Abstract
Newborn mammals are highly susceptible to respiratory infections. Although maternal antibodies (MatAb) offer them some protection, they may also interfere with their systemic immune response to vaccination. However, the impact of MatAb on the neonatal mucosal immune response remains incompletely described. This study was performed to determine the effect of ovalbumin (OVA)-specific MatAb on the anti-OVA antibody response in sera, nasal secretions and saliva from specific pathogen-free Vietnamese miniature piglets immunized at 7 or 14 days of age. Our results demonstrated that MatAb increased antigen-specific IgA and IgG responses in sera, and transiently enhanced an early secretory IgA response in nasal secretions of piglets immunized at 7 days of age. In contrast, we detected a lower mucosal (nasal secretion and saliva) anti-OVA IgG response in piglets with MatAb immunized at 14 days of age, compared with piglets with no MatAb, suggesting a modulatory effect of antigen-specific maternal factors on the isotype transfer to the mucosal immune exclusion system. In our porcine model, we demonstrated that passive maternal immunity positively modulated the systemic and nasal immune responses of animals immunized early in life. Our results, therefore, open the possibility of inducing systemic and respiratory mucosal immunity in the presence of MatAb through early vaccination.
Keywords: maternal antibodies, mucosal immunity, neonatal immunization, Vietnamese miniature potbellied piglets
Introduction
The mucosa of the upper respiratory airways interacts with a diverse array of microorganisms, antigens and allergens in its environment. A key defence mechanism is the local antibody immune response, characterized by the active transport of secretory IgA to the mucosal surface. This immunoglobulin binds antigens, preventing their attachment to epithelial cells by a mechanism called ‘immune exclusion’, neutralizing viral replication on epithelial cells, or mediating the transfer of antigens from the mucosal lamina propria to the mucosal surface. In addition, paracellular leakage of IgG antibodies contributes to immune exclusion. However, IgGs can activate inflammatory mechanisms that could cause damage to the mucosa in contrast to its importance in systemic compartments.1–3
In neonates, respiratory tract infections are frequent,4 yet few effective vaccines are available.5,6 Recent data indicate that neonatal immunization may generate a mucosal immune response, with the use of adequate immunization strategies.7 However, some evidence suggests that the presence of maternal antibodies (MatAb)8 may impair the systemic antigen-specific humoral immune response in neonates, though their effect on the mucosal compartments has not been fully explored.9 At the respiratory mucosa, the presence of MatAb has been associated with beneficial effects against infections,10 allergies and asthma.11 Little is known about the influence of MatAb in the nasal or oropharyngeal neonatal response following immunization, despite the fact that such antibodies could partially modulate the immune response against respiratory viruses.12
Pigs are frequently used as an experimental model because of their anatomical, physiological and genetic proximity to humans. Furthermore, pigs represent an excellent animal model for evaluating maternal passive immunity, because they have an epitheliochorial placenta, no transplacental transfer of MatAb, or of large molecules occurring during gestation.13,14 Accordingly, the survival of piglets depends upon their ingestion of colostrum during the first hours of life, which includes MatAb, proteins, and immune cells. IgG is the major isotype in sow colostrum, followed by IgA, and in piglets maternal IgG appears to down-regulate the neonatal systemic immune response. Nevertheless, few studies have supported a possible regulatory role by maternal IgG or IgA on the mucosal immune response to vaccination.15
To study the effect of MatAb on the mucosal immune response to neonatal immunization, we evaluated the anti-ovalbumin (OVA) IgA and IgG responses in sera, nasal secretions and saliva in piglets subcutaneously and intranasally immunized at 7 or 14 days of age, in the presence or absence of anti-OVA MatAb. We used this immunization protocol because the combination of parenteral priming, followed by mucosal booster immunization, has been shown to induce effective systemic and mucosal immune responses.16
Materials and methods
Animals
Specific pathogen-free, non-vaccinated Vietnamese miniature potbellied piglets, born from multiparous sows were obtained from the Production Unit of Experimental Laboratory Animals (UPEAL-Cinvestav, México D.F., México). The animals were kept in separate maternal rooms and weaned at 4 weeks of age. All handling and husbandry procedures followed institutional guidelines (NOM-062-ZOO-1999) approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL).
