Abstract
Objective
To determine the role of Ptc-1 in mediating pulsatile flow-induced changes in VSMC growth and vascular remodeling.
Approach and Results
In vitro, HCASMC were exposed to “normal” or pathological “low” pulsatile flow conditions for 24 h utilizing a perfused transcapillary flow system. Low pulsatile flow increased VSMC proliferation when compared to normal flow conditions. Inhibition of Ptc-1 by cyclopamine attenuated low flow-induced increases in Notch expression while concomitantly decreasing HCASMC growth to that similar of normal flow conditions. In vivo, ligation injury-induced low flow increased vSMC growth and vascular remodeling while increasing Ptc-1/Notch expression. Perivascular delivery of Ptc-1 siRNA by pluronic gel inhibited the pathologic low flow-induced increases in Ptc-1/Notch expression and the subsequent increase in vascular remodeling. In addition, this pathologic low flow-induced remodeling and was returned to normal flow control levels following ptc-1 gene knockdown.
Conclusions
These results suggest that pathological low flow stimulates SMC growth in vitro and vascular remodeling in vivo via Ptc-1 regulation of Notch signaling.
Introduction
Vascular remodeling occurs due to alterations in vascular smooth muscle cell (VSMC) proliferation and apoptosis and is a key event in the pathogenesis of vascular disease. Atherosclerosis, arteriosclerosis, vascular rejection, venous graft restenosis and coronary intervention are each characterized by increased VSMC growth and remodeling1,2,3, 4 that occurs at vessel branch points and bifurcations where blood flow is perturbed. This pathological “low flow” or pro-atherogenic flow type, which occurs at the curves and bifurcations of large artery and arteriole branch points, are the regions that remodel over time. Understanding how VSMC transduce pulsatile flow stimuli into intracellular biomechanical signals that promote VSMC growth and subsequent remodeling is unclear at present. We have previously shown that the biomechanical stimulation (shear stress and cyclic strain) of adult VSMC that contribute to the growth response of these cells involves Hedgehog and Notch signaling components 5,6,7. Moreover, we have recently shown that vascular–injury induced remodeling involves a marked up regulation of both Notch and Hedgehog signaling 5. However, how these signaling pathways co-ordinate in an altered hemodynamic environment to promote vascular remodeling in the pathogenesis of vascular disease remains unexplored.
Hedgehogs (Hh) are a class of 19-kDa morphogens that interact with heparin on the cell surface through an N-terminal basic domain and are tethered to the surface through cholesterol and fatty acyl modification 8. Sonic hedgehog (SHh) is the most widely expressed hedgehog during development where lack of SHh is embryonically lethal 8. Signaling occurs through interaction with the Patched receptors (Ptc1 and 2) that then activate the transcription factors Gli1, Gli2, and Gli3. The downstream targets of the Gli gene products include both Ptc and Gli themselves; thus, Ptc and Gli are both components and targets of the Hh signaling pathway. Hedgehog signaling, which has been well characterized as having an involvement in the development of embryonic lineages, has also been shown to promote VSMC growth and survival in adult tissue 9,10, 11. In addition, Notch signaling has also been implicated as a critical determinant of VSMC survival and vascular structure through modulation of signaling pathways that regulate growth 12,13–15. We, and others have recently demonstrated that Hh can regulate the expression of Notch target genes in a variety of cell types, supporting an interaction between these two pathways 5,7,11, 16, 17. Given these reports in the literature and our previous studies supporting biomechanical regulation of both Hh and Notch signaling components in VSMC in vitro and in the injury-induced remodeled vessel in vivo, we examined whether Ptc1 mediates pathological low flow-induced neointimal hyperplasia via regulation of the Notch signaling pathway.
