Summary
Epilepsy is one of the more prevalent neurological disorders in the world, affecting approximately 50 million people of different ages and backgrounds. Epileptic seizures propagating through both lobes of the forebrain can have permanent debilitating effects on a patient's cognitive and somatosensory brain functions. Epilepsy, defined by the sporadic occurrence of recurrent seizures (SRS), is often accompanied by inflammation of the brain. Pronounced increases in the expression of key inflammatory mediators (e.g. IL-1β, TNFα, cyclooxygenase-2, CXCL10) after seizures may cause secondary damage in the brain and increase the likelihood of repetitive seizures. The cyclooxygenase-2 (COX-2) enzyme is induced rapidly during seizures. The increased level of COX-2 in specific areas of the epileptic brain can help to identify regions of seizure-induced brain inflammation. A good deal of effort has been expended to determine whether COX-2 inhibition might be neuroprotective and represent an adjunct therapeutic strategy along with antiepileptic drugs to treat epilepsy. However, the effectiveness of COX-2 inhibitors on epilepsy animal models appears to depend on the timing of administration. With all of the effort placed on making use of COX-2 inhibitors as therapeutic agents for the treatment of epilepsy, inflammation, and neurodegenerative diseases there has yet to be a selective and potent COX-2 inhibitor that has shown a clear therapeutic outcome with acceptable side effects.
Keywords: Seizure, Neurodegeneration, Anti-convulsant, Prostaglandin, Blood-brain Barrier, cognitive deficit, EP2, EP1
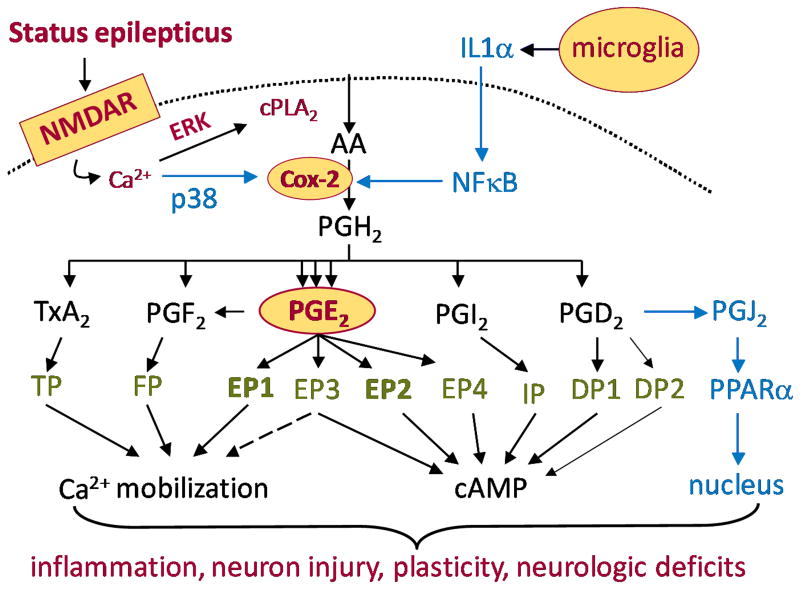
Injury or seizures can trigger multidimensional local inflammatory reactions in the brain primarily involving activated microglia and infiltrating monocytes but also reactive astrocytes and neurons. The roles of cyclooxygenase-2 (PTGS2, or COX-2) in the consequences of seizures, and in epilepsy, have received much attention since the finding that seizures induce COX-2 in hippocampal principal neurons within hours (Yamagata et al., 1993; Marcheselli & Bazan, 1996), partly via a pathway involving NMDA receptors (Yamagata et al., 1993). COX-2 is also induced within days of a seizure in astrocytes (Hirst et al., 1999), although non-aldehyde fixation is apparently needed to reveal astrocytic COX-2.
Cyclooxygenases 1 and 2 are membrane-associated proteins that catalyze the conversion of arachidonic acid to PGH2 in two steps mediated by separate enzymatic activities. First, the cyclooxygenase activity itself converts arachidonic acid to prostaglandin G2 (PGG2), and then a peroxidase activity reduces PGG2 to PGH2. PGH2 in turn is rapidly converted by specific synthases to one of five prostanoids: thromboxane A2, PGF2α, PGE2, prostacyclin (PGI2), or PGD2 (Figure 1). Four G-protein coupled receptors are activated by PGE2, and two by PGD2, whereas each of the other three prostanoids activates a single receptor. The thromboxane receptor TP and the EP3 receptor have multiple splice variants that differ in their C-terminal tails (Abramovitz et al., 2000), but the functions of these isoforms have not been uncovered. COX-2 is constitutively expressed at low to moderate levels in both cell bodies and dendritic spines of excitatory hippocampal neurons and is regulated strongly by synaptic activity (Kauffman et al., 1996). By contrast, perhaps all or at least some inhibitory interneurons in the hippocampus, such as those expressing somatostatin, do not express COX-2 (Serrano et al., 2011). Each COX-2 molecule undergoes suicide inactivation after converting about 400 arachidonate substrate molecules (Smith et al., 1996), which allows COX-2 to respond quite dynamically to fluctuating levels of neuronal activity. Cytosolic prostaglandin E synthase and microsomal prostaglandin E synthase-2 are constitutively expressed, whereas microsomal prostaglandin E synthase-1 is inducible and is often coupled to COX-2 (Murakami et al., 2000), such that PGE2 is a prominent product of induced COX-2. Most cells predominantly express a single prostanoid synthase and therefore only a single principal prostanoid (Fitzgerald, 2003), which aids analysis of a complex tissue response if the synthases can be localized. For example, although COX-2 is generally regarded as a pro-inflammatory enzyme, Gilroy et al. (1999) showed that COX-2 derived PGE2 released from infiltrating polymorphonuclear leukocytes is pro-inflammatory in the acute phase of carrageenin-induced pleurisy, whereas PGD2 released from infiltrating monocytes exerts an anti-inflammatory role during the resolution phase. Anti-inflammatory actions of non-neuronal COX-2 in the brain have been suggested by studies involving direct activation of the glial innate immune system by bacterial lipopolysaccharide (Aïd & Bosetti, 2011), which hinders the emergence of a simple picture of COX-2 function in epilepsy. Indeed, activation of a single PGE2 receptor (EP2) has recently been shown to exacerbate the rapid up-regulation of IL-6 and IL-1β in classically activated microglia but to blunt the production of TNF-α, IL-10, CCL3, and CCL4 (Quan et al., 2013). EP2 thus regulates innate immunity in the central nervous system in a nuanced manner by promoting many aspects of inflammation while dampening others.