Immunization of sows and piglets
Five sows were intramuscularly immunized 4 and 2 weeks before farrowing on each side of the neck with OVA (1 mg/kg, Sigma, St Louis, MO) in 0·9% saline, with mineral oil (GIBCO Vet LO7 0:1; GIBCO BRL, Grand Island, NY) as an adjuvant (20 : 1 antigen : adjuvant ratio) (OVA-seropositive sows; OVA+S); their litters were used as experimental models (OVA-seropositive piglets; OVA+P). Three non-immunized, OVA-seronegative sows (OVA–S) and their litters (OVA-seronegative piglets; OVA–P) were also used.
According to the level of anti-OVA IgA and IgG antibodies detected in colostrum and serum from the OVA+S, their litters were divided into experimental groups. One group of OVA+P (n = 8) was used to confirm the transference and decay with time of MatAb from the sows to their offspring.15 One OVA–P (n = 8) group was used as a non-transferred control. The other piglets were used for early immunization protocols.
Piglets from OVA+S and OVA–S were subcutaneously immunized (1 mg/kg OVA in 0·9% saline with adjuvant) at the base of the ear at 7 days (OVA+P 7D, n = 6, and OVA–P 7D, n = 5, respectively) or 14 days (OVA+P 14D, n = 8, and OVA–P 14D, n = 5) of age [day 0 post-immunization (i.e. 0 days p.i.)]. Ten days later (10 days p.i.), they received a second subcutaneous immunization as a booster, and finally at 20 days p.i. an intranasal booster (2 mg/kg OVA in 0·9% saline without adjuvant). An additional intranasal booster was given at 55 days p.i., and the groups were evaluated for an additional 14 days (Table 1).
Table 1.
List of experimental groups
| Group (number of piglets) | Immunization protocol SC/SC/IN/INB; age in days at immunization |
|---|---|
| Piglets from OVA–S | |
| OVA–P (n = 8) | Control (non-immunized) |
| OVA–P 7D (n = 5) | 7/17/27/62 |
| OVA–P 14D (n = 5) | 14/24/34/69 |
| Piglets from OVA+S | |
| OVA+P (n = 8) | Transfer group (anti-OVA MatAb) |
| OVA+P 7D (n = 6) | 7/17/27/62 |
| OVA+P 14D (n = 8) | 14/24/34/69 |
SC, subcutaneous; IN, intranasal; INB, IN boost; 7D, 7-day old piglets; 14D, 14-day-old piglets; OVA–S, OVA-seronegative sow; OVA–P, OVA-seronegative piglets; OVA–P 7D and OVA–P 14D, OVA-seronegative piglets immunized at 7 or 14 days of age with OVA; OVA+S, OVA-seropositive sow; OVA–P, OVA-seronegative piglets; OVA+P 7D and OVA+P 14D, OVA-seropositive piglets immunized at 7 or 14 days of age with OVA.
Sample collection
Blood samples were collected from the sows before priming and after farrowing. The anti-OVA MatAb kinetics of disappearance was determined weekly in sera from the eight non-immunized OVA+P, from 7 to 76 days of age. To evaluate piglet immune response after immunization, serum samples were collected at 0, 10, 20, 27, 34, 41, 48, 55, 62 and 69 days p.i. All blood samples were obtained by jugular venepuncture, centrifuged (350 g), and the resultant serum was stored at −20°.
Sow colostrum was collected under aseptic conditions within 4 hr after farrowing, treated with 20 μl/ml 10% dextran sulphate (Leuconostoc spp., Sigma, Oakville, ON, Canada) in a mixture of distilled water and 3M anhydrous CaCl2 (Sigma, St Louis, MO), and centrifuged at every step for 30 min at 2000 g. The middle layer (whey) was removed and stored at −20°.
Nasal secretions and saliva were also collected. For the individual and efficient collection of piglet mucosal secretions, sterile swabs were carefully rubbed into their nasal and oral cavities until wet. The collected swabs were kept at 4° in Eppendorf tubes until centrifuged at 5000 g at 4° for 5 min. The liquid obtained was collected and mixed with a cocktail of protease inhibitors (TPCK 50 μg/ml, TLCK 25 μg/ml, Sigma, Buchs, Switzerland, and PMSF 174 μg/ml, Sigma, Shanghai, China; 20 : 1 sample : cocktail) and stored at −20° until used. With this sampling method, we were able to detect nanograms of antigen-specific antibodies in mucosal secretions by quantitative ELISA.