Results
Ptc-1 Mediates Pulsatile Flow-induced Changes in HCASMC Growth in vitro Via a Notch-dependent Pathway
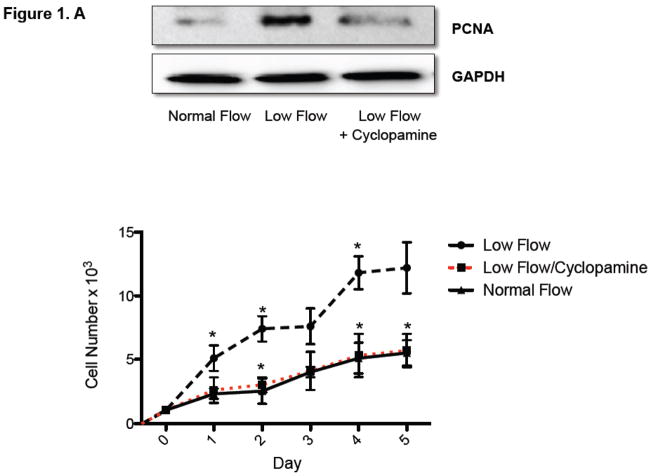
The effect of Ptc-1 inhibition on low pulsatile flow-induced Notch and HCASMC growth was determined using cells grown in a perfused transcapillary culture system under different flow conditions. Low flow enhanced PCNA expression and increased HCASMC cell number over time when compared to normal flow (Figure 1A). Ptc-1 inhibition with Cyclopamine (40μmol/L) treatment for 24 h resulted in a complete attenuation of low flow-induced increases in HCASMC growth as determined by PCNA expression (24 h) and cell counts over 5 days (Figure 1.A). In parallel studies, inhibition of Ptc-1 signaling for 24 h resulted in a decrease in low flow-induced Ptc-1 and Gli-2 mRNA expression by (80.0% ± 6 and 72.0% ± 4) respectively (Figure 1.B). Similarly, Cylopamine attenuated the low flow-induced increases in Notch receptor 1 and Notch target genes Hairy-related transcription factors, Hrt-1and 2 (Figure 1.B) to near control levels suggesting that low-flow-induced stimulation of Notch signaling is a Ptc-1-dependent event. The inhibitory effects of Cyclopamine on PCNA expression were comparable to the effects of Ptc1 knockdown with specific siRNA duplexes (Supplemental Figure 1).
Figure 1. Ptc-1 inhibition attenuates Low pulsatile flow-induced increases in Notch signaling and HCASMC Growth.
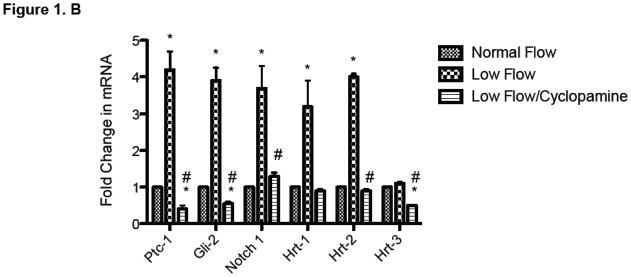
(A) (upper panel): Representative Western Blot of proliferating cell nuclear antigen (PCNA) protein expression in HCASMC exposed to normal or low flow conditions for 24 h with or without cyclopamine (40μmol/L). (A) (lower panel) Growth curves; cell counts of parallel triplicate wells were made on a daily basis. Representative experiment shown. (B) QRTPCR analysis of Hh/Notch signaling components in HCASMC following exposure to normal (0.3ml/min) and low (25ml/min) pulsatile flow conditions for 24 h in the perfused transcapillary culture system in the absence or presence of Cyclopamine (40μmol/L) treatment for 24 h. (Mean ± SEM, n=4).