Figure 1.
Schematic of the COX-2 signaling cascade following initiation of status epilepticus.
COX-2 involvement in seizures has been intensely investigated in in vitro and in vivo models of neuronal hyperexcitability and excitotoxicity by making use of two key tools: genetically manipulated mice that lack or over express COX-2 either globally or conditionally, and COX-2 inhibitors (selective and non-selective). The premise for these studies is that loss of COX-2 function may prove beneficial in reducing acute seizure severity, intensity, and frequency. This review will address a number of topics related to the roles of COX-2 and its prostanoid products in epilepsy, including; a) setting the seizure threshold both acutely and in chronic epilepsy, b) regulating the integrity of the blood-brain barrier and the p-glycoprotein transporter after seizures, and c) causing neuronal cell loss and inflammation following seizures.
COX-2 inhibitors: basis for selectivity
The structural basis for selectivity of the COX inhibitors has been revealed by X-ray and molecular modeling studies ( Kurumbail et al., 1996; Luong et al., 1996; Filizola et al., 1997). These studies showed that COX-1 and COX-2 isozymes share 60-65% sequence identity and a conserved overall structure including the substrate binding site and catalytic region. However, subtle differences at the substrate binding site lead to inhibitor selectivity. Both isozymes contain three distinct domains, an N-terminal EGF domain followed by a membrane interaction motif and a C-terminal catalytic domain that harbors the cyclooxygenase and peroxidase catalytic regions. The cyclooxygenase pocket is composed of a long hydrophobic channel that extends from the membrane binding domain to a nearby heme group, which is oxidized to initiate the cyclooxygenation reaction. COX-2 contains a valine at amino acid positions 434 and 523, whereas COX-1 has isoleucine at the corresponding positions. The difference in the nature of these amino acids produces a more flexible binding pocket in COX-2 compared to COX-1 (Kurumbail et al., 1996; Luong et al., 1996). Additional differences in amino acid sequence are noted at the N-terminal and C-terminal regions. For example, COX-2 lacks 17 amino acids in the N-terminus, but has an additional 18 amino acids in the C-terminus. These structural differences render the substrate binding site of COX-2 more accommodating of larger inhibitors (Figure S1) than that of COX-1.
Inhibitory potencies of non-steroidal anti-inflammatory drugs (NSAIDs) rely heavily on the type of assay performed. IC50 values for COX-1 and COX-2 inhibitors do not indicate the mechanism of enzyme inhibition, and vary with substrate concentration, incubation time and other assay-specific conditions. For this reason it is important to compare IC50 values among inhibitors under identical assay conditions. COX-1 and COX-2 selective inhibitors operate through at least four types of mechanism including irreversible inhibition (e.g. aspirin), reversible competitive inhibition (e.g. ibuprofen), slow time-dependent reversible inhibition (e.g. indomethacin and flurbiprofen) and slow time-dependent irreversible inhibition (e.g. celecoxib and rofecoxib). Thus, the selectivity (supplemental material) observed by these small molecules (some shown in Figure S1) is not determined simply by binding affinities, but is also partially attributed to enzyme kinetics.
Acute seizure threshold
Many studies have investigated the function of COX-2 in different seizure paradigms (Supplemental Table 1). A majority of these studies used pentylenetetrazol (PTZ) to induce seizures in rodents. For example, Dhir et al. (2006) examined the effects of COX-2 selective (rofecoxib, nimesulide) and nonselective inhibitors (aspirin, naproxen) on pentylenetetrazol (PTZ) induced convulsions. This study showed that oral administration of the COX-2 inhibitors in mice 45 minutes prior to an injection of a convulsion inducing dose of PTZ (80 mg/kg) increased the mean onset time of clonus, reduced seizure duration and speeded recovery to normal behavior and activity after PTZ seizures. Interestingly, oral administration of celecoxib (2 mg/kg, but not 0.2 or 20 mg/kg) 60 minutes prior to injection of pentylenetetrazol afforded an anticonvulsant action that was reversed by intracerebroventricular administration of PGE2 (10 ng) (Oliveira et al., 2008). If this finding can be repeated, it suggests that COX-2 activity may both facilitate and oppose seizure induction, and that facilitation requires activation of a PGE2 receptor. The selective COX-2 inhibitors rofecoxib and nimesulide were both shown to be more effective than the nonselective inhibitors. Akula et al. (2008) investigated the effects of acute intraperitoneal (i.p.) injections of rofecoxib on the PTZ induced seizure threshold in mice. A single i.p. injection of rofecoxib (2 and 4 mg/kg but not 1 mg/kg) 45 minutes prior to PTZ increased the seizure threshold for the onset of all phases of PTZ-induced convulsions and significantly decreased the incidence of PTZ induced convulsions. However, in a more recent study chronic oral administration of rofecoxib (30 mg/kg/day) for five days prior to PTZ was shown to have no effect on the incidence or severity of acute seizures induced by 40 or 55 mg/kg PTZ (Claycomb et al., 2011).
