Representative dot plots demonstrating the percentages of CD19+ IL-10+ cells, as detected by flow cytometry after intracellular staining. One representative figure from three independent experiments is shown.
Discussion
The immune response is negatively regulated by a number of phenotypically and functionally distinct cell populations and cytokines. In the T-cell department, the development of the most prominent Treg cells is dependent on TGF-β, and other cytokines, such as IL-4, IL-6 or IL-12, skew the TGF-β-dependent developmental programmes of Treg cells into other cell types, such as T helper type 9, 17, 22 or Th1-like cell populations.26,32–35
Among B cells, a subpopulation inhibiting the immune response by the production of IL-10 has been identified and described.3,4 In the present study we show that the development of IL-10-producing B cells (B10 cells) is regulated by distinct cytokines from the development of Treg cells. We found that the production of IL-10 is significantly enhanced if B cells are stimulated in the presence of IL-12 or IFN-γ. In contrast, the development of IL-10-producing B cells was significantly inhibited by TGF-β and IL-21. This pattern of regulatory activity of individual cytokines is distinct from their effect on the development of Treg cells. Namely, TGF-β, which inhibits Breg-cell development, has been shown to be the main factor responsible for the activation and functioning of Foxp3+ Treg cells.36,37 Another cytokine supporting the development of IL-10-producing B cells, IL-12, rather skews the development of Treg cells into other pro-inflammatory Th1-like lineages.27,34 Interferon-γ which strongly enhanced the development of IL-10-producing B cells, is not a factor determining the activation of Treg cells, although in one ex vivo setting the ability of IFN-γ to generate Foxp3+ Treg cells was described.38 The countering effect of IL-21 in the development of B10 cells in a population of B cells from naive mice has not been described, but it may be associated with the recently recognized role of IL-21 in states of immunological hyporeactivity.39 On the contrary, the involvement of IL-21 in expansion of Breg cells has been described in two models of autoimmunity.40,41 In the study of Yoshizaki et al.40 IL-21 itself induced a high (comparably as LPS) production of IL-10 without any other B-cell stimulation. In our model, IL-21 did not enhance IL-10 production above the background secretion, but rather inhibited IL-10 production in LPS-stimulated B cells. Our results are compatible with the findings of Tortola et al.42 who studied marginal zone B cells in a mouse model and found that IL-21 negatively regulated B-cell survival and antibody production. These discrepancies might reflect the different models used, the different compositions of the isolated B cells and the variable sensitivity of B cells to distinct activation signals.26
The regulatory effects of cytokines on IL-10 production by B cells did not correlate with their effects on B-cell proliferation. Although TGF-β and IL-21 inhibited B-cell proliferation, IL-12 and IFN-γ, which enhanced IL-10 production, did not affect B-cell proliferation. Rather, other cytokines (such as IL-4, IL-13) enhanced B-cell proliferation without any effect on IL-10 secretion. Hence, the immunoregulatory effects of cytokines on the development of IL-10-producing B cells appears to be independent of their effects on B-cell proliferation. Moreover, IL-12, IL-21, IFN-γ and TGF-β, which significantly influence IL-10 production by B cells, have a distinct pattern of effects on IL-10 production by T cells or macrophages. Another cytokine, which regulates IL-10 production by B cells, is IL-10 itself. We found that exogenous IL-10 added into cultures of LPS-stimulated B cells inhibited B-cell proliferation and also significantly suppressed IL-10 gene expression. In addition, blocking of endogenously synthesized IL-10 with a neutralizing anti-IL-10 mAb or blocking its effect with anti-IL-10R mAb enhanced B-cell proliferation and IL-10 production. These results suggest autoregulatory effects of endogenously synthesized IL-10 on the development of IL-10-secreting B cells. Our observations extend the results of Sindhava et al.,43 who showed in a model of Borrelia infection that stimulation of peritoneal B-1 cells through Toll-like receptor induced IL-10 production, and the secreted IL-10 mediated autoregulation of B-1 cells.