Pathological “low Flow” Promotes Neointimal Hyperplasia via a Ptc-1/Notch-dependent Pathway
Partial ligation of the carotid artery in a wildtype C57BL/6J mouse led to a 90% reduction in pulsatile flow when compared to sham operated controls as previously shown 5,19. This induced a reproducible remodeling response, assessed 2 weeks post ligation that included and an increase in SMC growth as compared to sham-operated vessels (Figure 2.A). Vascular remodeling was inhibited following perivascular delivery of Ptc-1 siRNA in mice when compared to perivascular delivery of control-scrambled siRNA (Figure 2B). The pathologic low flow-induced increase in SMC medial and neointimal volume was reduced to sham-operated levels following localized Ptc-1 gene knockdown (Figure 2.B) whereas the low flow-induced decreases in lumen volume were abrogated to sham-operated control levels following Ptc-1 knockdown (Figure 2.B). Low-flow-induced intimal medial thickness volume was significantly reduced following perivascular delivery of Ptc-1 siRNA (Figure 2.C).
Figure 2. Perivascular delivery of Ptc-1 siRNA inhibits injury-induced neointimal hyperplasia.
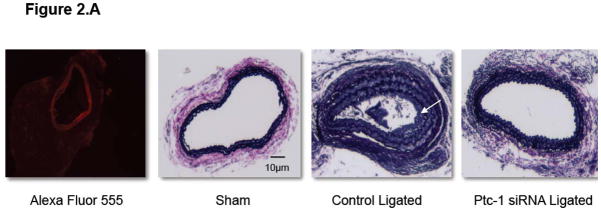
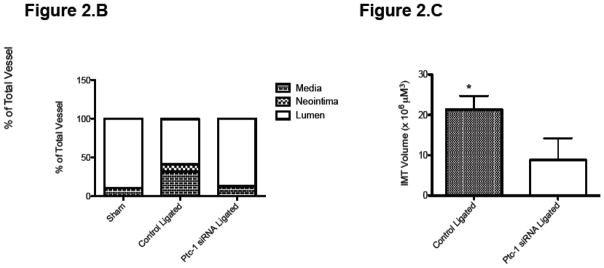
(A) Representative images of Alexa Fluor 555 tagged control siRNA in sham vessel 7 days post treatment, and Verhoeff-van Gieson stained sections of carotid artery from C57BI6/J mice 14 days after sham operation or ligation +/− Ptc-1 siRNA. (B) Carotid artery vessel media, neointima and lumen volumes (evaluated over a 1 mm carotid length) for sham, control ligated and Ptc-1 siRNA ligated groups. (C) Carotid artery Intima Medial thickening (IMT) volume for control ligated and Ptc-1 siRNA ligated. Data are mean ± SEM, 4 sections analyzed/animal, n=6 animals for each group.
Notch component and Ptc1 mRNA levels were determined in the carotid arteries of ligated animals and compared to sham. There was a significant increase in Notch1 and Hrt-2 mRNA levels in ligated vessels (Supplemental Figure 2). In addition, Ptc-1, Gli-2, Notch 1 and Hrt-2 mRNA levels were determined in the carotid arteries of sham, control scrambled siRNA ligated and Ptc-1 siRNA ligated vessels. The injury-induced low flow increases in Ptc-1, Gli-2, Notch 1 and Hrt-2 mRNA levels were reversed following selective knockdown of Ptc-1 by siRNA (Figure 3.A). Similarly, injury-induced increases in Ptc-1, Notch 1IC and PCNA protein were significantly attenuated following Ptc-1 gene knockdown (Figure 3.B). Parallel studies confirmed Ptc1 mediated inhibition of Notch1 stimulated HCASMC PCNA expression in vitro (Supplemental Figure 3).