Pretreatment with COX-2 inhibitors dampens the development of kindling produced by PTZ in rats (Dhir et al., 2007) or rapid electrical stimulation in rats or mice (Tu and Bazan, 2003; Takemiya et al., 2003), observations very likely explained by the aforementioned elevated seizure threshold by COX-2 inhibition. Conversely, administration of nimesulide or celecoxib to rats one hour prior to kainic acid (KA) strongly augmented kainic acid induced seizures (Kunz & Oliw, 2001a: Gobbo and O'mara, 2004). The KA seizures were more severe and prolonged in the inhibitor treated group, and acute mortality was also increased. Recently, it was demonstrated that conditional genetic ablation of the COX-2 gene limited to principal forebrain neurons does not alter the latency to reach electrographic status epilepticus (SE) or seizure intensity following an i.p. injection of pilocarpine compared to wildtype mice (Serrano et al., 2011), suggesting that the loss of COX-2 at least in these principal neurons does not alter the acute seizure threshold to pilocarpine. Together, these studies reveal some inconsistencies in regard to the role of COX-2 on acute seizures. In assessing the in vivo efficacy of a compound many factors should be considered such as the subject species, the convulsant, the vehicle of the test compound, the volume of fluid injected, the timing and route of administration. Any of these factors can influence the consequence of pharmacological manipulation of a target. Nevertheless, acute inhibition of COX-2 appears to be neutral or beneficial in most acute seizure models by increasing the acute seizure threshold. The corollary conclusion is that low constitutive expression of COX-2 in the brain reduces seizure threshold, which could be explained by the ability of PGE2 to elevate neuronal excitability (Chen and Bazan, 2005).
Blood-brain barrier disruption
The blood-brain barrier (BBB) is a selectively permeable interface between the CNS parenchyma and the blood. Tight junctions between adjacent endothelial cells restrict traffic of materials in the blood to the brain and force molecules to be transported by specific transcellular routes that allow uptake of essential molecules but restrict harmful bacteria or large circulating molecules such as antibodies. Disruption of the blood-brain barrier is a common feature in human temporal lobe epilepsy (Van Vliet et al., 2007). Many reports show an association between seizure activity and BBB leakiness (Roch et al., 2002; Ballabh et al., 2004; Neuwelt, 2004; Seiffert et al., 2004; van Vliet et al., 2007; Serrano et al., 2011; Jiang et al., 2013). Some anticonvulsants including phenytoin have shown poor penetration into the brain of rodents when administered chronically in mice or rats (Potschka & Loscher, 2001; Rizzi et al., 2002; Loscher & Postschka, 2005). Upregulation of p-glycoprotein (Pgp), which typically pumps drugs out of the brain, appears to underlie the development of resistance to phenytoin (Loscher & Potschka, 2005). Therefore, strategies for selective inhibition of the Pgp pump have been raised to improve pharmacotherapy in epilepsy (Brandt et al., 2006; van Vliet et al., 2006).
A series of studies has been performed to identify the target cells and molecules that regulate Pgp expression. Pgp expression was detected in endothelial cells, astrocytes, microglia and neurons (Decleves et al., 2000; Lee et al., 2001; Marroni et al., 2003), suggesting that antiepileptic drug resistance may be generated by the pumping of drugs out of neuronal and non-neuronal cells into the blood or the interstitial fluid. AEDs themselves were suggested to be regulators of Pgp expression. Recently, three AEDs (phenobarbital, carbamazepine and phenytoin) were shown to upregulate P-glycoprotein levels in capillary endothelial vessels in rats (Wen et al., 2008) and even more recently a study in mice provided in vivo evidence for the modulation of P-glycoprotein activity by AEDs (levetiracetam, topiramate and phenytoin (Moerman et al., 2011). The upregulation of P-glycoprotein observed in isolated mouse and rat brain capillaries exposed to glutamate appears to be dependent on COX-2 signaling as it is blocked by the selective COX-2 inhibitors celecoxib (Bauer et al., 2006), NS398 and indomethacin heptyl ester (Zibell et al., 2009). The COX inhibitors celecoxib (Zibell et al., 2009) and indomethacin (Bauer et al., 2006) also prevented seizure induced P-glycoprotein upregulation in rat brain capillaries in vivo in the pilocarpine status epilepticus model. Likewise, the COX-2 inhibitors SC-58236 and NS-398 promoted brain delivery of phenytoin in epileptic rats (van Vliet et al., 2010) associated with down-regulation of Pgp. Disruption of the BBB causes upregulation of COX-2 and subsequent induction of the Pgp in response to seizures (van Vliet et al., 2010). Further investigation revealed that pharmacological inhibition of the prostaglandin E2 receptor EP1 by SC-51089 prevents seizure-associated Pgp upregulation at the BBB (Pekcec et al., 2009), suggesting that blockade of the COX-2/EP1 signaling pathway could be a promising approach to control Pgp expression and to enhance access and efficacy of AEDs. However, it should be noted that in rats inhibition of EP1 by SC-51089 failed to prevent pilocarpine induced neurodegeneration in the hippocampal hilar formation (Pekcec et al., 2009).
Although direct inhibition of Pgp with tariquidar improves seizure control in rats treated with phenobarbital or phenytoin, pan inhibition of Pgp could disturb the protective nature of Pgp at the BBB leading to a deleterious effect (Brandt et al., 2006; van Vliet et al., 2006). An alternative would be to manipulate Pgp expression by regulating signaling molecules such as COX-2 that would not affect basal Pgp expression. Neuronal COX-2 induction and subsequent EP2 signaling coupled with BBB disruption may promote the epileptogenic process (Serrano et al., 2011; Jiang et al., 2013), which in turn leads to further upregulation of COX-2 (van Vliet et al., 2010). Even though studies targeting COX-2 suggest that COX-2 inhibition may be beneficial due to the role of this enzyme in epileptogenesis and pharmacotherapy, the adverse cardiovascular and cerebrovascular effects of COX-2 inhibitors severely limit these drugs as therapeutic agents.