OVA-specific quantitative ELISA
Serum, colostrum, nasal secretion and saliva OVA-specific IgA and IgG antibodies were measured using a quantitative ELISA test adapted in our laboratory. Ninety-six-well polystyrene microtitre plates (Costar®, Corning, NY) were coated with 2 μg/ml OVA (100 μl per well), in 50 mm carbonate–bicarbonate buffer, pH 9·6 (0·5 m Na2CO3, 0·5 m NaHCO3; J.T. Baker, Ecatepec, Mexico) and incubated overnight at 4°. The incubated plates were washed with PBS, pH 7·4 (137 mm NaCl, Sigma, St Louis MO; 1·4 mm KH2PO4, 4·3 mm Na2HPO4, J.T. Baker, Ecatepec, Mexico; 2·7 mm KCl, J.T. Baker, Center Valley, PA) containing 0·05% Tween-20 (Sigma, St Louis, MO) (PBS-T), and blocked with PBS-T for IgA and with 1% BSA (fraction V, Roche, Mannheim, Germany) for IgG for 60 min at 37°. Sera, colostrum, nasal secretions and saliva were fourfold diluted, starting with 1/50 for sera and 1/5 for mucosal secretions. After 60 min of incubation at 37°, the plate was washed with PBS-T and 100 μl of either anti-pig IgA or anti-pig IgG (Bethyl Laboratories, Montgomery, TX), at the adequate dilution, was placed into their respective wells. After 60 min of incubation at 37°, the wells were washed with PBS-T, and 100 μl of horseradish peroxidase-conjugated mouse (or goat) anti-pig IgA or IgG (Zymed Laboratories, San Francisco, CA) was added to each well. The reaction was visualized with 3,3′,5,5′-tetramethylbenzidine (Sigma, Shanghai, China) and stopped with 100 μl of 2 m H2SO4 (Sigma, St Louis, MO). The plates were read at 450 nm in a microplate reader (Thermo Scientific Multiskan EX, Suzhou, China). The results are expressed as optical densities (OD) in a predefined dilution. To eliminate the test background, the OD (triplicate) mean and standard deviation (SD) of the negative wells (without sample) were obtained and this value, +3 SD, was subtracted from each sample value. Negative controls from colostrum, sera and mucosal secretions were used to obtain the cut-off value above which the samples were considered positive. A standard curve of total anti-pig IgA or anti-pig IgG antibodies was run in each plate using a commercial standard serum (Bethyl Laboratories), following the manufacturer's instructions; ODs of the corrected samples were interpolated for transformation into quantitative data (concentration of anti-OVA IgA or IgG). Results are expressed in μg/ml for sera or ng/ml for mucosal secretions.
Statistical analysis
All statistical analyses were processed using SigmaPlot 12.0 software. A Student's t-test was used to detect differences in sows. The four experimental groups of immunized piglets, for each isotype and compartment, were first analysed by a two-way repeated measures analysis of variance (two-way RM anova), using the offspring from immunized or non-immunized sows as factor A, and the time post-immunization as factor B. In those instances where the F ratio was significant, differences among the means of the experimental groups were assessed using the Student–Newman–Keuls (SNK) test, to compare groups of the same age for each isotype at each time-point. Differences were considered statistically significant at P < 0·05.
Results
Anti-OVA antibodies in serum and colostrum of immunized sows
The induction of specific antibodies in sera and colostrum from OVA+S was evaluated. Significantly high levels of anti-OVA IgA (P < 0·001) (Fig. 1a) and IgG (P < 0·01) (Fig. 1b) antibodies were detected in serum of five OVA+S. Colostrums were collected after farrowing, and all OVA+S showed high levels of anti-OVA IgA (Fig. 1a), and IgG (Fig. 1b), whereas three OVA–S had no OVA-specific antibodies in their serum or colostrum (data not shown).
Figure 1.
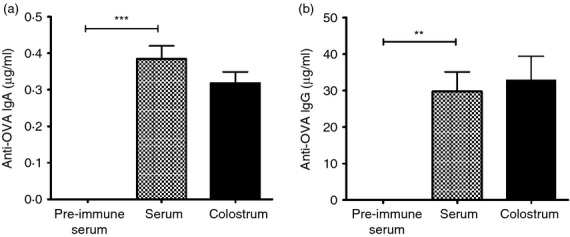
Anti-ovalbumin (OVA) IgA (a) and IgG (b) concentration in serum and colostrum of immunized sows. Five OVA-seronegative sows were bled and immunized with OVA 4 and 2 weeks before farrowing. Each bar represents the mean of the antibody concentration (μg/ml) in pre-immune serum, serum and colostrum of sows at farrowing ± SEM detected by ELISA. A Student's t-test was used to detect differences in sera, **P < 0·01, ***P < 0·001. Note that graph scales differ.