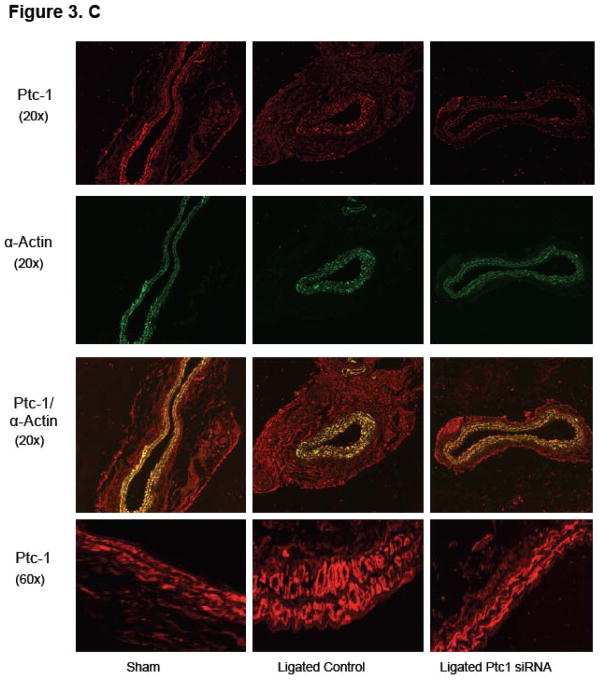
Figure 3. Perivascular delivery of Ptc-1 siRNA inhibits Notch-mediated Neointimal Hyperplasia.
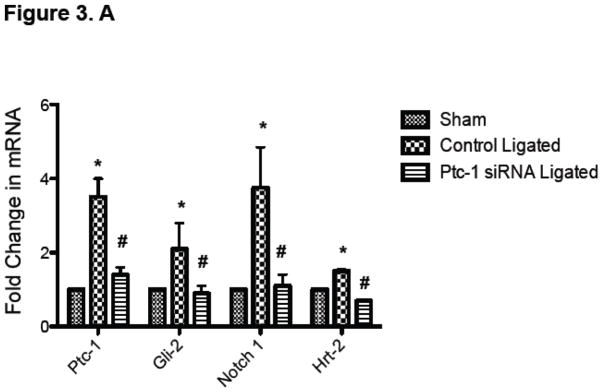
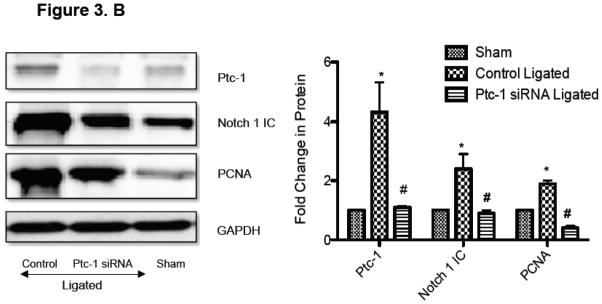
(A) QRTPCR analysis of Ptc-1, Gli-2, Notch 1 and Hrt-2 mRNA 14 days after carotid ligation +/− Ptc-1 siRNA. Data are normalized to GAPDH and represent the mean ± SEM, n=6. (B) Representative Western Blot and cumulative protein data of Ptc-1, Notch 1 IC and PCNA 14 days after carotid ligation +/− Ptc-1 siRNA. (N=6). (C) Photomicrographs of immunofluorescence staining for Ptc-1, SMC α-actin or dual staining for α-actin/Ptc-1 in carotid arteries 14 days after sham operation or ligation +/− Ptc-1 siRNA.
Immunohistochemical examination of fixed tissue sections of carotids from sham operated control vessels, scrambled siRNA ligated vessels and Ptc-1 siRNA ligated vessels revealed Ptc-1 expression in both the adventitia and SMC rich media by co-staining with SMC α-actin (Figure 3.C). Decreased SMC Ptc-1 concomitant with a reduction in IMT and a decrease in SMC growth following Ptc-1 gene knockdown was evident by dual immunofluorescence staining with α-actin (Figure 3.C).