Neurodegeneration and neuroinflammation
COX-2 is an important mediator of neuroinflammation. In a pilocarpine mouse model, for example, either conditional ablation of the COX-2 gene from principal forebrain neurons or post-seizure administration of a brain-permeant EP2 receptor antagonist dampened the delayed cytokine burst in the hippocampus that follows SE (Serrano et al., 2011; Jiang et al., 2013). COX-2 activation in the brain has been shown to promote delayed neuronal damage in rodent models of temporal lobe epilepsy (Manabe et al., 2004; Kawaguchi et al., 2005; Takemiya et al., 2006; Polaschek et al., 2010; Serrano et al., 2011). Transgenic mice overexpressing neuronal COX-2 have increased sensitivity to glutamate excitotoxicity in vitro and in vivo (Kelley et al., 1999). Blocking the seizure-induced increase in COX-2 function with selective inhibitors like rofecoxib reduced neuron death suggesting a neuroprotective effect of COX-2 inhibitors (Kunz & Oliw, 2001b; Kawaguchi et al., 2005; Hewett et al., 2006; Takemiya et al., 2006; Polaschek et al., 2010), though this appears to depend on the severity of the seizure activity (Holtman et al., 2009) and the schedule of drug administration, with the greatest neuroprotection afforded through multiple doses with a selective COX-2 inhibitor administered after SE induction. Pre-seizure administration of COX-2 inhibitors either did not prevent neuronal death (Takemiya et al., 2006) or caused increased mortality following kainic acid-induced seizures in rats (Kunz and Oliw, 2001a; Gobbo & O'Mara, 2004). A combination of pre- and post-treatment with the nonselective COX inhibitor naproxen was neuroprotective in a NMDA excitotoxicity model (Silakova et al., 2004). Even when neuroprotection is observed after COX-2 inhibition, selective COX-2 inhibitors do not prevent epileptogenesis or reduce the frequency of spontaneous seizures in animal models of epilepsy (Holtman et al., 2009).These findings, taken together, suggest that the COX-2 signaling cascade, as a whole, is not required for epilepsy development although it can be disease-modifying (Holtman et al., 2009; Polascheck et al., 2010). Similarly, other studies demonstrate that inhibition of COX-2 with celecoxib or SC-58236 after kainate-induced SE provided no significant improvement in neuronal damage or seizure activity (Gobbo & O'Mara, 2004; Holtman et al., 2009). By contrast, neuroprotection was observed following pre-treatment of rofecoxib prior to administration of kainic acid in rats (Kunz and Oliw, 2001b) or intrahippocampal injection of NMDA in mice (Hewett et al., 2006). The seemingly contradictory findings of the effect of COX-2 inhibitors on neurodegeneration and epileptogenesis in animal models might be due to the method of SE induction, the use of different COX-2 inhibitors with varied selectivity, variable treatment protocols and dosing schedules, as well as outcome measures (e.g. seizure intensity, cognitive deficits, cell death, etc) (Supplemental Table 1). Nevertheless, COX-2 inhibition appears to be a potentially valuable therapeutic strategy for reducing seizure-related neuronal damage.
COX-2 knockout mice have proven useful in substantiating the effects demonstrated with pharmacological inhibition of COX-2 before or after SE induction. Takemiya et al. (2006) reported that NS-398, a selective COX-2 inhibitor, administered repeatedly for 48 hours after kainic acid-induced seizures reduced hippocampal cell death and this effect was mimicked in COX-2 knockout mice. Similarly, Manabe et al. (2004) demonstrated that NS-398 can significantly reduce the size of an NMDA-induced neocortical lesion. Furthermore, the lesion produced by NMDA in COX-2 knockout mice was significantly smaller than that in wild-type mice. Jarvela et al. (2011) used organotypic hippocampal slice cultures to determine the effect of COX-2 inhibition on kainic acid-induced neuronal damage. Pretreatment with NS-398 did not prevent neuronal damage in this model. However, conditional ablation of the COX-2 gene restricted to principal forebrain neurons protected mice against hippocampal neurodegeneration 4 days after pilocarpine-induced SE (Serrano et al., 2011).
Despite conflicting results using a variety of selective COX-2 inhibitors in different epilepsy models, there is ample evidence to suggest that seizure-induced COX-2 plays a role in epilepsy-related neurodegeneration. Further studies are necessary to elucidate the extent and pattern of COX-2 involvement in neuronal damage, and to explore the role of COX-2 related neuroinflammation in delayed neurodegeneration. A clearer picture might emerge by focusing downstream in the COX-2 cascade. For example, EP2 receptor inhibition in the pilocarpine model is neuroprotective and completely recapitulates the protective effects of conditional ablation of COX-2 from forebrain neurons (Jiang et al., 2013), which points to a role for EP2 activation in seizure-induced neurodegeneration.
Spontaneous seizure frequency in the chronic state
COX-2 levels are increased in the brains of patients with epilepsy, especially temporal lobe epilepsy (TLE) and in animals that experience prolonged seizures (Desjardins et al., 2003; Serrano et al., 2011). Multiple studies using COX-2 inhibitors in animal models of epilepsy yielded controversial results regarding the involvement of COX-2 in spontaneous recurrent seizures (SRSs). For example, oral administration of the COX-2 inhibitor celecoxib (20 mg/kg) one day following a one-hour episode of SE induced by pilocarpine in rats reduced the number of animals that had SRSs by 33%, reduced the frequency of observed behavioral seizures by 65%, and reduced seizure duration by 52%, as monitored by video recording during the light period from 28 to 42 days after SE (Jung et al., 2006). In another study, intraperitoneal injection of the COX-2 inhibitor parecoxib (10 mg/kg) twice daily for 17 days, starting immediately after the onset of a 90-minute episode of pilocarpine-induced SE, had no effect on the incidence, frequency or duration of SRSs but reduced the severity of spontaneous seizures examined by continuous EEG/video monitoring (Polascheck et al., 2010). Treatment with another COX-2 selective inhibitor SC-58236 (10 mg/kg) by oral administration beginning one day before, shortly after, or 3-4 months after electrically induced SE in rats produced no anti-epileptogenic or anti-epileptic effect, but rather had severe adverse effects with a higher mortality after SE and after 2-weeks of chronic treatment (Holtman et al., 2009; Holtman et al., 2010). This disparity in the effectiveness of COX-2 inhibition on SRSs might be related to differences in the duration of SE experienced by the animals, by the dosing protocol of the COX-2 inhibitors (Supplemental Table 1), or by off-target toxicity.