Transfer of maternal antibodies to serum, nasal secretions and saliva of piglets
To confirm the transfer and decay time of MatAb from the sows to the offspring, eight non-vaccinated OVA+P were sampled weekly for the presence of anti-OVA MatAb. As expected, in serum OVA-specific IgA (Fig. 2a) and IgG (Fig. 2b) antibodies were absorbed, and gradually reduced in concentration with time. In contrast, anti-OVA IgA and IgG antibodies were not detected in nasal secretions or saliva in the same piglets at any time (data not show). No detectable levels of anti-OVA IgA or IgG antibodies were found in any of the eight OVA–P control serum, nasal secretion, or saliva samples.
Figure 2.
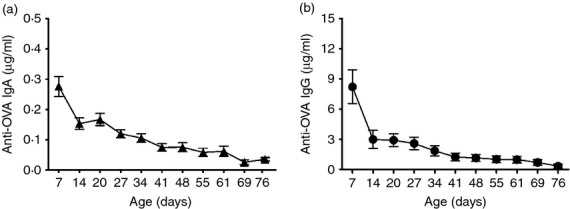
Transfer of anti-ovalbumin (OVA) IgA (a) and IgG (b) antibodies from an immunized sow to its offspring. Kinetics of serum anti-OVA IgA and IgG antibody concentrations from eight piglets (OVA+P) from an OVA-seropositive sow (OVA+S). Each symbol represents the mean (± SEM) of the antibody concentration of eight piglets, detected by ELISA. Note that graph scales differ.
Anti-OVA IgA and IgG responses in serum of OVA+P and OVA–P vaccinated groups
In sera, the IgA and IgG antibody responses were different among the four groups (P < 0·001) and with time (P < 0·001) (two-way RM anova). In piglets first immunized at 7 days old, a significantly higher level of anti-OVA IgA was detected in OVA+P 7D compared with OVA–P 7D (P < 0·001; SNK) (Fig. 3a). In contrast, the OVA+P 14D group showed higher levels of IgA than the OVA–P 14D group, but only at the beginning of the experiment due to the MatAb transfer (P < 0·001; SNK) (Fig. 3b). Anti-OVA IgG levels were significantly higher in the OVA+P 7D group compared with the OVA–P 7D (P < 0·001; SNK) group (Fig. 3c), but this difference was lost at the 14-day immunization (P = 0·187; SNK) (Fig. 3d). For both isotypes, the initial (< 20 days p.i.) anti-OVA IgA and IgG responses in the OVA+P group were primarily of maternal origin, and rapidly decreased with time (see Fig. 2a,b).
Figure 3.
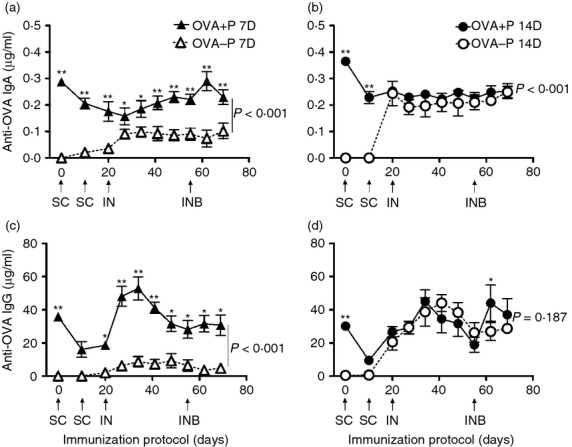
Serum anti-ovalbumin (OVA) IgA (a, b) and IgG (c, d) responses of 7-day-old (7D) or 14-day-old (14D) immunized piglets in the presence (OVA+P) or absence (OVA-P) of anti-OVA maternal antibodies (MatAb). Each triangle or circle represents the mean of the serum antibody concentration of five to eight piglets ± SEM, detected by ELISA. Arrows indicate the days and route of immunization. SC = subcutaneous, IN = intranasal, INB = IN boost. Significant differences were found among groups (P < 0·001) and in time (P < 0·001) by two-way repeated measures analysis of variance, for IgA and IgG. Thus, a Student–Newman–Keuls test was used to compare groups of the same age for each isotype (P shown in the graph) and at each time point (shown by asterisks, *P < 0·05, **P < 0·01). Note that graph scales differ.