To exclude the possibility that the different levels of IL-10 in B-cell cultures containing the regulatory cytokines were due to a different absorption of IL-10 by IL-10R, we determined the concentrations of IL-10 in the cultures containing anti-IL-10R mAb to prevent the binding of secreted IL-10 to its receptor. The results showed that the distinct levels of IL-10 observed in the presence of individual regulatory cytokines remained preserved. Furthermore, using RT-PCR we showed that the regulatory effects of IL-12, IL-21, IFN-γ and TGF-β on IL-10 production by B cells occur already on the level of IL-10 gene expression.
Theoretically, the cytokines could modulate IL-10 production in the cultures of LPS-stimulated B cells by an increase in level of IL-10 secretion of individual IL-10-producing cells, or alternatively the cytokines could regulate the number of cells producing IL-10. To distinguish between these two possibilities, an ELISPOT assay and visualization of individual CD19+ IL-10+ cells by flow cytometry were employed. The results showed that IL-12 and IFN-γ significantly increase the number of IL-10-producing cells, whereas the number of B cells secreting IL-10 was dramatically decreased in cultures containing IL-21 or TGF-β. These results demonstrated that the four cytokines modulate the development and the number of IL-10-secreting B cells after their stimulation through TLR.
The immunoregulatory activity of Breg cells has been demonstrated in various models of inflammation, autoimmunity, transplantation and anti-tumour immunity.4,19,44 In the majority of these models, the secretion of IL-10 has been shown to be the main mechanism of Breg-cell-mediated immunosuppression.4,9 Our results show that the development of B10 cells and IL-10 production by activated B cells are regulated by at least four cytokines. The involvement of all of these cytokines in the stimulation of immunity or in the activation of regulatory cells has been reported.27,34,38,39 Hence, there is a tightly regulated balance of the immune response resulting from the interaction of Treg cells, Breg cells and numerous cytokines. These cytokines maintain the balance between immunity and tolerance and can have opposite effects on the development of Treg cells and IL-10-producing Breg cells or can selectively influence only one regulatory cell population.
Acknowledgments
This work was supported by grant NT/14102 from the Grant Agency of the Ministry of Health of the Czech Republic, grants P304/11/0653 and P301/11/1568 from the Grant Agency of the Czech Republic, and projects MSM0021620858 and SVV 265211 from the Ministry of Education of the Czech Republic.
Disclosure
The authors have no financial or commercial conflicts of interest.
References
- 1.Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21:903–14. [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 3.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J Exp Med. 1997;186:1749–56. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. [Google Scholar]
- 5.Mauri C, Bosma A. Immune regulatory function of B cells. Ann Rev Immunol. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 6.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19+ CD24hi CD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Vlugt van der LE, Labuda LA, Ozir-Fazalalikhan A, et al. Schistosomes induce regulatory features in human and mouse CD1dhi B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS ONE. 2012;7:e30883. doi: 10.1371/journal.pone.0030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–63. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–72. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao G, Moore DJ, Lee KM, et al. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am J Transplant. 2010;10:796–801. doi: 10.1111/j.1600-6143.2010.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–98. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Eliav Y, Shin SU, Schreiber TH, Podack ER, Tadmor T, Rosenblatt JD. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer Immunol Immunother. 2013;62:87–99. doi: 10.1007/s00262-012-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinker MW, Lundy SK. Multiple mechanisms of immune suppression by B lymphocytes. Mol Med. 2012;18:123–37. doi: 10.2119/molmed.2011.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS. Allergen-specific transforming growth factor-β-producing CD19+ CD5+ regulatory B-cell (Br 3) responses in human late eczematous allergic reactions to cow's milk. J Interferon Cytokine Res. 2011;31:441–9. doi: 10.1089/jir.2010.0020. [DOI] [PubMed] [Google Scholar]