Discussion
Over the past decade we and others have investigated a role for the Notch and Hh signaling pathways in adult vascular cells and, in particular, how these pathways arbitrate cell fate decisions in normal and disease states 5,7,18,19,20. A fundamental role for these pathways in vascular development is well established, but their recapitulation in adult cells and subsequent pathological role warrants further exploration. To date we have determined that both Notch and Hh signaling pathways can mediate adult SMC growth and that these pathways are sensitive to biomechanical regulation in this cell type and in endothelial cells 7,21,22. Moreover, the biomechanically mediated changes in SMC growth are at least, in part, a Hh and/or Notch-dependent event. The novelty of our current study is that for the first time we show that the pathological flow-induced changes in SMC growth seen both in culture and in the injury-induced vessel occur via Ptc-1 regulation of downstream Notch signaling
We established in vitro that pathologic low flow induces a significant increase in pro-proliferative Hh signaling via ptc-1 regulation of the Notch pathway. Ptc-1 inhibition with cyclopamine resulted in decreased Notch signaling concomitant with a decrease in the low flow-induced SMC growth. Moreover, the importance of these findings was confirmed in vivo using a model of arterial injury where Hh/Notch signaling was significantly increased in the ligated low flow artery in comparison to the normal flow controls. To confirm that these pathologic flow-induced changes in Hh/Notch signaling were predominantly SMC-specific, antibodies specific for Hh/Notch signaling components were used in tandem with alpha-actin staining antibodies. In addition, Laser Capture Miicrodissection (LCM) was utilized to remove the SMC rich medial layer, which was then analyzed for Hh/Notch RNA expression (data not shown). Both techniques in parallel with whole vessel analysis confirmed that low flow results in increased Hh/Notch signaling which results in a pro-proliferative phenotype in these injured vessels promoting vascular remodeling.
We and others have previously established a functional relationship between Hh and Notch signaling and SMC growth in vitro and in vivo 5,16,17. Our previous studies had shown that normal vessels express Ptc1 primarily in adventitial cells but following vascular injury, medial and intimal SMCs express Ptc-1 in abundance in vivo 5. In this context, injury induced Hh stimulation leads to Notch 1 IC activation, which in turn results in robust stimulation of downstream Notch target gene expression within these lesions. It is these hrt’s, which feed into the proliferative (e.g. c-myc) and the anti-apoptotic machinery of the cell by regulating both pro-apoptotic Bax and anti-apoptotic Bcl-XL. This to our knowledge however is the first study that directly links Ptc-1 and Notch signaling together in controlling changes in SMC phenotype resulting from altered pulsatile flow.
In this study we utilized a perivascular delivery system of gene specific siRNAs by utilizing a pluronic gel for localized delivery 23,24, 25. Following pluronic gel delivery of Ptc-1 siRNA, which targets the desired siRNA to an area below the bifurcation of the ligated carotid artery, we achieved a significant inhibition of Ptc-1 mRNA and protein expression. Furthermore, this localized delivery of ptc-1 siRNA resulted in a marked inhibition of Notch signaling concomitant with decreased SMC medial growth and reduced low flow-induced remodeling. This study compliments recent work by Li et al that addressed the role of Notch 1 in neointimal formation following vascular injury. This study indicated a role for Notch 1, rather than Notch 3 in mediating SMC growth and neointimal formation after vascular injury through CHF1/Hey2 using heterozygous Notch1 mutants 26. In agreement, we demonstrate that Notch1 rather than Notch3 mRNA levels are elevated following injury, an effect that is abrogated following Ptc1 knockdown. While the level of neointimal hyperplasia was modest in our model, low flow was associated with significant vascular remodeling, albeit predominantly medial thickening. Vascular remodeling is a major manifestation of arteriosclerosis of the carotid artery, and it is an important predictor of cardiovascular events. The level of carotid remodeling has also a strong genetic component that underlies differences in neointimal hyperplasia amongst strains 27.
We have previously shown that the effect of VEGF-A knockdown on SHh-induced hrt-3 expression could be reversed after recovery with exogenous addition of recombinant VEGF-A 5. Taken together with our previous work, it is clear that the Hh pathway including Ptc-1, is an important regulatory molecule upstream of both VEGF-A and Notch in adult SMCs that govern SMC growth and survival 30,31. However, how Ptc-1 is specifically modified by biomechanical forces, which elicit this signaling cascade, warrants further investigation. In addition, it is possible that localized treatment of stenosed vessels with ptc-1 specific siRNA could represent a novel therapy for either restenosis after vein grafting, post angioplasty or atherosclerosis.