Anti-OVA IgA and IgG responses in nasal secretions of OVA+P and OVA–P vaccinated groups
The antigen-specific IgA and IgG responses were also measured in nasal secretions. The IgA response of OVA+P and OVA–P groups, first immunized at 7 or 14 days, were similar among the four groups (P = 0·053) (two-way RM anova), but differed with time (P < 0·001). Anti-OVA IgA of OVA+P 7D was significantly higher at days 20, 27, 34 and 41 post-immunization in comparison to OVA–P 7D (P < 0·05; SNK), but similar between groups (P = 0·449; SNK) (Fig. 4a). In contrast, the IgA response of animals first immunized at 14 days was similar between groups (P = 0·831; SNK) (Fig. 4b). On the other hand, the IgG response showed significant differences among the four groups (P = 0·013), and with time (P < 0·001) (two-way RM anova). The anti-OVA IgG response was low and similar between the groups first immunized at 7 days of age (P = 0·519; SNK) (Fig. 4c), but when the immunization started 7 days later, the anti-OVA IgG response of OVA+P 14D was significantly lower than that of OVA–P 14D (P = 0·010; SNK) (Fig. 4d).
Figure 4.
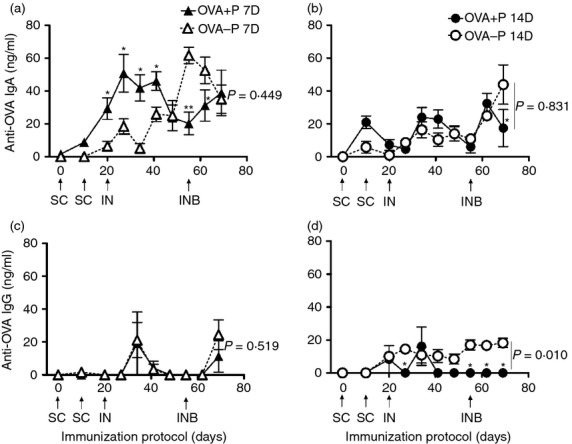
Anti-ovalbumin (OVA) IgA (a, b) and IgG (c, d) responses in nasal secretions of 7-day-old (7D) or 14-day-old (14D) immunized piglets in the presence (OVA+P) or absence (OVA–P) of anti-OVA maternal antibodies (MatAb). Each triangle or circle represents the mean of the antibody concentration in nasal secretions of five to eight piglets ± SEM, detected by ELISA. Arrows indicate the days and route of immunization. SC = subcutaneous, IN = intranasal, or INB = IN boost. By two-way repeated measures analysis of variance, significant differences were found with time (P < 0·001) for IgA; and among groups (P = 0·013) and with time (P < 0·001) for IgG. Hence, a Student–Newman–Keuls test was used to compare groups of the same age, for each isotype (P shown in the graph) at each time-point (shown by asterisks, *P < 0·05, **P < 0·01). Note that graph scales differ.
Anti-OVA IgA and IgG responses in saliva of OVA+P and OVA–P vaccinated groups
In saliva, the IgA response was different with time (P < 0·001), but not among the four experimental groups (P = 0·344) (two-way RM anova). No significant differences were measured for anti-OVA IgA between groups first immunized at 7 or 14 days of age (P = 0·493 and P = 0·235, respectively; SNK) (Fig. 5a,b). The anti-OVA IgA response of OVA+P 14D was significantly lower than the OVA–P 14D at 69 days p.i. only (P < 0·001; SNK) (Fig. 5b). The IgG response was different among the four experimental groups (P < 0·001) and with time (P < 0·001) (two-way RM anova). In piglets first immunized at 7 days of age, the anti-OVA IgG response was poor and no significant difference was found between groups (P = 0·400; SNK) (Fig. 5c). In contrast, the anti-OVA IgG response of OVA+P 14D was significantly lower than the OVA–P 14D group (P < 0·001; SNK) (Fig. 5d).