Comorbidities
Current cognitive comorbidities of epilepsy include attention deficit disorder (ADD), major depression and anxiety. Since COX-2 is induced following seizure activity in the brain and depression is a comorbidity of epilepsy, it remains a question as to how COX-2 functions in patients undergoing electroconvulsive seizure therapy for major depression. The function of COX-2 in major depression has been investigated in rodents, where COX-2 inhibitors alleviate memory loss following electroconvulsive seizures (Segi-Nishida, 2011). It would be worthwhile exploring whether COX-2 inhibition is beneficial to patients undergoing electroconvulsive treatment (Segi-Nishida, 2011).
Under normal conditions COX-2 is selectively expressed at moderate levels in several brain regions such as cerebral cortex, hippocampal CA3 region, and amygdala, likely a result of the activity-dependence of its expression (Yamagata et al., 1993). Moreover, COX-2 resides in dendritic spines of glutamatergic neurons, where excitatory neurotransmission and synaptic plasticity occur (Kaufmann et al., 1996), suggesting a role for neuronal COX-2 in modulating synaptic activity and therefore cognitive functions (Yang & Chen, 2008). In hippocampal slices a COX-2 inhibitor dampened both long-term potentiation (LTP) and long-term depression (LTD) (Murray & O'Connor, 2003; Shaw et al., 2003). PGE2, but not PGD2 or PGF2α, rescued the impaired long-term synaptic plasticity caused by COX-2 inhibition in hippocampal dentate granule neurons in vitro (Chen et al., 2002; Chen & Bazan, 2005), pointing to a potential role for one of the four PGE2 receptors.
Constitutive COX-2 expression helps refine homeostatic synaptic functions in neurons, whereas the rapid induction of COX-2 following brain injuries leads to neuropathologies presumably also through its involvement in synaptic modification (Yang & Chen, 2008). Indeed, a number of animal studies using selective COX-2 inhibitors indicate that COX-2 plays essential roles in spatial learning and memory (Teather et al., 2002; Rall et al., 2003; Shaw et al., 2003; Sharifzadeh et al., 2005). Cognitive impairments are often associated with epilepsy, especially temporal lobe epilepsy (TLE), when seizures initiate and propagate within the critical memory structures of the medial temporal lobe such as hippocampus and amygdala (LaFrance et al., 2008). Cognitive deficits are also commonly evidenced in animal models of epilepsy. For example, 9-12 weeks following pilocarpine induced SE, mice learned the escape platform location of the Morris water maze (MWM) task significantly more slowly than their non-seizure cohorts (Muller et al., 2009). Administration of the COX-2 inhibitor rofecoxib for two days after kainate had no effect on behavior in the MWM task (Kunz et al., 2005). Similarly, the COX-2 inhibitor parecoxib did not affect the behavioral and cognitive alterations associated with epilepsy (Polascheck et al., 2010). On the other hand conditional COX-2 ablation in principal forebrain neurons reduced the latency to find a previously learned target hole in a retrograde amnesia task in mice that had experienced pilocarpine-induced SE one month earlier, which was a selective effect in that their motor behavior and other cognitive measures were not changed (Levin et al., 2012).
Interestingly, celecoxib administered after but not prior to kainic acid injection significantly reduced the learning and object exploration deficits in a MWM task (Gobbo & O'Mara, 2004). As a first generation COX-2 inhibitor, celecoxib has a much lower selectivity for COX-2 over COX-1 compared to rofecoxib and parecoxib, thus a COX-2 independent action cannot be excluded in this study. Nonetheless, suppression of COX-2 activity by selective inhibitors or genetic manipulation reduces COX-2-promoted pathologies in the brain including neuroinflammation and neurodegeneration and this might potentially underlie reduced seizure-triggered cognitive deficits. However, the possibility that rofecoxib might also block the benefits of basal COX-2 in synaptic plasticity, learning and memory during the period of recovery should be considered. Further studies on the dosing of COX-2 selective inhibitors in combination with other anti-epileptic therapies would be needed to reevaluate COX-2 as a therapeutic target to improve cognitive functions following seizure attacks (see supplementary material). The downstream signaling molecules in the COX-2 cascade can serve as alternative therapeutic targets for reducing cognitive deficits following prolonged seizures.
Conclusions and future direction
Cyclooxygenase-2 is known to play a key role in the early inflammatory response to an insult, and consequently a significant role in post-seizure inflammation and hyperexcitability of the brain. Most investigators report that pretreatment of animals with a COX-2 inhibitor before a convulsant stimulus can dampen seizure intensity, which would be consistent with the excitatory effect of PGE2 generated by constitutively active COX-2. Post-seizure treatment, on the other hand, can be neuroprotective as expected from the strong proinflammatory role of induced COX-2. Although induction of this enzyme may be beneficial in the very early post-seizure period, delayed and prolonged induction appears to lead to damaging long-term consequences. To date none of the selective COX-2 inhibitors tested has reproducibly slowed epilepsy disease progression as measured by the appearance of spontaneous seizures.
Potential complications from chronic exposure to high doses of COX-2 inhibitors include non-selective inhibition of COX-1, an enzyme that can mediate beneficial effects, and suppression of the physiological regulation of synaptic plasticity by basal COX-2. Therefore, the dose and administration pattern may be critical to achieve beneficial effects of COX-2 inhibitors. The subject species, dosing amount and therapeutic window should all be explored with attention and reference to the induction pattern of COX-2. The risk of serious cardiovascular side effects that accompany chronic COX-2 inhibition makes it unlikely that chronic COX-2 inhibition is a viable therapeutic strategy, although short-term exposure might be useful.