In conclusion, we have shown for the first time that pulsatile flow mediates changes in SMC growth via regulation of Ptc-1 signaling and that local inhibition of Ptc-1 by siRNA prevents low flow-induced vascular remodeling. From this data and our previous studies it is tempting to speculate that a Hh/Notch axis may represent a novel therapeutic target for disease states where aberrant changes in SMC growth are key to disease etiology. A greater understanding of this signaling cascade within vascular lesions warrants further investigation.
Supplementary Material
Significance.
The on-set of atherosclerosis due to flow-limiting stenosis is a leading cause of heart attack which contributes to the 800,000 cardiovascular disease related deaths annually. The arterial remodeling responsible for this onset is characterized by a pathology where medial/neointimal thickening are the predominant feature. Changes in vascular smooth muscle cell growth (vSMC) play an important role during this remodeling, however the mechanisms remain unclear. As similar changes are also apparent during modeling of the embryonic vasculature, we and others have postulated that the control of vSMC growth and subsequent remodeling following vascular injury may share similar signaling patterns. This study has shown for the first time that pulsatile flow mediates changes in vSMC growth via regulation of Ptc-1 signaling allowing us to postulate that a Ptc-1/Notch axis represents a novel therapeutic target for disease states where aberrant changes in SMC growth are key to disease etiology.
Acknowledgments
Sources of Funding: This study was supported in part by funds from the National Institutes of Health (R00HL095650 to D.M.) and (R21AA020365 to E.R), American Heart Association (GIA0855865D to JPC) and Science Foundation Ireland (SFI-11/PI/1128 to PAC).
Footnotes
Disclosures: None
Bibliography
- 1.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz SM, Virmani R, Rosenfeld ME. The good smooth muscle cells in atherosclerosis. Curr Atheroscler Rep. 2000;2:422–429. doi: 10.1007/s11883-000-0081-5. [DOI] [PubMed] [Google Scholar]
- 3.Acampora KB, Nagatomi J, Langan EM, 3rd, LaBerge M. Increased synthetic phenotype behavior of smooth muscle cells in response to in vitro balloon angioplasty injury model. Annals of vascular surgery. 2010;24:116–126. doi: 10.1016/j.avsg.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: Its origin and pathophysiological significance. Hum Pathol. 1995;26:450–456. doi: 10.1016/0046-8177(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 5.Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, Collins N, Walls D, Redmond EM, Cahill PA. Sonic hedgehog induces notch target gene expression in vascular smooth muscle cells via vegf-a. Arterioscler Thromb Vasc Biol. 2009;29:1112–1118. doi: 10.1161/ATVBAHA.109.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O’Brien C, Walls D, Redmond EM, Cahill PA. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008;103:1370–1382. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- 7.Morrow D, Sweeney C, Birney YA, Guha S, Collins N, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. American Journal of Physiology-Cell Physiology. 2007;292:C488–C496. doi: 10.1152/ajpcell.00337.2005. [DOI] [PubMed] [Google Scholar]
- 8.Hooper JE, Scott MP. Communicating with hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 9.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang G, Zhang Z, Xu Z, Yin H, Bai L, Ma Z, Decoster MA, Qian G, Wu G. Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia. Biochim Biophys Acta. 2010;1803:1359–1367. doi: 10.1016/j.bbamcr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature medicine. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 12.Lai EC. Keeping a good pathway down: Transcriptional repression of notch pathway target genes by csl proteins. EMBO Rep. 2002;3:840–845. doi: 10.1093/embo-reports/kvf170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 14.Harper JA, Yuan JS, Tan JB, Visan I, Guidos CJ. Notch signaling in development and disease. Clin Genet. 2003;64:461–472. doi: 10.1046/j.1399-0004.2003.00194.x. [DOI] [PubMed] [Google Scholar]