Figure 5.
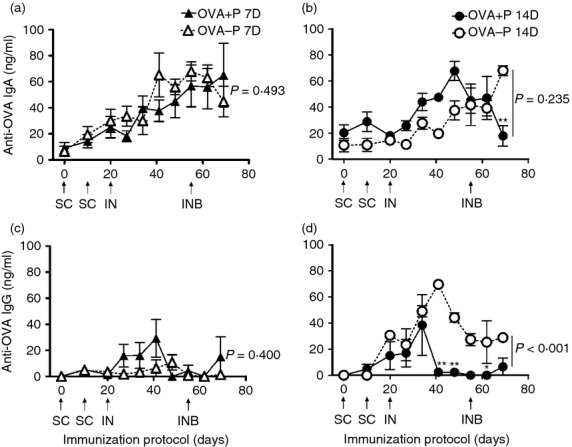
Anti-ovalbumin (OVA) IgA (a, b) and IgG (c, d) responses in saliva of 7-day-old (7D) or 14-day-old (14D) immunized piglets in the presence (OVA+P) or absence (OVA–P) of anti-OVA maternal antibodies (MatAb). Each triangle or circle represents the mean of the antibody concentration in the saliva of five to eight piglets ± SEM, detected by ELISA. Arrows indicate the day and route of immunization. SC = subcutaneous, IN = intranasal, or INB = IN boost. Two-way repeated measures analysis of variance found significant differences with time (P < 0·001) for IgA; and among groups (P < 0·001) and with time (P < 0·001) for IgG. Hence, a Student–Newman–Keuls test was used to compare groups of the same age, for each isotype (P shown in the graph) and at each time-point (shown by asterisks, *P < 0·05, **P < 0·01). Note that graph scales differ.
Discussion
The respiratory mucosa is a biological surface commonly exposed to antigenic challenges, making its antibody secretion essential for immune protection.1–3 However, minimal investigation into this mechanism in the neonatal and post-weaning periods has been performed in human and animal models.4 In recent years, the importance of maternal immunity on the induction and development of the immune response in the gastrointestinal mucosae has been revisited,7,11 but few reports have evaluated its role in the respiratory tract.9,12 Consequently, in this work we explored the effect of maternal immunity and age on the IgA and IgG immune responses in sera, nasal secretions and saliva after neonatal immunization, using a porcine model.
The transfer of MatAb from a sow to its offspring occurs during the first hours after farrowing,15 protecting the piglets against pathogens to which the sow has been previously exposed.10 Therefore, the intake of colostrum is important for the survival and development of the litter. In our experiment, we verified that this transfer of antigen-specific MatAb to piglet sera was indeed according to the level of immunity of the sows.
Neonatal piglet enterocytes undergo selective transcytosis for IgG, IgM and IgA from colostrum to serum, and it has been proposed that monomeric and dimeric IgA undergo reverse transudation from the neonatal blood to the respiratory tract of piglets.15,17 In our experiment, no antigen-specific MatAb were detected in mucosal secretions after birth; however, it is possible that MatAb levels in saliva and nasal secretions were sufficiently low to remain below the detection limit for our OVA-specific quantitative ELISA. Considering that maternally transferred secretory IgA have a secretory component in their structure, our results suggest that MatAb may have mainly a systemic role, whereby their secretion to the oronasal surfaces may be limited, which could have implications for mucosal immunization.
When we analysed the antigen-specific IgA and IgG immune responses in serum from OVA+P, we found that at 7 days, maternal immunity enhanced the systemic immune response. However, this enhancing effect was lost if the immunization protocol began 7 days later (at 14 days of age), suggesting that the stimulating factors15 are no longer available at that age, or that the immune maturation of the newborn immune system is already achieved.13,18 The enhancing effect could be explained in several ways: (i) A more efficient internalization and processing of MatAb–antigen immune complexes by antigen-presenting cells and follicular dendritic cells, followed by presentation to T cells;6,19 (ii) improved B-cell activation by cross-linking of the B-cell receptor with CR2 (CD21) molecules;12 (iii) newborn immune priming by maternal anti-idiotypic antibodies;20 (iv) insufficient MatAb to neutralize the antigen at the time of immunization may favour the immune response.8 Hence, we did not observe maternal interference for IgA or IgG responses in serum that have been found in other studies,6,8,15 probably because of differences in immunization protocol, age at immunization and/or type of antigen used. Therefore, further studies are needed to reach a consensus on the possibility of interference by maternal IgA and IgG.