An alternative therapeutic direction to COX-2 inhibitors would involve the downstream effector molecules in the COX-2 signaling cascade (i.e., prostanoid synthases and receptors), which should offer more selective targets for disease modification. Recently, attention has been given to such molecules. For example, the EP2 receptor appears to contribute to the development of neuropathologies following status epilepticus. The discovery of selective prostanoid receptor modulators may help avoid the complication of serious side effects by chronic inhibition of COX-2. Perhaps it is now time to switch the focus and effort from COX-2 to the key downstream players in the COX-2 cascade such as the prostanoid synthases and receptors, which offer more selective therapies to pathway specific components of neurological diseases. Modulators of prostanoid receptor function could also improve our understanding of the role of COX-2 in the brain immune response and inflammation, and consequentially the effects of COX-2 on the epileptic patient. This information will aid future research as well as the development of new treatment methods and medications.
Supplementary Material
Nonsteroidal anti-inflammatory drugs: Structures 1-5 are nonselective, but COX-1 preferring inhibitors. Structures 6-10 are selective COX-2 inhibitors. Drugs 7-8 have been withdrawn from the Unites States market, due to fatal cardiovascular side effects.
Acknowledgments
This work was supported by the Epilepsy Foundation (J.J.) and the National Institute of Neurological Disorders and Stroke (NINDS) grant K99 NS082379 (J.J.); the CounterACT program, National Institutes of Health (NIH), Office of the Director, and the NINDS grant U01 NS058158 (R.D.) and NIH grant R21 NS074169 (R.D.).
Abbreviations
- Pgp
p-glycoprotein
- EP1
prostaglandin E2 receptor 1
- EP2
prostaglandin E2 receptor 2
- COX-1
cyclooxygenase 1
- COX-2
cyclooxygenase 2
- PGE2
prostaglandin E2
- BBB
blood-brain barrier
- AED
anti-epileptic drug
- PTZ
pentylenetetrazol
- KA
kainic acid
- NMDA
N-methyl-D-aspartate
- NSAID
nonsteriodal anti-inflammatory drug
- MWM
morris water maze
- SRS
spontaneous recurrent seizure
- SE
status epilepticus
- IC50
half maximal inhibitory concentration
- TLE
temporal lobe epilepsy
- IL-1β
interleukin-1 beta
- TNFα
tumor necrosis factor alpha
- CXCL10
C-X-C motif chemokine 10
Footnotes
Disclosure: There are no conflicts of interest in relation to this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this manuscript is consistent with the Journal's guidelines.
References
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Aid S, Bosetti F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie. 2011;93:46–51. doi: 10.1016/j.biochi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akula KK, Dhir A, Kulkarni SK. Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor increases pentylenetetrazol seizure threshold in mice: possible involvement of adenosinergic mechanism. Epilepsy Res. 2008;78:60–70. doi: 10.1016/j.eplepsyres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Pekcec A, Toellner K, Miller DS, Potschka H. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol. 2008;73:1444–1453. doi: 10.1124/mol.107.041210. [DOI] [PubMed] [Google Scholar]
- Brandt C, Bethmann K, Gastens AM, Loscher W. The multidrug transporter hypothesis of drug resistance in epilepsy: Proof-of-principle in a rat model of temporal lobe epilepsy. Neurobiol Dis. 2006;24:202–211. doi: 10.1016/j.nbd.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Brideau C, Kargman S, Liu S, Dallob AL, Ehrich EW, Rodger IW, Chan CC. A human whole blood assay for clinical evaluation of biochemical efficacy of cyclooxygenase inhibitors. Inflamm Res. 1996;45:68–74. doi: 10.1007/BF02265118. [DOI] [PubMed] [Google Scholar]
- Brooks P, Emery P, Evans JF, Fenner H, Hawkey CJ, Patrono C, Smolen J, Breedveld F, Day R, Dougados M, Ehrich EW, Gijon-Banos J, Kvien TK, Van Rijswijk MH, Warner T, Zeidler H. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology (Oxford) 1999;38:779–788. doi: 10.1093/rheumatology/38.8.779. [DOI] [PubMed] [Google Scholar]
- Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Ford-Hutchinson AW, Forrest MJ, Gauthier JY, Gordon R, Gresser M, Guay J, Kargman S, Kennedy B, Leblanc Y, Leger S, Mancini J, O'Neill GP, Ouellet M, Patrick D, Percival MD, Perrier H, Prasit P, Rodger I, et al. Rofecoxib [Vioxx, MK-0966; 4-(4'-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999;290:551–560. [PubMed] [Google Scholar]
- Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;93:929–941. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Claycomb RJ, Hewett SJ, Hewett JA. Prophylactic, prandial rofecoxib treatment lacks efficacy against acute PTZ-induced seizure generation and kindling acquisition. Epilepsia. 2011;52:273–283. doi: 10.1111/j.1528-1167.2010.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decleves X, Regina A, Laplanche JL, Roux F, Boval B, Launay JM, Scherrmann JM. Functional expression of P-glycoprotein and multidrug resistance-associated protein (Mrp1) in primary cultures of rat astrocytes. J Neurosci Res. 2000;60:594–601. doi: 10.1002/(SICI)1097-4547(20000601)60:5<594::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Desjardins P, Sauvageau A, Bouthillier A, Navarro D, Hazell AS, Rose C, Butterworth RF. Induction of astrocytic cyclooxygenase-2 in epileptic patients with hippocampal sclerosis. Neurochem Int. 2003;42:299–303. doi: 10.1016/s0197-0186(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Rofecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor potentiates the anticonvulsant activity of tiagabine against pentylenetetrazol-induced convulsions in mice. Inflammopharmacology. 2006;14:222–225. doi: 10.1007/s10787-006-1535-3. [DOI] [PubMed] [Google Scholar]