- 15.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 17.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Bouaziz JD, Buanec Le H, Saussine A, Bensussan A. IL-10 producing regulatory B cells in mice and humans: state of the art. Curr Mol Med. 2012;12:519–27. doi: 10.2174/156652412800620057. [DOI] [PubMed] [Google Scholar]
- 19.Carter NA, Vasconcellos R, Rosser EC, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–79. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 20.Dass SE, Vital M, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. 2007;56:2715–8. doi: 10.1002/art.22811. [DOI] [PubMed] [Google Scholar]
- 21.Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm Bowel Dis. 2007;13:1365–8. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]
- 22.Lee KM, Kim JI, Stott R, et al. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12:2072–8. doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503–13. doi: 10.1038/ki.2010.162. [DOI] [PubMed] [Google Scholar]
- 24.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–61. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampropoulou V, Hoehlig K, Roch T, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–73. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 26.Jin H, Carrio R, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–65. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 27.Prochazkova J, Pokorna K, Holan V. IL-12 Inhibits the TGF-β-dependent T cell developmental programs and skews the TGF-β-induced differentiation into Th1-like direction. Immunobiology. 2012;217:74–82. doi: 10.1016/j.imbio.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Svobodova E, Krulova M, Zajicova A, Prochazkova J, Trosan P, Holan V. The role of mouse mesenchymal stem cells in differentiation of naive T cells into anti-inflammatory regulatory T cell and proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012;21:901–10. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–9. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 30.Krulova M, Pokorna K, Lencova A, Zajicova A, Fric J, Filipec M, Forrester JV, Holan V. A rapid separation of two distinct populations of corneal epithelial cells with limbal stem cell characteristics in the mouse. Invest Ophthalmol Vis Sci. 2008;49:3903–8. doi: 10.1167/iovs.08-1987. [DOI] [PubMed] [Google Scholar]
- 31.Trosan P, Svobodova E, Chudickova M, Krulova M, Zajicova A, Holan V. The key role of insulin-like growth factor I in limbal stem cell differentiation and corneal wound healing process. Stem Cells Dev. 2012;21:3341–50. doi: 10.1089/scd.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tofukuji S, Kuwahara M, Suzuki J, Ohara O, Nakayama T, Yamashita MY. Identification of a new pathway for Th1 cell development induced by cooperative stimulation with IL-4 and TGF-β. J Immunol. 2012;188:4846–57. doi: 10.4049/jimmunol.1103799. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Zhao J, Perlman S. Differential effects of IL-12 on Tregs and non-Treg T cells: roles of IFN-γ, IL-2 and IL-2R. PLoS ONE. 2012;7:e46241. doi: 10.1371/journal.pone.0046241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-γ-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–43. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25– naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng G, Wood KJ, Bushell A. Interferon-γ conditioning ex vivo generates CD25+ CD62L+ Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008;86:578–89. doi: 10.1097/TP.0b013e3181806a60. [DOI] [PubMed] [Google Scholar]
- 39.Petrelli A, Carvello M, Vergani A, et al. IL-21 is an antitolerogenic cytokine of the late-phase alloimmune response. Diabetes. 2011;60:3223–34. doi: 10.2337/db11-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–8. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Yang J, Chu Y, Wang J, Guan M, Zhu X, Xue Y, Zou H. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in lupus-prone MLR/lpr mice. PLoS ONE. 2013;8:e62855. doi: 10.1371/journal.pone.0062855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tortola L, Yadava K, Bachmann MF, Müller C, Kisielow J, Kopf M. IL-21 induces death of marginal zone B cells during chronic inflammation. Blood. 2010;116:5200–7. doi: 10.1182/blood-2010-05-284547. [DOI] [PubMed] [Google Scholar]
- 43.Sindhava V, Woodman ME, Stevenson B, Bondada S. Interleukin-10 mediated autoregulation of murine B-1 B-cells and its role in Borrelia hermsii infection. PLoS ONE. 2010;7:e11445. doi: 10.1371/journal.pone.0011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redfield RR, III, Rodriguez E, Parsons R, Vivek K, Mustafa MM, Noorchashm H, Naji A. Essential role for B cells in transplantation tolerance. Curr Opin Immunol. 2011;23:685–91. doi: 10.1016/j.coi.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