Given the importance of age on the development of systemic humoral immunity and on early immunization,6,13,18 it was confirmed that in the absence of antigen-specific MatAb, the serum antibody response of OVA–P 14D was higher than the OVA–P 7D group. We also demonstrated that the intranasal booster, given 2 weeks before the end of the experiment, maintained the immune response in all the experimental groups, suggesting the effective generation of memory clones which could last beyond the weaning period (28 days of age).
In nasal secretions, the transfer of antigen-specific maternal factors was able to transiently enhance the IgA response of OVA+P 7D, at least during the first 41 days p.i. It has been reported that maternal immunity may accelerate the maturation and activation of mucosal immunity, increasing antigen-specific immune exclusion,11 which may limit the antigen challenge, at least during this period. However, this effect disappeared in the 14-day groups, which could be explained by a more mature mucosa and a more effective barrier function.
On the other hand, the IgG response was low in nasal secretions of pigs immunized at 7 and 14 days of age, but an apparent down-regulation in the OVA+P 14D group was detected. This result suggests that maternal immunity may have differential effects between isotypes and age of immunization, favouring the IgA production above the IgG secretion in nasal mucosa. The priming of the immune system at the cervical lymph nodes may induce isotype-specific, circulating effector cells, which under the effect of the intranasal booster migrated to the mucosal effector sites, where the combination of the mechanisms explained above may have an effect on the activation, homing and function of mucosal effector B cells.21 The effect appears to be antigen-specific, because both groups of piglets (OVA+P and OVA–P) ingested colostrum, but only the OVA+P showed the modulatory response.
Finally, in saliva the immune response of piglets immunized at 7 or 14 days did not appear to be influenced by the presence of MatAb. However, a possible down-regulation in the IgG secretion from OVA+P 14D was also detected. These results agree with other published data, which showed that in intestinal tissue, MatAb induced a more pronounced suppression of IgG than IgA.22 Most of the IgG present in the salivary glands is derived from serum.2 However, in our porcine model, despite the high level of serum anti-OVA IgG in all 7-day and 14-day groups (except the OVA–P 7D) (Fig. 3), the low anti-OVA IgG level in saliva suggests a poor transudation of this antibody isotype, even for MatAb.
It seems that the induction of mucosal immunity in neonates may have important differences from systemic compartments in their regulatory mechanisms for activation, homing and effector function.4,21 Therefore, as has been reported elsewhere, the induction of mucosal immunity in neonates5,7,9,23,24 opens the possibility of effective protection at an age of high susceptibility to infections, even in the presence of MatAb.
In our experimental model, the five immunized sows gave highly uniform immune responses, indicating that the transfer of immunity to the offspring was also homogeneous. The use of non-vaccinated specific pathogen-free Vietnamese miniature piglets, in a relatively controlled environment (animal house instead of an open farm), reduced individual variability, allowing the use of a small number of animals in each group in agreement with institutional policies. Furthermore, it would be beneficial to evaluate the early immunization protocol using relevant antigens (i.e. bacteria, virus), the association between the level of immunity in the sow with the offspring, different ages (newborn) of immunization, longer periods of time (months, years), as well as using different experimental models.
In summary, our results demonstrated that MatAb increased the antigen-specific IgA and IgG responses in serum, and enhanced a transient early secretory IgA response in nasal secretions of piglets immunized at 7 days of age. At the same time, the nasal and salival IgG responses in the OVA+P 14D group were down-regulated by antigen-specific MatAb. These data suggest that antigen-specific maternal factors may have different effects on the newborn's immune response, according to age and immune compartment. This work provides evidence for the possibility of inducing mucosal and systemic specific antibody responses in piglets, through early immunization, even in the presence of MatAb, which could have implications for neonatal vaccination. Future studies should investigate in detail which factors of maternal immunity are able to enhance/down-regulate the neonatal mucosal immune response.
Acknowledgments
GBER and VLMA designed the study, carried out the experiments, analysed data and wrote the manuscript. GRCE, GEAL, REC and RGOI helped with the experiments and the care and handling of the animal model. We thank Dr Volker Gerdts and Dr Heather Wilson (VIDO, University of Saskatchewan, Canada) for critically reading the manuscript, Dr Martha Moreno-Lafont (National Polytechnic Institute, Mexico) for her help with statistical analysis, Manuel Flores and Daniel Casarrubias for their technical assistance, and Marcela Guzman for secretarial support. This work was partially supported by grants from CONACYT (60941) and the ICyTDF (323/09), Mexico. GBER received a scholarship (234097) from CONACYT.