- Dhir A, Naidu PS, Kulkarni SK. Effect of cyclooxygenase inhibitors on pentylenetetrazol (PTZ)-induced convulsions: Possible mechanism of action. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1478–1485. doi: 10.1016/j.pnpbp.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Dhir A, Naidu PS, Kulkarni SK. Neuroprotective effect of nimesulide, a preferential COX-2 inhibitor, against pentylenetetrazol (PTZ)-induced chemical kindling and associated biochemical parameters in mice. Seizure. 2007;16:691–697. doi: 10.1016/j.seizure.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Filizola M, Perez JJ, Palomer A, Mauleon D. Comparative molecular modeling study of the three-dimensional structures of prostaglandin endoperoxide H2 synthase 1 and 2 (COX-1 and COX-2) J Mol Graph Model. 1997;15:290–300. doi: 10.1016/s1093-3263(97)00107-1. [DOI] [PubMed] [Google Scholar]
- FitzGerald GA. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2:879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O'Mara SM. Post-treatment, but not pre-treatment, with the selective cyclooxygenase-2 inhibitor celecoxib markedly enhances functional recovery from kainic acid-induced neurodegeneration. Neuroscience. 2004;125:317–327. doi: 10.1016/j.neuroscience.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Hewett SJ, Silakova JM, Hewett JA. Oral treatment with rofecoxib reduces hippocampal excitotoxic neurodegeneration. J Pharmacol Exp Ther. 2006;319:1219–1224. doi: 10.1124/jpet.106.109876. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Young KA, Newton R, Allport VC, Marriott DR, Wilkin GP. Expression of COX-2 by normal and reactive astrocytes in the adult rat central nervous system. Mol Cell Neurosci. 1999;13:57–68. doi: 10.1006/mcne.1998.0731. [DOI] [PubMed] [Google Scholar]
- Holtman L, van Vliet EA, Edelbroek PM, Aronica E, Gorter JA. Cox-2 inhibition can lead to adverse effects in a rat model for temporal lobe epilepsy. Epilepsy Res. 2010;91:49–56. doi: 10.1016/j.eplepsyres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Holtman L, van Vliet EA, van Schaik R, Queiroz CM, Aronica E, Gorter JA. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res. 2009;84:56–66. doi: 10.1016/j.eplepsyres.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Jarvela JT, Ruohonen S, Kukko-Lukjanov TK, Plysjuk A, Lopez-Picon FR, Holopainen IE. Kainic acid-induced neurodegeneration and activation of inflammatory processes in organotypic hippocampal slice cultures: treatment with cyclooxygenase-2 inhibitor does not prevent neuronal death. Neuropharmacology. 2011;60:1116–1125. doi: 10.1016/j.neuropharm.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A. 2013;110:3591–3596. doi: 10.1073/pnas.1218498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;23:237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K, Hickey RW, Rose ME, Zhu L, Chen J, Graham SH. Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in CA3 hippocampus. Brain Res. 2005;1050:130–137. doi: 10.1016/j.brainres.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, Pasinetti GM. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am J Pathol. 1999;155:995–1004. doi: 10.1016/S0002-9440(10)65199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz T, Marklund N, Hillered L, Oliw EH. Assessment of the effects of the cyclooxygenase-2 inhibitor rofecoxib on visuospatial learning and hippocampal cell death following kainate-induced seizures in the rat. Brain Res Cogn Brain Res. 2005;25:826–832. doi: 10.1016/j.cogbrainres.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Kunz T, Oliw EH. Nimesulide aggravates kainic acid-induced seizures in the rat. Pharmacol Toxicol. 2001a;88:271–276. doi: 10.1034/j.1600-0773.2001.d01-116.x. [DOI] [PubMed] [Google Scholar]
- Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001b;13:569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- LaFrance WC, Jr, Kanner AM, Hermann B. Psychiatric comorbidities in epilepsy. Int Rev Neurobiol. 2008;83:347–383. doi: 10.1016/S0074-7742(08)00020-2. [DOI] [PubMed] [Google Scholar]
- Lee G, Schlichter L, Bendayan M, Bendayan R. Functional expression of P-glycoprotein in rat brain microglia. J Pharmacol Exp Ther. 2001;299:204–212. [PubMed] [Google Scholar]
- Levin JR, Serrano G, Dingledine R. Reduction in delayed mortality and subtle improvement in retrograde memory performance in pilocarpine-treated mice with conditional neuronal deletion of cyclooxygenase-2 gene. Epilepsia. 2012;53:1411–1420. doi: 10.1111/j.1528-1167.2012.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat Struct Biol. 1996;3:927–933. doi: 10.1038/nsb1196-927. [DOI] [PubMed] [Google Scholar]
- Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, Ross ME, Iadecola C. Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol. 2004;55:668–675. doi: 10.1002/ana.20078. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Bazan NG. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J Biol Chem. 1996;271:24794–24799. doi: 10.1074/jbc.271.40.24794. [DOI] [PubMed] [Google Scholar]
- Marroni M, Agrawal ML, Kight K, Hallene KL, Hossain M, Cucullo L, Signorelli K, Namura S, Bingaman W, Janigro D. Relationship between expression of multiple drug resistance proteins and p53 tumor suppressor gene proteins in human brain astrocytes. Neuroscience. 2003;121:605–617. doi: 10.1016/s0306-4522(03)00515-3. [DOI] [PubMed] [Google Scholar]
- Moerman L, Wyffels L, Slaets D, Raedt R, Boon P, De Vos F. Antiepileptic drugs modulate P-glycoproteins in the brain: a mice study with (11)C-desmethylloperamide. Epilepsy Res. 2011;94:18–25. doi: 10.1016/j.eplepsyres.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Muller CJ, Groticke I, Bankstahl M, Loscher W. Behavioral and cognitive alterations, spontaneous seizures, and neuropathology developing after a pilocarpine-induced status epilepticus in C57BL/6 mice. Exp Neurol. 2009;219:284–297. doi: 10.1016/j.expneurol.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh S, Kudo I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- Murray HJ, O'Connor JJ. A role for COX-2 and p38 mitogen activated protein kinase in long-term depression in the rat dentate gyrus in vitro. Neuropharmacology. 2003;44:374–380. doi: 10.1016/s0028-3908(02)00375-1. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. discussion 141-132. [DOI] [PubMed] [Google Scholar]