- 15.Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM, Cahill PA. Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a cbf-1/rbp-jk dependent pathway. Faseb J. 2004;18:1421–1423. doi: 10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- 16.Schreck KC, Taylor P, Marchionni L, Gopalakrishnan V, Bar EE, Gaiano N, Eberhart CG. The notch target hes1 directly modulates gli1 expression and hedgehog signaling: A potential mechanism of therapeutic resistance. Clin Cancer Res. 2010;16:6060–6070. doi: 10.1158/1078-0432.CCR-10-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ. Sonic hedgehog and notch signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS One. 2011;6:e14680. doi: 10.1371/journal.pone.0014680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow D, Sweeney C, Scheller A, Walls D, Cummins P, Cahill PA. Notch receptor ligand-signalling role in vascular smooth muscle cell proliferation. Faseb Journal. 2003;17:A59–A59. [Google Scholar]
- 19.Wang W, Campos AH, Prince CZ, Mou Y, Pollman MJ. Coordinate notch3-hairy-related transcription factor pathway regulation in response to arterial injury. Mediator role of platelet-derived growth factor and erk. J Biol Chem. 2002;277:23165–23171. doi: 10.1074/jbc.M201409200. [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Prince CZ, Hu X, Pollman MJ. Hrt1 modulates vascular smooth muscle cell proliferation and apoptosis. Biochem Biophys Res Commun. 2003;308:596–601. doi: 10.1016/s0006-291x(03)01453-0. [DOI] [PubMed] [Google Scholar]
- 21.Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res. 2005;96:567–575. doi: 10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- 22.Morrow D, Cullen JP, Cahill PA, Redmond EM. Cyclic strain regulates the notch/cbf-1 signaling pathway in endothelial cells: Role in angiogenic activity. Arterioscler Thromb Vasc Biol. 2007;27:1289–1296. doi: 10.1161/ATVBAHA.107.142778. [DOI] [PubMed] [Google Scholar]
- 23.Indolfi C, Avvedimento EV, Rapacciuolo A, Esposito G, Di Lorenzo E, Leccia A, Pisani A, Chieffo A, Coppola A, Chiariello M. In vivo gene transfer: Prevention of neointima formation by inhibition of mitogen-activated protein kinase kinase. Basic Res Cardiol. 1997;92:378–384. doi: 10.1007/BF00796211. [DOI] [PubMed] [Google Scholar]
- 24.Zhou B, Margariti A, Zeng L, Habi O, Xiao Q, Martin D, Wang G, Hu Y, Wang X, Xu Q. Splicing of histone deacetylase 7 modulates smooth muscle cell proliferation and neointima formation through nuclear beta-catenin translocation. Arterioscler Thromb Vasc Biol. 2011;31:2676–2684. doi: 10.1161/ATVBAHA.111.230888. [DOI] [PubMed] [Google Scholar]
- 25.Smolock EM, Korshunov VA, Glazko G, Qiu X, Gerloff J, Berk BC. Ribosomal protein l17, rpl17, is an inhibitor of vascular smooth muscle growth and carotid intima formation. Circulation. 2012;126:2418–2427. doi: 10.1161/CIRCULATIONAHA.112.125971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Takeshita K, Liu PY, Satoh M, Oyama N, Mukai Y, Chin MT, Krebs L, Kotlikoff MI, Radtke F, Gridley T, Liao JK. Smooth muscle notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119:2686–2692. doi: 10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: The “glagov phenomenon” is genetically determined. Circulation. 2004;110:220–226. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 30.Shawber CJ, Funahashi Y, Francisco E, Vorontchikhina M, Kitamura Y, Stowell SA, Borisenko V, Feirt N, Podgrabinska S, Shiraishi K, Chawengsaksophak K, Rossant J, Accili D, Skobe M, Kitajewski J. Notch alters vegf responsiveness in human and murine endothelial cells by direct regulation of vegfr-3 expression. J Clin Invest. 2007;117:3369–3382. doi: 10.1172/JCI24311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siekmann AF, Covassin L, Lawson ND. Modulation of vegf signalling output by the notch pathway. Bioessays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.