Glossary
- 7D
7-day-old piglets
- 14D
14-day-old piglets
- MatAb
maternal antibodies
- OVA
ovalbumin
- OVA–S
OVA-seronegative sow
- OVA–P
OVA-seronegative piglets
- OVA–P 7D
OVA-seronegative piglets immunized at 7 days of age with OVA
- OVA–P 14D
OVA-seronegative piglets immunized at 14 days of age with OVA
- OVA+S
OVA-seropositive sow
- OVA+P
OVA-seropositive piglets
- OVA+P 7D
OVA-seropositive piglets immunized at 7 days of age with OVA
- OVA+P 14D
OVA-seropositive piglets immunized at 14 days of age with OVA
Disclosures
The authors declare no conflict of interest.
References
- 1.Brandtzaeg P, Jahnsen FL, Farstad IN, Haraldsen G. Mucosal immunology of the upper airways: an overview. Ann N Y Acad Sci. 1997;830:1–18. doi: 10.1111/j.1749-6632.1997.tb51875.x. [DOI] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann N Y Acad Sci. 2007;1098:288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- 3.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–67. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 4.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 5.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 6.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–12. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 9.Jónsdóttir I. Maturation of mucosal immune responses and influence of maternal antibodies. J Comp Pathol. 2007;137(Suppl. 1):S20–6. doi: 10.1016/j.jcpa.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Elahi S, Buchanan RM, Babiuk LA, Gerdts V. Maternal immunity provides protection against pertussis in newborn piglets. Infect Immun. 2006;74:2619–27. doi: 10.1128/IAI.74.5.2619-2627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine. 2003;21:3382–8. doi: 10.1016/s0264-410x(03)00338-4. [DOI] [PubMed] [Google Scholar]
- 12.Crowe JE., Jr Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin Infect Dis. 2001;33:1720–7. doi: 10.1086/322971. [DOI] [PubMed] [Google Scholar]
- 13.Butler JE, Sun J, Wertz N, Sinkora M. Antibody repertoire development in swine. Dev Comp Immunol. 2006;30:199–221. doi: 10.1016/j.dci.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2012;20:50–7. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol. 2009;33:384–93. doi: 10.1016/j.dci.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14:430–45. doi: 10.1128/CMR.14.2.430-445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nechvatalova K, Kudlackova H, Leva L, Babickova K, Faldyna M. Transfer of humoral and cell-mediated immunity via colostrum in pigs. Vet Immunol Immunopathol. 2011;142:95–100. doi: 10.1016/j.vetimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Markowska-Daniel I, Pomorska-Mól M, Pejsak Z. The influence of age and maternal antibodies on the postvaccinal response against swine influenza viruses in pigs. Vet Immunol Immunopathol. 2011;142:81–6. doi: 10.1016/j.vetimm.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Siegrist CA, Barrios C, Martinez X, Brandt C, Berney M, Córdova M, Kovarik J, Lambert PH. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur J Immunol. 1998;28:4138–48. doi: 10.1002/(SICI)1521-4141(199812)28:12<4138::AID-IMMU4138>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RW. On the maternal transmission of immunity: a ‘molecular attention’ hypothesis. Biosystems. 1995;34:87–105. doi: 10.1016/0303-2647(94)01444-c. [DOI] [PubMed] [Google Scholar]
- 21.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TV, Yuan L, Azevedo MS, et al. High titers of circulating maternal antibodies suppress effector and memory B-cell responses induced by an attenuated rotavirus priming and rotavirus-like particle-immunostimulating complex boosting vaccine regimen. Clin Vaccine Immunol. 2006;13:475–85. doi: 10.1128/CVI.13.4.475-485.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–46. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polewicz M, Gracia A, Garlapati S, et al. Novel vaccine formulations against pertussis offer earlier onset of immunity and provide protection in the presence of maternal antibodies. Vaccine. 2013;31:3148–55. doi: 10.1016/j.vaccine.2013.05.008. [DOI] [PubMed] [Google Scholar]