- Oliveira MS, Furian AF, Royes LF, Fighera MR, Fiorenza NG, Castelli M, Machado P, Bohrer D, Veiga M, Ferreira J, Cavalheiro EA, Mello CF. Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res. 2008;79:14–21. doi: 10.1016/j.eplepsyres.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Patrignani P, Panara MR, Greco A, Fusco O, Natoli C, Iacobelli S, Cipollone F, Ganci A, Creminon C, Maclouf J, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Ther. 1994;271:1705–1712. [PubMed] [Google Scholar]
- Pekcec A, Unkruer B, Schlichtiger J, Soerensen J, Hartz AM, Bauer B, van Vliet EA, Gorter JA, Potschka H. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther. 2009;330:939–947. doi: 10.1124/jpet.109.152520. [DOI] [PubMed] [Google Scholar]
- Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson Gd, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- Polascheck N, Bankstahl M, Loscher W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol. 2010;224:219–233. doi: 10.1016/j.expneurol.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Potschka H, Loscher W. In vivo evidence for P-glycoprotein-mediated transport of phenytoin at the blood-brain barrier of rats. Epilepsia. 2001;42:1231–1240. doi: 10.1046/j.1528-1157.2001.01901.x. [DOI] [PubMed] [Google Scholar]
- Quan Y, Jiang J, Dingledine R. EP2 receptor signaling pathways regulate classical activation of microglia. J Biol Chem. 2013;288:9293–9302. doi: 10.1074/jbc.M113.455816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall JM, Mach SA, Dash PK. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003;968:273–276. doi: 10.1016/s0006-8993(03)02248-0. [DOI] [PubMed] [Google Scholar]
- Rizzi M, Caccia S, Guiso G, Richichi C, Gorter JA, Aronica E, Aliprandi M, Bagnati R, Fanelli R, D'Incalci M, Samanin R, Vezzani A. Limbic seizures induce P-glycoprotein in rodent brain: functional implications for pharmacoresistance. J Neurosci. 2002;22:5833–5839. doi: 10.1523/JNEUROSCI.22-14-05833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch C, Leroy C, Nehlig A, Namer IJ. Magnetic resonance imaging in the study of the lithium-pilocarpine model of temporal lobe epilepsy in adult rats. Epilepsia. 2002;43:325–335. doi: 10.1046/j.1528-1157.2002.11301.x. [DOI] [PubMed] [Google Scholar]
- Schlichtiger J, Pekcec A, Bartmann H, Winter P, Fuest C, Soerensen J, Potschka H. Celecoxib treatment restores pharmacosensitivity in a rat model of pharmacoresistant epilepsy. Br J Pharmacol. 2010;160:1062–1071. doi: 10.1111/j.1476-5381.2010.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segi-Nishida E. Exploration of new molecular mechanisms for antidepressant actions of electroconvulsive seizure. Biol Pharm Bull. 2011;34:939–944. doi: 10.1248/bpb.34.939. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011;31:14850–14860. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh M, Naghdi N, Khosrovani S, Ostad SN, Sharifzadeh K, Roghani A. Post-training intrahippocampal infusion of the COX-2 inhibitor celecoxib impaired spatial memory retention in rats. Eur J Pharmacol. 2005;511:159–166. doi: 10.1016/j.ejphar.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O'Mara SM. Deficits in spatial learning and synaptic plasticity induced by the rapid and competitive broad-spectrum cyclooxygenase inhibitor ibuprofen are reversed by increasing endogenous brain-derived neurotrophic factor. Eur J Neurosci. 2003;17:2438–2446. doi: 10.1046/j.1460-9568.2003.02643.x. [DOI] [PubMed] [Google Scholar]
- Silakova JM, Hewett JA, Hewett SJ. Naproxen reduces excitotoxic neurodegeneration in vivo with an extended therapeutic window. J Pharmacol Exp Ther. 2004;309:1060–1066. doi: 10.1124/jpet.103.063867. [DOI] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Tacconelli S, Capone ML, Sciulli MG, Ricciotti E, Patrignani P. The biochemical selectivity of novel COX-2 inhibitors in whole blood assays of COX-isozyme activity. Curr Med Res Opin. 2002;18:503–511. doi: 10.1185/030079902125001335. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, Yamagata K. Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res. 2006;56:103–110. doi: 10.1016/j.neures.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, Maru E. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat. 2003;71:205–216. doi: 10.1016/s1098-8823(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, Perkins WE, Rogers RS, Shaffer AF, Zhang YY, Zweifel BS, Seibert K. 4-[5-Methyl-3-phenylisoxazol-4-yl]- benzenesulfonamide, valdecoxib: a potent and selective inhibitor of COX-2. J Med Chem. 2000;43:775–777. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002;9:41–47. doi: 10.1101/lm.43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B, Bazan NG. Hippocampal kindling epileptogenesis upregulates neuronal cyclooxygenase-2 expression in neocortex. Exp Neurol. 2003;179:167–175. doi: 10.1016/s0014-4886(02)00019-5. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, van Schaik R, Edelbroek PM, Redeker S, Aronica E, Wadman WJ, Marchi N, Vezzani A, Gorter JA. Inhibition of the multidrug transporter P-glycoprotein improves seizure control in phenytoin-treated chronic epileptic rats. Epilepsia. 2006;47:672–680. doi: 10.1111/j.1528-1167.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, Aronica E, Gorter JA, Potschka H. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010;58:404–412. doi: 10.1016/j.neuropharm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Wen T, Liu YC, Yang HW, Liu HY, Liu XD, Wang GJ, Xie L. Effect of 21-day exposure of phenobarbital, carbamazepine and phenytoin on P-glycoprotein expression and activity in the rat brain. J Neurol Sci. 2008;270:99–106. doi: 10.1016/j.jns.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des. 2008;14:1443–1451. doi: 10.2174/138161208784480144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibell G, Unkruer B, Pekcec A, Hartz AM, Bauer B, Miller DS, Potschka H. Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology. 2009;56:849–855. doi: 10.1016/j.neuropharm.2009.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nonsteroidal anti-inflammatory drugs: Structures 1-5 are nonselective, but COX-1 preferring inhibitors. Structures 6-10 are selective COX-2 inhibitors. Drugs 7-8 have been withdrawn from the Unites States market, due to fatal cardiovascular side effects.